Abstract
Objectives
To present an index case and review the histologic and electron microscopic findings in chloroquine (CQ) and hydroxychloroquine (HCQ) myopathy, focusing primarily on cardiomyopathy. CQ and HCQ are antimalarial drugs with disease-modifying activity in rheumatic diseases (DMARD) and now are among the most widely used DMARDs. Although they are rare, severe adverse effects caused mainly by deposition of intracellular metabolites in both cardiac and skeletal muscle have been described. Currently, both CQ and HCQ have been proposed to have efficacy for patients with coronavirus disease 2019, and several large centers in the United States and other countries have started clinical trials.
Methods
A case of HCQ cardiotoxicity diagnosed on an endomyocardial biopsy is presented. A review of the pathology archives was performed to identify additional cases of CQ or HCQ myopathy, and histologic changes were recorded. A brief literature review with an emphasis on pathologic findings in myopathies was performed.
Results
Including the index case, 4 cases of CQ or HCQ myopathy were identified. Light microscopic findings included vacuolated myopathy, and electron microscopic findings included myeloid bodies and curvilinear inclusion bodies.
Conclusion
CQ and HCQ myopathy can present following long-term administration of the drug. The pathologic findings are nonspecific and overlap with other vacuolated myopathies, necessitating careful correlation of the histologic changes with the patient’s medical history.
Keywords: Chloroquine, Hydroxychloroquine, Cardiomyopathy, Myopathy, COVID-19
Key Points.
This review identified the common light and electron microscopic findings in hydroxychloroquine and chloroquine cardiomyopathy.
Light microscopic findings commonly include vacuolar myopathy, which invokes a broad differential diagnosis.
Accurate diagnosis can be achieved by careful correlation of light and electron microscopic findings with clinical history.
Historically, chloroquine (CQ) and hydroxychloroquine (HCQ) have been used for the treatment and prophylaxis of malaria. The mechanism of action of CQ and HCQ has not been completely elucidated; however, studies show inhibition of both cellular functions and molecular pathways involved in immune activation.1 CQ and HCQ have also been shown to have utility as disease-modifying antirheumatic drugs (DMARD) for the treatment of rheumatic diseases including rheumatoid arthritis, systemic lupus erythematosus, antiphospholipid syndrome, and primary Sjögren syndrome, among others.1,2 In addition to the antimalarial and antirheumatologic properties, potential antiviral activity of CQ and HCQ has been identified.3 Several small clinical trials and uncontrolled case series are emerging in the literature on CQ and HCQ use in patients with coronavirus disease 2019 (COVID-19).4-6
CQ and HCQ are typically well tolerated, and after several years in clinical use as antimalarial drugs and DMARDs, their accumulated safety data are reassuring, but they are not without notable side effects. The most common side effects are gastrointestinal, including nausea and vomiting, whereas more serious adverse effects include retinopathy, ventricular arrhythmias, cardiomyopathies, and skeletal muscle toxicity.7-11 These serious effects are thought to be cumulative but reversible and occur after long-term use.2,12 HCQ is a CQ derivative with an additional hydroxyl group and is approximately 40% less toxic than CQ in animal models. CQ overdose in adults has a mortality risk of about 20%, whereas HCQ is 2- or 3-fold less toxic.
Recently, we diagnosed chronic HCQ-induced cardiotoxicity in a patient with heart failure and a history of long-term HCQ use who then underwent cardiac transplantation. Given the recent rise in prominence of both CQ and HCQ as potential therapy for the emerging COVID-19 pandemic, we present this index case, a short series of cases from the pathology archives, and a review of the literature on pathologic changes observed in CQ- or HCQ-induced myopathies. To the best of our knowledge, this report is only the third of a patient requiring cardiac transplant because of CQ or HCQ toxicity.13,14
Case Presentation
The patient, a 60-year-old woman, was referred to an academic medical center for evaluation for possible cardiac transplant. The patient had a history of connective tissue disease, originally diagnosed as scleroderma approximately 20 years before admission. She was treated by HCQ 200 mg twice daily and nonsteroidal anti-inflammatory drugs, with adequate symptomatic control. Two years before admission, the patient presented with myocardial infarction (MI) and was found to have complete occlusion of the distal left anterior descending coronary artery. Cardiac magnetic resonance imaging showed limited infarct and overall normal left ventricle systolic function, and the patient was medically managed. A subsequent angiogram showed restoration of flow. She was discharged with no wall motion abnormality on an echocardiogram. Two months after her MI, she continued to have shortness of breath and peripheral edema and was diagnosed with heart failure with preserved ejection fraction.
