Abstract
Background
Coronavirus disease 2019 (COVID-19) spreading from Wuhan, Hubei province in China, is an expanding global pandemic with significant morbidity and mortality. Even though respiratory failure is the cardinal form of severe COVID-19, concomitant cardiac involvement is common. Myocarditis is a challenging diagnosis due to heterogeneity of clinical presentation, ranging from mild symptoms to fatal arrhythmia and cardiogenic shock (CS). The aetiology is often viral and endomyocardial biopsy (EMB) is the gold standard for definite myocarditis. However, the diagnosis is often made on medical history, clinical presentation, magnetic resonance imaging, and blood tests.
Case summary
We present a 43-year-old man with mixed connective tissue disease treated with hydroxychloroquine who rapidly developed CS 4 days from symptom onset with fever and cough, showing positive polymerase chain reaction nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA. While computed tomography of the thorax was normal, high-sensitivity troponin T was elevated and electrocardiogram showed diffuse ST elevation and low voltage as signs of myocardial oedema. Echocardiography showed severe depression of left ventricular function. The myocardium recovered completely after a week with mechanical circulatory support (MCS). EMB was performed but could neither identify the virus in the cardiomyocytes, nor signs of inflammation. Still the most probable aetiology of CS in this case is myocarditis as a sole symptom of COVID-19.
Discussion
COVID-19 patients in need of hospitalization present commonly with respiratory manifestations. We present the first case of fulminant myocarditis rapidly progressing to CS in a COVID-19 patient without respiratory failure, successfully treated with inotropes and MCS.
Keywords: Case report, COVID-19, Myocarditis, Cardiogenic shock, Mechanical circulatory support, Endomyocardial biopsy
Learning points
Coronavirus disease 2019 (COVID-19) may come with a wide variety of clinical manifestations, even without respiratory symptoms.
Mechanical cardiac support can be life-saving by relieving the heart and preventing multiorgan failure as in this case even without antiviral therapy, but prevents further diagnostic evaluation with magnetic resonance.
Endomyocardial biopsy can provide the nature of the aetiological agent and should be considered in cases with fulminant myocarditis. When taken, preservation should be made so that different modalities for analysing are not precluded. In this case preservation in formaldehyde made electron microscopy non optional.
Introduction
A large number of coronavirus disease (COVID-19) patients displaying cardiac involvement have been reported since the first cases of COVID-19 in Wuhan, China in December 2019. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen is a positive-sense single-stranded RNA virus with transmission primarily via respiratory droplets.1 The clinical course is characterized by fever, cough, fatigue, anosmia, and ageusia, sometimes complicated by acute respiratory distress syndrome (ARDS).2 Fulminant myocarditis is a rare critical clinical condition with poor in-hospital outcome, whose main characteristic is a rapidly progressive clinical course with the need for haemodynamic support. Aetiology includes a variety of triggers, often viral infections. Myocardial injury is common in patients with COVID-19, including myocarditis, myocardial infarction, and stress-induced cardiomyopathy. COVID-19-related myocarditis can be caused by a combination of direct viral injury and cardiac damage due to the host’s immune response.
