Abstract
Background
Various patient demographic and clinical characteristics have been associated with poor outcomes for individuals with coronavirus disease 2019 (COVID-19). To describe the importance of age and chronic conditions in predicting COVID-19-related outcomes.
Methods
Search strategies were conducted in PubMed/MEDLINE. Daily alerts were created.
Results
A total of 28 studies met our inclusion criteria. Studies varied broadly in sample size (n = 21 to more than 17,000,000). Participants’ mean age ranged from 48 years to 80 years, and the proportion of male participants ranged from 44% to 82%. The most prevalent underlying conditions in patients with COVID-19 were hypertension (range: 15%–69%), diabetes (8%–40%), cardiovascular disease (CVD) (4%–61%), chronic pulmonary disease (1%–33%), and chronic kidney disease (range 1%–48%). These conditions were each associated with an increased in-hospital case fatality rate (CFR) ranging from 1% to 56%. Overall, older adults have a substantially higher case fatality rate (CFR) as compared to younger individuals affected by COVID-19 (42% for those <65 vs 65% > 65 years). Only one study examined the association of chronic conditions and the risk of dying across different age groups; their findings suggested similar trends of increased risk in those < 65 years and those > 65 years as compared to those without these conditions.
Conclusions
There has been a traditional, single-condition approach to consideration of how chronic conditions and advancing age relate to COVID-19 outcomes. A more complete picture of the impact of burden of multimorbidity and advancing patient age is needed.
Keywords: COVID-19, Epidemiology, Multimorbidity
The first known cases of pneumonia identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1), or COVID-19, were identified in Wuhan, China in December 2019. COVID-19 has unfortunately spread rapidly throughout the world since that time (2). As of November 23, 2020, the number of cases in the United States has increased to more than 11.8 million with more than 250,000 deaths attributed to COVID-19 (3). Based on recent CDC reports, approximately 8 out of every 10 COVID-19-related deaths in the United States have been among adults aged 65 years and older (4).
Some early case reports and small clinical studies suggested a greater impact of COVID-19 in older adults with underlying chronic conditions as compared to younger individuals without these conditions (5–8). Numerous studies have shown that COVID-19-positive older adults presenting with specific conditions such as diabetes, CVD, and obesity are at a higher risk for hospitalization and mortality than older adults without these chronic conditions (6,9–12).
The overall aim of this literature review was to summarize the rapidly evolving literature on the patient characteristics associated with poor outcomes for individuals with coronavirus disease 2019 (COVID-19), specifically focusing on the relative importance of age and burden of multiple chronic conditions, with a specific focus on their interaction, in predicting COVID-19-related outcomes.
Method
Search Strategy and Information Sources
This rapid review followed the basic Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). “Rapid reviews “are literature reviews that use methods to accelerate or streamline traditional [systematic review] processes” in order to meet the needs and timelines of the end users (health care institutions, health professionals, and patient associations).” In keeping with the methodology of a rapid review, it was conducted with a shortened timeline and omitted stages of the PRISMA systematic review process, but the process maintained transparent and used reproducible methods (14). A health sciences librarian (C.C.) developed the search strategies and conducted unique searches in PubMed/MEDLINE. Major concepts included, but were not limited to, severe acute respiratory syndrome coronavirus 2, COVID-19, mortality, case fatality rate (CFR), hospitalization, chronic conditions, comorbidity, diabetes mellitus, CVD, chronic lung disease, obesity, and aged and older adults.
A combination of medical subject heading (MeSH) terms and text words was created to search PubMed. The full search algorithm is detailed in Supplementary Methods 1. Due to the evolving COVID-19 situation, a PubMed alert was set up using the original search strategy to capture relevant and significant newly published literature. Daily monitoring of these results contributed to a selection of studies being included until October 30, 2020. Bibliographic references were hand searched to identify additional possible studies to include.
Inclusion and Exclusion Criteria
Studies were included if (i) the articles were published between December 1, 2019 and October 30, 2020, (ii) cases of COVID-19 were confirmed, (iii) patients’ demographic and clinical characteristics were described, (iv) prevalence of chronic conditions were included, and the (v) articles were written in English.
Data Abstraction
A detailed data abstraction form was developed a priori allowing 2 reviewers to independently abstract data in duplicate from these studies. Data abstraction began when there was a minimum of 80% agreement between reviewers in the sample of pilot studies; any disagreements were resolved either by consensus or by consulting a third reviewer.
