Abstract
Purpose
Acute-on-chronic liver failure (ACLF) is a clinical syndrome with high short-term mortality, unclear mechanism and controversial diagnosis criteria. The Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) study has been conducted in China to fill the gaps. In the first phase (the CATCH-LIFE investigation cohort), 2600 patients were continuously recruited from 14 national nationwide liver centres from 12 different provinces of China in 2015–2016, and a series of important results were obtained. To validate the preliminary results, we designed and conducted this multicentre prospective observational cohort (the CATCH-LIFE validation cohort).
Participants
Patients diagnosed with chronic liver disease and hospitalised for acute decompensation (AD) or acute liver injure were enrolled, received standard medical therapy. We collected the participants’ demographics, medical history, laboratory data, and blood and urine samples during their hospitalisation.
Findings to date
From September 2018 to March 2019, 1370 patients (73.4% men) aged from 15 to 79 years old were enrolled from 13 nationwide liver centres across China. Of these patients, 952 (69.5%) had chronic hepatitis B, 973 (71.1%) had cirrhosis and 1083 (79.1%) complicated with AD at admission. The numbers and proportions of enrolled patients from each participating centre and the patients’ baseline characteristics are presented.
Future plans
A total of 12 months is required for each participant to complete follow-up. Outcome information (survival, death or receiving liver transplantation) collection and data cleansing will be done before June 2020. The data in the CATCH-LIFE validation cohort will be used for comparison between the new ACLF diagnostic criteria derivated from the CATCH-LIFE investigation cohort with existing ones. Moreover, future proteomic and metabolic omics analyses will provide valuable insights into the mechanics of ACLF, which will promote the development of specific therapy that leads to decrease patients’ mortality.
Registration
Keywords: hepatology, epidemiology, hepatobiliary disease
Strengths and limitations of this study.
The Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) validation cohort makes the CATCH-LIFE study the unique acute-on-chronic liver failure (ACLF) related study with two independent multicentre prospective cohorts, which provides ample statistical power to clarify certain controversial portions of the ACLF’s definitions and diagnostic criteria.
The participants in the study have typical characteristics of ACLF in hepatitis B virus high-endemic areas.
The availability of proteomics and metabolomics may illuminate the unclear mechanism of ACLF and provides opportunities to discover novel markers for diagnosis and outcome prediction.
The 28-day hospitalisation of participants will clarify the natural course of ACLF.
The participating centres of this study are highly coincident with the centres that participated in the CATCH-LIFE investigation study, which could generally limit the effectiveness of the validation.
Introduction
Patients with chronic liver disease and acute deterioration requiring hospitalisation include some potential victims of a dangerous clinical syndrome—acute-on-chronic liver failure (ACLF). ACLF is characterised by chronic liver disease and rapid progression of liver injury, culminating in multiple organ failures and high short-term mortality (over 50% in 90 days).1–3 However, as a possible short-term fatal syndrome, up to 13 definitions4 and several different diagnostic criteria of ACLF1 5–7 exist, causing clinician confusion rather than guidance. Only the diagnostic criteria derived from solid evidence and representative data should be applied in clinical practice.
The first evidence-based ACLF diagnostic criterion was proposed in 2013. The European Association for the Study of the Liver-Chronic Liver Failure Consortium (EASL-CLIF), through the CLIF Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) study in Europe, modified the Sequential Organ Failure Assessment8 score, showed that the failure of 6 organs/system (liver, coagulation, renal, bran, circulation and respiratory) is closely related to the short-term mortality of ACLF patients, and designed the EASL-CLIF Consortium Organ Failure score (OFs) system.1 9 10 Nevertheless, the CANONIC study only covered aetiologies of Western-type ACLF. Alcoholism and hepatitis C virus (HCV) are the main aetiologies of Western-type ACLF,10 while hepatitis B virus (HBV) accounts for most Eastern-type ACLF.11 12 There are also significant differences between Eastern-type and Western-type ACLF in precipitating events, pathogenesis and clinical characteristics, OF type distribution and so on.13 14 Therefore, in East, Southeast and Central Asia where HBV is highly endemic,15 it is unwise to directly introduce diagnostic criteria based on data collected from HBV low-endemic regions.15
The Chinese HBsAg-positive population is estimated to be 86 million, accounting for 30% of HBsAg carriers worldwide and 60% of HBV high-endemic areas,15 which makes China the optimum source of representative data for Eastern-type ACLF. In the beginning of 2015, the Chinese Acute on Chronic Liver Failure (Ch-CLIF) Consortium launched the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) investigation study (NCT02457637). From January 2015 to December 2016, 2600 potential ACLF patients were continuously recruited into the investigation cohort from 14 nationwide liver centres across China. The detailed design and description of the study was published elsewhere.16 Then we described the mathematical meaning of ‘organ failure’, established ‘CATCH-LIFE OFs’ for Eastern-type ACLF diagnosis, developed a prognostic prediction model for patients’ stratification, and obtained other preliminary results on ACLF’s mechanism via multi-omics analysis. All these results shall be milestones in the field, if being validated. Validation from an external cohort is the most convincing type of evidence. However, there is no qualified cohort available currently.