Before her admission, it was discovered that the patient had new-onset complete heart block following a syncopal event, and a pacemaker was placed. Echocardiogram showed massive tricuspid valve regurgitation, diastolic left ventricular dysfunction, systolic and diastolic right ventricular dysfunction, and restrictive cardiomyopathy. 99m-Tc pyrophosphate echocardiogram showed findings suspicious for transthyretin-associated familial cardiac amyloidosis with abnormal localization of activity within the left ventricular myocardium. By the time of the current admission, the patient had developed cardiogenic shock and a balloon pump was placed. A diagnostic endomyocardial biopsy was performed, and 14 days after admission she underwent cardiac transplant.
Light and Electron Microscopic Findings in Endomyocardial Biopsy From the Index Case
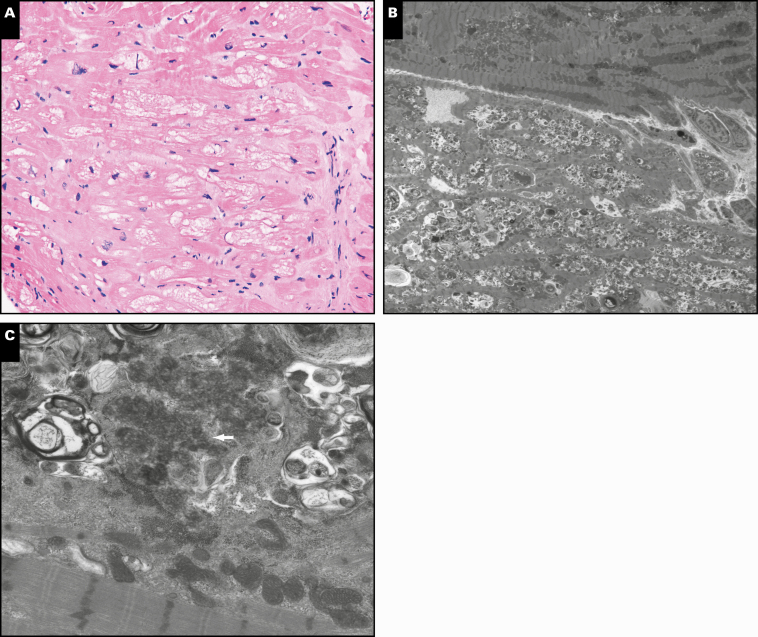
H&E sections revealed myocyte vacuolization, mild myocyte hypertrophy, and focal interstitial fibrosis Image 1A. No necrosis or myofiber inflammation was identified. Electron microscopy showed extensive lysosomal inclusions with partly whorled lamellar (myeloid) bodies, amorphous cytoplasmic debris, and curvilinear inclusions Image 1B and Image 1C. Amyloid deposition was not identified by light microscopy utilizing Congo red stain, nor was it identified on electron microscopy.
Image 1.
A, Endomyocardial biopsy showing vacuolated cardiomyocytes, mild myocyte hypertrophy, and interstitial fibrosis (H&E, ×200). B, At low power, the performed ultrastructural studies show extensive lysosomal-type vacuoles and debris as correlates of the vacuolated changes seen on the formalin-fix, paraffin-embedded sections (×8,000). C, At higher power the ultrastructural studies show that the lysosomal vacuoles and debris are associated with myelinoid debris and curvilinear bodies (arrow, ×12,000).
Gross and Light Microscopic Findings in Explanted Heart From the Index Case
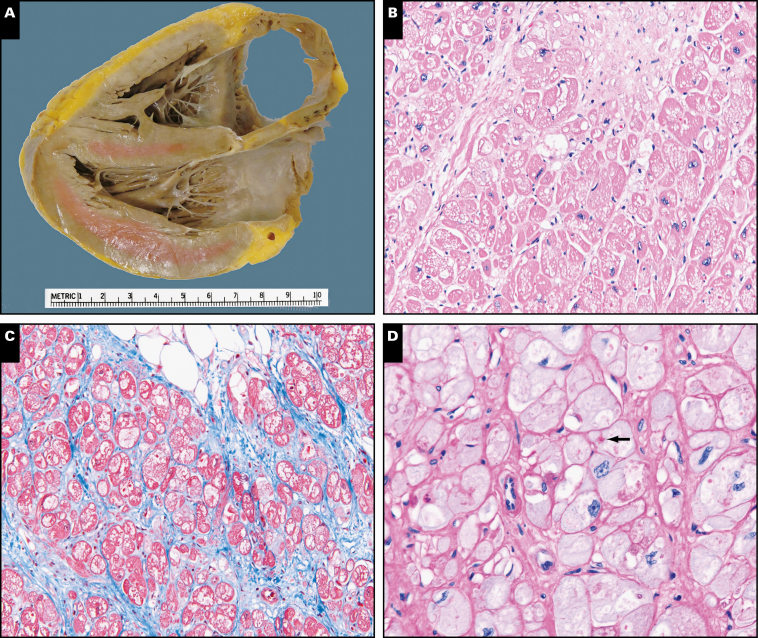
The explanted heart weighed 338 g and exhibited a smooth serosal surface with a mild to moderate amount of epicardial fibroadipose tissue. The coronary arteries were patent, with only minimal calcific atherosclerosis (maximum stenosis of 20% in the right main coronary artery). A 4-chamber cut did not reveal obvious dilation of the chambers Image 2A. A 1.0-cm fibrotic area was identified at the apex, consistent with healed infarct. The right ventricular wall thickness was 0.6 cm, and the left ventricular wall thickness was 1.4 cm. Histologic examination revealed diffuse transmural vacuolar change, moderate myocyte hypertrophy, and mild to moderate interstitial fibrosis Image 2B. Repeated Congo red stain for amyloid was negative. Trichrome stain highlighted interstitial fibrosis, and periodic acid Schiff (PAS) stain with diastase revealed a few PAS-positive vacuoles Image 2C and Image 2D.
