Abstract
Purpose of Review
Because the incidence of type 2 diabetes and prediabetes in children is rising, routine screening of those at risk is recommended. In 2010, the ADA made the recommendation to include hemoglobin A1c (HbA1c) as a diagnostic test for diabetes, in addition to the oral glucose tolerance test or fasting plasma glucose. Our objective was to assess the pediatric literature with regard to HbA1c test performance and discuss advantages and disadvantages of use of the test for diagnostic purposes.
Recent Findings
HbA1c has a number of advantages, including elimination of the need for fasting, lower variability, assay standardization, and long-term association with future development of diabetes. It also has many drawbacks. It can be affected by a number of non-glycemic factors, including red blood cell turnover, hemoglobinopathies, medications, race, and age. In particular, it performs differently in children compared with adults, generally with lower sensitivity for prediabetes (as low as 0–5% in children vs 23–27% in adults) and lower area under the receiver operating characteristic curve (AUC) (0.53 vs 0.73 for prediabetes), and it has lower efficacy at a higher cost, compared with other tests of glycemia. Finally, HbA1c may perform very differently across diverse populations according to race/ethnicity; in Chinese populations, the proportion of individuals classified with prediabetes based on HbA1c predominates compared with IFG (77% for HbA1c vs 27.7% for IFG), whereas in US populations, it is the opposite (24.8% for HbA1c vs 80.1% for FPG).
Summary
HbA1c is controversial because although it is convenient, it is not a true measure of glycemia. The interpretation of HbA1c results requires a nuanced understanding that many primary care physicians who are ordering the test in greater numbers do not possess. Alternative markers of glycemia may hold promise for the future but are not yet endorsed for use in practice. Further studies are needed to determine appropriate thresholds for screening tests and the long-term impact of screening and identification.
Keywords: Diabetes, Fasting plasma glucose, Oral glucose tolerance test, Hemoglobin A1c
Introduction
Type 2 diabetes (T2D) is increasingly common in children, with the greatest increase in incidence among non-Hispanic black and native American youth [1•]. Early T2D can have a long asymptomatic period, leading to under-recognition [2]. Complications, including elevated blood pressure, microalbuminuria, and dyslipidemia, occur early in adolescents with T2D [3, 4]. Although longitudinal studies of diabetes prevention in children have been limited, some evidence suggests that aggressive lifestyle interventions improve glucose abnormalities in obese children [5].
Due to the potential benefit from intervention at an early, asymptomatic stage of disease and the alarming report of a 30-fold increase in T2D in youth in the preceding 20 years [6], screening for T2D in youth was first recommended by the American Diabetes Association (ADA) and the American Academy of Pediatrics (AAP) in 2000 [2, 7]. The resulting guideline recommended that overweight (BMI > 85th percentile for age and sex) youth be screened for diabetes in the presence of two of the following risk factors: signs or conditions associated with insulin resistance (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome), family history of T2D in a first- or second-degree relative, and higher-risk race/ethnicity (American Indian, African-American, Hispanic, Asian/Pacific Islander). Screening was recommended to begin at the earlier of age 10 years or the onset of puberty and repeated every 2 years. The initial guideline in 2000 advised that a 2-h post-glucose (2-h PG) from an oral glucose-tolerance test (OGTT), fasting plasma glucose (FPG), or an elevated random plasma glucose accompanied by symptoms consistent with diabetes be used to diagnose diabetes, but stated that OGTT was not recommended for routine clinical use.
In an effort to optimize uptake of screening practices due to the convenience of the non-fasting test, in 2010, the ADA guidelines incorporated hemoglobin A1c (HbA1c) as a preferred test for diagnosing diabetes and prediabetes in adults and children [8]. The guidelines were not without controversy, because of a lack of studies regarding its test performance in the pediatric population at that time.
In this review, we will highlight studies that have subsequently been performed in pediatric populations to assess the test performance of hemoglobin A1c, describe arguments for and against use of the test, and discuss promising alternate diabetes screening methodologies that have not yet been endorsed or incorporated into clinical practice.
Diagnostic Definitions of Diabetes
Currently, the American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes (ISPAD) support the use of 2-h PG from an OGTT, FPG, and HbA1c as diagnostic criteria for diabetes. Table 1 shows the threshold values for defining prediabetes and diabetes for these tests.
Table 1.