Then, we designed and conducted this CATCH-LIFE validation cohort study. The overall study aim is to validate the preliminary results of the CATCH-LIFE investigation cohort study, including possible results obtained in the future.
Details are as follows:
Initially, the two cohorts of the CATCH-LIFE study will be used to describe patients’ epidemiological characteristics, discover risk factors of the mortality and evidence-based cut-off values of organ failure.
Subsequently, in clinical application:
To compare CATCH-LIFE OFs with existing ACLF diagnostic criteria and find the most appropriate criteria for Eastern-type ACLF.
To estimate the cut-off values for organ failure of ACLF in HBV high-endemic areas.
To validate the prognostic prediction model established for assessing patient outcomes.
The objective of this section is to ensure the authenticity, reliability and integrity of the clinical data collected.
In experimental research:
To explore the mechanism of ACLF via multi-omics.
To validate the proteomic and metabolic kits for early diagnosis and outcome prediction.
The objective of this section is to ensure the quality of bio-specimens during collection, storage and transport.
Cohort description
Overview
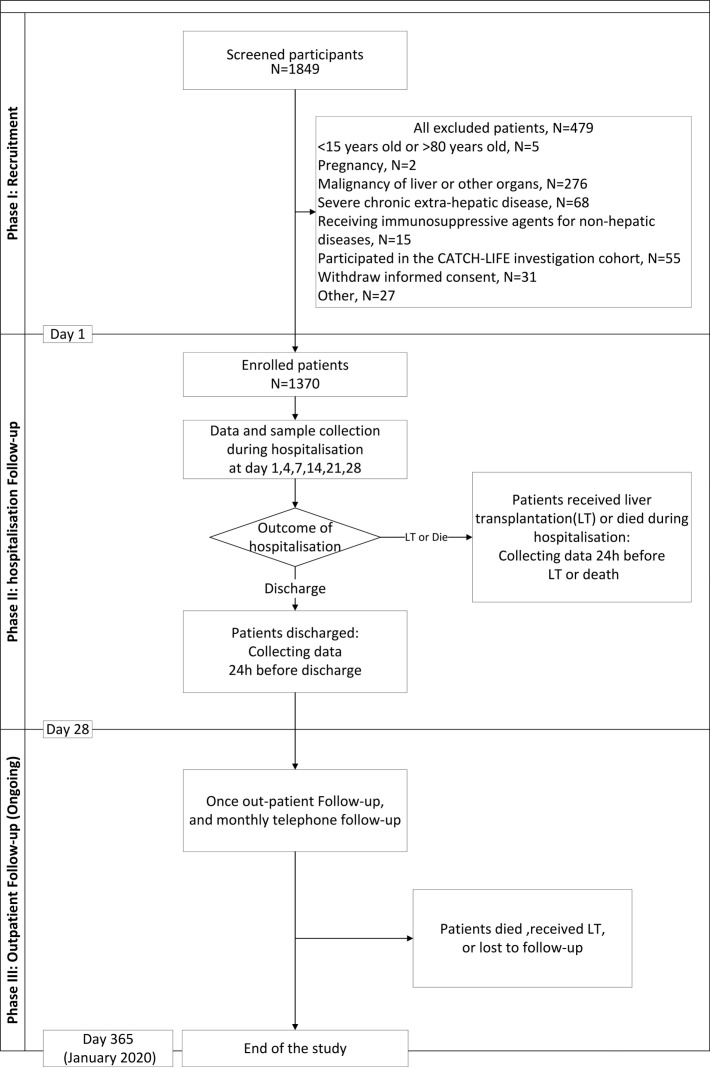
The CATCH-LIFE validation study is a multicentre prospective observational cohort study conducted in 13 nationwide liver centres from different provinces of China. All participating centres met the qualifications (online supplemental appendix 1). Patients diagnosed with chronic liver disease and hospitalised for acute deterioration were enrolled. Data were collected according to the case-report forms (online supplemental appendix 2). The study had three processes: recruitment, hospitalisation follow-up and post-discharge follow-up (figure 1). All-cause death, survival and undergoing liver transplantation (LT) were considered the endpoints. Recruitment began in September 2018 and ended in January 2019. The follow-up is ongoing and will last for 12 months.
