Abstract
Background
Coronavirus disease 2019 (COVID-19) continues to cause significant morbidity and mortality worldwide. Correctional and detention facilities are at high risk of experiencing outbreaks. We aimed to evaluate cohort-based testing among detained persons exposed to laboratory-confirmed cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in order to identify presymptomatic and asymptomatic cases.
Methods
During 1–19 May 2020, 2 testing strategies were implemented in 12 tiers or housing units of the Cook County Jail, Chicago, Illinois. Detained persons were approached to participate in serial testing (n = 137) and offered tests at 3 time points over 14 days (day 1, days 3–5, and days 13–14). The second group was offered a single test and interview at the end of a 14-day quarantine period (day 14 group) (n = 87).
Results
224 detained persons were approached for participation and, of these, 194 (87%) participated in ≥1 interview and 172 (77%) had ≥1 test. Of the 172 tested, 19 were positive for SARS-CoV-2. In the serial testing group, 17 (89%) new cases were detected, 16 (84%) on day 1, 1 (5%) on days 3–5, and none on days 13–14; in the day 14 group, 2 (11%) cases were identified. More than half (12/19; 63%) of the newly identified cases were presymptomatic or asymptomatic.
Conclusions
Our findings highlight the utility of cohort-based testing promptly after initiating quarantine within a housing tier. Cohort-based testing efforts identified new SARS-CoV-2 asymptomatic and presymptomatic infections that may have been missed by symptom screening alone.
Keywords: SARS-CoV-2, COVID-19, correctional facilities, serial testing
Our findings suggest that early cohort-based testing in detained persons helped identify new SARS-CoV-2 asymptomatic and presymptomatic infections that may have been missed by symptom screening alone. Frequency of testing may be dependent on status of outbreak in the facility.
The United States has the highest incarceration rate of any nation in the world—431 detained persons per 100 000 population—with an estimated 2.3 million people confined on any given day [1]. High rates of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), have been reported in correctional and detention facilities across the United States [2]. Correctional and detention facilities are prone to outbreaks of COVID-19 for a variety of reasons, including congregate and crowded living conditions making social distancing, quarantine, and medical isolation challenging [3, 4]. Additionally, movement of staff across housing units and turnover among detained persons increase opportunities for SARS-CoV-2 introduction and transmission [4]. Early testing strategies relied on symptom assessments and were ineffective in identifying presymptomatic and asymptomatic infections [5].
The Cook County Jail (CCJ) in Chicago, Illinois, is one of the largest single-site jails in the United States, with an average daily census of approximately 6000 detained persons and a maximum capacity of 7500 [6]. Prior to the COVID-19 pandemic, CCJ housed detained persons in 6 divisions, composed of celled and dormitory housing areas [7]. The first laboratory-confirmed SARS-CoV-2 case in CCJ was identified on 18 March [8]. The Chicago Department of Public Health (CDPH) provided COVID-19 control and mitigation recommendations based on Centers for Disease Control and Prevention (CDC) guidance [9], aligned with the existing practices already in effect at the jail.
Infection-prevention and -control (IPC) interventions practiced have been described elsewhere [8]. At the time of investigation, CCJ was utilizing temperature and symptoms screening and testing detained persons with a point-of-care assay for SARS-CoV-2 at intake. Additionally, enhanced sanitation and disinfection practices, isolation of suspected and confirmed SARS-COV-2 cases in single cells, cohorting of other positive detained persons, quarantining all exposed detained persons, social distancing, universal masking of staff and detained persons, and minimization of detained persons and staff movement were implemented. Although the number of symptomatic laboratory-confirmed cases declined from early April to early May 2020, the use of testing strategies to identify asymptomatic cases was considered to reduce further transmission of COVID-19 in the facility [8].
During 1–19 May 2020, in partnership with CDPH, Cook County Health (CCH), Cook County Sheriff’s Office (CCSO), and Cermak Health Services (CHS) of Cook County Health, a CDC team conducted an epidemiologic investigation at CCJ. The investigation objectives were to evaluate the utility of serial testing as a method of identifying presymptomatic and asymptomatic cases and to describe symptomology among persons identified during the investigation.
