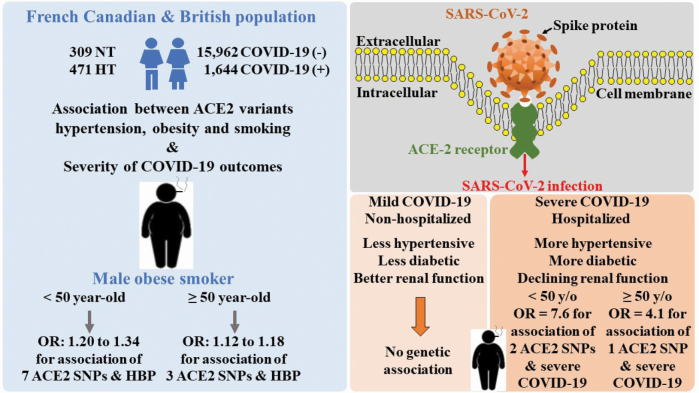
Abstract
BACKGROUND
Angiotensin-converting enzyme 2 (ACE2) has been identified as the entry receptor for coronaviruses into human cells, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19). Since hypertension (HT) is a leading comorbidity in non-survivors of COVID-19, we tested for association between ACE2 gene and HT in interaction with specific pre-existing conditions known to be associated with COVID-19 severity.
METHODS
Genetic analysis of ACE2 gene was conducted in French-Canadian (FC) and British populations.
RESULTS
In FC individuals, the T allele of the single nucleotide polymorphism rs2074192 of ACE2 gene was a risk factor for HT in adult obese males [odds ratio (OR) = 1.39, 95% confidence interval (CI) 1.06–1.83)] and even more so in obese males who smoked (OR = 1.67, CI: 1.24–2.55), but not in lean males, non-smoker males or females. The T allele was significantly associated with severity of HT and with earlier penetrance of HT in obese smoking males. Significant interaction between the T allele and obesity was present in both sexes. The association of ACE2 (rs233575) genotype with blood pressure was also seen in adolescents but the interaction with obesity was present only in females. Several variants in ACE2 gene were found to be associated with HT in obese, smoking males in British individuals of the UK Biobank. In addition, we observed more severe outcomes to COVID-19 in association with ACE2 risk alleles in obese, smoking males.
CONCLUSIONS
This is the first report that ACE2 variants are associated with earlier penetrance and more severe HT and with more severe outcomes of COVID-19 in obese smoking males.
Keywords: ACE2, blood pressure, COVID-19, hypertension, obesity, SARS–CoV-2, sex, smoking
Graphical Abstract
Graphical Abstract.
The severity of infection to coronaviruses including that of COVID-19 is known to be associated with age, sex, and specific pre-existing conditions.1,2 Most mortality occurs in the elderly and is almost double in males (4.7%) compared to females (2.8%). It is 13.2% for individuals with cardiovascular diseases (CVD), 9.2% in patients with diabetes, 8.4% in those with hypertension, 8.0% in patients with chronic respiratory diseases and 6.6% in those with cancer. Death rate of individuals with pre-existing conditions is nearly 10-fold higher than in individuals with no pre-existing conditions. Similar to the 2003 SARS outbreak, hypertension (HT) preceding hospitalization was the most frequent comorbidity in non-survivors, in addition to diabetes and cardiovascular disease, particularly in current smokers in COVID-19 cohorts.3,4 History of HT, smoking, and obesity were also prevalent in COVID-19 hospitalized patients and non-survivors in the New York city area.5 The SARS coronaviruses, SARS–CoV, and the new SARS–CoV-2, which causes COVID-19, bind to angiotensin-converting enzyme 2 (ACE2).3 ACE2 protein and gene (ACE2) were initially studied in rodent models of HT6 and human studies demonstrated genetic association with HT7–9 and in individuals with cardiovascular disease and type 2 diabetes.10,11 Comparative mapping showed that Ace2 co-localizes with quantitative trait locus for HT in stroke-prone hypertensive rats12 and Sabra salt-sensitive rats.13
We reported previously an association between genetic variants in ACE2 with blood pressure (BP) in a cohort of adolescents, including the single nucleotide polymorphism (SNP) rs2074192 located in intron 16 of ACE2 on chromosome X.14 This SNP has been associated with BP and HT in candidate-gene studies, yet was overlooked in most genome-wide association studies (GWAS) that typically do not analyze sex chromosomes.
Experimental and human studies of HT in the context of COVID-19 were the topic of a recent editorial comment.15 A review documenting all genetic factors involved in Coronavirus host-susceptibility to infection and complications did not report any clear genetic association between ACE2 polymorphisms and susceptibility or severity to COVID-19 in the general population.16
In the present study, we evaluated the possible associations between genetic polymorphisms of ACE2 with co-morbidities related to COVID-19. Our hypothesis is that HT, obesity, and smoking act as effect modifiers of the impact of ACE2 in COVID-19 severity, that these effects may be age- and sex-variable.
Methods
Study populations
We initially studied French-Canadian (FC) individuals recruited in the Saguenay-Lac-St-Jean area of the Canadian province of Quebec. This population is known for its Mendelian diseases caused by a founder effect due to fast demographic growth.17,18 Our sample consisted of multigenerational families, ascertained by the presence of an affected sibpair with HT and dyslipidemia, and with both parents of FC origin.19 Since family information was not used here, we increased the number of hypertensive or normotensive individuals by including unrelated individuals. The sample size was 780 individuals. HT was defined as having systolic BP (SBP) or diastolic BP (DBP) ≥140/90 mm Hg on 2 occasions or currently taking anti-HT medication as in all our previous studies..19,20 Our sample was divided into 2 categories: being able or not to withdraw anti-hypertensive medication. The latter group represented more severe HT, arrhythmias, and participants with history of myocardial infarction.