Figure 1.
Flowchart of the study procedures. CATCH-LIFE, Chinese Acute-on-Chronic Liver Failure; LT, liver transplantation.
bmjopen-2020-037793supp001.pdf (495.6KB, pdf)
Selection of centres
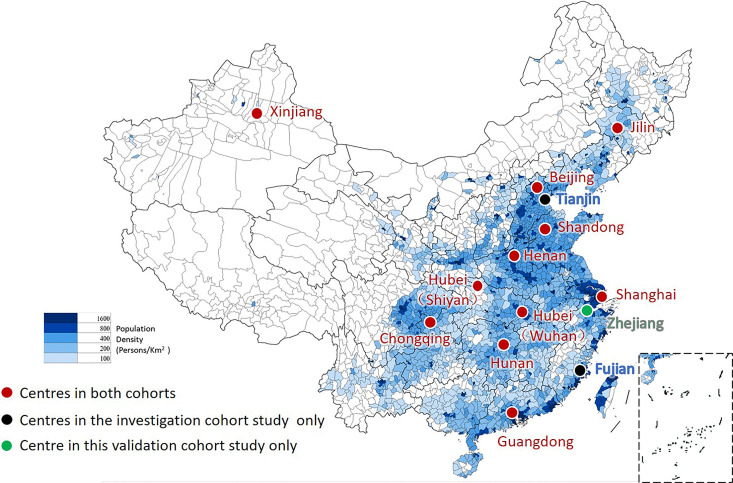
Thirteen centres from 11 different provinces (Shanghai, Beijing, Chongqing, Hunan, Hubei, Guangdong, Zhejiang, Shandong, Jilin, Henan and Xinjiang) participated the CATCH-LIFE validation cohort. Their locations, together with the population density of China, are shown in figure 2. Twelve of the 13 centres also participated in the CATCH-LIFE investigation cohort (shown as red dots in figure 2). The First Affiliated Hospital of Zhejiang University in Zhejiang province (shown as the green dot in figure 2) is accepted as a new centre. Two centres (in Tianjin and Fujian provinces) participated in the investigation cohort but are not active in this study (shown as blue dots in figure 2). Despite subtle changes, the distribution of the centres remains close to the population distribution of China; 12/13 centres are in Southeastern China, representing 94% of the Chinese population, and 1/13 centres is in Northwestern China, representing 6% of the population.
Figure 2.
The distribution of centres and the population density of China. Thirteen centres from 11 different provinces participated the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) validation cohort. Red dots indicate the 12 of the 13 participating centres that also participated in the CATCH-LIFE investigation cohort. The green dot is the new participating centre in Zhejiang province. Blue dots are two centres in Tianjin and Fujian provinces participated in the investigation cohort but are not active in this study. The distribution of the centres accords with the population distribution of China.
Study population and recruitment
The study included patients with chronic liver disease (various aetiologies, including cirrhosis or non-cirrhosis conditions) and an exacerbation requiring hospitalisation, referred to as ‘acute-on-chronic liver disease’. In another word, ACLF patients with high short-term mortality and other unstable chronic liver disease patients with low risk of death are both enrolled. The following are detailed inclusion and exclusion criteria.
Inclusion criteria
Patients who met all the following criteria were included.
Chronic liver disease with or without cirrhosis, including chronic viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, autoimmune liver disease, metabolic liver disease and chronic drug-induced liver disease. The duration of underlying non-cirrhotic chronic liver disease should be longer than 6 months.
Acute liver injury (serum alanine aminotransferase or aspartate transaminase over three times the upper limit of the normal level or total bilirubin (TB) over 2 mg/dL within 1 week before recruitment) or acute decompensation (AD) (hepatic encephalopathy, ascites, gastrointestinal bleeding, bacterial infection within 1 month before recruitment).
Inpatients: patients hospitalised or under emergency observation >24 hours.
Exclusion criteria
Patients who met any of the following criteria were excluded.
(i) <15 years old or >80 years old; (ii) pregnancy; (iii) malignancy of liver or other organs (including leukaemia); (iv) chronic obstructive pulmonary disease level IV; (v) New York Heart Association (NYHA) Functional Class ≥3; (vi) myocardial infarction within 3 months before admission; (vii) diabetes with severe complications; (viii) chronic kidney disease with end-stage renal failure; (ix) receiving immunosuppressive agents for non-hepatic diseases; (x) patients who participated in the CATCH-LIFE investigation cohort study.