Image 2.
A, Explanted heart gross image of a 4-chamber cut showing a mild amount of epicardial fibroadipose tissue and no obvious dilation of the atria or ventricles. B, Explanted heart showing diffuse vacuolization of the cardiac myocytes (H&E, ×200). C, Explanted heart with moderate interstitial fibrosis (blue; trichrome, ×200). D, Explanted heart showing periodic acid Schiff-diastase resistant bodies (arrow) within the vacuolated spaces (×200).
Pathology Archive Review
After diagnosis of the index case, a search of the pathology archives at the University of Chicago was performed. The aim was to find other cases of CQ or HCQ myopathy in either heart or skeletal muscle. The queried years were from 1990 to 2020, and a total of 4 potential additional cases were identified. One other case of cardiac CQ or HCQ toxicity was found and was characterized by rimmed vacuolar myopathy and curvilinear bodies. Three unequivocal cases of HCQ or CQ toxicity in skeletal muscle were found. The findings in skeletal muscle were similar to those seen in heart biopsies: fiber vacuolation, basophilic stippling, and myocyte hypertrophy. A full description of the archived cases can be found in Table 1.
Table 1.
Pathologic Findings Identified in Archived CQ- or HCQ-Induced Myopathies
| Case | CQ/HCQ Use | Specimen | Light Microscopic Findings | Special Stains | Electron Microscopic Findings |
|---|---|---|---|---|---|
| 1 (index case) | HCQ, >20 y | Cardiac biopsy | Vacuolar myopathy | Congo red, negative | Myeloid and curvilinear bodies |
| 2 | HCQ, 9-11 y | Cardiac biopsy | Vacuolar myopathy Mild to moderate myocyte hypertrophy and minimal interstitial fibrosis |
Congo red, negative | Abnormal lysosomal debris with whorled lamellar structure (myeloid body), debris is also associated with cytoplasmic glycogen Curvilinear inclusions Hypercontraction changes Lipofuscin deposits |
| 3 | HCQ, duration unknown | Right thigh muscle biopsy | Vacuolar myopathy | None performed | Lysosomal membranous debris Membrane-bound glycogen |
| 4 | HCQ, duration unknown | Quadriceps biopsy | Vacuolar myopathy | Trichrome: Increased granular staining Diffusely increased acid phosphatase reactivity |
Myeloid bodies |
| 5 | HCQ, 6 y | Left vastus lateralis Left deltoid |
Vacuolar myopathy Focal perimysial mononuclear inflammatory cell infiltrates |
Not performed | Myeloid and curvilinear bodies |
CQ, chloroquine; HCQ, hydroxychloroquine.
Discussion
Multiple mechanisms of actions for quinine drugs have been proposed; regardless of mechanism, the final problem lies with the accumulation of metabolites in the cytosol, which leads to several deleterious downstream effects. The first and most accepted mechanism is the disruption of the lysosomal autophagy pathway. CQ/HCQ is lysosomotropic and accumulates in lysosomes causing an increase in pH, which inhibits lysosomal enzymes, like hydroxylases.14 This impairs macroautophagy, a process by which cellular organelles and proteins are degraded by lysosomal enzymes.15 CQ and HCQ inhibit lysosome-autophagosome fusion and prevent endosomal acidification, which consequently leads to the accumulation of metabolic products in the cytosol.16 Autophagy also has secondary deleterious effects on mitochondrial function including a decrease in oxidative phosphorylation and increased mitochondrial DNA damage.17 Inhibition of autophagy results in increased apoptosis, leading to myofiber necrosis—a feature seen in both cardiac and skeletal muscle biopsies.18 The other proposed mechanism of HCQ toxicity is direct binding of the hydrophobic region of the HCQ molecule membrane phospholipids, causing neutralization of phosphate groups and displacement of calcium. This pathway also leads to myofiber necrosis through alterations in the cell membrane.1,17,19
HCQ cardiotoxicity can happen in both acute and chronic settings Table 2. Although chronic toxicity is a well-recognized side effect of the drug, the acute toxicity can be just as devastating, if not more so, but the number of studies is limited.20-24 In acute settings, side effects are caused primarily by HCQ acting as a Vaughan Williams class 1a antiarrhythmic with “quinidine-like” effect. HCQ causes the blockage of sodium, calcium, and potassium channels of the cardiac myocyte.2 Sodium channel blockade leads to a widened QRS-interval and increases the risk of developing tachyarrhythmias. Potassium channel blockade delays ventricular repolarization and prolongs the QT interval, increasing the risk of ventricular arrhythmias, including Torsade de pointes. The risk of acute toxicity is highest shortly after drug use, given high circulating drug levels, before its distribution to the tissue. Recently, in a double-blinded, randomized, phase 2b clinical trial for COVID-19 patients, the high CQ dose arm—in which the patients were given a total of 12 g CQ over a period of 10 days—had to be halted because of acute cardiotoxicity from arrhythmias and QTc prolongation compared with the baseline electrocardiogram.25 Treatment with CQ or HCQ should be reconsidered in cases of previously known myocardial disease.