ACE2 genotypes of rs2074192 were available for 352 males (59% hypertensive) and 428 females (61% hypertensive) from 122 families. A second randomly selected sample from the Heart and Health Study (Santé du Coeur du Québec), from the same geographic region, consisted of 36 unrelated males (25% hypertensive) and 48 females (38% hypertensive).21 Smoking status was determined as either “ever smoker” or “never smoker,” with “ever smoker” being a current regular or previous regular smoker. Obesity was defined as body mass index (BMI) ≥ 28 kg/m2 according to FC study protocol.21
The third independent sample of FCs was a sample of 525 adolescents (283 females and 242 males, age: 12–18 years, Supplementary Table S2 online) from the Saguenay Youth Study (SYS), a population-based study aimed at investigating the etiology and early stages of common cardiometabolic and brain diseases.22 The cohort was recruited via high schools. Both maternal and paternal grandparents were of FC ancestry born in the region. The adolescents studied here were those in whom rs233575 (see the following paragraphs) in ACE2 was genotyped and in whom BP and BMI were measured. Due to the X-chromosome location of ACE2, all females who were heterozygotes for the genotype (n = 153) were excluded from further study; the final sample size was 133 females (homozygotes) and 242 males. Overweight/obesity was defined as BMI ≥85th age- and sex-specific percentile.23 Puberty status was evaluated with a validated questionnaire.24 Effect of cigarette smoking was not studied in this adolescent sample.
The UK Biobank population (mainly of White British origin) constituted our fourth sample where we also evaluated data from 17,606 individuals who had been tested for COVID-19 and of which 1,644 individuals were positive for COVID-19 infection.25 Positive individuals were further divided into mild positive (n = 657) and severe positive (n = 987) according to information about hospitalization provided by the UK Biobank management team. The term severe follows the definition used by jamanetwork.com of New York as “sufficiently medically ill to require hospital admission.” 5 Furthermore, from the 502,492 subjects included in UK Biobank, 144,753 had HT and 30,437 had diabetes.
Genotyping
We genotyped SNP rs2074192 in intron 16 of ACE2 gene, known for its strongest association with HT8–11,14 in 780 members of FC families and 85 unrelated subjects.
A 296 bp nucleotide fragment containing both SNPs was amplified by polymerase chain reaction using primers 5′GCCTTGCAACCTAGATTAGGT3 ′ and 5′ATTGGCATTTGGAGGTAGCAC3′, and 2 variants were revealed by the single-strand conformational polymorphism technique. Four chromosomes of each strand conformational polymorphism variant were sequenced in males and 3 haplotype alleles were found. One strand conformational polymorphism variant consisted of 2 alleles which were distinguished by restriction enzyme SspI. Male genotypes consisted of 2 alleles (T or C), while females presented with 3 genotypes (T/T, T/C, and C/C). Since T/T and T/C genotypes demonstrated a similar tendency for association with HT (odds ratio [OR] = 1.74, confidence interval [CI]: 0.87–3.48 and OR = 1.45, CI: 0.98–2.18) by the chi-square test, they were pooled in all analyses. Genotypes were in Hardy–Weinberg equilibrium among normotensive and hypertensive females.
In SYS adolescents, rs233575 in ACE2 was genotyped with the Human610-Quad BeadChips (Illumina, San Diego, CA). This SNP is located 176 bp apart from the SNP rs2074192, which was studied in 2 adult samples but not genotyped in SYS adolescents. Both SNPs are in intron 16 of ACE2 (Supplementary Figure S1 online) and in partial linkage disequilibrium (LD) (r2 = 0.37–0.47, D′ = 1 in various European subpopulations). Their genomic context was examined with ENCODE,26 GTEx,27 and HaploReg28 (Supplementary Figures S1 and S2 online).
Samples from the UK Biobank were genotyped with the UK BioBank Axiom arrays (Affymetrix, Santa Clara, CA). Genotype calling was performed by Affymetrix on two closely related purpose-designed arrays. Approximately 50,000 participants were run on the UK BiLEVE Axiom array (Resource 149600) and the remaining ~450,000 were run on the UK Biobank Axiom array (Resource 149601). We analyzed 12 SNPs within ACE2 gene for their association with HT and severity to COVID-19 in the UK Biobank participants. They were selected from Disgenet database (https://www.disgenet.org/) for their association with cardiovascular disease phenotypes. These SNPs were extracted from .bgen files using Plink2 software (https://www.cog-genomics.org/plink/2.0/). Linkage disequilibrium between SNPs was calculated by ld-estimator package (https://pypi.org/project/ld-estimator/) in Python (Supplementary Figure S5 online).
Statistical methods
Descriptive statistics include means and SDs for continuous variables and proportions for categorical variables. Bivariate comparisons were made at the pre-specified 2-sided alpha-level of 0.05, using Student’s t-test or the chi-square test, as appropriate. All association analyses were performed separately for males and females. Differences in mean values of obesity measures between ACE2 allele T and C carriers were estimated by multiple linear regression, while the association of HT with ACE2, and its interactions with obesity and smoking, were analyzed using multiple logistic regression. In all regression analyses, the generalized estimating equations approach was used to account for the dependence between individuals from the same family.29
Kaplan–Meier survival analyses were carried-out by kaplanmeier package (https://pypi.org/project/kaplanmeier/) in Python.
In the SYS adolescents, we used linear regression model to test whether ACE2 genotype (rs233575) is associated with SBP and DBP in interaction with sex and overweight/obesity. Both models were adjusted for age, puberty stage, and height.
In the UK Biobank, OR, SE, and P values as well-as probabilities of binary phenotypes were calculated using “emmeans” Package in R (https://cran.r-project.org/web/packages/emmeans/emmeans.pdf).
Ethics
The study was approved by the Ethics Committees at Sagamie Hospital in Chicoutimi, the CHUM in Montréal and the Hospital for Sick Children in Toronto.
Results
ACE2 and HT in FC individuals
Age, BP, selected anthropomorphic phenotypes, smoking habits are compared, separately for males and females, in Supplementary Table S1 online. Table 1 shows the impact of risk allele (T) of SNP rs2074192 as being more significantly associated with several features of HT in males compared to females as well as specific features of obesity (such as subscapular skinfold) which we have previously described as having higher genetic determinants.19
Table 1.