Results
Search Results
A total of 70 studies were identified in our initial literature search. Among these, 31 studies were removed based on abstract review, leaving 39 studies for detailed review. Of these 39 studies, 28 met our inclusion criteria and were included in this review (Supplementary Figure 1). Studies were excluded if they included less than 10 patients, did not report the prevalence of comorbidities, did not report adverse outcomes, or were not published in the English language.
Study Characteristics
The characteristics of the reviewed studies are given in Table 1. Thirteen studies were case series (5,7,8,10,15–23), 14 were retrospective cohort investigations (6,9,11,12,16,24–33), and 1 was a prospective cohort study (34). Ten studies were conducted in China (10,12,16,18,24,30–32,34), and 9 of these studies utilized data gathered from patients in Wuhan (10,12,16,18,24,30–32,34). Eleven were U.S.-based studies (3 in Washington State (5,15,35), 2 in California (27,28), 1 in Georgia (25), 1 in Michigan (23), 1 in New York (21), and 3 nationwide) (22,26,29). Four studies were based in Italy (6,8,17,33), 1 in Korea (7), and 2 in the United Kingdom (9,11).
Table 1.
Study Characteristics by Country
| Author/s | Country | Study Sample (n=) | Inpatient/ICU | Age, Mean, Years (range) | Male |
|---|---|---|---|---|---|
| Chen et al. | China | 99 | 1 hospital | 55.5 (21–82) | 68% |
| Chen et al. | China | 274 | 1 hospital | Nonsurvivors 68 (IQR 62–77) Survivors 51 (IQR 37–66) |
Nonsurvivors 73% Survivors 55% |
| Du et al. | China | 179 | 1 hospital | 57.6 (18–87) | 54% |
| Guan et al. | China | 1590 | 575 hospitals in China | 48.9 ± 16.3 | 57% |
| Huang et al. | China | 41 | 1 hospital | 49 (IQR 41–58) | 73% |
| Liu et al. | China | 56 | 1 hospital | Older: 68 (IQR 65–70) Younger: 47 (IQR 36–51) |
Older 67% Younger 50% |
| Wu et al. | China | 201 | 1 hospital | 51 (IQR 43–60) | 64% |
| Yan et al. | China | 193 | 1 hospital | 64 (49–73) | 59% |
| Zhang et al. | China | 663 | 1 hospital | 55.6 (23–95) | 48% |
| Zhou et al. | China | 191 | 2 hospitals | 56 (18–87) | 62% |
| Grasselli et al. | Italy | 1591 | 1 ICU patients from 72 hospitals | 63 (14–91) | 82% |
| Graselli et al. | Italy | 3988 | ICU network | 63 (IQR 55–69) | 79.9% |
| Iaccarino et al. | Italy | 1591 | 26 hospitals | Nonsurvivors 80 Survivors 65 |
Nonsurvivors 67% Survivors 64% |
| Onder et al. | Italy | Subsample: 1625 | 19 Italian regions and 2 provinces | 79.5 (SD 8.1) | 70% |
| Korean Society | Republic of Korea | 54 | Country wide | 75.5 (35–93) | 61% |
| Atkins et al. | United Kingdom | 269,070 | UK Biobank | 73.1 (65–86) | 45% |
| Williamson et al. | United Kingdom | 17,278,392 | General practice network | No mean age included | 50% |
| Arentz et al. | United States | 21 | ICU at 1 Hospital | 70 (43–92) | 52% |
| Buckner et al. | United States | 105 | 3 hospitals | 69 (23–97) | 50% |
| Gold et al. | United States | 305 | 8 hospitals | 60 (23–95) | 49% |
| Harrison et al. | United States | 31 461 | 24 health care organizations | 50 (18–90) | 46% |
| McMichael et al. | United States | 129 | Skilled nursing facility | Residents: 81 (54–100) Staff: 42.5 (22–79) |
Patients: 37% Staff: 21% |
| Myers et al. | United States | 377 | 21 hospitals; inpatient/ICU | Overall: 61 (IQR 50–73) Inpatient: 60 (IQR 49–72) ICU: 63(IQR 53–73) |
Overall: 56% Inpatient: 52% ICU: 65% |
| Richardson et al. | United States | 5700 | 12 Hospitals | 63 (0–107) | 60% |
| Stokes et al. | United States | 1,320,488 | Country wide | 48 (IQR 33–63) | 49% |
| Suleyman et al. | United States | 463 | 5 hospitals and 9 EDs in Michigan | 57.5 (SD 16.8) | 44% |
| Tartof et al. | United States | 6916 | Integrated health care system | 49 (IQR 36–60) | 45% |
| Wortham et al. | United States | 62 813 | Country wide | Case-based surveillance 78 (IQR 67–87) Supplemental surveillance 75 (IQR 64–84) |
56% |
Note: ICU = Intensive care unit; IQR = Interquartile range.