We present a case of fulminant myocarditis rapidly progressing to cardiogenic shock (CS) without pulmonary manifestations in a COVID-19 patient.
Timeline
| Laboratory measurements, circulatory and respiratory support | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Day 0 | Day 1a | Day 1b | Day 4 | Day 5 | Day 6 | Day 7 | Day 9 | Day 11 | Day 12 | |||
| Haemoglobin, g/L | 137–170 | 161 | 159 | 154 | 131 | 125 | 107 | 96 | 97 | 97 | 94 | ||
| White blood cell count ×109/L | 3.5–8.8 | 9.7 | 8.5 | 16.2 | 9.5 | 11.4 | 15 | 14.4 | 11.5 | 11.4 | 16 | ||
| Platelet count ×109/L | 140–350 | 245 | 236 | 197 | 139 | 82 | 65 | 70 | 59 | 88 | 131 | ||
| Lymfocyte count ×109/L | 1.1–4.8 | 1.8 | NA | NA | 0.7 | NA | 0.5 | 1.2 | 1.6 | NA | |||
| Sodium, mmol/L | 137–145 | 136 | 129 | 134 | 134 | 136 | 135 | 142 | 155 | 154 | NA | ||
| Potassium, mmol/L | 3.5–4.4 | 3.6 | 4.5 | 4.4 | 5 | 4.2 | 4.5 | 4 | 4 | 4.1 | NA | ||
| Creatinine, µmol/L | 60–105 | 73 | 77 | 69 | 83 | 166 | 105 | 98 | 142 | 128 | 127 | ||
| C-reactive protein, mg/L | <10 | 4 | 6 | <5 | 9 | 88 | 134 | 44 | 25 | NA | 38 | ||
| Procalcitonin, µg/L | <0.5 | 0.06 | 0.06 | 0.1 | 0.9 | 9 | 17 | 10 | 2.6 | 0.6 | 0.4 | ||
| Creatine kinase-MB, ng/mL | <5 | NA | NA | 111 | 37 | NA | NA | NA | NA | NA | NA | ||
| High-sensitivity troponin T, ng/L | <15 | 590 | 730 | 1620 | 1820 | NA | NA | NA | NA | NA | NA | ||
| N-terminal pro-brain natriuretic peptide, ng/L | <300 | NA | 6100 | 10100 | 17300 | 28600 | NA | 3400 | NA | NA | NA | ||
| Interleukin-6, ng/L | <7 | NA | NA | 30 | 300 | NA | 35 | 46 | NA | NA | 25 | ||
| D-dimer, mg/L | <0.20 | NA | <0.5 | 0.56 | 0.67 | 0.19 | 0.28 | 0.24 | 0.54 | NA | NA | ||
| Lactate dehydrogenase, µkat/L | <3.5 | NA | 5.1 | NA | NA | 6.4 | NA | 2 | NA | NA | NA | ||
| Ferritin, µg/L | 34–275 | NA | 220 | NA | 261 | NA | 318 | NA | NA | NA | NA | ||
| Lactate, mmol/L | 0.5–2.2 | NA | 3.4 | 5.1 | 4.7 | 4 | 5.8 | NA | 0.9 | 0.9 | 0.6 | ||
| Impella | x | x | x | x | x | x | |||||||
| VA-ECMO | x | x | x | x | x | ||||||||
| Intubated | x | x | x | x | x | x | x | ||||||
Case presentation
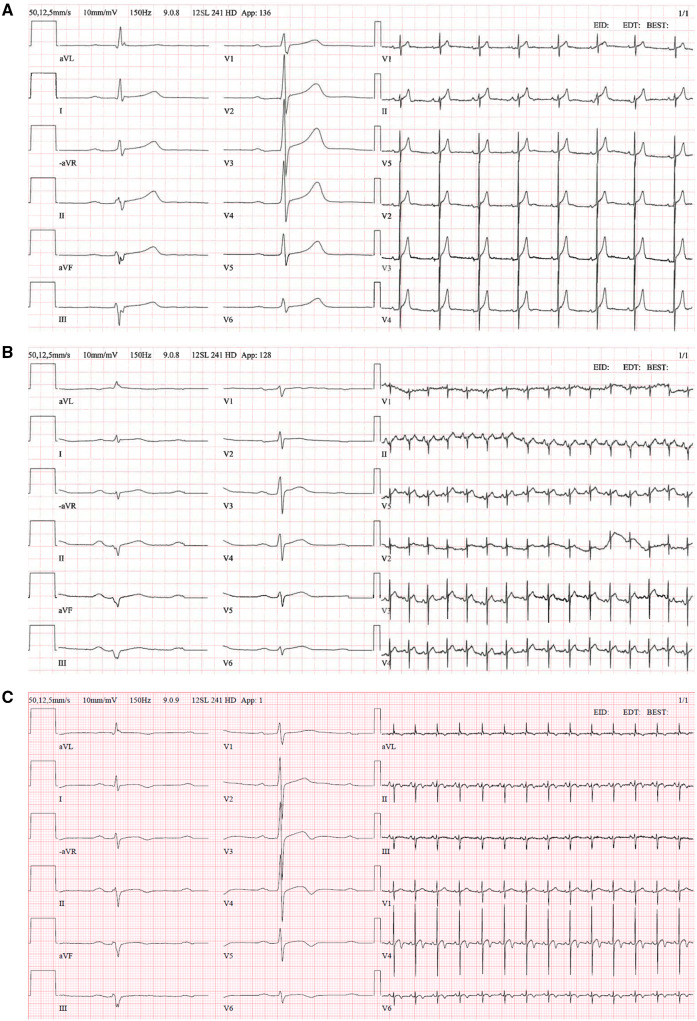
A 43-year-old non-smoking male with a history of Mixed Connective Tissue Disease (MCTD) presented to the emergency room due to chest pain. His drug history was hydroxychloroquine 400 mg once daily (for the past 5 months), and he was otherwise healthy in excellent physical condition. The patient presented with a 4 day history of fever and cough. The patient’s wife was also suffering from similar symptoms. Physical examination revealed no murmurs or rales, systolic blood pressure (BP) of 100 mmHg, a heart rate of 130 b.p.m., and oxygen saturations of 90–100% on 10–15 L/min of oxygen. Electrocardiograms showed sinus tachycardia and diffuse ST-segment elevation. All electrocardiograms are presented in Figure 1A–C.