Every patient received standard medical therapy and was informed that the choice to participate in the study would not affect their therapeutic regimen. All consenting patients included in the study provided written informed consent. At any stage, if a patient revokes consent, he/she would be withdrawn from the study and not recruited into the study again.
Follow-up and data collection
A total of 12 months is required for each participant to complete hospitalisation follow-up and regular post-discharge follow-up. All-cause death and 12-month survival were considered the endpoints; receiving LT was considered a competitive event versus death. Loss to follow-up was considered a censoring event.
Tables 1 and 2 show the details and schedule of data collection during the follow-up. Modularity is the main feature of our data collection schedule. All data elements were divided into 10 modules, and different combinations of modules were collected on days 1, 4, 7, 14, 21 and 28 (or the day before discharge or LT/death for patients hospitalised less than 28 days), making it easier for researchers in data collection and management.
Table 1.
Broad categories and data elements collected in the Chinese Acute-on-Chronic Liver Failure validation cohort study
| Broad categories | Data elements |
| Demographic data | Age, sex, ethnicity, identity number, postal code, address, mobile number, education status and insurance status |
| Medical history | Aetiology and duration of chronic liver disease, type of present and/or previous acute decompensation or acute liver injury, possible predisposition (HBV reactivation, infection, recent alcohol intake, etc) and history of other chronic disease (hypertension, diabetes, etc) |
| Basic and vital signs | Height, weight, body mass index, temperature, heart rate, blood pressure and oxygen saturation (read from pulse oximeters) |
| Laboratory tests | Routine blood test (HGB, WBC, PLT count and neutrophil/lymphocyte ratio), liver function (ALT, AST, TB, AKP, γ-GT, albumin, prealbumin), renal function test (creatinine, BUN), blood-gas analysis and electrolytes (pH, sodium, potassium), coagulation series (prothrombin time, INR, D-dimer), others (blood ammonia, C reactive protein, procalcitonin, AFP, CA199, fasting blood glucose) |
| Hepatitis virus tests | HBV (HBV-DNA, HBsAg, HBsAb, HBeAg, HBeAb, HBcAb), HCV, HAV and HEV antibodies (IgM) |
| Optional laboratory tests (if necessary) | Thromboelastogram, cytokine, serum amyloid A, serum ferritin; ascites test (if patients take paracentesis): RBC count, WBC, count and proportion of polynuclear cell; autoimmune liver disease test; evaluation of Bacterial infection (sputum, blood, midstream urine, ascites, bile culture) |
| Imaging examination | Abdominal B ultrasound, abdominal CT/MRI scan, fibro-scan |
| Organ failure assessment | Liver, coagulation, respiratory, renal, brain, circulation failure |
| Hospitalisation summary | Medication (starting and ending times and dosage of antibiotics, glucocorticoids and proton pump inhibitor), hospitalisation duration and expenses |
| Status/outcome | Survival, liver transplantation (LT), death, lost to follow-up, re-hospitalised, malignancy detected, including the time of outcome, pathology results of the removed liver (for LT) or cause of death |
AFP, alpha-fetoprotein; AKP, alkaline phosphatase; BUN, blood urea nitrogen; CA199, carbohydrate antigen; γ-GT, gamma-glutamyl transferase; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; HGB, haemoglobin; INR, international normalised ratio; PLT, platelet; RBC, red blood cell; WBC, white cell count.
Table 2.
Data collection schedule of the Chinese Acute-on-Chronic Liver Failure validation cohort study
| Time after recruitment | Hospitalisation follow-up | Post-discharge follow-up (ongoing) | |||||||
| Broad categories | Day 1 | Day 4 | Day 7 | Day 14 | Day 21 | Day 28 | Prior to death/LT*/discharge | Outpatient follow-up | Monthly telephone follow-up |
| Demographic data | √ | ||||||||
| Medical history | √ | √ | v | ||||||
| Basic and vital signs | √ | √ | √ | √ | √ | √ | √ | t | |
| Laboratory tests | √ | √ | √ | √ | √ | √ | √ | √ | |
| Hepatitis virus tests | √ | ||||||||
| Optional laboratory tests | If necessary | ||||||||
| Imaging examination | √ | ||||||||
| Organ failure assessment | √ | √ | √ | √ | √ | √ | √ | ||
| Hospitalisation summary | √ | ||||||||
| Status/outcome | √ | √ | √ | ||||||
LT, liver transplantation.