Study sample sizes ranged from 21 (5) to 17,278,392 (11) patients. Five out of 28 studies had less than 100 participants (5,7,10,18,24), 11 studies had between 100 and 1000 participants (8,12,15,16,23,25,30–32,34,35), 10 studies had between 1000 and 1,000,000 participants (6,9,10,21,26–29,33), and 2 studies had more than 1,000,000 participants (11,22). Participants’ age varied widely among the included studies with the mean age ranging from 48 years (22) to 80 years (8).
Prevalence of Chronic Conditions
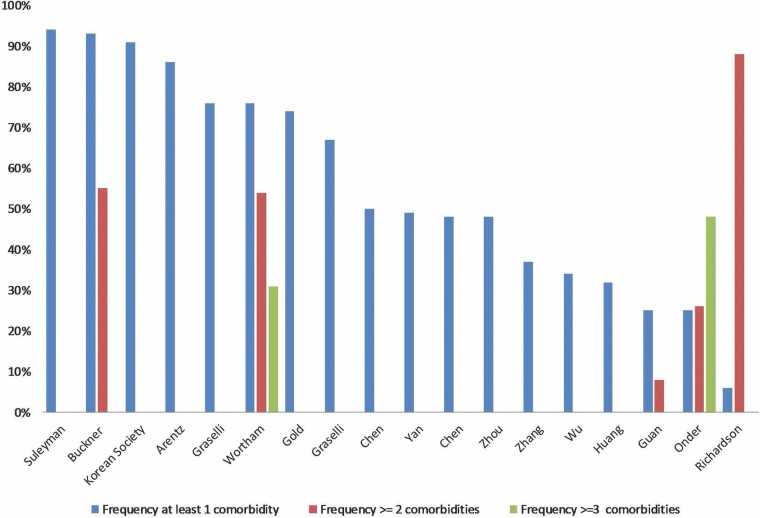
Eighteen studies reported the percentage of patients presenting with one or more chronic conditions (Figure 1) (5–8,10,12,15–18,21–25,29–32). Three studies reported the average number of previously diagnosed chronic conditions at the time of hospitalization for COVID-19 (8,9,23). Three studies provided information using the Charlson Comorbidity index. One reported a median score of 4, noting that 88% of patients had more than one comorbidity (8). The second study reported a Charlson comorbidity index of 1, 2, and >=3 in 17%, 8%, and 15% of the study cohort, respectively (26). The third study reported an overall median score for the study sample of 2.8, a median score of 4.4 in nonsurvivors, and 2.6 in survivors (33).
Figure 1.
Prevalence of number of comorbidities reported by study.