Characteristics of French Canadians by sex and ACE2 (rs2074192) genotype
| Males | Females | |||||
|---|---|---|---|---|---|---|
| T (n = 129) | C (n = 223) | P value | T/T + C/T (n = 235) | C/C (n = 193) | P value | |
| Demographic characteristics | ||||||
| Age, yr; mean (SD) | 50 (14) | 48 (13) | 0.17 | 53 (14) | 51 (15) | 0.22 |
| Cardiovascular parameters | ||||||
| SBP, mm Hg; mean (SD) | 138 (19) | 133(19) | 0.04 | 135 (21) | 133 (23) | 0.61 |
| DBP, mm Hg; mean (SD) | 88 (12) | 86 (11) | 0.13 | 81 (12) | 82 (10) | 0.71 |
| MAP, mm Hg; mean (SD) | 104 (12) | 101 (12) | 0.02 | 98 (13) | 98 (12) | 0.66 |
| PP, mm Hg; mean (SD) | 49 (15) | 46 (11) | 0.06 | 52 (13) | 49 (15) | 0.12 |
| Hypertensive, n (%) | 86 (67) | 123 (55) | 0.03 | 154 (65) | 108 (56) | 0.04 |
| HT medication, n (%) | 65 (50) | 76 (34) | <0.01 | 128 (54) | 99 (51) | 0.51 |
| Obesity parameters | ||||||
| TBF skinfold, %; mean (SD) | 26 (5) | 25 (6) | 0.05 | 38 (6) | 37 (7) | 0.33 |
| Waist, cm; mean (SD) | 97 (11) | 96 (11) | 0.38 | 86 (15) | 84 (13) | 0.12 |
| Hip, cm; mean (SD) | 100 (7) | 99 (7) | 0.40 | 102 (11) | 101 (10) | 0.22 |
| Waist/hip ratio; mean (SD) | 1 (0.1) | 1 (0.1) | 0.50 | 0.8 (0.1) | 0.8 (0.1) | 0.20 |
| Subscapular skinfold, mm; mean (SD) | 26 (10) | 23 (10) | 0.01 | 27 (12) | 26 (13) | 0.50 |
| Suprailiac skinfold, mm; mean (SD) | 25 (12) | 22 (13) | 0.03 | 26 (11) | 25 (13) | 0.43 |
| BMI, kg/m2; mean (SD) | 27 (5) | 27 (4) | 0.55 | 27 (5) | 26 (5) | 0.25 |
| Obese,an (%) | 52 (40) | 81 (36) | 0.45 | 80 (34) | 60 (31) | 0.43 |
| Smoking parameters | ||||||
| Ever smokers,bn (%) | 73 (57) | 131 (59) | 0.76 | 98 (42) | 85 (44) | 0.95 |
P values of descriptive statistics are based on Students t-test for differences in means and the χ2 test for differences in proportions. Significant P values are indicated in bold. Abbreviations: ACE2, angiotensin-converting enzyme 2; BMI, body mass index; DBP, diastolic blood pressure; HT medication, anti-hypertensive medication; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; TBF, total body fat.
aObesity defined as BMI ≥ 28 kg/m2; missing values for obesity: 8 males, 11 females.
bPrevious or current smokers; missing values for smoking: 51 males, 61 females.
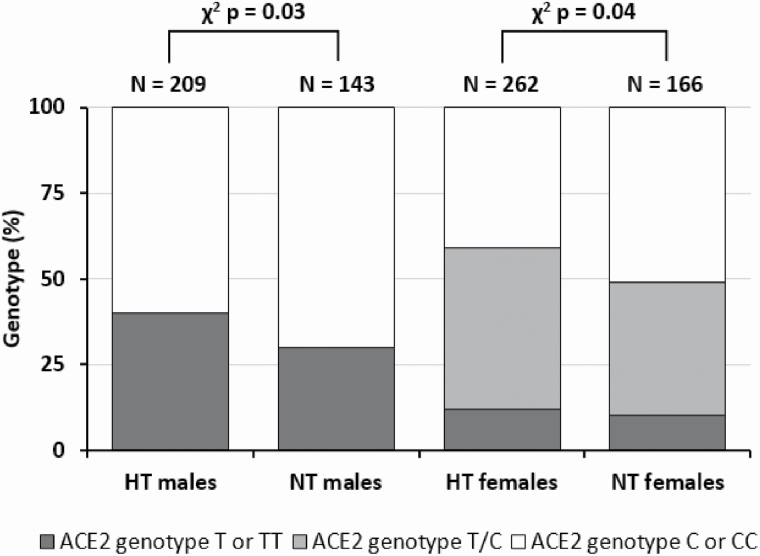
Figure 1 shows that T allelic frequencies were similar in males (37%) and females (33%, P = 0.2), and that the association between SNP rs2074192 and HT was significant in both males and females (P = 0.03 and P = 0.04, respectively).
Figure 1.
Distribution of ACE2 (rs2074192) genotypes and hypertension among males and females of French Canadians. Abbreviations: ACE2, angiotensin-converting enzyme 2; HT, hypertensive; NT, normotensive.
To assess whether the association between genotypes and BP phenotypes was modified by obesity, we selected normotensive and hypertensive individuals who were not taking anti-hypertensive medication and divided them into obese (BMI ≥28, obesity threshold used previously19) and non-obese sub-groups, in the 2 sexes separately. BP differed significantly between genotypes only among obese individuals of both sexes, with no difference among non-obese individuals (Table 2). The differences between genotypes were 10 mm Hg for SBP, 8 mm Hg for DBP, and 9 mm Hg for MAP in obese males, with respective P = 0.07, <0.01, and <0.01 values. Among obese females, the differences were 14 mm Hg (P = 0.02), 6 mm Hg (P < 0.01), and 8 mm Hg (P = 0.01), respectively. These results were obtained with the generalized estimating equations regression model adjusted for age and accounted for family clusters. Accordingly, for both sexes and most BP measures, there were statistically significant interactions between the genotypes and obesity.
Table 2.