Table 2.
Cardiac Complications Caused by Chloroquine or Hydroxychloroquine (Adapted From Chatre et al9)
| Measure | Change |
|---|---|
| Electrocardiogram |
Atrioventricular block (first, second, or third degree) Sick sinus syndrome |
| Right and/or left bundle-branch block | |
| Ischemic changes or myocardial infarction | |
| Quinidine-like effect | |
| Echocardiogram |
Valvular regurgitation (mitral, aortic, or tricuspid) Right heart failure or pulmonary artery wall hypertrophy |
| Left ventricular hypertrophy, biventricular hypertrophy, restrictive cardiomyopathy | |
| Ventricular hypokinesia | |
| Diastolic dysfunction | |
| Decreased left ventricular ejection fraction | |
| Valvular regurgitation (mitral, aortic, or tricuspid) |
A systematic review of the literature showed that the cumulative duration of HCQ causing chronic cardiotoxicity is about 7 years.26 Arrhythmias are the most common acute cardiac side effect, likely secondary to quinidine-like effects. In comparison, chronic rhythm abnormalities are caused by direct damage of the conduction system. The mechanism of damage includes intracellular deposition of metabolites, disruption of autophagy, myofiber necrosis, and mitochondrial damage in the conduction system. Patients with rheumatoid arthritis who used HCQ for longer than 14 years had a more than 3-fold increase in the risk of developing chronic heart failure compared with the general population.7 Left ventricular hypertrophy or biventricular or biatrial dilatation with concentric hypertrophy with a restrictive filling pattern can be seen with long-term CQ or HCQ use.9 Other cardiopulmonary adverse effects include hypokinesia (9.4%), pulmonary arterial hypertension (3.9%), and valvular dysfunction (7.1%). Typically, the damage is proposed to depend on both cumulative dosage and the time over which the dosage is received.9
The histomorphology of the tissue damage caused by CQ or HCQ Table 3 is identical in all striated muscles, whether cardiac or skeletal.27 Vacuolated myopathy is the most consistent morphologic feature on H&E.9 The vacuoles in CQ and HCQ toxicity are clear with a rim (have granular basophilic material around the vacuole). These autophagic vacuoles are membrane-bound vacuoles that contain cytoplasmic degradation products and exhibit acid phosphatase activity (can be highlighted by acid phosphatase enzymatic stain) indicative of lysosomal dysfunction. More common histochemical techniques may be useful in establishing a diagnosis of CQ or HCQ toxicity, including PAS stain.9 The rim of these vacuoles can also be emphasized by either LC3 or p62 immunohistochemistry (not done in our patient).28,29 Neither LC3 nor p62 positivity is pathognomonic for CQ or HCQ toxicity and serves only as a marker for autophagy. Vacuolation may yield an appearance of myocyte hypertrophy and may lead to disorganization of the myofibrillar architecture.
Table 3.
Histologic and Ultrastructural Findings, Pathophysiology, and Differential Diagnosis in CQ/HCQ Cardiotoxicity
| Histology | Pathophysiology | Differential Diagnosis |
|---|---|---|
| Light microscopy | ||
| Myocyte vacuolization (100%) | Lysosomal storage of glycolipids (PAS positive) | Storage disorders (Fabry, Pompe) Ischemic heart disease Drug toxicity (amiodarone, cobalt, perhexiline maleate) Danon disease Mitochondrial disorders |
| Myofiber necrosis | Secondary to autophagic function | Ischemic heart disease |
| Myocyte hypertrophy | Not known | Storage disorders |
| Interstitial fibrosis | Not known | Amyloid |
| Electron microscopy | ||
| Lamellar structures (myeloid bodies/zebra bodies) (83%) | Accumulated glycolipids and glycoproteins | Fabry disease |
| Curvilinear body (68%) | Accumulated autophagolysosomes with poorly digested intralysosomal contents | Neuronal ceroid lipofuscinosis |
| Mitochondrial abnormalities | Damage of mitochondrion secondary to autophagic dysfunction | Mitochondrial myopathies |
| Abundant secondary lysosomes | Ineffective lysosomal machinery | Autophagic myopathies |
CQ, chloroquine; HCQ, hydroxychloroquine.