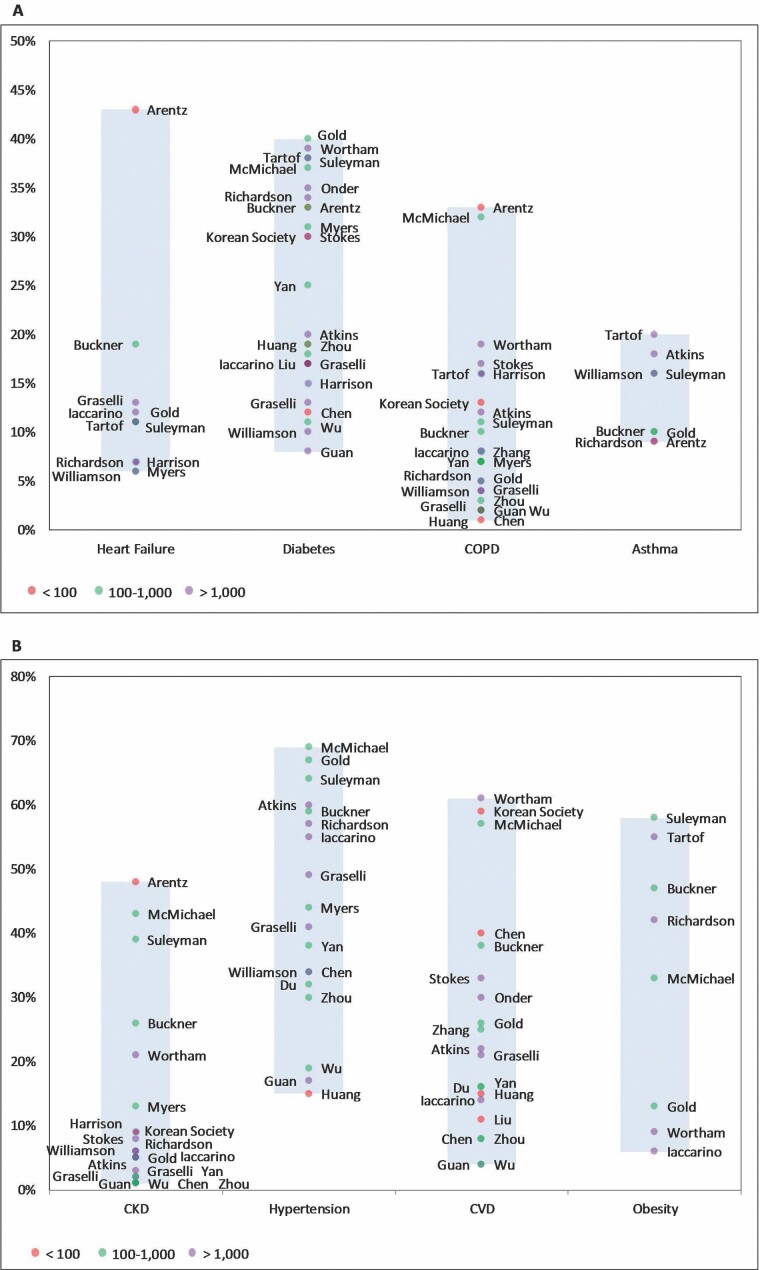
A majority of studies (n = 23) reported the prevalence of particular chronic conditions present in study participants (Figure 2) (5–10,12,15–18,21–23,25,26,28–30,32,33,36,37). The most commonly reported chronic conditions were hypertension (6,9–11,15–21,23,25,27,28,30–33) with a prevalence ranging from 15% (18) to 69% (20), diabetes mellitus (5–11,15,16,18–30,32–34,38) ranging from 8% (10) to 40% (25), CVD (6–10,12,15,16,18–20,22,24,25,29–34) ranging from 4% (10) to 61% (29), chronic obstructive pulmonary disease (COPD) (5–7,9–11,15,16,18,20–24,26–30,32,33) ranging from 1% (24) to 33% (5), and chronic kidney disease (5–7,9–11,15,16,20–23,26,27,29,30,32,33) with a prevalence ranging from 1% (10,16,29,31) to 48% (5).
Figure 2.
Prevalence of individual chronic conditions reported by study.
Association Between Comorbidities and Risk of Dying
All but 2 studies reported overall case-fatality rates during hospitalization for COVID-19 (Supplementary Table 1) (5–11,15,17–26,28,30–34,39). Case fatality rates ranged from less than 1% (7) to 56% (31) during a follow-up period of 28 days post-hospital admission.
Thirteen studies reported in-hospital CFRs related to particular chronic conditions (Supplementary Table 1) (6,10,11,17,22,26,28,30–32,34,39). The most common conditions associated with high CFRs were hypertension, CVD, diabetes, COPD, chronic kidney disease, and heart failure (HF). There was a considerable variation in the CFRs associated with each particular condition, as an example CFRs for those with hypertension ranged from 0.1% (11) to 78% (31), CFRs for those individuals with diabetes ranged from 0.2% (11) to 81% (31), and CFRs for those with chronic kidney disease ranged from 0.4% (11) to 100% (16,32).
Fourteen studies reported CFRs across different age groups (Supplementary Table 1) (6–8,10–12,16,21,25,26,28,34). Case fatality rates for those aged <65 years ranged from 0% (11) to 42% (6); and for those aged ≥ 65 years ranged from 0% (11) to 56% (6). Only one study reported CFRs by age group and compared those with and without underlying conditions (22). The CFRs during COVID-19 hospitalization for those presenting with at least one comorbidity were 8%, 17%, 32%, and 50% for individuals aged 50–59 years, 60–69, 70–79, and > 80 years old, respectively (22).