METHODS
Study Population and Facility Details
During May 2020, CCJ had a lower average daily population, approximately 4000 detainees [6]. This was due to decompression efforts by the criminal justice system [7, 8]. The facility and an early COVID-19 outbreak in CCJ have been described elsewhere [8]. The CCJ is divided into separate divisions, which comprise celled and dormitory housing areas. Each division has smaller housing units (“tiers”), which are residential units structured as dormitories or celled living units. Celled living units were primarily double occupancy but were utilized as single occupancy during the outbreak. Most dormitory-style living units housed between 38 and 48 detained persons at full capacity. When possible, this number was decreased by approximately 50% to adhere to social-distancing guidelines.
Individuals with laboratory-confirmed SARS-CoV-2 infection were removed from their tier and moved to isolation celled units or cohort dormitory housing for a minimum of 21 days. Exposed persons remained in the tier and were quarantined together and monitored daily for abnormal vital signs and COVID-19–related symptoms for a minimum of 14 days.
Investigation Design
Housing units were selected for inclusion in the investigation if at least 1 detained person had a positive SARS-CoV-2 test and the unit was placed on quarantine. The investigation team employed 2 cohort-based SARS-CoV-2 testing strategies within quarantined tiers where a known case of COVID-19 resided (ie, close-contact exposure): (1) 1 group was offered a test at 3 time points (serial testing cohort) and (2) a second group was offered a single test at the end of quarantine (day 14 group). All detained persons in the day 14 group were already near the end of their quarantine period at the time of this investigation. Since there are anecdotal reports of correctional facilities performing testing on the last day of quarantine (before lifting restrictions), the utility of these 2 testing strategies was assessed to assist in prioritization of testing resources.
Serial testing was conducted at 3 time points—day 1, days 3–5, and days 13–14—which were selected based on the median SARS-CoV-2 incubation period of 5 days [10]. At each time point, detained persons were offered a test and brief interview to assess current symptoms and had the option of declining either or both. Day 14 testing occurred on the final day (day 13–14) of quarantine. After detained persons verbally consented, the investigation team conducted face-to-face interviews using a standardized questionnaire. Symptoms assessed were self-report of fever, cough, dyspnea, chills, myalgia, headache, rhinorrhea, nasal congestion, pharyngitis, anosmia (loss of smell), ageusia (loss of taste), abdominal pain, nausea, vomiting, and diarrhea (≥3 loose stools in a 24-hour period). Additionally, at each time point, body temperature readings were taken from the temporal region of the forehead using a nontouch infrared thermometer. Questionnaires for the day 1 (serial testing cohort) and day 14 group included variables on demographics, COVID-19–related symptoms (in the past 2 weeks and 2 months), medical history, potential exposures to known COVID-19 cases, and daily movement within the facility during the last 2 months. Detained persons with positive test results at any of the 3 time points were removed from the tier and transferred to an isolation tier. The 14-day quarantine timeline would be reset from the day a new laboratory-confirmed positive result was detected. Detained persons with negative test results remained eligible for subsequent tests and symptom assessments until the individual declined, received a positive test result, or completed the 14-day quarantine period.
Those with positive test results were isolated and medically monitored by nursing staff. The CDC team reassessed symptoms approximately 1 week later, and if anyone was symptomatic, it was immediately reported to the nursing manager. Detained persons included in the serial testing cohort who declined both an interview and testing were not eligible for subsequent visits by the CDC team. Persons who agreed to at least 1 component (ie, interview or testing) were offered an interview and testing at subsequent visits.
Laboratory Testing
All specimens for SARS-CoV-2 testing were collected by flocked nasopharyngeal (NP) swabs. The NP swabs collected at the facility were stored in a cooler at 4°F and transported to the referral hospital laboratory (John H. Stroger, Jr, Hospital of Cook County) within 12 hours. The laboratory performed 1-step, real-time reverse transcriptase–polymerase chain reaction (RT-PCR) on all samples, using the SARS-CoV-2 m2000 RealTime system RT-PCR (Abbott Laboratories, IL, USA) [11]. All results were reported to the facility clinical staff within 24 hours of specimen submission, and if positive, detained persons were notified of their individual test result and immediately isolated.