Another ubiquitous feature of vacuolated myopathies is an increase in the number of inclusion bodies and vacuoles as seen on ultrastructural examination (Table 3).29 The rimmed vacuoles are not truly empty; rather, they consist of 15- to 18-nm tubulofilaments and are surrounded by a rim composed of autophagic material, as confirmed by the punctate staining pattern of LC3 and p62 immunohis-tochemistry.29,30 Ultrastructural examination by electron microscopy demonstrates the accumulation of lamellar lysosomal inclusions (also known as myeloid bodies), curvilinear bodies, abundant secondary lysosomes, and megamitochondria in CQ and HCQ toxicity.30
Vacuolated myopathies have been extensively described in a multitude of diseases in which autophagic impairment is the key cause.29 They have the same histologic features (fiber vacuolation, basophilic stippling) and an increase in the number of LC3- and p62-positive puncta.29 The common differential diagnosis of CQ or HCQ toxicity in the heart includes storage disorders like Fabry disease and adult-onset Pompe disease (acid maltase deficiency), drug-induced myopathy (amiodarone, cobalt, and perhexiline maleate), genetic diseases (Danon disease and other mitochondrial disorders), and ischemic heart disease.29-32 The differential diagnosis for skeletal muscle biopsies is similar but also includes adult-onset Pompe disease, autophagic myopathies, and inclusion body myositis.31,33
On electron microscopy, the presence of myeloid and zebra bodies in CQ and HCQ toxicity confounds the differential with other diseases. In the setting of Fabry disease, myeloid and zebra bodies are inclusions consisting of whorled layers of alternating dense and pale material composed of accumulated glycolipids and glycoproteins due to inhibition of intralysosomal α-galactosidase A activity. Although these inclusions are highly characteristic of Fabry disease, they are nonspecific and may be seen in other metabolic disorders and the majority of patients with CQ or HCQ toxicity. Cases of combined Fabry disease and connective tissue diseases, including systemic lupus erythematosus, have been described, leading to misdiagnosis of Fabry disease as a rheumatologic disease.34,35 This misdiagnosis has resulted in some cases of Fabry disease being treated as a rheumatologic disorder with HCQ or CQ therapy.36 Because of the clinical similarity between the 2 conditions, Fabry disease must be precluded by screening, especially if there is family history, neurologic or kidney involvement, or the presence of only myeloid bodies and no curvilinear bodies on biopsy. However, like most storage disorders, biochemical tests and genotyping are needed for a definite diagnosis of Fabry disease, and biopsy plays only a supportive role.
Also observed on electron microscopy in cases of HCQ and CQ toxicity are curvilinear inclusion bodies, which are “comma-shaped” structures.30 The differential diagnosis of curvilinear inclusion bodies includes neuronal ceroid lipofuscinosis, a rare group of hereditary neurodegenerative lysosomal storage diseases that result from excessive accumulation of lipopigments (lipofuscin) in the body’s tissues.37,38 Although not necessarily true for all types of neuronal ceroid lipofuscinosis or HCQ or CQ toxicity, in CLN3 disease (the most frequent neuronal ceroid lipofuscinosis), curvilinear inclusion bodies are structurally composed of autophagolysosomes with poorly digested intralysosomal accumulations of subunit C of mitochondrial ATP synthase.30 The presence of curvilinear inclusion bodies is helpful because they have not been described in Fabry diseases.34 It should be borne in mind that these bodies are somewhat challenging to identify because they require higher magnification (>×30,000) on electron microscopy and a pathologist who is familiar with these structures.39 These inclusion bodies have also been described in the retina,25 in peripheral nerve ganglia, and in skeletal muscle.27 However, the presence of curvilinear bodies is not a uniform finding in cases of HCQ-induced toxicity (present in only 68% of patients).9 When present, they serve as an important diagnostic clue for CQ and HCQ toxicity because they are not seen in Fabry disease and other toxic cardiomyopathies.34
Another disease that can affect both cardiac and skeletal muscle is adult-onset Pompe disease, a glycogenosis due to deficiency of lysosomal acid α-glucosidase, causing glycogen storage disease type 2 (acid maltase deficiency). Biopsies show a PAS-positive vacuolopathy.40 Again, the pathologist’s role is key to suggest this differential diagnosis, as the disease is rare, but clinical findings are vague and nonspecific. A description of a patient with HCQ or CQ toxicity incorrectly diagnosed as acid maltase deficiency and treated with enzyme replacement has been published.41 The diagnosis of acid maltase deficiency can be confirmed using an acid α-glucosidase activity enzyme activity assay and the specific mutation identified by sequencing. Because enzyme replacement therapy is now approved by the US Food and Drug Administration for this disease, it is vital to render the correct diagnosis.
