We read the study by Paterson et al. (2020) entitled ‘The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings’ with great interest. They described a cohort of 43 patients, 23.25% of whom had encephalopathy and 18.6% strokes with a high prevalence of inflammatory CNS syndromes (27.9%). Nine of 12 cases demonstrated acute disseminated encephalomyelitis; this is a higher frequency than that found in other studies. We aimed to compare these results to a sample from a Latin American middle-income country.
Since the first case was identified in December 2019, the SARS-CoV-2 infection has been an increasing cause of morbimortality. Presently, some countries have experienced stabilization in the number of cases. However, the pandemic is still spreading in South America. Brazil presently has one of the highest rates of new patient infections globally and this is accompanied by an increasing number of deaths. Initially, COVID-19 was linked to a life-threatening pulmonary insufficiency. However, many other organ-specific manifestations have been observed, given that the ACE-2 receptors, the sites of cellular invasion by the virus, are expressed in multiple extrapulmonary tissues such as pneumocytes in the alveolar epithelium, endothelial lung cells, heart, kidneys, bowel and brain. In the latter, infection occurs mainly in the cerebral cortex and brainstem (Gupta et al., 2020; Tay et al., 2020; Zubair et al., 2020).
We have reviewed all of the cases admitted to the Hospital Santa Marcelina (with a catchment area of 3 million people from Sao Paulo, Brazil) who underwent neurological consultation by physicians of other specialties, from 1 March to 30 June 2020. In this observational study in a single-tertiary general hospital, we observed that 5.24% of patients exhibited neurological complications (63 of 1201 COVID-19 inpatients).
The corresponding author reviewed all the neurological patients. All treatments followed our general guidelines for neurological diseases. Evidence of SARS-CoV-2 was defined as confirmed COVID-19 if the PCR results were positive using real-time reverse transcription PCR methods in nasopharyngeal or oropharyngeal swab specimens and probable COVID-19 when typical symptoms of fever and cough and a chest CT showing ground glass aspects were present in the absence of PCR evidence.
We classified the neurological syndromes as cerebrovascular diseases (ischaemic or haemorrhagic stroke, central venous thrombosis), encephalopathy (impairment of consciousness without localization signs, presenting as confusion, lethargic status, delirium or coma); meningitis and meningoencephalitis (encephalopathy with inflammatory CSF), epilepsy, demyelinating disease, and peripheral syndromes (involving muscles, neuromuscular junction, nerves or roots). Patients were evaluated according to previous illnesses, and the information was collected from the family and thorough analysis of medical records. The outcome was hospital discharge or death. This was a retrospective, observational, non-interventional study, and all data were collected anonymously. This study received approval from our ethical committee, and we were waived from requiring informed consent.
The statistical analysis was performed using JASP software version 0.13 (JASP Team, 2020). We used descriptive statistics to characterize the sample. Qualitative variables were compared using the chi-square test and quantitative variables using parametric (independent samples t-test) or non-parametric tests (Mann-Whitney test) where appropriate. A P-value of <0.05 was considered significant. Confidence intervals (CI) were calculated if indicated, and logistic regressions were used to determine factors linked to mechanical ventilation and outcome (discharge or death).
We evaluated and followed up 63 inpatients with a median age of 60 years [minimum and maximum: 18–87 years; interquartile range (IQR): 22.5 years]; there was no gender prevalence [32 males (50.8%; mean age 59.8 ± 15.7 years) and 31 females (54.9 ± 15.9 years)] with no difference by age (P = 0.219). Forty-three (68.3%) patients met the criteria for confirmed SARS-CoV-2 infection and 20 (31.7%) for probable infection. Seventeen patients (27%) had known previous neurological diseases, but the majority (73%) had new-onset neurological events during the SARS-CoV-2 infection period, including those with pre-existing neurological disorders.
All clinical syndromes are depicted in Fig. 1. The most frequent complication was a stroke in 30 patients (25 ischaemic and 5 haemorrhagic) (47.6%) with a median age of 62 years (non-stroke patients were a median of 49 years of age). These patients were significantly older (P = 0.022) with no gender predominance. Comparisons between stroke and non-stroke patients showed a significant difference in ferritin levels (P = 0.027), but no differences were observed in C-reactive protein (CRP) (P = 0.224), creatine phosphokinase (CPK) (P = 0.622), lactate dehydrogenase (LDH) (P = 0.054) or D-dimer (P = 0.347) levels. Both groups with and without stroke had high levels of systemic inflammation.
Figure 1.