Data Analyses
Data from the paper-based questionnaires were entered into secured REDCap electronic data-capture tools hosted at CDC [12, 13]. Univariate and bivariate analyses were used to calculate counts and percentages of demographic characteristics, self-reported underlying medical conditions, self-reported symptoms, test results, and refusals by day of testing for both testing strategies. Demographic, clinical, and symptom variables were further stratified by interview and test day and overall test result (ie, positive vs negative). Underlying medical conditions were grouped into 12 categories by medical classifications [14].
Reported symptoms were classified into the Council for State and Territorial Epidemiologists (CSTE) COVID-19 case definition based on CSTE clinical criteria A and/or B. The CSTE clinical criteria A were as follows—at least 2 of the following symptoms: fever (measured or subjective), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s). The CSTE clinical criteria B were at least 1 of cough or shortness of breath [15]. Cases of COVID-19 identified in this investigation were classified as symptomatic (reporting ≥1 criteria A or B symptom[s] with an onset date before the specimen collection date resulting in a positive test), asymptomatic (no criteria A or B symptoms reported), presymptomatic (≥1 criteria A or B symptom[s] reported with an onset within 7 days after the specimen collection date resulting in a positive test and none reported before the specimen collection date resulting in a positive test), or unknown (reporting unknown to ≥1 criteria A or B symptom). All analyses were performed using statistical software SAS version 9.4 (SAS Institute, Cary, NC, USA), R 3.6.1 (R Foundation for Statistical Computing; 2019) and the networkD3 package (version 0.4; https://cran.r-project.org/web/packages/networkD3/networkD3.pdf) software.
Ethics
This investigation was part of the ongoing public health response to COVID-19; thus, CDC’s Human Research Protection Office determined the activity to meet the requirements of public health surveillance as defined in 45 CFR (Code of Federal Regulations) 46.102(l)(2) and exempt from human subjects’ research regulations. The CDPH, CCH, CHS, and CCSO approved this project prior to initiation. At each encounter with detained persons, the CDC team provided an overview of the study and procedures and answered any questions before offering an interview or testing. Verbal consent was obtained from each detained person prior to enrollment. Data-collection forms were approved under the Office of Management and Budget (OMB: 0920–1011).
RESULTS
A total of 224 detained persons from 12 quarantined units were approached for participation in the investigation: 137 persons in 7 units from the serial testing group and 87 persons in 5 units from the day 14 group. Across both groups, 195 (87%) agreed to participate in at least 1 component: 171 (88%) consented to both interview and testing, 23 (12%) to the interview only, and 1 (1%) to testing only; 29 (13%) detained persons refused to participate in the interview or testing.
Of participants interviewed, most were men (181/193; 94%) and under the age of 50 (168/193; 87%) (Table 1). The majority (67%) of participants self-identified as non-Hispanic Black, 19% as Hispanic, and 6% as non-Hispanic White. Underlying medical conditions were self-reported by 65% of participants; respiratory diseases (32%) were the most common. A history of smoking tobacco was reported by 77% (Table 1).
Table 1.
Demographic Characteristics of Detained Persons Tested for SARS-CoV-2, by Test Result: Cook County Jail, Chicago, Illinois—May 2020