ADA definitions of dysglycemia (diabetes and prediabetes) [9]
| 2-h plasma glucose on OGTT (mg/dL) |
Fasting plasma glucose (mg/dL) |
Hemoglobin A1c (%) | Random glucose (mg/dL)a |
|
|---|---|---|---|---|
| Prediabetes | 140–199 | 100–125 | 5.7–6.4 | |
| Diabetes | ≥ 200 | ≥ 126 | ≥ 6.5 | ≥ 200 |
Symptoms consistent with diabetes
Rationale for the Diagnostic Tests
Historically, the OGTT and FPG were used as the gold-standard definitions for defining diabetes. The glycemic thresholds were based on epidemiologic studies in the adult literature that demonstrated an increased risk of diabetes-related retinopathy beyond thresholds of 200 mg/dL for the OGTT and 126 mg/dL for the fasting plasma glucose [10]. OGTT is advantageous in that it identifies the greatest proportion of individuals with prediabetes or diabetes [11], but it does have disadvantages, including the requirement that patients have to fast for the test, the length of time required for the test (minimum of 2 h), and lack of familiarity with how to order and interpret OGTTs among pediatricians/family physicians. Advantages of FPG include the fact that it is a one-time blood draw, and most providers are familiar with ordering and interpreting the test, but its main disadvantage is the need for fasting.
In 2010, the ADA modified its guidelines to include HbA1c as a diagnostic test for diabetes, in part to overcome some of the disadvantages of OGTT and FPG. The diagnostic definition represented a shift in its clinical application, as HbA1c had been used in clinical practice since the 1980s predominantly for monitoring ongoing management among individuals already diagnosed with diabetes [12, 13].
Reasons to Support the Adoption of HbA1c
The rationale for the shift was based on a number of considerations. First, HbA1c does not require patients to fast prior to testing, which is convenient for both patients and providers. Second, it has lower variability compared with glucose measures [14]. Third, the existence of the National Glycohemoglobin Standardization Program (NGSP) ensured standardization of the assays to those of the Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS). Fourth, HbA1c was linked to the long-term development of diabetes complications in epidemiologic studies in adult studies [10, 15], although at the time that the guidelines were adopted in 2010, long-term data on children was unavailable.
The utility of HbA1c measurement in childhood as a marker of risk for future development of type 2 diabetes in adulthood was recently demonstrated. Vijayakumar et al. followed a group of American Indian children and adolescents longitudinally over a period of 42 years [16••]. At baseline, they identified children with glucose levels in the prediabetes range based on an HbA1c (5.7–6.4%), an FPG (100–125 mg/dL), or a 2-h PG (140–199 mg/dL), and tracked them for future development of type 2 diabetes, as defined using ADA criteria (HbA1c ≥ 6.5%, FPG ≥ 126 mg/dL, or 2-h PG ≥ 200 mg/dL). They found that children with prediabetes had a higher incidence of diabetes compared with children who did not have prediabetes, regardless of test type (Table 2). Area under the receiver operating characteristic curve (AUC) was 0.70 for HbA1c, 0.63 for FPG, and 0.73 for 2-h PG, and there were no significant differences in the AUC curves between HbA1c and FPG or 2-h PG.
Table 2.
Test performance of prediabetes thresholds for detecting clinical development of diabetes in a longitudinal study
| Definition | Total, n |
Cases, n |
Percent of cases |
Population | Characteristics | Sensitivity | Specificity | PPV | NPV | AUC ROC | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pediatrics | |||||||||||
| Clinical diagnosis of diabetes at follow-up | 2095 | 227 | 10.8% | US American Indian population | HbA1c ≥ 5.7% (baseline prevalence 3.0%) | Boys 8%, girls 19% | Boys 100%, girls 99% | – | – | 0.67 (boys 0.65, girls 0.72) | Vijayakumar et al. [16••] |
| FPG ≥ 100 (baseline prevalence 9.2%) | Boys 14%, girls 19% | Boys 89%, girls 95% | – | – | 0.59 (boys 0.58, girls 0.67) | ||||||
| 2-h PG ≥ 140 (baseline prevalence 8.1%) | Boys 13%, girls 32% | Boys 96%, girls 93% | – | – | 0.67 (boys 0.68, girls 0.75) | ||||||
| Adults | |||||||||||
| Clinical diagnosis of diabetes at follow-up | 2005 | 536 | 26.7% | US American Indian population | HbA1c ≥ 5.7% (baseline prevalence 8.4%) | Men 17%, women 15% | Men 97%, women 97% | – | – | Males 0.66, females 0.63 | Vijayakumar et al. [16••] |
| FPG ≥ 100 (baseline prevalence 21.1%) | Men 34%, women 32% | Men 82%, women 90% | – | – | Males 0.64, females 0.66 | ||||||
| 2-h PG ≥ 140 (baseline prevalence 17.3%) | Men 24%, women 37% | Men 94%, women 91% | – | – | Males 0.65, females 0.70 |
This was a pivotal study, as it was one of the first epidemiologic studies to provide validation for the utility of the OGTT, FPG, and HbA1c for predicting future diabetes development in a pediatric cohort. However, as the authors noted in their limitation section, the generalizability to populations with lower rates of diabetes was uncertain, given that this was an American Indian population with a very high prevalence of diabetes compared with the general population. Furthermore, they also questioned the generalizability to populations with hemoglobinopathies, revealing one of the problems with using HbA1c as a diagnostic test in the pediatric community.