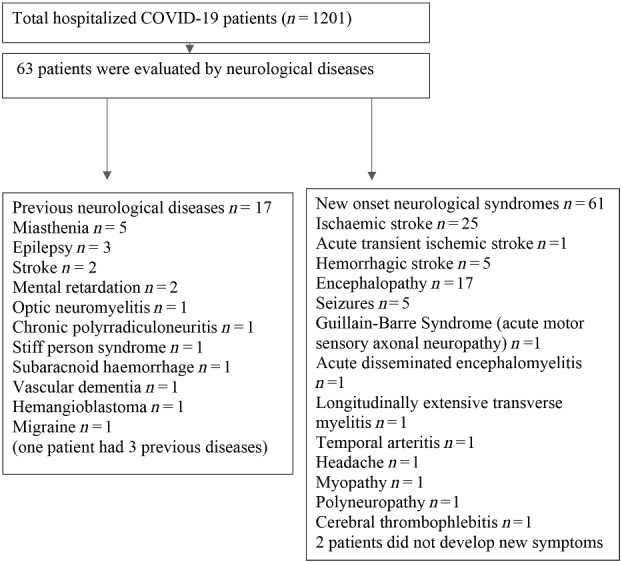
Distribution of neurological manifestations.
Encephalopathy was the second most frequent complication, present in 17 patients (27%) with a median age of 51 years (minimum–maximum: 27–86 years). Non-encephalopathic patients had a median age of 60 years (minimum–maximum : 18–87 years). Sixteen of 17 patients had EEG records presenting predominant theta activity (n = 7); diffuse attenuation (n = 5); predominant delta activity (n = 1); slow posterior dominant rhythm (n = 1); and normal activity (n = 1). None of them had focal lesions on the cerebral CT scan. There was no difference between encephalopathic and non-encephalopathic patients regarding CPK (P = 0.726), ferritin (P = 0.321), LDH (P = 0.103) and D-dimer (P = 0.165) levels or by gender or age. Only the CRP levels were higher in the encephalopathic group, but both groups depicted high levels for all systemic inflammatory markers.
The comorbidities and demographics are shown in Table 1. The most frequent comorbidity was systemic arterial hypertension (SAH) in 52.4% of patients, followed by diabetes mellitus in 23.8%. SAH was more frequent in males (χ2 = 6.986, P = 0.008), and in older patients (t = 2.806, P = 0.007; 95% CI: 3.060 to 8.237). Patients with previous records of stroke were older (t = 2.403, P = 0.019; 95% CI 2.875 to 31.352); tobacco addiction showed a tendency toward significance among older patients (t = 1.959, P = 0.055; 95% CI −0.202 to 19.692). There was no gender or age predominance among the other comorbidities.
Table 1.
Characteristics and comorbidities of 63 inpatients with COVID-19 and neurological diseases
|
n (%) |
Age (mean ± SD, years) |
Ratio female: male | |||
|---|---|---|---|---|---|
| Comorbidities | Yes | No | Yes | No | |
| SAH | 33 (52.4) | 30 (47.6) | 62.5 (12.1)* | 51.8 (17.7)* | 11:22* |
| Diabetes | 15 (23.8) | 48 (76.2) | 62.4 (15.3) | 55.8 (15.8) | 8:7 |
| Renal failure | 4 (6.3) | 59 (93.7) | 58.5 (14.5) | 57.4 (16.0) | 2:2 |
| Tobacco use | 12 (19) | 51 (81) | 65.3 (6.3) | 55.5 (16.8) | 6:6 |
| Pulmonary disease | 2 (3.0) | 61 (97) | 72.5 (20.5) | 57.0 (15.6) | 1:1 |
| Pre-existing stroke | 5 (7.9) | 58 (92.1) | 73.2 (5.4)# | 56.1 (15.7)# | 3:2 |
| Obesity | 3 (4.8) | 60 (95.2) | 33.3 (14.5)& | 58.6 (15.0)& | 2:1 |
| Neoplasm | 5 (7.9) | 58 (92.1) | 52.8 (15.1) | 57.8 (16.0) | 3:2 |
| Cardiac arrythmia | 2 (3.2) | 61 (96.8) | 64.0 (7.1) | 57.2 (16.0) | 1:1 |
| Cardiopathy | 6 (9.5) | 57 (90.5) | 55.8 (22.4) | 57.6 (15.2) | 1:5 |
| Alcohol abuse | 4 (6.3) | 59 (93.7) | 53.0 (13.4) | 57.7 (16.0) | 1:3 |
| Severity | |||||
| Mechanical ventilation | 30 (47.6) | 33 (52.4) | 59.7 (16.7) | 55.4 (14.9) | 15:15 |
| Outcome | Death | Discharge | Death | Discharge | |
| 29 (46.0) | 31 (49.2) | 58.9 (16.6) | 56.6 (14.8) |
16:13 (death) 13:18 (discharge) |
|
SAH = systemic arterial hypertension.
P = 0.007; χ2 = 6.986; #P = 0.019; &P = 0.006.
Mechanical ventilation was necessary for 33 patients with severe COVID-19 (52.4%). No comorbidity was related to progress toward orotracheal intubation. The in-hospital mortality rate was 46% for neurological patients (29 patients); 31 were discharged, and three have remained hospitalized since 30 June 2020. Comparing survivors and non-survivors by logistic regression, death was more frequent among patients who received mechanical ventilation (estimate 3.556, standard error 0.886, z = 4.013, P < 0.001), and no other comorbidity influenced outcome.