Eleven out of the 28 included studies examined the association between underlying conditions and the risk of dying during hospitalization for COVID-19 (Table 2) (6,9–12,26,28,30–32,34). Among these studies, 9 out of 12 examined the association between hypertension and the risk of dying (6,9–11,28,30,32–34); risks ranged from an hazard ratio (HR) of 0.89 (95% confidence interval [CI] 0.85; 0.93) (11) to an odds ratio (OR) of 4.08 (95% CI 1.58; 10.5) (34). Ten out of 12 studies (6,9–11,26,28,30–33) examined the association between diabetes and the risk of dying; risks ranged from RR 1.23 (95% CI 0.77; 1.95) (28) to OR 2.85 (95% CI 1.35; 6.05) (32). The association between COPD and the risk of dying was examined in 8 out of 12 studies (6,9–11,26,28,32,33); the risks of dying ranged from RR 1.05 (95% CI 0.67; 1.65) (28) to OR 2.68 (95% CI 1.42; 5.05) (10). Four of the 12 studies (9,12,33,34) examined the association between CVD and risk of dying during the COVID-19 hospitalization; the risks of dying ranged from HR 0.95 (0.74; 1.21) (9) to OR 2.46 (95% CI 0.76;8.04).(34) Lastly, 5 out of 12 studies (6,9,11,26,33) examined the association of chronic kidney disease with the risk of dying; these risks ranged from HR 1.49 (0.96; 2.31) (9) to HR 2.78 (95% CI 2.19; 3.53]) (6).
Table 2.
In-hospital Risk of Dying Associated With Particular Chronic Conditions
| Author | Hypertension | CVD | Diabetes | Pulmonary Disease | Kidney Disease | Heart Failure | |
|---|---|---|---|---|---|---|---|
| Odds ratio | Atkins | 1.38 (1.13;1.68) | 0.95 (0.74;1.21) | 1.73 (1.36; 2.22) | 1.58 (1.17; 2.15) | 1.49 (0.96;2.31) | |
| Du | 4.08 (1.58;10.5) | 2.46 (0.76;8.04) | |||||
| Harrison | 1.11 (0.96;1.27) | 1.24 (1.08;1.43) | 2.13 (1.84;2.48) | 1.42 (1.21;1.87) | |||
| Zhang | 2.44 (1.50;3.95) | ||||||
| Zhou | 3.05 (1.57;5.92) | 2.85 (1.35;6.05) | 5.40 (0.96;30.4) | ||||
| Hazard ratio | Graselli | 1.68 (1.53;1.84) | 1.66 (1.47;1.88) | 2.03 (1.59;2.59) | 2.78 (2.19;3.53) | 1.66 (1.48;1.87) | |
| Guan | 1.58 (1.07;2.32) | 1.59 (1.03;2.45) | 2.68 (1.42;5.05) | ||||
| Williamson | 0.89 (0.85;0.93) | 1.95 (1.83;2.07) | 1.63 (1.55;1.71) | 2.52 (2.33;2.72) | 1.17 (1.12;1.22) | ||
| Wu | 1.70 (0.92;3.14) | 1.58 (0.80;3.13) | |||||
| Yan | 1.53 (1.02; 2.30) | ||||||
| Relative risk | Tartof | 1.29 (0.83;2.02) | 1.23 (0.77;1.95) | 1.05 (0.67;1.65) | 0.89 (0.58;1.35) |
Note: CVD = Cardiovascular disease.
Ten out of the 12 studies reported an association between older age and increased mortality in individuals with COVID-19 (Supplementary Table 2) (6,9,11,12,23,26,28,30,32,34). Four of the 12 studies examined the risk of dying using age as a stratified variable (6,9,11,28). One of the studies reported risk of dying, HR 1.91 (95% CI 1.63; 2.24); 2.98 (95% CI 2.56; 3.46); and 4.25 (95% CI 3.68; 4.92) for individuals aged 56–63 years old, 64–69 years, and 70 years and older, respectively (6). Another study reported risk of dying, HR 2.40 (95% CI 2.16; 2.66); 6.08 (95% CI 5.52; 6.69); and 20.61 (95% CI 18.72; 22.70) for individuals aged 60–69 years; 70–79 years; and ≥ 80 years, respectively, as compared to those aged < 60 years (11). Only one study that included the Charlson index reported the association between Charlson index scores and adverse outcomes (33). Charlson Index score index was a logarithmic multiplier when the risk of death was assessed by increasing by one point the score, starting from the score of 2 (33). No actual ORs were reported in this study (33).