The duration of hospitalisation follow-up depended on the patient’s condition and generally did not exceed 28 days. During hospitalisation, patients’ demographic data, contact details, history of disease, clinical/laboratory data, organ failure assessment (online supplemental appendix 2) and extra bio-specimens (whole blood, plasma and urine) were collected on day 1. Some data elements were retaken at days 4, 7, 14, 21 and 28 (or the day before discharge if the patient was hospitalised for less than 28 days). For patients who died or underwent LT, available data 24 hours prior to death/LT were collected. At the end of hospitalisation, patient status (discharge, death or LT) was recorded. The time and the main cause of death or the time of LT and the pathology results of the removed liver were recorded as well. Other important information, particularly hospitalisation duration and expenses, and specific medications were also noted. Whether patients had cirrhosis was diagnosed by imaging examination after enrolment according to signs of dysmorphia and relation to portal hypertension.17
The patients’ post-discharge follow-up was performed via outpatient visits and telephone calls. The time of outpatient visits was not fixed but was recommended to be 4 weeks after discharge. Telephone follow-up was performed monthly for health guidance and patient status check (survival, death or LT). If a patient was alive, the research staff would ask whether any complications (ascites growth, bacterial infection, gastrointestinal bleeding, hepatic encephalopathy and jaundice) occurred or if any malignancy was determined. If a patient died, then the time and the main cause of death was noted. If a patient underwent LT, the location and date of the procedure was recorded.
Ascertainment of AD
According to the concepts of liver-specific complications2 and decompensation events18 in cirrhosis, the CATCH-LIFE study define the following five complications ‘overt ascites’, ‘hepatic encephalopathy (HE)’, ‘gastrointestinal bleeding (variceal bleeding)’, ‘jaundice’ and ‘bacterial infection’ within 1 month before recruitment as AD in the CATCH-LIFE study. Ascites manifested by moderate symmetrical distension of abdomen or with marked abdominal distension19 was the criterion of overt ascites. Moreover, the most depth of ascites ≥50 mm reported by ultrasound was also was defined as overt ascites. Gastrointestinal bleeding was defined by the development of an upper and/or lower gastrointestinal variceal bleeding due to cirrhosis and portal hypertension. The criterion and severity classification of HE was referred West-Haven HE grade.20 The criterion for jaundice was TB >5 mg/dL. Spontaneous bacterial peritonitis, pneumonia, sepsis, urinary tract infection, and cellulitis and any other type of acute bacterial infection were included in bacterial infection, which was defined by laboratory tests and imaging evidence.
Quality control
Electronic data capture system
All elements of the patients’ clinical data were collected through the CRF and integrated into an electronic data capture (EDC) system. The functions of the system include more than electronification. In addition to data storage, security, backup and export, the system has a built-in logical verification system. The logical verification includes unfilled prompts, abnormal value prompts, contradictory prompts and hiding unnecessary parts automatically (such as automatically hiding ‘microbial culture results’ for non-infected patients). Moreover, any traces of the modification of the data is retained. The EDC system maintains the reliability, completeness and accuracy of the data and is helpful in audit trials, management of data-related questions and source data validation.
Personnel training
Complete and timely training of personnel was conducted before the EDC system was implemented. The data manager (DM), principal investigator (PI) and data entry personnel were granted corresponding system rights.
Internal verification
(i) EDC logical verification and data entry personnel self-examination was performed; (ii) the PI and DM performed inspections; (iii) a telephone check-in was conducted weekly; (iv) the PI meeting was conducted every 4 months; (v) on-site verification was conducted when recruitment was completed (March 2019), consisting of eligibility check, extreme value verification, critical case review (such as cases diagnosed with ACLF) and core data elements review.
Raw data traceability archiving
The photographs or screen captures of medical records were taken and preserved as raw data, including medical history, progress notes, vital signs, physical examination, laboratory test results, imaging/pathology data, medication and medical orders. Participants were not identified by name, and confidentiality of the information derived from the medical records was preserved. All related raw data pictures from every centre were stored on their own hard disk, and a classified copy was sent to the responsible centre every quarter. All data had three backups. Pictures of the raw data were only used for backup and backtracking, and all centres (including the coordinating centre) did not have access to the picture data from other centres.
Quality assessment (external verification)
A third-party company was responsible for data management, audit and inventory.
The database was sent to the data centre of the EASL-CLIF Consortium for quality verification.
Storage and transport of bio-specimens
All biospecimens containing blood, plasma and urine samples were stored at −80°C. At the end of March 2019, all bio-specimens were transported via cold chain (−80°C) to the biological sample bank in Shanghai Renji Hospital (plasma and urine) and Chongqing Southwest Hospital (peripheral blood mononuclear cell (PMBC) DNA isolated from blood samples).