Can HbA1c Really Be Considered a Diagnostic Test?
When the ADA released the guidelines in 2010, it recommended HbA1c as a diagnostic test, not just a screening test, which is significant because unlike glucose levels, HbA1c is not a direct measure of glycemia. It is an indirect measure represented by the proportion of total hemoglobin with glucose attached to the N-terminal valine of the beta chain, and it reflects blood glucose levels over the last 2–3 months based on red blood cell turnover [17, 18]. As a result, there are a number of non-glycemic factors that can affect HbA1c levels.
Non-Glycemic Factors That Alter HbA1c
The scientific literature documents a number of non-glycemic factors that alter HbA1c, including disorders that affect blood cell turnover, hemoglobinopathies, medications, race, and age.
Red Blood Cell Turnover
Disorders that lead to high red cell turnover (including cystic fibrosis [19], hemolysis, or hemorrhage with subsequent transfusion [20]) can lead to falsely low HbA1c levels. In contrast, decreased red cell turnover leads to prolonged exposure of the erythrocyte to glucose and may result in falsely elevated HbA1c. Conditions with decreased red cell turnover and the potential for falsely elevated HbA1c include iron deficiency anemia [21-23] and spherocytosis [24].
Hemoglobinopathies
Hemoglobin variants, which are single amino acid substitutions in the B chain, can also interfere with HbA1c measurement [25]. The most common variants globally are hemoglobins S, C, D, and E. Unfortunately, the interference of the variants will vary depending on the A1c method. For example, HbAS and HbAC, but not HbAE and HbAD, can lead to false elevations with the Bayer (Metrika) A1CNOW assay. Yet, HbAE and HbAD and not HbAS or HbAC can lead to false elevations with the Bio-Rad Variant II Turbo A1c. This is an important consideration, as in the USA, it has been estimated that over 10% of non-Hispanic black individuals aged 18 or older have either the HbC or the HbS trait, and unfortunately, most providers, and even endocrinologists, are not typically aware of which assay their lab is using or which variants directly impact their particular assay results [26]. Although the NGSP provides guidance to determine whether or not a method shows clinically significant interference due to a hemoglobin variant, the measured value may vary up to 7% at 6 and/or 9% HbA1c from the NGSP reference method of ion-exchange high-performance liquid chromatography [27]. This translates to a potentially clinically significant range around 6% (5.6–6.4%) that may lead to a diagnosis of prediabetes or not.
Medications
Medications can also lower or raise HbA1c through a variety of mechanisms. HbA1c may be falsely lowered by erythrocyte destruction (dapsone, ribavirin, antiretrovirals, trimethoprim-sulfamethoxazole), altered hemoglobin (hydroxyurea) or glycation (vitamins C or E, aspirin), and may be falsely elevated by assay interference, which may occur in the setting of large doses of aspirin or chronic opiate use [28].
Differences by Race: HbA1c 0.3–0.4% Higher in African American Individuals
Studies in adults have documented significant differences in HbA1c by race, independent of glycemia. A number of epidemiologic studies have shown that even among non-diabetic populations, African Americans have significantly higher HbA1c compared with whites [29-31]. In a community-based study of cardiovascular disease, HbA1c level was 0.3 to 0.4% higher in black compared with white persons with no reported diabetes even after adjustment for age and adiposity [32]. Similarly, in the Diabetes Prevention Program, HbA1c levels were 6.2% for blacks vs 5.8% for whites (p < 0.0001) even after adjusting for age, sex, BMI, blood pressure, and factors more directly related to glycation, including fasting glucose, glucose area under the curve, corrected insulin response, and insulin sensitivity [29]. Such differences in HbA1c could reflect inter-individual differences in a catalyst for glycation such as erythrocyte 2,3-disphophoglycerate [33]. The findings may also reflect differences in erythrocyte survival, potentially related to higher rates of under-recognized hemaglobinopathies in African American populations. Although the identified differences in HbA1c by race are relatively small, they could impact the classification of an individual as diabetic or prediabetic, impacting clinical management if a provider relies on HbA1c as the sole diabetes screening test.
Differences by Age: Higher HbA1c With Age
Germane to the pediatric population, there are significant differences in HbA1c by age regardless of glycemic status. Pani et al. looked at differences by age in HbA1c among non-diabetic adults in the Framingham Heart Study and the National Health and Nutrition Examination Survey (NHANES) [34]. Even after adjusting for sex, BMI, FPG, and 2-h PG, there was a significant difference by age; for example, the 97.5th percentile for HbA1c was 6.0% for those aged 40 years compared with 6.6% for those aged 70 years in the Framingham cohort, and the 97.5th percentile for HbA1c was 5.6% (< 40 years) vs 6.2% (70 years) in NHANES. These differences appear to extend to the pediatric age range as well. Several studies of pediatric patients have highlighted the poor sensitivity of HbA1c using standard ADA-defined thresholds to identify prediabetes, as discussed in greater detail below [16••, 35, 36•]. This suggests that there may be age-specific differences in glycation relative to traditional tests of glycemia such as OGTT, with younger individuals having a lower HbA1c for a given 2-h plasma glucose.