Association Between Comorbidity Burden and Mortality by Age Group
Only one study included in our literature review reported the association between the burden of multimorbidity and risk of dying during hospitalization for COVID-19 further stratified by the patient’s age (Table 3) (10). This study included 1590 confirmed COVID-19 cases hospitalized across China (mean age: 49 years; 43% women). The most prevalent chronic conditions in this cohort were hypertension (17%) and diabetes (8%) and, 8% of the study sample reported having 2 or more chronic conditions. Among individuals aged <65 years, risks of dying were 2 times and 3 times higher for those with one and 2 or more chronic conditions as compared to those < 65 years without comorbidities, respectively (10). The risks of dying during hospitalization for those 65 years and older were 1.8 and 2.7 times higher for those with one and 2 or more chronic conditions as compared to those ≥65 years without comorbidities, respectively (Table 3) (10). One study reported the association between more prevalent chronic conditions and the risk of dying across different age groups. The study included 31 461 (mean age 50 years; 54.5% women) COVID-19 patients hospitalized in 24 health care organizations across the United States (26). The most common comorbidities were chronic pulmonary disease (17.5%) and diabetes mellitus (15.0%) (26). Among individuals less than 70 years old, the risk of dying was 1.6, 1.4 and twice the risk in those presenting with HF, pulmonary disease, and renal disease as compared to those without these chronic conditions, respectively. The risks of dying in those ≥ 70 years were 1.3 and 1.9 times higher in those with HF and renal disease, as compared to those without these conditions, respectively (26). Somewhat similar results were reported in a recent investigation that examined the association of obesity and risk of dying at 21 days after hospital admission among 6916 patients with COVID-19 (mean age: 49 years; 45% men) at Kaiser Permanente Southern California (28). The risk of dying was most striking among those aged 60 years or younger, with a 12 times higher risk of dying in individuals ≤60 years and a BMI of ≥45 kg/m2 as compared to those in the same age group with a BMI of less than 24 kg/m2 (28). The risk of dying for those older than 60 years was 3 times higher for those in the highest BMI group as compared to those with a BMI of less than 24 kg/m2 (28).
Table 3.
Association Between Comorbidity Burden or BMI and Risk of Dying During Hospitalization for COVID-19 by Age Group
| Author | Risk of Dying by Age Group | |||
|---|---|---|---|---|
| Guan et al. | <65 years | ≥65 years | ||
| 1 morbidity | ≥2 morbidities | 1 morbidity | ≥2 morbidities | |
| Hazard ratio | 2.21 (1.23; 3.96) | 3.33 (1.56; 7.13) | 1.80 (0.91; 3.55) | 2.72 (1.41; 5.27) |
| Tartof et al. | ≤60 | >60 | ||
| BMI 40–44 kg/m2 | BMI ≥ 45 kg/m2 | BMI 40–44 kg/m2 | BMI ≥ 45 kg/m2 | |
| Relative risk | 17.14 (3.37; 87.27) | 12.35 (2.28; 66.77) | 1.25(0.43; 3.61) | 3.03 (1.15; 8.00) |
| Harrison et al. | 50–69 years | |||
| Heart failure | Pulmonary disease | Diabetes | Kidney disease | |
| OR | 1.62 (1.21; 2.18) | 1.35 (1.07; 1.71) | 1.17 (0.93; 1.47) | 2.23 (1.72; 2.89) |
| 70–90 years | ||||
| Heart failure | Pulmonary disease | Diabetes | Kidney disease | |
| OR | 1.34 (1.10; 1.64) | 1.12 (0.93; 1.35) | 0.98 (0.82; 1.18) | 1.88 (1.57; 2.25) |
Note: No comorbidity, 18.5–24 kg/m2, no heart failure, no pulmonary disease, no diabetes, no kidney disease are the referent groups, respectively. BMI = Body mass index; OR = Odds raio.