ACE2 (rs2074192) genotypes and BP of French Canadians in subgroups defined by sex and obesity
| Blood pressure measures, mm Hg; mean (SD) | Interaction Pa | Obese (BMI ≥ 28) | Non-obese (BMI < 28) | ||
|---|---|---|---|---|---|
| T | C | T | C | ||
| Males (n = 205) b | |||||
| SBP | 0.07 | 138 (23) | 128 (12)NS | 131 (16) | 127 (14)NS |
| DBP | <0.01 | 93 (10) | 85 (8)* | 83 (10) | 83 (10)NS |
| MAP | <0.01 | 108 (14) | 99 (9)* | 99 (11) | 97 (11)NS |
| PP | 0.86 | 45 (16) | 42 (10)NS | 48 (14) | 45 (10)NS |
| Females (n = 198) b | |||||
| SBP | 0.02 | 137 (19) | 123 (16)* | 120 (15) | 120 (16)NS |
| DBP | <0.01 | 86 (9) | 80 (7)* | 76 (11) | 77 (10)NS |
| MAP | 0.01 | 102 (10) | 94 (8)* | 90 (11) | 91 (11)NS |
| PP | 0.27 | 49 (12) | 44 (14)NS | 44 (11) | 42 (10)NS |
Significant P values are indicated in bold. Abbreviations: ACE2, angiotensin-converting enzyme 2; DBP, diastolic blood pressure; GEE, generalized estimating equations; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
a Interaction between the genotype and obesity tested in the GEE regression model adjusted for age and accounting for family clusters.
b Analysis restricted to participants in whom anti-HT medication was withdrawn.
* and NS indicate whether the difference in mean BP between the 2 genotypes is statistically significant at α = 0.05 (*) or non-significant (NS).
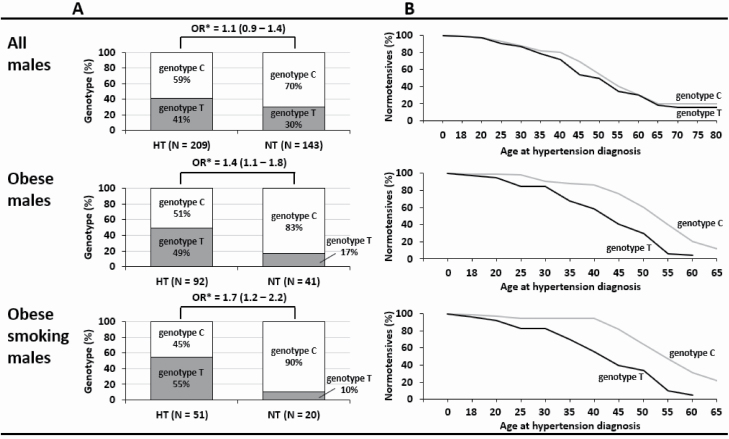
We then compared the ACE2 genotype frequencies in male subgroups. Figure 2A shows that T genotype represents a significant risk for HT in obese OR = 1.4 (95% CI: 1.1–1.8) and more so in obese males who smoke (OR = 1.7, 95% CI: 1.2–2.2). Kaplan–Meier analysis showed that the T genotype predisposes to earlier onset of HT among obese and obese, smoking males (Figure 2B). For example, at age of 45 years, over 50% of obese and smoking males with genotype T have HT compared with only 10% of genotype C carriers.
Figure 2.
Risk of hypertension among genotype T male carriers of French Canadians in subgroups determined by (A) obesity and smoking. (B) Kaplan–Meier curve for time to diagnosis. *OR estimated in logistic regression models that take into account family clusters. Abbreviations: HT, hypertensive; NT, normotensive; OR, odds ratio.
Supplementary Figure S3A online shows that the T genotype tends to be more frequent among hypertensive males taking anti-hypertensive medications (as a sign of HT severity and past outcome events) than among those not taking any. Similarly, a higher T/T genotype frequency was observed among females taking 2 or more antihypertensive medications to control their BP. In addition, in the group of subjects with no contraindication to withdraw medication, there was an under-representation of the risk allele T (Supplementary Figure S3B online). The difference was significant in females (P = 0.002).
The characteristics of the adolescent sample is presented in Supplementary Table S2 online. In adolescents, the ACE2 genotype at rs233575 (A/G) was associated with SBP and DBP in interaction with overweight/obesity and sex (Supplementary Figure S4 online). This 3-way interaction was nearly significant for SBP (P = 0.09) and significant for DBP (P = 0.04). For both SBP and DBP, the interaction was due to the A allele being associated with higher BP mainly in overweight/obese females; in these females, AA vs. GG genotype was associated with higher SBP by 8 mm Hg (P = 0.005) and higher DBP by 10 mm Hg (P = 0.003; Supplementary Figure S4 online).
ACE2 and HT in British individuals
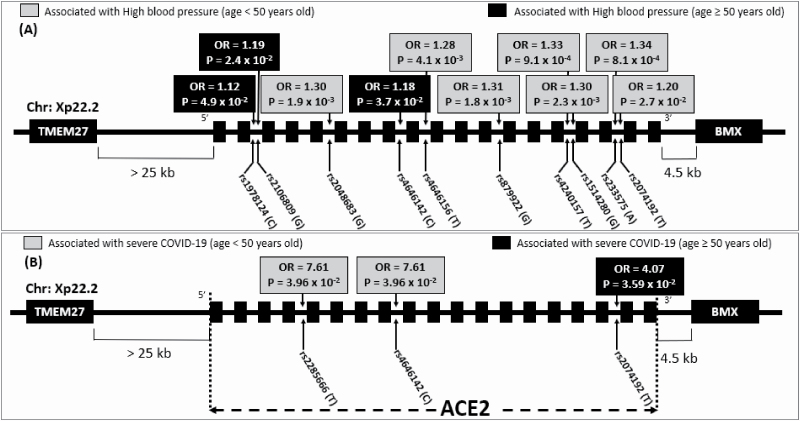
We used 12 SNPs spanning ACE2 gene (Supplementary Figure S5 online) to assess further the relationship between ACE2 and HT in relation to age, sex, obesity, and smoking in the British participants of the UK Biobank. Table 3 summarizes the association results of the 12 SNPs and HT in all males, obese, and obese smoking males less than the age of 50. Half (6/12) of the SNPs, including the SNP rs233575 analyzed in FC adolescents, exhibited significant (after Bonferroni correction) association with HT in obese smoking, but not in all or in obese males. The 6 SNPs (Figure 3A) are in linkage disequilibrium with high r2 (Supplementary Figure S5 online) in the upstream region of ACE2 gene. The association with HT was nearly significant for SNP rs2074192 studied in adult FC, supporting the relevance of our initial observation in FC in this large cohort of UK Biobank. Three SNPs (rs2106809, rs1978124, and rs4646142) downstream of ACE2 gene are associated with HT in older individuals (Supplementary Table S3 online).