Differences in Test Performance Among Children Compared With Adults: HbA1c Has a Sensitivity as Low as 0–5% for Detection of Prediabetes in Children
Since 2010, there have been a number of studies conducted in pediatric populations to evaluate the test performance of HbA1c compared against the traditional gold standard tests. These have been conducted in representative US populations, convenience populations of US overweight and obese children, and European populations of overweight/obese children.
Table 3 shows the cross-sectional test performance characteristics of HbA1c for diagnosing diabetes and prediabetes as defined using OGTT or FPG, for both children and studies from adults of representative samples.
Table 3.
Test performance of HbA1c for detecting diabetes or prediabetes, as defined by OGTT or FPG
| Definition | Total, n | Cases, n | Percent of cases |
Population | Characteristics | Sensitivity | Specificity | PPV | NPV | AUC ROC | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test performance of HbA1c ≥ 6.5% for detecting diabetes in cross sectional studies | |||||||||||
| Pediatrics | |||||||||||
| 2-h OGTT ≥ 200 | 1,156 | 31 | 2.70% | US convenience sample | 100% obese, 35% AA, 29% H | 32% | 99.5% | 62.5% | 98% | 0.89 | Nowicka et al. [35] |
| FPG ≥ 126 | 1,156 | 4 | 0.35% | US representative sample | 49% obese, 51% overweight, 17% AA, 13% H | 75.0% (30.1–95.4) | 99.9% (99.5–100.0) | 75% | 99.9% | 0.88 | Lee et al. [37] |
| Diabetes (FPG ≥ 126 mg/dL and/or 2-h glucose ≥ 200 mg/dL) | 4,848 | 53 | 1.10% | German health system | 16% overweight, 46% obese, 38% extremely obese | 32% | 99.0% | 22% | 99.0% | 0.97 | Ehehalt et al. [36•] |
| Adults | |||||||||||
| 2-h OGTT ≥ 200 | 1,476 | 91 | 6.2% | US representative sample | 31% obese, 34% overweight, 11% AA, 8% H | 30.8% (22.2–40.9) | 99.6% (99.2–99.9) | 85% | 96% | – | Lee et al. [37] |
| FPG ≥ 126 | 6,751 | 240 | 3.6% | US representative sample | 34% obese, 32% overweight, 10% AA, 8% H | 53.8% (47.4–60.0) | 99.5% (99.3–99.6) | 79% | 98% | 0.93 | Lee et al. [37] |
| FPG ≥ 126 | 13,166 (12,485 + 691) | 255 prevalent, 1,308 incident during 7-year follow-up | 11.9% | US representative sample + US longitudinal community-based sample | Obese 25–28% (A1c<6.5%), 55–60% (A1c ≥ 6.5%); AA 21–28% (A1c < 6.5%), 50–61% (A1c ≥ 6.5%) | 47% | 98%% | – | – | 0.89 | Selvin et al. [39] |
| FPG ≥ 126 | 6,559 | 54 | 0.8% | US representative sample | Obesity distribution not defined; 27% AA, 27% H | 42.8% | 99.6% | – | – | 0.9 | Rohlfing et al. [38] |
| Test performance of HbA1c ≥ 5.7% to detect impaired fasting glucose, impaired glucose tolerance, or prediabetes in cross sectional studies | |||||||||||
| Pediatrics | |||||||||||
| Dysglycemia 2-h OGTT ≥ 140 | 1,156 | 347 | 30% | US convenience sample | 100% obese, 35% AA, 29% H | 34% | 83% | 49% | 72% | 0.62* | Nowicka et al. [35] |
| 254 | 20 | 35.90% | US convenience sample | 22% overweight, 78% obese, 30% AA, 0% H | 32% | 74% | 45% | 62% | 0.54 | Lee et al. [46] | |
| 149 | 24 | 16% | US convenience sample | 100% obese, 71% H, ~8% B | 75% | 58% | 25% | 92% | 0.74 | Brar et al. [40] | |
| 267 | 20 | 7.5% | US representative sample | 47% obese, 53% overweight, 18% AA, 15% H | 0.0% (0.0–16.1) | 97.6% (94.8–98.9) | 0% | 92% | 0.53 (0.39–0.67) | Lee et al. [37] | |
| Impaired fasting glucose FPG 100–125 | 933 | 59 | 6.3% | Chinese representative sample | Obesity and race distribution not defined; mean BMI (kg/m2): 22.2 (boys), 20.9 (girls) | 17% | 82% | 6% | 94% | 0.53* | Li et al. [41] |
| 1,156 | 181 | 15.7% | US representative sample | 49% obese, 51% overweight, 17% AA, 13% H | 5.0% (2.6–9.2) | 98.3% (97.2–98.9) | 35% | 85% | 0.61 (0.56–0.65) | Lee et al. [37] | |
| Adults | |||||||||||
| Dysglycemia 2-h OGTT ≥ 140 | 1,476 | 227 | 15.4% | US representative sample | 31% obese, 34% overweight, 11% AA, 8% H | 26.9% (21.5–33.0) | 87.2% (85.2–88.9) | 28% | 87% | 0.73 (0.70–0.76) | Lee et al. [37] |
| Diabetes: FPG > = 126 | 6,751 | 2,100 | 31.1% | US representative sample | 34% obese, 32% overweight, 10% AA, 8% H | 23.1% (21.3–25.0) | 91.1% (90.3–91.9) | 54% | 72% | 0.74 (0.72–0.75) | Lee et al. [37] |
Prediabetes only