| SARS-CoV-2 Test Results (n = 169) | |||
|---|---|---|---|
| Demographic Characteristics | Interview Results (n = 193)a | Positive (n = 19) | Negative (n = 150) |
| Age, median (IQR), years | 33 (25–43) | 41 (30–49) | 32 (25–42) |
| Age category, n (%) | |||
| ≤29 years | 77 (40) | 4 (21) | 62 (41) |
| 30–49 years | 91 (47) | 11 (58) | 69 (46) |
| ≥50 years | 25 (13) | 4 (21) | 19 (13) |
| Sex, n (%) | |||
| Men | 181 (94) | 19 (100) | 139 (93) |
| Women | 12 (6) | 0 (0) | 11 (7) |
| Race/ethnicity, n (%) | |||
| Hispanic | 36 (19) | 3 (16) | 29 (19) |
| White, non-Hispanic | 12 (6) | 1 (5) | 10 (7) |
| Black, non-Hispanic | 130 (67) | 14 (74) | 100 (67) |
| Other,b non-Hispanic | 15 (8) | 1 (5) | 11 (7) |
| Previous tobacco use (ever used), n (%) | 148 (77) | 16 (84) | 113 (75) |
| Daily | 119 (62) | 10 (53) | 92 (61) |
| Less than daily | 29 (15) | 6 (32) | 21 (14) |
| Not at all | 44 (23) | 3 (16) | 36 (24) |
| Underlying medical condition,c n (%) | 126 (65) | 10 (53) | 98 (65) |
| Respiratory disease | 62 (32) | 8 (42) | 44 (29) |
| Asthma | 53 (28) | 6 (32) | 38 (25) |
| COPDd | 8 (4) | 2 (11) | 6 (4) |
| Cardiovascular disease | 32 (17) | 4 (21) | 26 (17) |
| Hypertension | 28 (15) | 4 (21) | 23 (15) |
| Other cardiovascular disease/conditione | 5 (3) | 0 (0) | 4 (3) |
| BMI (kg/m2), n (%) | 58 (30) | 1 (5) | 49 (33) |
| Obesity: 30.0 to <40.0 | 52 (27) | 0 (0) | 44 (29) |
| Severe obesity: ≥40.0 | 6 (3) | 1 (5) | 5 (3) |
| Immunocompromising disease/condition,f n (%) | 5 (3) | 2 (11) | 3 (6) |
| Hemoglobin disorder,g n (%) | 4 (2) | 1 (5) | 3 (6) |
| Type 2 diabetes, n (%) | 7 (4) | 1 (5) | 4 (3) |
| Other chronic disease/condition,h n (%) | 7 (4) | 1 (5) | 5 (3) |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IQR, interquartile range; NP, nasopharyngeal swab; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aA total of 224 detained persons were approached for interview and NP collection: 111 detained persons agreed to interview from the serial cohort on day 0; 82 agreed to interview from the day 14–only group. Of these 193 detained persons interviewed (on day 0 for serial testing cohort; day 14 for day 14–only group), 169 agreed to NP collection on day 0, days 3–5, or days 13–14. Twenty-four detained persons refused testing and interview; 2 agreed to testing only. One additional person agreed to interview on days 3–4 only, and so is excluded from Table 1.
bAmerican Indians/Alaska Natives (1), other race (8), multiple races (6).
cTotals will not sum to 193; these categories are not mutually exclusive. Classification and subcategories were created based on CDC guidance [14]. They are inclusive of the conditions, diseases, or disorders most commonly reported by detained persons.
dCOPD including emphysema and chronic bronchitis.
eInclusive of heart arrythmia, Type II heart block (Mobitz type II), mitral valve prolapse, congenital heart abnormality, and history of heart attack.
f Inclusive of HIV/AIDS, corticosteroid use, and autoimmune disease.
g Inclusive of sickle cell disease.
hInclusive of hepatitis C, diverticulitis, gout, and history of cancer.
Interview and test participation varied by group, unit, and day (Table 2). Forty percent (89/224) of detained persons refused a test at least once. Of the serial testing cohort, 31% (43/137) detained persons refused testing on day 1. Among detained persons who approved testing or interview at least once, there were no differences between refusal rates by sex, underlying medical conditions, or symptom reporting across time points; however, a higher proportion of younger participants (<30 years) refused interview or testing on days 3–5 and days 13–14.
Table 2.