With respect to the study by Paterson et al. (2020), we observed only two patients presenting an immune disorder during their COVID infection period: a 44-year-old hypertensive female with lethargy and delayed awakening showing diffuse attenuation activity on EEG and a 37-year-old hypertensive male. The MRI of the former showed images suggestive of demyelinating lesions, and she was given a diagnosis of acute disseminated encephalomyelitis. The man had tetraparesis with pyramidal signs and a longitudinally extensive transverse myelitis on MRI (hyperintensity on T2-weighted images, spinal cord oedema from the upper cervical to thoracolumbar transition and inflammatory CSF with no oligoclonal bands, Supplementary Fig. 1). Both patients had no other recognizable cause for their syndromes. In contrast, we had more than twice the rate of stroke (47.6%) and a similar rate of encephalopathy. The percentage of stroke seen is compatible with other studies, but the numbers are heterogeneous, varying from 1.7% in Spain (Romero-Sánchez et al., 2020) to 3.5% and 5.7% in China (Mao et al., 2020; Xiong et al., 2020) and 62% in the UK (Varatharaj et al., 2020). In Brazil, one study in a university hospital dedicated to COVID-19 care has shown a prevalence of encephalopathy (44.4%) and stroke (16.7%) (Studart-Neto et al., 2020).
Twenty-seven per cent of our COVID-19 patients had a pre-existing neurological disease, with myasthenia in 29%. Two of these myasthenic patients developed stroke and one encephalopathy. In a review, the rate of previous neurological illnesses varied from 0% to 40% with a pooled percentage of 8%; cerebrovascular disease was the main comorbidity (16%) (Herman et al., 2020).
We have shown retrospective data, with limitations due to the design and the inclusion of moderate and severe COVID-19 patients. Neurological findings differ between reports, depending on the evaluated symptomatology, medical specialty, and severity of the illness. Most studies are from high-income countries with different vascular risk factors and nutritional status and older age. Here, we aimed to determine the prevalence of neurological complications in patients with SARS-CoV-2 infection, characterizing the observed clinical syndromes and outcomes in a tertiary hospital in a middle-income country with a heterogeneous population with high levels of vascular risk factors. Neurological complications could be caused by many concomitant factors: endothelial lesions, prothrombotic states, inflammatory storm and a sequela of systemic complications (bacterial infections, kidney insufficiency, etc.). It is also possible to detect the actions caused by viral spread in the olfactory nerve or through the breakdown of the blood–brain barrier by systemic inflammation and consequent cytokine invasion (Ellul et al., 2020; Helms et al., 2020; Solomon et al., 2020; Wiersinga et al., 2020). Data are insufficient to determine with certainty the main causes, as patients may remain in intensive care settings with severe systemic illness, resulting in considerable challenges to neuroimaging and neurophysiological examination.
Brazil offers free and universal health coverage through a unified health system with both federal and local governments responsible for the health care of COVID-19 patients. Successful and negative experiences and lessons learned from studies and data from low- and middle-income countries and Latin America are relevant to suggesting possible mechanisms linked to neurological manifestations of COVID-19. These studies may assist with the provision of better care and control of the pandemic regionally and globally.
Data availability
All study data will be available from the corresponding author upon reasonable request.
Funding
No funding was received towards this work.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Contributor Information
Sonia M D Brucki, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Luiza A Corazza, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Apolônio P de Queiroz, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Maraysa P Barros, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
João F S Tatsch, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Ivy L Riso, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Nardiel A Batista, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Gregori Manfroi, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Luis A Sawada, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Luana L R Batista, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Meire Argentoni Baldocchi, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Julian L de Freitas, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Mariana B Aidar, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
Maria S G Rocha, Department of Neurology, Hospital Santa Marcelina, Sao Paulo, Brazil.
References
- Ellul MA Benjamin L Singh B Lant S Michael BD Easton A, et al. Neurological associations of COVID-19. The Lancet Neurology 2020; 19: 767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; 382: 2268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology 2020; 95: 77–84. [DOI] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, . et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, . et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020; 143: 3104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen, Á, Layos-Romero A, García-García J, . et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 2020; 95: e1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji, SS, Keller K, Ali AS, . et al. Neuropathological features of COVID-19. N Engl J Med 2020; 383: 989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studart-Neto A Guedes BF Tuma RLE Camelo Filho AE Kubota GT Iepsen BD, et al. Neurological consultations and diagnoses in a large, dedicated COVID-19 university hospital. Arq Neuro-Psiquiatr 2020; 78: 494–500. [DOI] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nature Rev Immunol 2020; 20: 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, . et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020; 7: 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19). JAMA 2020; 324: 782. [DOI] [PubMed] [Google Scholar]
- Xiong W, Mu J, Guo J, Lu, L, Liu D, Luo J, . et al. New onset neurologic events in people with COVID-19 infection in three regions in China. Neurology 2020; 95: e1479–87. [DOI] [PubMed] [Google Scholar]
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019. JAMA Neurol 2020; 77: 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data will be available from the corresponding author upon reasonable request.