Regarding detection of diabetes, studies in pediatric populations of overweight and obese populations have reported sensitivity estimates of 32% at the recommended threshold of 6.5% [35, 36•]. In contrast, adult studies have reported sensitivities that are higher, ranging from 30 to 53% [37-39]. Our study of a nationally representative population yielded a sensitivity estimate of 75%, but the wide confidence intervals (30.1–95.4) make it difficult to compare, which is likely due to the low prevalence of diabetes (0.35%) [37]. AUC estimates varied between 0.88 and 0.97 for children, and 0.89–0.93 for adults. In our nationally representative sample, we also directly compared AUC for adults vs children, demonstrating a trend for lower AUC for children (AUC 0.88, 95% CI 0.66 to 1.00) vs adults (AUC 0.93, 95% CI 0.91 to 0.95), but again, the differences were not statistically significant due to the small number of cases in children [37].
Regarding detection of prediabetes, we directly compared test performance characteristics of children compared with adults for a nationally representative population. We found both lower sensitivity and lower AUC for HbA1c for children compared with adults regardless of the gold-standard definition. At a threshold of FPG ≥ 100 mg/dL, sensitivity was 5% in children vs 23.1% in adults, and AUC was 0.61 for children vs 0.74 in adults. At a threshold of 2-h PG ≥ 140 mg/dL, sensitivity was 0% for children vs 26.9% for adults, and AUC was 0.53 for children vs 0.73 for adults [37].
Use of Different HbA1c Thresholds for Children?
The age-specific differences in HbA1c regardless of glycemic status and differences in HbA1c test performance for children vs adults raise the question of whether lower HbA1c thresholds should be used for pediatric populations. For example, in their longitudinal study, Vijayakumar reported that the sensitivity of an HbA1c threshold of 5.7% (the current prediabetes definition) was only 8% in boys and 19% in girls, and therefore highlighted a threshold of 5.4% on their ROC curve, which would result in a higher sensitivity (40% in the high-risk group) [16••]. Based on their clinical population of German overweight and obese children, Ehehalt et al. proposed a lower optimized threshold of 6.0% for detecting diabetes in children, which would result in a sensitivity of 94%, specificity of 93%, a PPV of 12%, a NPV of 99.9%, and an AUC of 0.97 [36•]. Similarly, Nowicka et al. in their population of overweight and obese children also proposed a lower HbA1c threshold of 5.8% with a sensitivity of 68% and a specificity of 88%. A lower threshold could lead to earlier detection of at-risk children, providing a greater opportunity for intervention, but alternatively, if lower thresholds are adopted, this could lead to an excess of false positive results and unnecessary referrals to pediatric endocrinologists, which is reportedly occurring right now, even at the higher thresholds [35].
Elevated HbA1c With Normal Glucose Tests: True Pathology vs Assay Interference?
Since the guidelines were issued, a number of Pediatric Endocrinologists in the USA have been anecdotally reporting an excess of patients referred from primary care physicians for prediabetes based on an HbA1c level between 5.7 and 6.4%, whom they subsequently evaluate with formal OGTT testing revealing normal IFG and 2-h PG levels (Pediatric Endocrine Listserv). Epidemiologic studies have shown that IFG, OGTT, and HbA1c do not necessarily identify identical populations of individuals [11]; therefore, it is possible for a child to have prediabetes based on an elevated HbA1c alone, but it is unclear whether this represents a problem with the HbA1c assays or a true epidemic of prediabetes.
Figure 1a shows an overlapping Venn diagram of individuals who tested positive for diabetes using FPG, HbA1c, or both from the Vijayakumar study, demonstrating the lack of perfect concordance between the two tests; FPG detected the greatest proportion of individuals with diabetes (80.1%) compared with HbA1c (24.8%). This data is consistent with findings from nationally representative populations, which have also reported a higher proportion of individuals identified by the FPG vs the HbA1c [11]. Compare this breakdown to a study by Li et al. looking at prediabetes outcomes in an exclusively Chinese population of children (Fig. 1b), which showed the exact opposite; HbA1c detected a much higher proportion of individuals with prediabetes (77%) compared with IFG (27.7%) [41].