Discussion
The reviewed literature suggests a high prevalence of chronic conditions in patients with COVID-19 and a significant association between chronic conditions and adverse outcomes in this population. In this rapid review, we found a significant prevalence of chronic conditions in individuals hospitalized with COVID-19; the most frequent morbidities reported were hypertension, diabetes mellitus, CVD, chronic pulmonary disease, and chronic kidney disease. Findings from our review also suggest a higher risk of dying during in men versus women hospitalized with COVID-19. Whereas our findings highlight the high prevalence of chronic conditions in patients hospitalized with COVID-19, and the association of these conditions with an increased risk of dying, very limited data are currently available on the magnitude and impact of burden of multimorbidity on the risk of dying in older adults. Only one of the studies included in our review examined the interaction between comorbidities and the risk of dying stratified by age group (<65 years and 65 years and older individuals) (10). Findings of this investigation suggest a similar increased risk of dying in those < than 65 and those aged 65 years and older as compared to those without comorbidities, but with a higher impact in the younger group. Somewhat similar trends were reported in 2 recent investigations, one that examined the association of chronic conditions and the risk of dying in COVID-19 patients across 24 health care organizations across the United States (26) and another that examined the association of obesity and risk of dying in COVID-19 patients at Kaiser Permanente Southern California (28). This was the only study included in our review that examined the association of obesity as a risk factor for adverse outcomes in patients hospitalized with COVID-19 stratified by age group. The studies by Harrison et al. and Tartof et al. suggest a higher impact in the association of obesity, HF, pulmonary disease, diabetes and renal disease, and the risk of dying in the younger group as compared to those without these chronic conditions (26,28). However, due to the considerable variation in existing approaches to characterizing the burden of multimorbidity in older adults with COVID-19, any comparison across different studies with the objective of making inferences on the association of burden of multimorbidity and adverse outcomes based on the current literature is challenging. Clinicians and health care delivery systems need clinically actionable approaches for characterizing and understanding the implications of multimorbidity to guide the care of patients with COVID-19.
As the COVID-19 pandemic continues, there is an increasing risk of overwhelming health care facilities and jeopardizing patient care. Findings from this literature review highlight the importance of future areas of research. Different approaches have been implemented to assess multimorbidity in COVID-19 patients; however; it is still unclear which is the best approach for characterizing and understanding the implications of multimorbidity in older adults with COVID-19. One important limitation of some multimorbidity assessment approaches is the lack of incorporating severity of illness distinctions for chronic conditions. Some chronic conditions which predict worse disease progression have already been identified, including hypertension, diabetes, COPD, and chronic kidney disease. However, there is no information on which dyads or triads of chronic conditions are more prevalent in COVID 19 patients and how these dyads or triads might impact adverse outcomes (40). Lastly, a high proportion of individuals with COVID-19 are older adults; however, there is no information on how very prevalent geriatric syndromes, namely mobility impairment, functional limitation or frailty, impact adverse outcomes. Data on the interplay between multimorbidity, mobility/functional limitation, and the risk of adverse events in older patients with COVID-19 are necessary to improve the care of this vulnerable population.
Study Strengths and Limitations
This literature review included 28 peer-reviewed publications and MMWR reports reviewed internally. The methods used throughout included a comprehensive search strategy of multiple databases. A number of limitations of our review must be acknowledged, however, in interpreting the present results. This review was limited to studies published in English. The extent to which our inability to review studies published in languages other than English affected our findings is unknown. Since our review included only peer-reviewed publications and MMWR reports reviewed internally at the CDC, there is a potential for introducing possible publication bias. Variability in definitions of risk factors and the potential for differential ascertainment of chronic conditions, chart review versus patient reports, possibly contributed to the heterogeneity in the affected comparisons. Most of the studies included in our review described very sick hospitalized populations. Inasmuch, the findings from these studies might be not generalizable to community-based settings. Differing lengths of follow-up may have also resulted in heterogeneity, and studies that were deemed to have an inadequate length of follow-up may have missed events and biased the results toward smaller effect sizes. Finally, since more than half of the included studies were from early reports in Wuhan, China, the generalizability of these findings to other race/ethnic groups may be limited. Future studies should examine potential racial and ethnic differences in the magnitude and impact of multimorbidity on mortality in older adults hospitalized with COVID-19.