Figure 1.
(A) Electrocardiogram 2019, normal sinus rhythm, left axis deviation. (B) Electrocardiogram at admission, sinus tachycardia, low voltage and diffuse ST-segment elevation in limb and precordial leads. (C) Electrocardiogram Day 24 at Coronary Care Unit during rehabilitation period.
Thoracic computed tomography (CT) scan revealed no pathology. High sensitivity Troponin T (hsTnT) was 590 ng/L [upper limit of normal (ULN) 15 ng/L] and the patient was initially treated as non-ST-elevation myocardial infarction (NSTEMI) with aspirin, ticagrelor, and fondaparinux. Later, perimyocarditis was suspected and cefotaxime and colchicine were administered. Treatment with cefotaxime was not the hospitals’ policy, treatment was initiated to cover for a possible bacterial infection. The nasopharyngeal swab polymerase chain reaction (PCR) for SARS-CoV-2 was positive. During the night, the patient was stable with 3 L of O2 (saturation 100%), BP 110/75 mmHg, and heart rate 95 b.p.m.
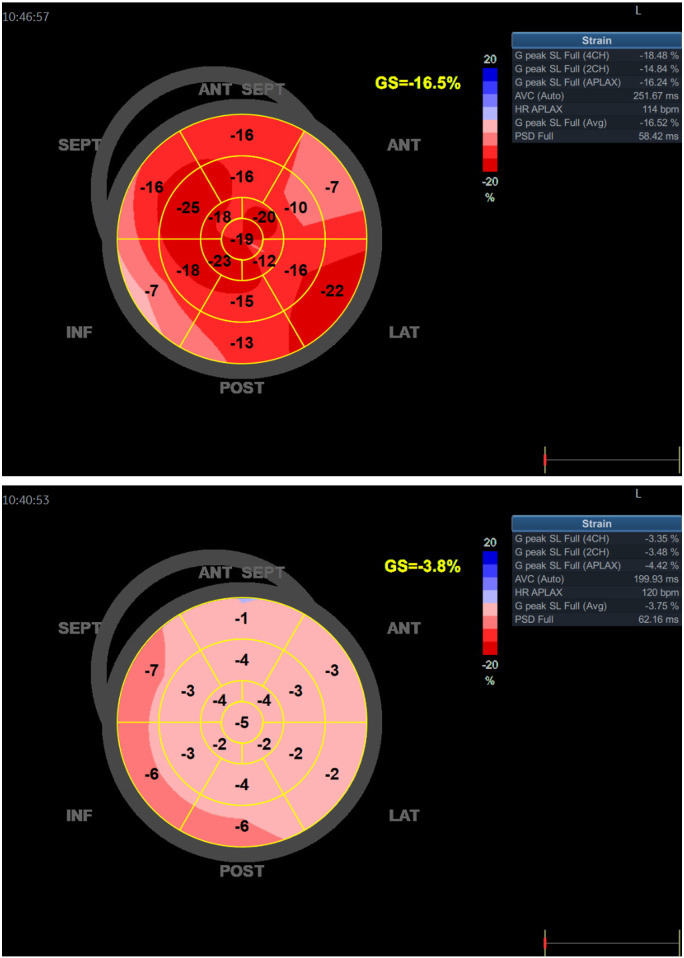
Blood samples on the second day of admission showed an elevated N-terminal pro-brain natriuretic peptide (NT-ProBNP) of 6100 ng/L (ULN <300 ng/L). Echocardiography showed a normal sized left ventricle with severely depressed systolic function, ejection fraction (EF) 10–15%. A 5 mm pericardial effusion was found along the right ventricular wall. The central venous saturation (ScvO2) was 53% and the patient was referred to the local Coronary Care Unit (CCU). Upon arrival at the CCU, the patient was somnolent but did express significant anxiety, and complained of chest and abdominal pain. He showed signs of CS with cold extremities, oliguria, heart rate 120–130 b.p.m., and BP 85/70 mmHg. Levosimendan and norepinephrine infusions were started together with IV fluid. ScvO2 had dropped to 36%. Immediate echocardiography confirmed the previous findings (Figure 2) and the patient was referred to the cardiothoracic intensive care unit (ICU). Inotropic support was enhanced with epinephrine and milrinone. He received a Swahn-Ganz pulmonary artery catheter as well as mechanical circulatory support (MCS) (Impella® CP Smart Assist, Abiomed, Aachen, Germany), initially at 3.8 L/min later increased to 4.2 L/min. Mixed venous gas saturation (SvO2) improved to 57%. However, cardiac index remained low between 1.8 and 2.5 L/m2 and norepinephrine in high doses was needed to maintain mean arterial BP above 60 mmHg. During the night, he was bleeding profoundly from the MCS access site and received massive transfusions with 48E erythrocytes, 22E fresh frozen plasma and 3E platelets. Hydroxychloroquine treatment was terminated upon his arrival to ICU.