Neuromyotoxicity due to HCQ toxicity was first reported in 1965 and is thought to be rare. One prospective study estimated the incidence of HCQ myopathy to be 1.9 per 1,000 patient years.42 Patients with preexisting renal or hepatic disease or advanced age or those receiving chronic drug therapy are more susceptible to developing myopathy and neuropathy secondary to both CQ and HCQ use. HCQ myopathy presents with proximal muscle weakness and normal to mildly elevated creatine kinase levels. Neuromuscular drug toxicity leading to ventilatory failure has also been described.7,43 Clinically, this drug toxicity may be underrecognized given similarity between the symptoms of the disease and drug toxicity. One study suggests the use of lactate dehydrogenase levels as a surrogate marker to detect muscle damage.44 Although electromyography is not sensitive for the diagnosis of CQ or HCQ toxicity, it is nonetheless suitable for monitoring disease evolution from a subclinical to a clinical stage in patients with antimalarial myopathy. The gold standard to confirm the diagnosis of antimalarial myopathy is muscle biopsy. Histologic features, albeit nonspecific, include vacuolar myopathy, predominantly in type 1 myofibers.45-47 It should be noted that the vacuoles arise at least 6 months after the onset of muscle symptoms, so early biopsy may not be diagnostic. Electron microscopy shows curvilinear bodies in the cytoplasm. These can be associated with glycogen, lipofuscin, lysosomes, and myelin figures similar to the findings in the cardiac muscle. The curvilinear bodies are located between myofibrils and at perinuclear zones. Fibers contain large amounts of glycogen, and myelin figures are abundant.
Numerous patients are treated with CQ and HCQ every year; it is unclear why only some patients experience these severe adverse events.42 The presentation of patients with CQ- or HCQ‐induced cardiomyopathy is varied and diverse.7,43 Possible risk factors Table 4 might include long‐term exposure, higher doses, and the use of CQ instead of HCQ; however, a review by Tönnesmann et al27 showed that cumulative dose and the duration of treatment (6-12 years) were not particularly high in the 2 patients who required transplantation. These findings make it difficult to predict which patients are at higher risk of developing toxicity. If cardiomyopathy does develop, it is impossible to foretell which patient will respond to the withdrawal of the drug. Given the variability in clinical presentations, it is postulated that genetic polymorphisms may make some patients more susceptible to CQ and HCQ toxicity.44 A case of hypertrophic cardiomyopathy due to MYBPC3 mutation and HCQ toxicity on endomyocardial biopsy has been described.42 The predictive factors of drug response in different cases are most probably genetic but have not been elucidated.
Table 4.
Risk Factors for Increased Hydroxychloroquine Toxicity
| Female sex |
| Age >60 y |
| Renal dysfunction |
| Lupus nephritis |
| Underlying cardiac disease |
| Prolonged use |
| High dose |
| Decreased exercise |
| Systemic inflammation |
| COX inhibition (nonsteroidal anti-inflammatory drug use) |
CQ- or HCQ-induced cardiomyopathy can be fatal unless it is diagnosed at an early stage.2 If there are multiple confounders in the clinical history, endomyocardial biopsy with an ultrastructural electron microscopy examination is indicated.43,45 Although endomyocardial biopsy is invasive, it remains the gold standard for the diagnosis of CQ and HCQ cardiomyopathy. In addition, the biopsy can be useful for excluding other causes of unexplained cardiomyopathies, such as the cardiac manifestations of connective tissue diseases or adult-onset storage disorders.43,45 Following diagnosis, the drug should be discontinued immediately and replaced with a noncardiotoxic DMARD.45 If the drug is withdrawn quickly enough, complete or at least partial reversal of cardiomyopathy is the most likely outcome.44,45 However, the possibility that a patient with congestive heart failure will recover from New York Heart Association class 4 to class 1 without transplantation has not yet been described in the literature. In patients in whom presentation is confounding and the diagnosis is delayed, severe morbidity such as placement of a pacemaker or even transplantation can be the outcome.13,26,44
Even though there is tissue deposition of the drug, antimalarial myopathy is reversible. Monitoring of HCQ tissue levels after drug withdrawal showed that all tissue levels fell rapidly, by approximately 80% in the first 8 days and by another 50% in the next 7 days, so there was a decrease of approximately 90% in 15 days.48 Treatment cessation in patients with moderate to severe clinical myopathy tended to normalize muscle weakness, muscle enzyme disturbances, and electromyographic changes at 6-month follow-up.45 An observation by Eadie and Ferrier suggests that, overall, patients on higher doses seemed to develop myopathic symptoms more slowly than those on lower doses.49 This could be interpreted as suggesting that individual susceptibility was a more important factor than cumulative toxicity. An animal model showed that CQ-induced myopathy can be treated not only by halting drug use but also by decreasing the autophagy or glycogen synthesis, as glycogen synthase is suggested to play a direct role in regulating autophagy.50
In conclusion, this article presented a case of HCQ cardiotoxicity necessitating transplant and found similar changes identifiable in archival tissue. HCQ and CQ toxicity in both the cardiac and skeletal muscle shows striking light and electron microscopic findings, but the differential diagnosis is broad, and accurate diagnosis requires careful pathologic examination and clinical correlation.