SARS-CoV-2 Testing Uptake and Results in 12 Quarantined Tiers by Testing Strategy, Tier, and Testing Day: Cook County Jail, Chicago, Illinois—May 2020
| Day 1 | Days 3–5 | Days 13–14 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tier | No. of Days Since Index Case to Day 1 | Total,a No. in Each Cohort | Overall Attack Rate,b % | Testing Eligible, n | Testing Total, n | Testing Refusal, n (%) | Positive Result, n (%) | Testing Eligible, n | Testing Total, n | Testing Refusal, n (%) | Positive Result, n (%) | Testing Eligible, n | Testing Total, n | Testing Refusal, n (%) | Positive Result, n (%) |
| Serial cohort | |||||||||||||||
| 1 | 2 | 16 | 13 | 16 | 13 | 3 (19) | 1 (8) | 14 | 10 | 4 (29) | 1 (10) | 12 | 5 | 7 (58) | 0 (0) |
| 2 | 2 | 22 | 9 | 22 | 11 | 11 (50) | 2 (18) | 17 | 8 | 9 (53) | 0 (0) | 15 | 8 | 7 (47) | 0 (0) |
| 3 | 6 | 20 | 5 | 20 | 8 | 12 (60) | 1 (13) | 13 | 1 | 12 (92) | 0 (0) | 12 | 1 | 11 (92) | 0 (0) |
| 4 | 3 | 16 | 6 | 16 | 16 | 0 (0) | 1 (6) | 15 | 14 | 1 (7) | 0 (0) | 15 | 12 | 3 (20) | 0 (0) |
| 5 | 4 | 22 | 0 | 22 | 11 | 11 (50) | 0 (0) | 12 | 6 | 6 (50) | 0 (0) | 12 | 6 | 6 (50) | 0 (0) |
| 6 | 1 | 21 | 43 | 21 | 21 | 0 (0) | 9 (43) | 12 | 11 | 1 (8) | 0 (0) | 11 | 11 | 0 (0) | 0 (0) |
| 7 | 1 | 20 | 10 | 20 | 14 | 6 (30) | 2 (14) | 14 | 6 | 8 (57) | 0 (0) | 14 | 4 | 10 (71) | 0 (0) |
| Subtotal | 137 | … | 137 | 94 | 43 (31) | 16 (17) | 97 | 56 | 41 (42) | 1 (2) | 91 | 47 | 44 (48) | 0 (0) | |
| Day 14 cohort | |||||||||||||||
| 8 | 1 | 20 | 5 | … | … | … | … | … | … | … | … | 20 | 15 | 5 (25) | 1 (7) |
| 9 | 1 | 17 | 6 | … | … | … | … | … | … | … | … | 17 | 16 | 1 (6) | 1 (6) |
| 10 | 7 | 14 | 0 | … | … | … | … | … | … | … | … | 14 | 11 | 3 (21) | 0 (0) |
| 11 | 5 | 16 | 0 | … | … | … | … | … | … | … | … | 16 | 15 | 1 (6) | 0 (0) |
| 12 | 1 | 20 | 0 | … | … | … | … | … | … | … | … | 20 | 19 | 1 (5) | 0 (0) |
| Subtotal | 87 | … | … | … | … | … | … | … | … | … | 87 | 76 | 11 (13) | 2 (3) | |
Abbreviations: NP, nasopharyngeal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aIn cohort 4, day 0/1, 3 NP swabs were collected but no results found due to tube leakage—included in Testing Eligible, included in Testing Total, excluded from Testing Refusal. In cohort 3, day 0/1, 1 NP was collected but no results found due to incorrect order—included in Testing Eligible, included in Testing Total, excluded from Testing Refusal. In cohort 3, day 3/4, 1 NP was collected but no result found due to mislabeling—included in Testing Eligible, included in Testing Total, excluded from Testing Refusal.
bOverall attack rate is calculated by dividing the total number of new positive cases by the total detained persons eligible for testing over the entire 14-day period.
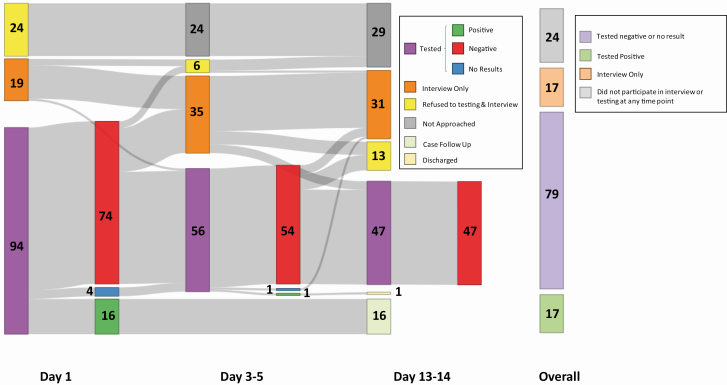
Among the 137 detained persons approached for the serial testing cohort, 96 (70%) were tested during at least 1 of the 3 time points (Figure 1). Of these, 17 (18%) had positive test results, 78 (81%) had negative test results, and 1 (1%) did not receive a test result due to specimen spillage in transport (Table 2). Among the 17 persons with a positive test result, 16 (94%) were positive on day 1 and 1 (6%) was positive on days 3–5 (Table 2). No new positives (0/47) were identified through serial testing efforts on days 13–14. Among the 87 detained persons approached for a single test (day 14 group), 82 (94%) agreed to participate; 76 (93%) were tested and interviewed. Of the 76 tested, 2 (3%) had a positive test result. Considering both testing strategies, 19 (11%) of the 172 persons across 8 units (6 units using the serial testing approach and 2 units using the single test approach) tested positive for SARS-CoV-2 at any point. Estimated attack rates over the entire follow-up period ranged from 5% to 43% within tiers.