Table 3.
Odds ratios of 12 ACE2 variants for high blood pressure and frequencies of hypertension for all males, obese males, and obese smoking males in UK Biobank participants below age of 50 years old
| Age < 50 years old | ||||||
|---|---|---|---|---|---|---|
| Males | Obese males | Obese smoking males | ||||
| rs ID (RA/nRA) | OR (SE) | P value | OR (SE) | P value | OR (SE) | P value |
| rs2074192 (T/C) | 1.02 (0.02) | NS | 1.02 (0.03) | NS | 1.20 (0.07) | 2.7 × 10 −2 |
| rs233575 (A/G) | 1.04 (0.02) | NS | 1.04 (0.03) | NS | 1.34 (0.06) | 8.1 × 10 −4 |
| rs1514280 (G/A) | 1.03 (0.02) | NS | 1.04 (0.03) | NS | 1.30 (0.07) | 2.3 × 10 −3 |
| rs4240157 (T/C) | 1.03 (0.02) | NS | 1.04 (0.03) | NS | 1.33 (0.06) | 9.1 × 10 −4 |
| rs879922 (G/C) | 1.03 (0.02) | NS | 1.04 (0.03) | NS | 1.31 (0.07) | 1.8 × 10 −3 |
| rs4646156 (T/A) | 1.05 (0.02) | 2.6 × 10 −2 | 1.04 (0.03) | NS | 1.28 (0.07) | 4.1 × 10 −3 |
| rs4646188 (G/A) | 0.99 (0.03) | NS | 0.99 (0.05) | NS | 1.15 (0.11) | NS |
| rs4646142 (C/G) | 1.02 (0.03) | NS | 1.02 (0.04) | NS | 1.10 (0.10) | NS |
| rs2048683 (G/T) | 1.05 (0.02) | 1.8 × 10 −2 | 1.05 (0.03) | NS | 1.30 (0.07) | 1.9 × 10 −3 |
| rs2285666 (T/C) | 1.03 (0.03) | NS | 1.03 (0.04) | NS | 1.09 (0.10) | NS |
| rs2106809 (G/A) | 1.01 (0.03) | NS | 1.02 (0.04) | NS | 1.07 (0.10) | NS |
| rs1978124 (C/T) | 1.02 (0.02) | NS | 1.00 (0.03) | NS | 1.13 (0.07) | NS |
Significant P values are indicated in bold. Abbreviations: ACE2, angiotensin-converting enzyme 2; nRA, non risk allele; NS, non significant; OR, odds ratio; RA, risk allele.
Figure 3.
Association of ACE2 variants with (A) high blood pressure and (B) severe COVID-19 within obese smoking males of UK Biobank participants below and above 50 years old. Abbreviations: ACE2, angiotensin-converting enzyme 2; OR, odds ratio.
Table 4.
Some characteristics of mild and severe positive COVID-19 among UK Biobank participants separated by sex.
| COVID-19 | P value or OR (±SE) | P value or OR (±SE) | ||||
|---|---|---|---|---|---|---|
| Mild positive (n = 657) | Severe positive (n = 987) | |||||
| Males (49.3%) | Females (50.7%) | Males (57.1%) | Females (42.9%) | Males, (S) vs. (M) | Females, (S) vs. (M) | |
| Age, yr; mean (SE) | 54.8 (0.5) | 53.1 (0.5) | 58.7 (0.3) | 56.2 (0.4) | <0.0001 | <0.0001 |
| Hypertensive, (%) | 68.5 | 55.5 | 70.6 | 56.3 | 1.12 (0.17) | 1.03 (0.16) |
| Diabetic, (%) | 9.3 | 7.3 | 15.3 | 8.8 | 1.77 (0.42) | 1.22 (0.34) |
| BMI, kg/m2; mean (SE) | 28.5 (0.3) | 28.5 (0.3) | 29.1 (0.2) | 28.9 (0.3) | 0.15 | 0.64 |
| eGFR < 60 mL/min/1.73 m2, (%) | 2.2 | 1.7 | 4.1 | 3.3 | 1.92 (0.89) | 1.97 (0.95) |
| UACR > 30 µg/mg, (%) | 15.2 | 20.7 | 25.3 | 22.2 | 1.89 (0.58) | 1.10 (0.34) |
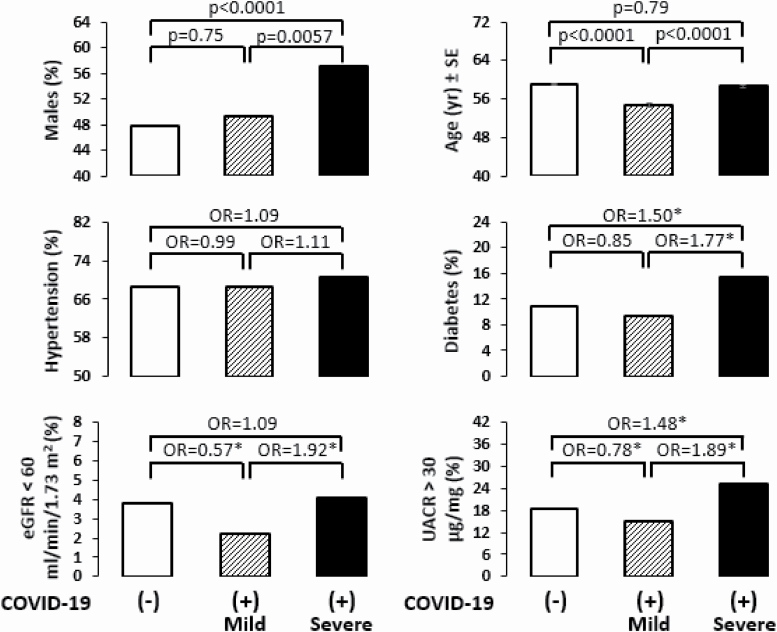
Some of the values for certain characteristic are not available for all participants. Significant P values are indicated in bold. Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate calculated using CKD-EPI formula; M, mild positive COVID-19; OR, odds ratio; S, severe positive COVID-19; UACR, Urinary albumin creatinine ratio.