Fig. 1.
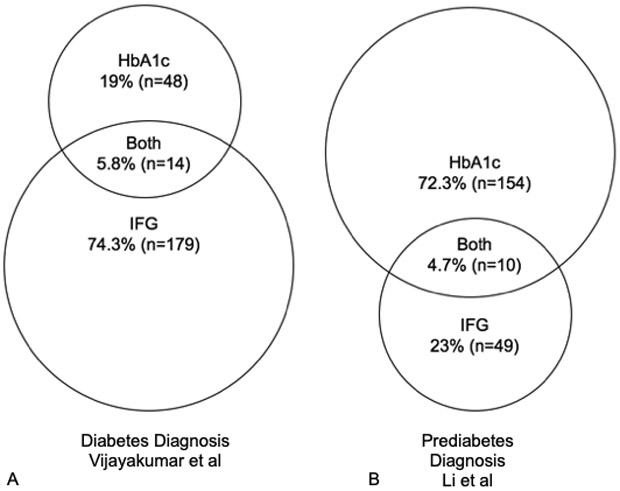
Diabetes or prediabetes diagnoses identified by IFG vs HbA1c. Data from studies by Vijayakumar et al. [16••] and Li et al. [39]
It is unclear whether these represent true differences in prediabetes for different populations of race/ethnicity or if this could be due to hemoglobin variants in Asian populations leading to falsely elevated HbA1c results, but more studies are clearly needed to explore this issue across multi-ethnic populations. Since the release of the guidelines, there have been published case reports of false positive results using HbA1c, particularly in Asian populations [42]. For example, clinicians in Japan described a 33-year-old Japanese woman with an HbA1c level of 7.2% at routine screening, but with a normal FPG (93 mg/dL) and 2-h PG (92 mg/dL), and a 58-year-old Japanese man with an HbA1c level of 7.3%, but with a normal FPG (96 mg/dL) and 2-h PG (119 mg/dL). The authors had to perform two different types of chromatograms to finally detect a hemoglobin variant; both patients were heterozygous for a hemoglobin variant, Hb Toranomon [β112 (G14) Cys → Trp], which led to interference with the assay and false elevation of HbA1c values, rather than true pathology.
The Risk of Misclassification and Lack of Awareness by Primary Care Providers
In addition to false positive cases, there have also been false negative cases in which HbA1c was not truly reflective of diabetes. One case report describes a 32-year-old male who had intermittent polyuria and polydipsia with HbA1c levels of 5.7 and 5.8% despite FPG levels of 211 and 225 mg/dL; he went undiagnosed for several years before seeing an endocrinologist [43] based on the low HbA1c levels, and was later revealed to be heterozygous for Hemoglobin Leiden which is associated with mild hemolytic anemia, leading to falsely low HbA1c levels.
This is a clear example of how primary care physicians may not be aware of all of the non-glycemic factors that influence HbA1c and therefore may have difficulty with interpretation of test results. Despite this lack of understanding, they nonetheless are ordering HbA1c with greater frequency. We conducted a survey of diabetes screening practices among pediatricians and family physicians in 2011. At that time, only 38% of providers were aware of the new ADA-recommended HbA1c screening guidelines. However, a majority (67%) reported that because of the guidelines, they would change their screening practices, leading to an estimated total of 84% of physicians with intentions to order HbA1c [44].
Cost
One additional barrier to use of HbA1c as a diagnostic test for diabetes in youth is cost. We conducted a cost-effectiveness analysis of a one-time US screening program for diabetes in overweight children 10–17 years, to compare the total costs, effectiveness, and efficiency (cost per case identified) of a number of different screening strategies for identifying children with diabetes and dysglycemia, including different thresholds of HbA1c compared to other tests like random glucose, and the 50-g 1-h glucose challenge test [45]. We found that HbA1c, when used as the primary screening strategy followed by OGTT for positive screens, was less effective at identifying children with diabetes and dysglycemia, and cost more per case identified. For example, the cost per case of diabetes was $518,000 for an HbA1c threshold of 6.5% compared with $192,000 for an OGTT; the cost per case of dysglycemia was $938 for an HbA1c threshold of 5.7%, compared with $390–$763 for a range of other screening tests. Cost-effectiveness is an important consideration for setting screening policy.
Future Alternatives?
Table 4 provides a comparison of test performance for additional tests that have been evaluated as potential screening tools for diabetes and prediabetes in children. Definitions of prediabetes and diabetes have not been formally established for the adult and pediatric populations using these alternative tests, but we do note that random plasma glucose and 1-h non-fasting plasma glucose after 50-g oral glucose challenge are promising tests, given AUC values that are statistically superior to HbA1c (AUC 0.66 and 0.68 vs 0.45, respectively). Possible thresholds to consider include cutoffs of 110 or 120 mg/dL for a 1-h GCT, which would result in sensitivities of 63 and 44% and false positive rates of 37 and 19%, respectively, or cutoffs of 100 or 110 mg/dL for a random glucose, which would result in sensitivities of 55 and 30% and false positive rates of 33 and 12%, respectively [46].