Patient and public involvement
Participants of the CATCH-LIFE validation cohort or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Findings to date
In total, 1370 patients from 13 centres were enrolled in the CATCH-LIFE validation cohort study, and the number of enrolled patients from each centre in each month are presented in online supplemental appendix 3. The top five centres with the largest numbers of enrolled patients were Beijing Ditan Hospital (n=199), Chongqing Southwest Hospital (n=178), Hunan Xiangya Hospital (n=167), Shanghai Ren Ji Hospital (n=162) and Guangzhou Nanfang Hospital (n=125). The average monthly enrolment number was 274.
We collected the patients’ plasma, PBMC DNA and urine on day 1 of admission and stored them at −80°C. Of the 1370 patients enrolled, plasma samples were obtained at least once from 1114 patients, and two or more samples were obtained from 463 patients; PMBC DNA was obtained from 977 patients. At the end of March 2019, all PBMC DNA samples were transported to Chongqing Southwest Hospital for a genome-wide association study test; other samples (plasma and urine) were sent to Renji Hospital for proteomic and metabolic tests.
Table 3 shows the patients’ demographic data and the condition estimation on the first day of admission. Overall, 73.7% of the patients were men, and the mean age of the patients was 49.5 years, including 71.1% (n=973) of cirrhotic patients and 413 (28.9%) of non-cirrhotic patients; 69.5% (n=952) patients had chronic HBV-related liver diseases. The proportion of patients with AD is 79.1% (n=1083). Jaundice (44.6%) was the most common observed AD event, followed by overt ascites (40.7%), gastrointestinal bleeding (16.4%), infection (15.9 %) and HE (7.7%).
Table 3.
The baseline characteristics on the first day of admission
| Baseline characteristics | |
| Demographic data | |
| Male sex, n (%) | 1006 (73.4%) |
| Age (years) median (IQRs) | 49.0 (40.0–59.0) |
| HBV-related, n (%) | 952 (69.5%) |
| Cirrhosis, n (%) | 973 (71.1%) |
| Laboratory data, median (IQRs) | |
| Total bilirubin (mg/dL) | 3.9 (1.5–13.7) |
| INR | 1.41 (1.17–1.79) |
| Serum creatinine (mg/dL) | 0.78 (0.65–0.96) |
| ALT (U/L) | 82 (29–383) |
| AST (U/L) | 101 (46–265) |
| γ-GT (U/L) | 82 (38–158) |
| AKP (U/L) | 125 (92–63) |
| Albumin (g/L) | 32.3 (28.1–37.0) |
| CRP (mg/L) | 7.3 (3.1–14.6) |
| WBC (×109/L) | 4.95 (3.69–7.06) |
| Hb (g/L) | 118 (94–136) |
| Platelet count (×109/L) | 96.0 (61.0–150.0) |
| Serum sodium (mmol/L) | 138 (136–141) |
| Patients with AD | 1083 (79.1%) |
| Type of AD | |
| Overt ascites | 558 (40.7%) |
| Gastrointestinal bleeding | 224 (16.4%) |
| HE | 105 (7.7%) |
| Jaundice | 611 (44.6%) |
| Infection | 218 (15.9%) |
| Score | |
| MELD score | 15 (10–22) |
| Child-Pugh score | 8 (7–10) |
| Child-Pugh grade | |
| Child-Pugh A, n (%) | 261 (19.1%) |
| Child-Pugh B, n (%) | 533 (38.9%) |
| Child-Pugh C, n (%) | 576 (42.0%) |
AD, acute decompensation; AKP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C reactive protein; γ-GT, γ-glutamyl transferase; Hb, haemoglobin; HBV, hepatitis B virus; HE, hepatic encephalopathy; INR, international standardisation ratio; MELD, the Model for End-stage Liver Disease; WBC, white cell count.
Strengths and limitations
The CATCH-LIFE validation cohort has several strengths. First, compared with the CANONIC study (n=1343)1 and Chinese Group on the Study of Severe Hepatitis B (n=1322)7 from China, the study scale is a larger multicentre, prospective cohort of ACLF patients in the world. This cohort made the whole CATCH-LIFE study a unique ACLF-related study with two large independent multicentre prospective cohorts and 3970 patients. It provides plenty of data and solid evidence in related fields. Second, as the largest HBV high-endemic country, China is the optimum location for Eastern-type ACLF research. The centre distribution of this study was kept consistent with the population density distribution in China, so its data have epidemiological characteristics of patients with Eastern-type ACLF. Third, intensive quality control and quality assessment strategies were applied to ensure the authenticity, reliability and integrity of the clinical data collected. Standardised procedures were conducted in the bio-specimen’s collection, storage, transport, processing and analysis to ensure the validity. Finally, we are engaged with using emerging new technologies and exploring the mechanics of ACLF, including genomics, proteomics and metabolomics. Such applications will provide insight of this fatal disease.