References
- 1. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155-166. [DOI] [PubMed] [Google Scholar]
- 2. Abbasi S, Tarter L, Farzaneh-Far R, et al. Hydroxychloroquine: a treatable cause of cardiomyopathy. J Am Coll Cardiol. 2012;60:786. [DOI] [PubMed] [Google Scholar]
- 3. Savarino A, Boelaert JR, Cassone A, et al. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020. doi: 10.1101/2020.03.16.20037135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [DOI] [PubMed] [Google Scholar]
- 7. Siddiqui AK, Huberfeld SI, Weidenheim KM, et al. Hydroxychloroquine-induced toxic myopathy causing respiratory failure. Chest. 2007;131:588-590. [DOI] [PubMed] [Google Scholar]
- 8. Srinivasa A, Tosounidou S, Gordon C. Increased incidence of gastrointestinal side effects in patients taking hydroxychloroquine: a brand-related issue? J Rheumatol. 2017;44:398. [DOI] [PubMed] [Google Scholar]
- 9. Chatre C, Roubille F, Vernhet H, et al. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919-931. [DOI] [PubMed] [Google Scholar]
- 10. Stein M, Bell MJ, Ang LC. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27:2927-2931. [PubMed] [Google Scholar]
- 11. Dogar MU, Shah NN, Ishtiaq S, et al. Hydroxychloroquine-induced restrictive cardiomyopathy: a case report. Postgrad Med J. 2018;94:185-186. [DOI] [PubMed] [Google Scholar]
- 12. Tönnesmann E, Stroehmann I, Kandolf R, et al. Cardiomyopathy caused by longterm treatment with chloroquine: a rare disease, or a rare diagnosis? J Rheumatol. 2012;39:1099-1103. [DOI] [PubMed] [Google Scholar]
- 13. Costedoat-Chalumeau N, Hulot JS, Amoura Z, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology. 2007;107:73-80. [DOI] [PubMed] [Google Scholar]
- 14. Vallecillo-Hernández J, Barrachina MD, Ortiz-Masiá D, et al. Indomethacin disrupts autophagic flux by inducing lysosomal dysfunction in gastric cancer cells and increases their sensitivity to cytotoxic drugs. Sci Rep. 2018;8:3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook KL, Wärri A, Soto-Pantoja DR, et al. Hydroxychloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer [published correction appears in Clin Cancer Res. 2016 Jun 1;22(11):2825]. Clin Cancer Res. 2014;20:3222-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaanine AH, Gordon RE, Nonnenmacher M, et al. High-dose chloroquine is metabolically cardiotoxic by inducing lysosomes and mitochondria dysfunction in a rat model of pressure overload hypertrophy. Physiol Rep. 2015;3. doi: 10.14814/phy2.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Redmann M, Benavides GA, Berryhill TF, et al. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017;11:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boya P, Gonzalez-Polo RA, Poncet D, et al. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927-3936. [DOI] [PubMed] [Google Scholar]
- 19. Queyriaux B, Carlioz R, Perrier E, et al. Cardiovascular effects linked to the use of chloroquine. Ann Cardiol Angeiol. 2001;50:285-292. [DOI] [PubMed] [Google Scholar]
- 20. Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44:173-175. [DOI] [PubMed] [Google Scholar]
- 21. Fragasso G, Sanvito F, Baratto F, et al. Cardiotoxicity after low-dose chloroquine antimalarial therapy. Heart Vessels. 2009;24:385-387. [DOI] [PubMed] [Google Scholar]
- 22. Abdin A, Pöss J, Kandolf R, et al. Hydroxychloroquine-induced cardiomyopathy in a patient with limited cutaneous systemic sclerosis. Clin Res Cardiol. 2017;106:234-236. [DOI] [PubMed] [Google Scholar]
- 23. Zerbib Y, Guillaumont MP, Touati G, et al. Early cardiotoxicity of hydroxychloroquine. Rev Med Interne. 2016;37:209-211. [DOI] [PubMed] [Google Scholar]
- 24. O’Laughlin JP, Mehta PH, Wong BC. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep Cardiol. 2016;2016:4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borba M, Sampiao F, Sampaio V, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). Preprint posted online April 14, 2020. doi: 10.1101/2020.04.07.20056424 [DOI]