Table 4.
Test performance of potential alternate screening tests for diabetes and dysglycemia in youth
| Definition | Total, n | Cases, n | Percent of cases | Population | Characteristics | AUC ROC | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Diabetes | ||||||||
| Fructosamine (FA) | Diabetes 2-h OGTT ≥ 200 | 117 | Not defined | US convenience sample | Obesity distribution not defined (median BMI Z score 2.3), 17% AA, 59% H | 0.85 | Chan et al. [49] | |
| Glycated albumin | 0.92 | |||||||
| 1,5-Anhydroglucitol (1,5-AG) | 0.87 | |||||||
| Dysglycemia (prediabetes and diabetes) | ||||||||
| Fructosamine (FA) | Dysglycemia 2-h OGTT ≥ 140 | 254 | 56 | 22% | US convenience sample | 22% overweight, 78% obese, 30% AA, 0% H | 0.55 | Lee et al. [37] Diabetes Care |
| Fructosamine (FA) | 117 | – | 40%a | US convenience sample | Obesity distribution not defined (median BMI Z score 2.3), 17% AA, 59% H | 0.5 | Chan et al. [49] | |
| Glycated albumin | 117 | – | 40%a | 0.54 | ||||
| 1,5-Anhydroglucitol (1,5-AG) | 117 | – | 40%a | 0.61 | ||||
| Random plasma glucose (RPG) | 254 | 56 | 22% | US convenience sample | 22% overweight, 78% obese, 30% AA, 0% H | 0.66 | Lee et al. [37] Diabetes Care | |
| 1-h glucose challenge test (1-h GCT) | 254 | 56 | 22% | 0.68 | ||||
OGTT oral glucose tolerance test
Not provided
Additional markers of short-term or intermediate glycemia have also been studied, including 1,5-anhydroglucitol (1,5-AG), reflecting the past 48 h–2 weeks [47], and glycated albumin (GA) and fructosamine (FA), reflecting the past 2–4 weeks [48]. One study of overweight children found that 1,5-AG, GA, and FA each had ROC AUC of greater than 0.85 for diagnosing diabetes, but this was based on a very small number of cases; AUC for diagnosing dysglycemia was much lower (0.50–0.62) [49]. Further studies in additional populations are clearly needed to assess the utility of these biomarkers.
Conclusions
The ADA recommendation to include HbA1c as a diagnostic test for diabetes, in addition to FPG and OGTT, is controversial. Potential drawbacks include suboptimal test performance and costs, lack of awareness of its limitations by primary care physicians, and false positive and negative results. Although the convenience of collecting HbA1c may result in expanded screening of the population, it remains imperfect as a screening test for diabetes in children. Other non-fasting tests such as the 1-h GCT or a random glucose may achieve improved test performance at lower cost.
Acknowledgments
M.E. Vajravelu is supported by NIH/NIDDK training grant T32 DK007314. J.M. Lee is supported by R01 HD074559.
Footnotes
Conflict of Interest M.E. Vajravelu declares that she has no conflict of interest. J.M. Lee reports receiving consulting fees from Unitio and grant funding from Lenovo.
Human and Animal Rights and Informed Consent This article does not contain any studies with animal subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of outstanding importance
- 1.•.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29.This study highlights the rising incidence of type 2 diabetes in youth, which was greatest among minority racial and ethnic groups.
- 2.American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics. 2000;105(3):671–80. [DOI] [PubMed] [Google Scholar]
- 3.Williams DE, Cadwell BL, Cheng YJ, Cowie CC, Gregg EW, Geiss LS, et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999-2000. Pediatrics. 2005;116(5):1122–6. [DOI] [PubMed] [Google Scholar]
- 4.Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37(2):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22(2):345–54. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Type 2 diabetes in children and adolescents.. Diabetes Care 2000;23(3):381–389. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. 12. Children and adolescents: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S126–S36. [DOI] [PubMed] [Google Scholar]
- 10.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus*. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–97. [DOI] [PubMed] [Google Scholar]
- 11.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14(11):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. 2011:1–25. [PubMed] [Google Scholar]
- 14.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–51. [DOI] [PubMed] [Google Scholar]
- 15.Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K, et al. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care. 2011;34(1):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.••.Vijayakumar P, Nelson RG, Hanson RL, Knowler WC, Sinha M. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care. 2017;40(1):16–21.This study provides the first longitudinal evidence of the utility of HbA1c as a predictor of type 2 diabetes when used in American Indian youth.