There are two limitations in this study. First, the centres of this study are highly coincident with the centres that participated in the CATCH-LIFE investigation study, which could generally limit the effectiveness of the external validation. Nevertheless, for these two studies, the 3-year interval in recruitment, the high internal heterogeneity in composition and no intersections in participants limited the significance of this limitation. Given that the advantages of the centres’ geographical distribution and the efficiency gains from the job familiar research staff, the centre selection strategy has merits as well. Second, as a cohort in the HBV high-endemic area, the study included a few hundred non-HBV-related patients (only 30% in total), and if further stratified by specific aetiologies, their data would be insufficient and cause potential bias. However, the aetiology of these patients (mainly alcoholic liver disease) matched Western-type ACLF; thus, they can be considered as a subgroup to compare and summarise the similarities and differences between the Western and Eastern types of ACLF, with efforts to arrive at a shared definition.
In summary, we successfully established a qualified external validation cohort for the CATCH-LIFE study in HBV high-endemic area and presented the clinical features of Eastern type ACLF through the large-scale prospective cohorts. The CATCH-LIFE study will make a considerable contribution to the exploration of ACLF mechanisms and the establishment evidence-based diagnostic criteria.
Supplementary Material
Acknowledgments
We thank the following Chinese (Acute on) Chronic Liver Failure Consortium (Ch-CLIF.C) members and participants for the contributions to this study: Department of Gastroenterology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University—Shan Yin, Bo Zeng, Liuying Chen, Shijin Wang; Centre of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University—Qun Zhang, Yixin Hou, Yuxin Li, Yunyi Huang; Department of Infectious Diseases, Southwest Hospital, Third Military Medical University (Army Medical University)—Shuning Sun, Wenting Tan, Xiaomei Xiang, Yunjie Dan; Department of Infectious Disease, Hunan Key Laboratory of Viral Hepatitis, Xiangya Hospital, Central South University—Jun Chen, Chengjin Liao, Xiaoxiao Liu; Department of Infectious Diseases, Institute of Infection and Immunology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology—Jing Liu, Ling Xu, Shue Xiong, Yan Xiong, Congcong Zou; Hepatology Unit, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University—Beiling Li, Guotao Zhong, Xiuhua, Jiang Congyan Zhu; Department of Hepatology, First Hospital of Jilin University—Chang Jiang, Xiaoyu Wen, Na Gao, Chunyan Liu; Department of Infectious Disease, Taihe Hospital, Hubei University of Medicine—Qing Lei, Sen Luo; Department of Infectious Disease, The First Hospital of Zhejiang University—Haotang Ren; Department of Liver Intensive Care Unit, Shanghai Public Health Clinical Centre, Fudan University —Xue, Mei Jiefei Wang, Liujuan Ji; Department of Infectious Diseases and Hepatology, Second Hospital of Shandong University—Tao Li, Xuanqiong Fang Li Jing, Wang Ziyu; Liver Disease Centre, First Affiliated Hospital of Xinjiang Medical University—Rongjiong Zheng Fangrong Jie, Nan Li; Department of Infectious Disease, Henan Provincial People’s Hospital—Huiming Jin; and Chinese Evidence-based Medicine Centre and CREAT Group, West China Hospital, Sichuan University—Jing Tan, Yan Ren.
Footnotes
XS and HL contributed equally.
Contributors: LQ, XW, GD, YH, JC, ZM contributed equally and share first authorship. HL, XW, GD, YH, JC, XZ, ZM, YS, ZQ, FL, YG and XL obtained funding. XS and HL designed the study. XW, GD, YH, JC, XZ, ZM, YS, ZQ, FL, YG, XL, JL, WG, YZ, TW, DW and FD collected the data. LQ drafted the manuscript. HL contributed to the critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors have read and approved the final manuscript. XW, GD, YH, JC, XZ, ZM, YS, ZQ, FL, YG and XL are the study guarantors.