Figure 2.
(A and B) Longitudinal strain at admission and after 15 Days.
The next day the patient was anaesthetized, intubated and surgical revision of the access site was performed as well as endomyocardial biopsy (EMB) via the right internal jugular vein. Milrinone was terminated and vasopressin added because of vasodilatation. Hydrocortisone, 100 mg three times/day, was started due to clinical suspicion of Addison crisis, as he periodically had been on cortisone due to MCTD. The biopsies showed no histopathological signs of myocarditis and the myocardial samples tested negative for SARS-CoV-2 RNA. Repeated PCR tests for SARS-CoV-2 were positive, both in tracheal secretion and a few days afterwards even in urine culture. In total, the patient was positive for SARS-CoV-2 in three different tests.
Despite massive inotropic and vasopressor support as well as Impella®, pre-shock signs remained. It was felt that this mandated an upgrade of MCS to include veno-arterial extracorporeal membrane oxygenation (VA-ECMO, Cardiohelp™ Maquet Cardiovascular, Bridgewater, NJ, USA) in combination with the Impella®. Within hours the circulation stabilized (initially blood flow of 3.7 L/min, sweep gas flow of 2.5 L/min at 100% oxygen) and inotropic support could gradually be decreased.
A new CT scan could not demonstrate any pulmonary infiltrates, and laboratory parameters improved. Within 24 h after the VA-ECMO institution, renal function improved.
After 3 days, while weaning MCS, transthoracic echocardiography confirmed biventricular systolic improvement. After a multidisciplinary meeting on Day 7, the patient was successfully weaned off MCS, the left ventricular function normalized (Figure 2B), and he was later moved to the CCU. At Day 25, he was referred to his local county hospital being treated for an infection of unclear focus.
Discussion
We describe a case of isolated fulminant SARS-CoV-2 associated myocarditis rapidly progressing to CS in need of and successfully treated with temporary MCS. Our main finding reflects that which has been previously reported by Inciardi et al.,3 that severe cardiac involvement may be the only clinical manifestation of SARS-CoV-2.
The first case report of myocarditis associated with COVID-19 was a 63-year-old male who after travelling to Wuhan, developed fever, shortness of breath and chest tightness.4 He was considered to have fulminant myocarditis (EF 32%) along with ARDS and needed respiratory support by veno-venous (VV) ECMO.1 Later, a similar case was reported from Lombardy, with SARS-CoV-2 associated myocarditis in a 69-year old man with ARDS requiring mechanical ventilation.5 Magnetic resonance imaging (MRI) showed regional subepicardial late gadolinium enhancement suggestive of myocarditis. In addition, several mild cases of myocarditis without need of circulatory or respiratory support have been reported.6,7 EMB was not performed in these cases.
Hydroxychloroquine has been reported as causing cardiotoxicity in rare cases, mainly after many years of treatment.8 The rapid progress of fulminant heart failure together with the fact that our patient had only been treated for 5 months made this differential diagnosis unlikely. Although MCTD is recognized as being associated with pre-capillary pulmonary hypertension and pericarditis, the lack of association with fulminant myocarditis, as in this case, pointed us away from MCTD as a factor.9 The initial clinical suspicion of NSTEMI was rejected as the patient rapidly deteriorated. The patient had no history of angina symptoms and he exercised regularly. The reduced QRS amplitudes were interpreted as a sign of myocardial oedema that together with signs of acute heart failure made myocarditis plausible, as well as the echocardiographic findings of severe global reduction of the left ventricular function without any regional differences. The need for MCS precluded the possibility of an MRI scan, and strengthened the decision for EMB, especially in the absence of respiratory symptoms and lymphopenia. Unfortunately, the biopsies did not result in histological evidence for myocarditis and preservation in formaldehyde precluded further analysis with electron microscopy that has been described in a case report by Sala et al.10
Even though we cannot prove SARS-CoV-2 as the aetiology, which is the main limitation of this case report, the fast recovery of systolic function while on MCS strongly supports viral myocarditis. It is of importance to highlight that the patient tested positive to SARS-CoV-2 three times in total. To the best of our knowledge, this is the first case of isolated fulminant myocarditis in a patient testing positive for COVID-19 needing MSC because of CS. We have found one other case report on a COVID-19 patient treated with Impella® and VA-ECMO, but this was due to ARDS decompensating chronic heart failure.11 Although VV-ECMO is an established treatment for ARDS, including COVID-19 with serial published reports,12,13 there are few published cases of VA-ECMO and other MCS and no case without concomitant respiratory failure. In viral myocarditis, MCS can be lifesaving, in its ability to support the circulation, thereby preventing multi-organ failure, and allowing the myocardium, most often, to heal spontaneously.14 If other supportive or antiviral treatments should be offered to SARS-CoV-2 associated fulminant myocarditis is yet to be proven,15 and it is of importance to note that our patient recovered without antiviral treatments.