- 17.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–73. [DOI] [PubMed] [Google Scholar]
- 18.Sacks DB, John WG. Interpretation of hemoglobin A1c values. JAMA 2014;311(22):2271–2, 2272. [DOI] [PubMed] [Google Scholar]
- 19.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem. 2011;57(2):344–6. [DOI] [PubMed] [Google Scholar]
- 21.Brooks AP, Metcalfe J, Day JL, Edwards MS. Iron deficiency and glycosylated haemoglobin A. Lancet. 1980;2(8186):141. [DOI] [PubMed] [Google Scholar]
- 22.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112(3):126–8. [DOI] [PubMed] [Google Scholar]
- 23.Hardikar PS, Joshi SM, Bhat DS, Raut DA, Katre PA, Lubree HG, et al. Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young Indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care. 2012;35(4):797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold JG, McGowan HJ. Delay in diagnosis of diabetes mellitus due to inaccurate use of hemoglobin A1C levels. J Am Board Fam Med. 2007;20(1):93–6. [DOI] [PubMed] [Google Scholar]
- 25.Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3(3):446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer LM, Adams JG 3rd, Steinberg MH, Miller IE, Stokes N. Screening for sickle cell trait: the veterans administration National Sickle Cell Program. Am J Hematol. 1987;24(4):429–32. [DOI] [PubMed] [Google Scholar]
- 27.NGSP. HbA1c Assay Interferences 2017. [Available from: http://www.ngsp.org/interf.asp.
- 28.Unnikrishnan R, Anjana RM, Mohan V Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care. 2007;30(10):2453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman WH, Dungan KM, Wolffenbuttel BH, Buse JB, Fahrbach JL, Jiang H, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(5):1689–94. [DOI] [PubMed] [Google Scholar]
- 31.Cohen RM. A1C: does one size fit all? Diabetes Care. 2007;30(10): 2756–8. [DOI] [PubMed] [Google Scholar]
- 32.Eberhardt MS, Lackland DT, Wheeler FC, German RR, Teutsch SM. Is race related to glycemic control? An assessment of glycosylated hemoglobin in two South Carolina communities. J Clin Epidemiol. 1994;47(10):1181–9. [DOI] [PubMed] [Google Scholar]
- 33.Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta. 1997;260(1):49–64. [DOI] [PubMed] [Google Scholar]
- 34.Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham offspring study and the National Health and nutrition examination survey 2001-2004. Diabetes Care. 2008;31(10):1991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin a(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011;34(6):1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Ehehalt S, Wiegand S, Korner A, Schweizer R, Liesenkotter KP, Partsch CJ, et al. Diabetes screening in overweight and obese children and adolescents: choosing the right test. Eur J Pediatr. 2017;176(1):89–97.This multicenter observational study of nearly 5,000 overweight and obese youth found that the optimal threshold for identifying diabetes using HbA1c was 6.0%, lower than suggested by current standards.
- 37.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011;158(6):947–52 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23(2):187–91. [DOI] [PubMed] [Google Scholar]
- 39.Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care. 2011;34(1):84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brar PC, Mengwall L, Franklin BH, Fierman AH. Screening obese children and adolescents for prediabetes and/or type 2 diabetes in pediatric practices: a validation study. Clin Pediatr. 2014;53:771–776. [DOI] [PubMed] [Google Scholar]
- 41.Li P, Jiang R, Li L, Li L, Wang Z, Li X, et al. Diagnostic performance of hemoglobin A1c for prediabetes and association with cardiometabolic risk factors in Chinese adolescents without diabetes. J Investig Med. 2012;60(6):888–94. [DOI] [PubMed] [Google Scholar]
- 42.Tomita M, Kabeya Y, Okada K, Shirai M, Asai S, Katsuki T, et al. Normal glucose tolerance with high HbA1c levels observed in two cases of Hb variants. Diabetol Int. 2010;1(2):104–7. [Google Scholar]
- 43.Arakaki RF, Changcharoen B. Glycemic assessment in a patient with HB Leiden and type 2 diabetes. AACE Clin Case Rep. 2016;2(4):e307–e10. [Google Scholar]
- 44.Lee JM, Eason A, Nelson C, Kazzi NG, Cowan AE, Tarini BA. Screening practices for identifying type 2 diabetes in adolescents. J Adolesc Health. 2014;54(2):139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu EL, Kazzi NG, Lee JM. Cost-effectiveness of screening strategies for identifying pediatric diabetes mellitus and dysglycemia. JAMA Pediatr. 2013;167(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care. 2011;34(12):2597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGill JB, Cole TG, Nowatzke W, Houghton S, Ammirati EB, Gautille T, et al. Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycemia: a U.S. trial of the GlycoMark assay. Diabetes Care. 2004;27(8): 1859–65. [DOI] [PubMed] [Google Scholar]
- 48.Selvin E, Francis LM, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34(4):960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia—1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr Diabetes. 2016;17(3): 206–11. [DOI] [PubMed] [Google Scholar]