Funding: This work was supported by grants from the National Key Research and Development Program of China (No. 2017YFC0908100, 2017YFC0908103). This study was partly supported by the National Science and Technology Major Project (2018ZX10302206, 2018ZX10723203, 2017ZX10202202), Shanghai Municipal Education Commission–Guofeng Clinical Medicine and Shanghai Municipal Government Funding (grant 16CR1024B), the National Natural Science Foundation of China (81270533, 81271884, 81930061, 81401665, 81461130019, 81470038, 81470869, 81473641, 81571978, 81660333, 81670576, 81700561, 81870425, GZ1263), the Foundation for Innovative Research groups of Natural Science Foundation of Hubei Province of China (2018CFA031) and Shandong Province Natural Science Foundation (No. ZR2019PH052).
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Renji Hospital Ethics Committee at Shanghai Jiao Tong University School. This study did not involve any biological material sourced from executed prisoners. All the transplanted livers that the participants of the CATCH-LIFE validation cohort study have received were ethically sourced. The transplanted livers were voluntarily donated from civilians and allocated by the China Organ Transplant Response System (COTRS), or sourced from living-related party liver transplantation (LRLT) approved by the ethics committee, and a written informed consent was obtained from every doner or his/her legal surrogates. A documentation, indicating that the executed prisoners are not sources of organ transplants, had been sent to the BMJ-Open. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Provenance and peer review: Not commissioned; externally peer reviewed
Data availability statement: Data are available upon reasonable request. Data are available upon reasonable request via email: aclf_group@163.com.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37. 10.1053/j.gastro.2013.02.042 [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet 2015;386:1576–87. 10.1016/S0140-6736(15)00309-8 [DOI] [PubMed] [Google Scholar]
- 3.Jalan R, Yurdaydin C, Bajaj JS, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 2014;147:4–10. 10.1053/j.gastro.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Wlodzimirow KA, Eslami S, Abu-Hanna A, et al. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int 2013;33:40–52. 10.1111/j.1478-3231.2012.02790.x [DOI] [PubMed] [Google Scholar]
- 5.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL). Hepatol Int 2009;3:269–82. 10.1007/s12072-008-9106-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL) 2014. Hepatol Int 2014;8:453–71. 10.1007/s12072-014-9580-2 [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018;67:2181–91. 10.1136/gutjnl-2017-314641 [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the Working group on sepsis-related problems of the European Society of intensive care medicine. Intensive Care Med 1996;22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS, O’Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250–6. 10.1002/hep.27077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo V, Moreau R, Jalan R, et al. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol 2015;62:S131–43. 10.1016/j.jhep.2014.11.045 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Xia Q, Zeng B, et al. Submassive hepatic necrosis distinguishes HBV-associated acute on chronic liver failure from cirrhotic patients with acute decompensation. J Hepatol 2015;63:50–9. 10.1016/j.jhep.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Yang Y, Hu Y, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 2015;62:232–42. 10.1002/hep.27795 [DOI] [PubMed] [Google Scholar]
- 13.Hernaez R, Solà E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut 2017;66:541–53. 10.1136/gutjnl-2016-312670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj JS, Moreau R, Kamath PS, et al. Acute-on-Chronic liver failure: getting ready for prime time? Hepatology 2018;68:1621–32. 10.1002/hep.30056 [DOI] [PubMed] [Google Scholar]
- 15.Dusheiko G, Agarwal K. Delineating the global challenges of hepatitis B virus infection. Lancet Gastroenterol Hepatol 2018;3:372–3. 10.1016/S2468-1253(18)30093-1 [DOI] [PubMed] [Google Scholar]
- 16.Gu W-Y, Xu B-Y, Zheng X, et al. Acute-on-Chronic liver failure in China: rationale for developing a patient registry and baseline characteristics. Am J Epidemiol 2018;187:1829–39. 10.1093/aje/kwy083 [DOI] [PubMed] [Google Scholar]
- 17.Aubé C, Bazeries P, Lebigot J, et al. Liver fibrosis, cirrhosis, and cirrhosis-related nodules: imaging diagnosis and surveillance. Diagn Interv Imaging 2017;98:455–68. 10.1016/j.diii.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. 10.1016/S0140-6736(14)60121-5 [DOI] [PubMed] [Google Scholar]
- 19.Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International ascites Club. Hepatology 2003;38:258–66. 10.1053/jhep.2003.50315 [DOI] [PubMed] [Google Scholar]
- 20.Blei AT, Córdoba J, Practice Parameters Committee of the American College of Gastroenterology . Hepatic encephalopathy. Am J Gastroenterol 2001;96:1968–76. 10.1111/j.1572-0241.2001.03964.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037793supp001.pdf (495.6KB, pdf)