Lead author biography

Dr Joanna-Maria Papageorgiou graduated from Sofia Medical University, Bulgaria in 2005. Her clinical carrier began as a Resident Doctor in Internal Medicine at Höglandssjukhuset, Eksjö, Sweden. She completed her Cardiology residency programme in University Hospital of Linköping, Sweden in 2016. With a keen interest in Heart Failure, Pulmonary Arterial Hypertension, and Heart Transplantation, she is currently a clinical fellow of Cardiology at University Hospital of Linköping. Her current research focuses on Heart Failure and Pulmonary Arterial Hypertension.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We acknowledge the senior consultant clinical physiologist Meriam Aneq Åström, helping in preparing the echocardiography material accompanying this publication. We also acknowledge the senior consultant cardiologist Jesper Schüllerqvist, taking care of the patient at the local county hospital the first days of disease as well as during the rehabilitation period. He has been helpful in gathering material to this manuscript from the first days of hospital stay. Finally, we acknowledge Kjell Jansson, head of the Department of Clinical Physiology as well as senior consultant cardiologist with expertise in advanced heart failure, who was engaged in patient care during the critical period of CS with MCS need, guiding in writing this case report with senior advising.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: H.v.d.W. has previously received personal fee from Orion Pharma and J-M.P. has previously received personal fee from Orion Pharma as well as Novartis. None of the other authors had any competing interests.
Funding: none declared.
Contributor Information
Joanna-Maria Papageorgiou, Department of Cardiology, Linköping University Hospital, SE-58185 Linköping, Sweden.
Henrik Almroth, Department of Cardiology, Linköping University Hospital, SE-58185 Linköping, Sweden.
Mattias Törnudd, Department of Cardiothoracic and Vascular Surgery in Linköping and Department of Health, Medicine and Caring Sciences, Unit of Cardiovascular Sciences, Linköping University Hospital, SE-58185 Linköping, Sweden.
Henriëtte van der Wal, Department of Cardiology, Linköping University Hospital, SE-58185 Linköping, Sweden.
Georgia Varelogianni, Department of Clinical Physiology in Linköping and Department of Health, Medicine and Caring Sciences, Unit of Cardiovascular Sciences, Linköping University Hospital, SE-58185 Linköping, Sweden.
Sofia Sederholm Lawesson, Department of Cardiology in Linköping and Department of Health, Medicine and Caring Sciences, Unit of Cardiovascular Sciences, Linköping University Hospital, SE-58185 Linköping, Sweden.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D. et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX. et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020;48:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doyen D, Moceri P, Ducreux D, Dellamonica J et al.. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet 2020;395:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paul JF, Charles P, Richaud C, Caussin C, Diakov C et al.. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging 2020;21:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim IC, Kim JY, Kim HA, Han S et al.. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J 2020;41:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joyce E, Fabre A, Mahon N.. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013;2:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ungprasert P, Wannarong T, Panichsillapakit T, Cheungpasitporn W, Thongprayoon C, Ahmed S. et al. Cardiac involvement in mixed connective tissue disease: a systematic review. Int J Cardiol 2014;171:326–330. [DOI] [PubMed] [Google Scholar]
- 10. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D. et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bemtgen X, Kruger K, Supady A, Durschmied D, Schibilsky D, Bamberg F. et al. First successful treatment of COVID-19 induced refractory cardiogenic plus vasoplegic shock by combination of pVAD and ECMO—a case report. ASAIO J 2020;66:607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs JP, Stammers AH, St Louis J, Hayanga JWA, Firstenberg MS, Mongero LB. et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: experience with 32 patients. ASAIO J 2020;66:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sultan I, Habertheuer A, Usman AA, Kilic A, Gnall E, Friscia ME. et al. The role of extracorporeal life support for patients with COVID-19: Preliminary results from a statewide experience. J Card Surg 2020;35:1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB. et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 15. Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY. et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.