ACE2 and COVID-19 in British individuals
Age and BMI were the two notable differences between British individuals who tested negative or positive to COVID-19 infection in both sexes (Supplementary Table S4 online). Among the positive individuals, 57% of males had severe outcomes compared to 43% of females (Table 4). Males who had severe outcomes requiring hospitalization were generally older (P = 1.4 × 10−9) than those with mild outcomes, who did not require hospitalization. Comparison of infected males who had severe vs. mild outcomes showed significant clinical differences including higher percentage of individuals with HT, diabetes, albuminuria, and low estimated glomerular filtration rate (Figure 4). Furthermore, individuals who had mild symptoms to COVID-19 were significantly younger, had less: HT, diabetes, declining renal function compared to people testing negative to COVID-19. Noticeably, SNP rs2074192 was nominally significantly associated (OR = 4.07; P = 0.036) with more severe outcomes of COVID-19 within obese smoking males of 50 years or older (Figure 3B). The association remained the same after adjustment for HT.
Figure 4.
Some characteristics of negative and positive COVID-19 separated by severity in UK Biobank male participants. *Significant odds ratio. Abbreviations: eGFR, estimated glomerular filtration rate; OR, odds ratio; UACR, urinary albumin creatinine ratio.
Discussion
The association between hypertension and obesity has been known for decades and its neurohumoral and renal mechanisms are well established including activation of the renin-angiotensin-aldosterone system. In the FC population, we have demonstrated distinct genomic architecture between hypertensive families with and without obesity, and this feature persists in the depth of 26 generations.30 The ACE2 genotype represents a significant risk for HT particularly in obese males who smoke in whom the T genotype of rs2074192 predisposes to earlier onset of HT among obese and obese, smoking males (Figure 2B).
Our observation in FC was confirmed in the UK Biobank cohort where the 2 SNPs rs2074192 and rs233575 analyzed in adult and FC adolescents, were shown to be associated with HT in obese smoking males also in this cohort.
Patel et al. suggested a novel role for ACE2 in heart disease associated with obesity, whereas an ACE2 deficit results in epicardial fat inflammation and cardiac insulin resistance.31 Touyz expanded the possibility that an ACE2 defect may result in a generalized inflammatory response32 a notion potentially relevant in context of COVID-19 late-stage multiorgan failure.1
A recent analysis of ACE2 gene expression in multiple tissues of thousands of individuals reported important ethnical variations, decline with aging and lower expression in T2D individuals and after inflammatory cytokine treatment.33
We examined the genomic context of SNPs rs233575 and rs2074192 with ENCODE,26 GTEx,27 and HaploReg v4.1.28 Both SNPs are located in intron 16 within a regulatory element characterized by high H3K27Ac signal (indicates regulatory-factor binding,34Supplementary Figure S1A online). Both SNPs alter binding of regulatory factors, including ARID5A which is an RNA-binding protein that plays a key a role in posttranscriptional events, such as splicing, and is stimulated by inflammation (Supplementary Figure S2 online).35 Of note, exon 17 of ACE2 is expressed at a substantially lower level than its neighboring exons 16 and 18 in tissues with high expression of ACE2 (e.g., testes, kidney, heart, and adipose tissue; Supplementary Figure S1B online). Whether the intronic region of ACE2 tagged by our 2 SNPs is involved in the regulation of alternative splicing, i.e., the generation of transcripts without exon 17, requires further exploration.
Our data suggest that the ACE2 locus has an impact on HT in obese individuals rather than on obesity itself (Tables 2 and 3). Genotype T hypertensive males have higher mean values of anthropomorphic measures related with fat and its distribution than genotype C carriers, while among normotensives the association shows an opposite tendency. Such a tendency of opposite association in hypertensive vs. normotensive males indicates that once genotype T male carriers gain weight, they develop HT. A higher HT risk among obese, male smokers with T genotype is consistent with our study hypothesis that individuals with the T allele lose the protective effect of the C allele against HT and are therefore more likely to develop HT when risk factors, such as obesity and smoking, are present. They could be also more at risk of developing severe outcomes after COVID-19 infection at older age.
The impact of smoking on HT was previously demonstrated in the context of genetic variation in ACE1. The interaction of ACE1 with smoking, similar to the ACE2-smoking interaction found in this study, can be explained by the fact that both RAAS components (if not in imbalance) and smoking increase the degradation of nitric oxide and the production of free radicals, causing endothelial damage and impaired vasodilatation. We have previously shown that smoking can interact with genetic determinants of certain stroke types.36 We have also reported, in the same population studied here, a cluster encompassing genes associated to HT, obesity, smoking, and response to stress, jointly driven by neuronal plasticity genes in a sex-specific matter.37 Specific mechanism of association with ACE2 polymorphism, HT, and smoking requires further exploration.