Figure 1.
Flow diagram of the number of detained persons tested, interviewed, and refused in the serial testing cohort: Cook County Jail, Chicago, Illinois—May 2020. Serial testing was conducted in 7 tiers (housing units) at 3 time points over 14 days (day 1, days 3–5, and days 13–14). Detained persons who were cohorted and placed under quarantine due to exposure to a recent laboratory-confirmed case were approached to participate in the investigation. Eligible detained persons either interviewed and tested, only interviewed, or refused both. Detained persons with a positive test result were isolated and followed for clinical observation: those with negative test result were contacted again on later time points.
Among the 194 detained persons who agreed to the interview, 139 (72%) did not report symptoms and 52 (27%) reported symptoms; for the remaining 3 (2%) respondents, reported symptoms were unknown or missing (Table 3). Of the detained persons reporting symptoms, 28 (54%) had symptom onset during the 2 weeks prior to specimen collection, 18 (35%) had symptom onset more than 2 weeks prior, 2 (4%) had an unknown onset date, and 4 (8%) were presymptomatic. Among the 19 detained persons with a SARS-CoV-2–positive test result, a substantial proportion were asymptomatic (63%) at the time of specimen collection. One-third of detained persons were asymptomatic at the time of testing and later developed symptoms. Among the 7 detained persons with positive test results who reported symptoms at the time of specimen collection, 57% reported symptom onset during the prior 2 weeks and 43% reported symptom onset more than 2 weeks prior. A total of 151 detained persons had a negative test result for SARS-CoV-2, with one-quarter reporting at least 1 symptom in the preceding 2 months (Table 3).
Table 3.
Symptom Status of Detained Persons Tested for SARS-CoV-2 by Testing Strategy and Test Results: Cook County Jail, Chicago, Illinois—May 2020
| SARS-CoV-2 Test Results (n = 170)b | |||||||
|---|---|---|---|---|---|---|---|
| Interview Results (n = 194)a | Positive (n = 19) | Negative (n = 151) | |||||
| Symptom Status | Serial Testing Cohort (n = 112) | Day 14 Testing Cohort (n = 82) | Day 1 (n = 16) | Days 3–5 (n = 1) | Days 13–14c (n = 2) | Serial Testing Cohort (n = 77) | Day 14 Testing Cohort (n = 74) |
| Asymptomatic,d n (%) | 77 (69) | 62 (76) | 5 (31) | 1 (100) | 2 (100) | 55 (71) | 56 (76) |
| Presymptomatic,e n (%) | 4 (4) | NA | 4 (25) | 0 (0) | 0 (0) | 0 (0) | NA |
| Symptomatic,f n (%) | 29 (26) | 19 (23) | 7 (44) | 0 (0) | 0 (0) | 21 (27) | 17 (23) |
| Onset in past 2 weeks | 15 (52) | 13 (68) | 4 (57) | … | … | 10 (48) | 11 (65) |
| Onset >2 weeks ago | 14 (48) | 4 (21) | 3 (43) | … | … | 11 (52) | 4 (24) |
| Onset unknown | 0 (0) | 2 (11) | 0 (0) | … | … | 0 (0) | 2 (12) |
| Unknown,g n (%) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
Abbreviations: COVID-19, coronavirus disease 2019; CSTE, Council of State and Territorial Epidemiologists; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aA total of 194 detained persons agreed to interview during at least 1 time point throughout the course of the investigation.
bA total of 171 agreed to testing and interview during at least 1 time point throughout the course of the interview. One did not receive final test results due to specimen spillage and is excluded from the SARS-CoV-2 Test Results section.
cTwo positives were identified in the single test day 14 group.
dPersons reporting no CSTE criteria A or B COVID-19 symptoms in the 2 weeks prior to testing. Criteria A: fever, subjective fever, chills, myalgia, headache, sore throat, loss of taste or smell. Criteria B: cough or shortness of breath.
ePersons who reported onset of symptoms after the date of specimen collection that resulted in a positive test.
fPersons reporting ≥1 CSTE criteria A or B COVID-19 symptom.
gPersons reporting “unknown” to ≥1 CSTE criteria A or B COVID-19 symptom.