Conclusions
There has been a traditional, siloed, single-condition approach to consideration of how chronic conditions and advancing age relate to COVID-19 outcomes. A more complete picture of the impact of burden of multimorbidity and the interaction with advancing patient age is needed.
Funding
This work was supported by the National Institute on Aging (grant numbers R33AG057806 and R01AG062630 to J.G. and M.T.) and the National Heart, Lung, and Blood Institute (R01HL35434 and U01HL105268 to R.G.).
Author Contributions
Study concept and design: M.T. and J.H.G.; Acquisition of data: M.T., C.D., T.H., and C.C. Preparation of manuscript: M.T., C.D., T.H., J.H.G., and R.J.G. Critical revision of the manuscript: J.H.G., R.J.G., C.C., M.T., and C.D.
Conflict of Interest
None declared.
Supplementary Material
References
- 1. Coronaviridae Study Group of the International Committee on Taxonomy of V. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi:10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---30-november-2020. Accessed April 23, 2020.
- 3. WHO. Coronavirus Disease (COVID-19) Situation Report – 168 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- 4. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): Older Adults. https://www.cdc.gov/coronavirus/2019-ncov/community/retirement/checklist.html. Accessed November 22, 2020.
- 5. Arentz M, Yim E, Klaff L, et al. . Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi:10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grasselli G, Greco M, Zanella A, et al. . Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi:10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis on 54 mortality cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35(12):e132. doi:10.3346/jkms.2020.35.e132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. doi:10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 9. Atkins JL, Masoli JAH, Delgado J, et al. . Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224–2230. doi:10.1093/gerona/glaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan WJ, Liang WH, Zhao Y, et al. . Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547 . doi:10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson EJ, Walker AJ, Bhaskaran K, et al. . OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;11:430–436. doi:10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Wang X, Jia X, et al. . Risk factors for disease severity, unimprovement, and mortality of COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020. doi:10.1016/j.cmi.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. doi:10.1371/journal.pmed.1000097 [PMC free article] [PubMed] [Google Scholar]
- 14. Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10. doi:10.1186/2046-4053-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckner FS, McCulloch DJ, Atluri V, et al. . Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis. 2020;71:2167–2173. doi:10.1093/cid/ciaa632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen T, Wu D, Chen H, et al. . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grasselli G, Zangrillo A, Zanella A, et al. . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi:10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020. doi:10.1016/j.jinf.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMichael TM, Clark S, Pogosjans S, et al. . COVID-19 in a long-term care facility - king county, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:339–342. doi:10.15585/mmwr.mm6912e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson S, Hirsch JS, Narasimhan M, et al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020. doi:10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stokes EK, Zambrano LD, Anderson KN, et al. . Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi:10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suleyman G, Fadel R, Malette K, et al. . Clinical characteristics and morbidity associated with Coronavirus Disease 2019 in a series of patients in metropolitan detroit. JAMA. 2020. doi:10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gold J, Wong K, Szablewski C, et al. . Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545–550. doi:10.15585/mmwr.mm6918e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrison S, Fazio-Eynullayeva E, Lane D, Underhill P, Lip G. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020. doi:10.1371/journal.pmed.1003321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323:2195–2198. doi:10.1001/jama.2020.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tartof S, Qian L, Hong V, et al. . Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. doi:10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wortham JM, Lee JT, Althomsons S, et al. . Characteristics of PERSONS WHO DIED with COVID-19 - United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:923–929. doi:10.15585/mmwr.mm6928e1 [DOI] [PubMed] [Google Scholar]
- 30. Wu C, Chen X, Cai Y, et al. . Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. doi:10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan Y, Yang Y, Wang F, et al. . Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1). doi:10.1136/bmjdrc-2020-001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M; et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the italian society of hypertension. Hypertension. 2020;72(2):366–372. doi:10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 34. Du RH, Liang LR, Yang CQ, et al. . Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5). doi:10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagner AH, Kiwala S, Coffman AC, et al. . CIViCpy: a python software development and analysis toolkit for the CIViC knowledgebase. JCO Clin Cancer Inform. 2020;4:245–253. doi:10.1200/CCI.19.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi:10.1002/jmv.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang JJ, Dong X, Cao YY, et al. . Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020. doi:10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 38. Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020. doi:10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang J, Zheng Y, Gou X, et al. . Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi:10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tinetti M, Fried T, Boyd C. Designing healthcare for the most common chronic condition-multimorbidity. JAMA. 2012;307:2493–2494. doi:10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.