A stronger impact of the ACE2–obesity–smoking interaction in hypertensive males compared to females may be attributed to different causes. One could be the ACE2 localization on chromosome X. It is known that the female X chromosomes undergoes inactivation which is not always random, some regions remain transcriptionally active to a certain level, thus leaving 2 active alleles in females compared to only 1 in males. The involvement of chromosome X in HT is also supported by the fact that about 50% of Turner syndrome females present with cardiovascular outcomes.38
The same argument can be made for the susceptibility to severe COVID-19 outcomes. In UK Biobank, 56% of males had severe outcomes to COVID-19 while 54% of females had mild outcomes (Figure 4). Noticeably individuals with mild outcomes seemed to have better renal function compatible with recent findings of COVID-19–related dysfunction of proximal tubule.39
Perspectives
The enzyme ACE2 degrades angiotensin II and facilitates the entry of coronavirus into cells. The present report identifies age- and sex-dependent associations of different ACE2 alleles with HT, obesity, and smoking. Recent evidence indicates that the pathogenicity of COVID-19 infection is greater in older individuals, and in individuals with HT, obesity, and smokers. We are showing here an association between ACE2 polymorphisms and severity of outcomes to COVID-19 in infected individuals of the UK Biobank. Although we do not have quantitative estimates of ACE2 activity, the current results raise the possibility of a genetic contribution to the morbidity and mortality attributed to COVID-19 infection.
Supplementary Material
Acknowledgments
The French-Canadian study was supported by the Canadian Institutes of Health Research # 8892 (PH, JT), US Public Health Service Grant P50 HL-54998 (AC, PH, TK), OPTITHERA (PH, JT), Medpharmgene (PH, JT), and Consortium Québécois du Médicament (PH, JT). The Saguenay Youth Study has been funded by the Canadian Institutes of Health Research (TP, ZP), Heart and Stroke Foundation of Canada (ZP), and the Canadian Foundation for Innovation (PH, JT, ZP). This research has been conducted using the UK Biobank Resource. The access to UK Biobank was provided as part of the UK Biobank projects 49731 and 59642. PH was a Canada Research Chair in Predictive Genomics and JH is a FRQS scholar Junior 1, scholar supported by IVADO. The authors wish to thank Malgorzata Labuda for the initial analyses of the French-Canadian families.
Disclosure
The authors declared no conflict of interest.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Articles Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, Liu S, Yang J-K. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Heal2020; 8: 1– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita K, Ding N, Kou M, Hu X, Chen M, Gao Y, Honda Y, Zhao D, Dowdy D, Mok Y,. Ishigami J, Appel LJ. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Glob Heart2020; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu W, Tao Z-W, Wang L, Yuan M-L, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu H-G, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl)2020; 133: 1032– 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; The Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020; 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002; 417:822–828. [DOI] [PubMed] [Google Scholar]
- 7. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res 2020; 24:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan Z, Wu G, Yue M, Ye J, Chen Y, Xu B, Shu Z, Zhu J, Lu N, Tan X. Hypertension and hypertensive left ventricular hypertrophy are associated with ACE2 genetic polymorphism. Life Sci 2019; 225:39–45. [DOI] [PubMed] [Google Scholar]
- 9. Wu X, Zhu B, Zou S, Shi J. The association between ACE2 gene polymorphism and the stroke recurrence in Chinese population. J Stroke Cerebrovasc Dis 2018; 27:2770–2780. [DOI] [PubMed] [Google Scholar]
- 10. Liu C, Li Y, Guan T, Lai Y, Shen Y, Zeyaweiding A, Zhao H, Li F, Maimaiti T. ACE2 polymorphisms associated with cardiovascular risk in Uygurs with type 2 diabetes mellitus. Cardiovasc Diabetol 2018; 17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng N, Zhang Y, Ma J, Li H, Zhou F, Qu Y. Association of polymorphisms of angiotensin I converting enzyme 2 with retinopathy in type 2 diabetes mellitus among Chinese individuals. Eye (Lond) 2015; 29:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilbert P, Lindpaintner K, Beckmann JS, Serikawa T, Soubrier F, Dubay C, Cartwright P, De Gouyon B, Julier C, Takahasi S. Chromosomal mapping of two genetic loci associated with blood-pressure regulation in hereditary hypertensive rats. Nature 1991; 353:521–529. [DOI] [PubMed] [Google Scholar]
- 13. Yagil C, Sapojnikov M, Kreutz R, Zürcher H, Ganten D, Yagil Y. Role of chromosome X in the Sabra rat model of salt-sensitive hypertension. Hypertension 1999; 33:261–265. [DOI] [PubMed] [Google Scholar]
- 14. Malard L, Kakinami L, O’Loughlin J, Roy-Gagnon MH, Labbe A, Pilote L, Hamet P, Tremblay J, Paradis G. The association between the angiotensin-converting enzyme-2 gene and blood pressure in a cohort study of adolescents. BMC Med Genet 2013; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens 2020; 33:373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. LoPresti M, Beck DB, Duggal P, Cummings DAT, Solomon BD. The role of host genetic factors in coronavirus susceptibility: review of animal and systematic review of human literature. Am J Hum Genet 2020; 107:381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laberge AM, Michaud J, Richter A, Lemyre E, Lambert M, Brais B, Mitchell GA. Population history and its impact on medical genetics in Quebec. Clin Genet 2005; 68:287–301. [DOI] [PubMed] [Google Scholar]
- 18. Labuda M, Labuda D, Korab-Laskowska M, Cole DE, Zietkiewicz E, Weissenbach J, Popowska E, Pronicka E, Root AW, Glorieux FH. Linkage disequilibrium analysis in young populations: pseudo-vitamin D-deficiency rickets and the founder effect in French Canadians. Am J Hum Genet 1996; 59:633–643. [PMC free article] [PubMed] [Google Scholar]
- 19. Hamet P, Merlo E, Seda O, Broeckel U, Tremblay J, Kaldunski M, Gaudet D, Bouchard G, Deslauriers B, Gagnon F, Antoniol G, Pausová Z, Labuda M, Jomphe M, Gossard F, Tremblay G, Kirova R, Tonellato P, Orlov SN, Pintos J, Platko J, Hudson TJ, Rioux JD, Kotchen TA, Cowley AW Jr. Quantitative founder-effect analysis of French Canadian families identifies specific loci contributing to metabolic phenotypes of hypertension. Am J Hum Genet 2005; 76:815–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Gharbawy AH, Kotchen JM, Grim CE, Kaldunski M, Hoffmann RG, Pausova Z, Gaudet D, Gossard F, Hamet P, Kotchen TA. Predictors of target organ damage in hypertensive blacks and whites. Hypertension 2001; 38:761–766. [DOI] [PubMed] [Google Scholar]
- 21. Joffres MR, Hamet P, MacLean DR, L’italien GJ, Fodor G. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens 2001; 14:1099–1105. [DOI] [PubMed] [Google Scholar]
- 22. Pausova Z, Paus T, Abrahamowicz M, Bernard M, Gaudet D, Leonard G, Peron M, Pike GB, Richer L, Séguin JR, Veillette S. Cohort profile: the Saguenay youth study (SYS). Int J Epidemiol 2017; 46:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen.2020.. https://www.cdc.gov/healthyweight/bmi/calculator.html. Accessed 25 June 2020.