- 26. Freihage JH, Patel NC, Jacobs WR, et al. Heart transplantation in a patient with chloroquine-induced cardiomyopathy. J Heart Lung Transplant. 2004;23:252-255. [DOI] [PubMed] [Google Scholar]
- 27. Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434-442. [DOI] [PubMed] [Google Scholar]
- 28. Daniels BH, McComb RD, Mobley BC, et al. LC3 and p62 as diagnostic markers of drug-induced autophagic vacuolar cardiomyopathy: a study of 3 cases. Am J Surg Pathol. 2013;37:1014-1021. [DOI] [PubMed] [Google Scholar]
- 29. Lee HS, Daniels BH, Salas E, et al. Clinical utility of LC3 and p62 immunohistochemistry in diagnosis of drug-induced autophagic vacuolar myopathies: a case-control study. PLoS One. 2012;7:e36221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Margeta M. Autophagy defects in skeletal myopathies. Annu Rev Pathol. 2020;15:261-285. [DOI] [PubMed] [Google Scholar]
- 31. Engel AG. Acid maltase deficiency in adults: studies in four cases of a syndrome which may mimic muscular dystrophy or other myopathies. Brain. 1970;93:599-616. [DOI] [PubMed] [Google Scholar]
- 32. Raben N, Takikita S, Pittis MG, et al. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand. Autophagy. 2007;3:546-552. [DOI] [PubMed] [Google Scholar]
- 33. Nogalska A, Terracciano C, D’Agostino C, et al. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol. 2009;118:407-413. [DOI] [PubMed] [Google Scholar]
- 34. Rahman P, Gladman DD, Wither J, et al. Coexistence of Fabry’s disease and systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:475-478. [PubMed] [Google Scholar]
- 35. Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34:236-242. [DOI] [PubMed] [Google Scholar]
- 36. Chatre C, Filippi N, Roubille F, et al. Heart involvement in a woman treated with hydroxychloroquine for systemic lupus erythematosus revealing Fabry disease. J Rheumatol. 2016;43:997-998. [DOI] [PubMed] [Google Scholar]
- 37. Boldrini R, Biselli R, Santorelli FM, et al. Neuronal ceroid lipofuscinosis: an ultrastructural, genetic, and clinical study report. Ultrastruct Pathol. 2001;25:51-58. [DOI] [PubMed] [Google Scholar]
- 38. Dolman CL, Chang E. Visceral lesions in amaurotic familial idiocy with curvilinear bodies. Arch Pathol. 1972;94:425-430. [PubMed] [Google Scholar]
- 39. Mammen AL. Which nonautoimmune myopathies are most frequently misdiagnosed as myositis? Curr Opin Rheumatol. 2017;29:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vinnakota S, Zyl MV, Geske J. Hydroxychloroquine cardiomyopathy in a patient with gene positive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2019;73:abstract 2148. [Google Scholar]
- 41. Shukla S, Gultekin SH, Saporta M. Pearls and oysters: hydroxychloroquine-induced toxic myopathy mimics Pompe disease: critical role of genetic test. Neurology. 2019;92:e742-e745. [DOI] [PubMed] [Google Scholar]
- 42. Esdaile JM. More thoughts about antimalarials: should one prescribe chloroquine? J Rheumatol. 1999;26:1868. [PubMed] [Google Scholar]
- 43. Seguin P, Camus C, Leroy JP, et al. Respiratory failure associated with hydroxychloroquine neuromyopathy. Eur Neurol. 1995;35:236-237. [DOI] [PubMed] [Google Scholar]
- 44. Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 2014;30:1706-1715. [DOI] [PubMed] [Google Scholar]
- 45. Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Estes ML, Ewing-Wilson D, Chou SM, et al. Chloroquine neuromyotoxicity. clinical and pathologic perspective. Am J Med. 1987;82:447-455. [DOI] [PubMed] [Google Scholar]
- 47. Hughes JT, Esiri M, Oxbury JM, et al. Chloroquine myopathy. Q J Med. 1971;40:85-93. [PubMed] [Google Scholar]
- 48. Janssen L, Allard NAE, Saris CGJ, et al. Muscle toxicity of drugs: when drugs turn physiology into pathophysiology. Physiol Rev. 2020;100:633-672. [DOI] [PubMed] [Google Scholar]
- 49. Eadie MJ, Ferrier TM. Chloroquine myopathy. J Neurol Neurosurg Psychiatry. 1966;29:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zirin J, Nieuwenhuis J, Perrimon N. Role of autophagy in glycogen breakdown and its relevance to chloroquine myopathy. PLoS Biol. 2013;11:e1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]