- 24. Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988; 17:117–133. [DOI] [PubMed] [Google Scholar]
- 25. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018; 562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kheradpour P, Kellis M. Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments. Nucleic Acids Res 2014; 42:2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, Foster B, Moser M, Karasik E, Gillard B, Ramsey K, Sullivan S, Bridge J, Magazine H, Syron J, Fleming J, Siminoff L, Traino H, Mosavel M, Barker L, Jewell S, Rohrer D, Maxim D, Filkins D, Harbach P, Cortadillo E, Berghuis B, Turner L, Hudson E, Feenstra K, Sobin L, Robb J, Branton P, Korzeniewski G, Shive C, Tabor D, Qi L, Groch K, Nampally S, Buia S, Zimmerman A, Smith A, Burges R, Robinson K, Valentino K, Bradbury D, Cosentino M, Diaz-Mayoral N, Kennedy M, Engel T, Williams P, Erickson K, Ardlie K, Winckler W, Getz G, DeLuca D, MacArthur D, Kellis M, Thomson A, Young T, Gelfand E, Donovan M, Meng Y, Grant G, Mash D, Marcus Y, Basile M, Liu J, Zhu J, Tu Z, Cox NJ, Nicolae DL, Gamazon ER, Im HK, Konkashbaev A, Pritchard J, Stevens M, Flutre T, Wen X, Dermitzakis ET, Lappalainen T, Guigo R, Monlong J, Sammeth M, Koller D, Battle A, Mostafavi S, McCarthy M, Rivas M, Maller J, Rusyn I, Nobel A, Wright F, Shabalin A, Feolo M, Sharopova N, Sturcke A, Paschal J, Anderson JM, Wilder EL, Derr LK, Green ED, Struewing JP, Temple G, Volpi S, Boyer JT, Thomson EJ, Guyer MS, Ng C, Abdallah A, Colantuoni D, Insel TR, Koester SE, Little AR, Bender PK, Lehner T, Yao Y, Compton CC, Vaught JB, Sawyer S, Lockhart NC, Demchok J, Moore HF. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013; 45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40:D930–D934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986; 73:13–22. [Google Scholar]
- 30. Pausova Z, Gaudet D, Gossard F, Bernard M, Kaldunski ML, Jomphe M, Tremblay J, Hudson TJ, Bouchard G, Kotchen TA, Cowley AW, Hamet P. Genome-wide scan for linkage to obesity-associated hypertension in French Canadians. Hypertension 2005; 46:1280–1285. [DOI] [PubMed] [Google Scholar]
- 31. Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD, Oudit GY. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 2016; 65:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Touyz RM. Protecting the heart in obesity: role of ACE2 and its partners. Diabetes 2016; 65:19–21. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, Gong W, Han J-DJ. Individual variation of the SARS‐CoV‐2 receptor ACE2 gene expression and regulation. Aging Cell2020; 19:e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 2007; 39:311–318. [DOI] [PubMed] [Google Scholar]
- 35. Nyati KK, Zaman MM, Sharma P, Kishimoto T. Arid5a, an RNA-binding protein in immune regulation: RNA stability, inflammation, and autoimmunity. Trends Immunol 2020; 41:255–268. [DOI] [PubMed] [Google Scholar]
- 36. Krajcoviechova A, Wohlfahrt P, Mayer O Jr, Vanek J, Hajkova J, Hlinovsky D, Kvasnicka T, Tremblay J, Hamet P, Filipovsky J, Kvasnicka J, Cifkova R. Tobacco smoking strongly modifies the association of prothrombin G20210A with undetermined stroke: consecutive survivors and population-based controls. Atherosclerosis 2015; 240:446–452. [DOI] [PubMed] [Google Scholar]
- 37. Nikpay M, Šeda O, Tremblay J, Petrovich M, Gaudet D, Kotchen TA, Cowley AW Jr, Hamet P. Genetic mapping of habitual substance use, obesity-related traits, responses to mental and physical stress, and heart rate and blood pressure measurements reveals shared genes that are overrepresented in the neural synapse. Hypertens Res 2012; 35:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elsheikh M, Casadei B, Conway GS, Wass JA. Hypertension is a major risk factor for aortic root dilatation in women with Turner’s syndrome. Clin Endocrinol (Oxf) 2001; 54:69–73. [DOI] [PubMed] [Google Scholar]
- 39. Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z, Penaloza A, De Greef J, Yildiz H, Pothen L, Yombi JC, Dewulf J, Scohy A, Gérard L, Wittebole X, Laterre P-F, Miller SE, Devuyst O, Jadoul M, Morelle J, Aboubakar F, Acid S, Amini N, Bailly S, Beauloye C, Castanares-Zapatero D, Coche E, Collienne C, Cornette P, De Brauwer I, Dechamps M, Dupriez F, Froidure A, Garnir Q, Gerber B, Ghaye B, Gilard I, Gohy S, Grégoire C, Hantson P, Jacquet L-M, Kabamba B, Kautbally S, Lanthier N, Larbaoui F, Liistro G, Maes F, Montiel V, Mwenge B, Pierard S, Pilette C, Pouleur AC, Sogorb A, Starkel P, Rodriguez-Villalobos H, Thoma M, Van Caeneghem O, Vancraeynest D. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020; 98:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.