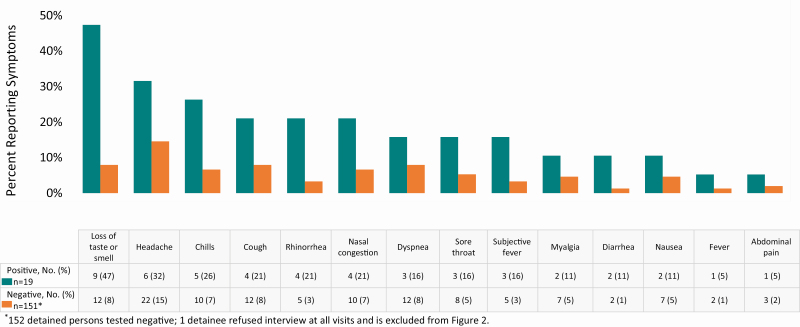
Among the 19 detained persons with a positive test result for SARS-CoV-2, the most commonly reported symptoms in the 2 weeks prior to testing were loss of taste or smell (47%), headache (32%), and chills (26%) (Figure 2). Of the 151 detained persons interviewed with negative test results, commonly reported symptoms in the preceding 2 weeks included headache (15%), loss of taste or smell (8%), cough (8%), dyspnea (8%), chills (7%), and nasal congestion (7%).
Figure 2.
Number and percentage of detained persons reporting symptoms in 2 weeks prior to testing, by SARS-CoV-2 test result: Cook County Jail, Chicago, Illinois—May 2020. *152 detained persons tested negative; 1 detainee refused interview at all visits and is excluded from Figure 2. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Cohort-based SARS-CoV-2 testing of quarantined detained persons at one of the largest single-site jails in the United States led to the prompt identification and isolation of asymptomatic and presymptomatic persons with SARS-CoV-2 infection, and likely reduced transmission. Twelve of the 19 detained persons with a SARS-CoV-2–positive test result were asymptomatic (63%) at the time of specimen collection; 4 of these developed symptoms after testing. Symptom screening alone would have been ineffective in identifying new cases, given that a high percentage of detained persons with positive test results for SARS-CoV-2 did not report COVID-19 symptoms at the time of specimen collection. Early identification and isolation of SARS-CoV-2 infection, through cohort-based testing, including strategies already in use at CCJ, reduces the likelihood of transmission, especially in congregate living facilities [8].
The overall estimated attack rate in selected tiers (11%) was lower than previous reports in CCJ (98% in 1 dormitory) and other correctional facilities [8, 16]. This likely reflects success in mitigating transmission through prevention measures adopted by CCJ management. Among the 7 quarantined tiers where cohort-based serial testing was implemented, 17% of enrolled detained persons tested positive for SARS-CoV-2 infection during the 14-day quarantine period; estimated attack rates within tiers ranged from 5% to 43%. However, the attack rates may be underestimated for a variety of reasons, including refusals and potential misclassification of previous infections [17]. Finally, we cannot rule out that detained persons may have already recovered from a SARS-CoV-2 infection prior to the investigation.
While 2 cases were identified in the day 14 group, cohort-based testing closest to the start of quarantine had the highest yield and identified the majority of SARS-CoV-2 infections (16/19; 84%). One case was identified on days 3–5 and none on day 14. This is consistent with evidence suggesting that serial testing in congregate settings such as correctional facilities can identify new cases before symptom onset and potentially prior to their infectious period, allowing them to be isolated early and interrupting transmission [17]. Additionally, the short testing turnaround time at the CCJ-affiliated laboratory made implementation of control strategies feasible.
The majority of the newly detected cases were asymptomatic or presymptomatic. However, among detained persons with a positive test result who reported at least 1 symptom, headache and loss of taste or smell were the most commonly reported symptoms. Among detained persons with a negative test result, 25% reported symptoms compatible with COVID-19. While nonspecific symptoms may be explained by circulation of other respiratory infections or seasonal allergies, the large number of detained persons with negative test results who reported loss of taste or smell might suggest possible false-negative test results or confabulation of symptoms or prior COVID-19 infection.
We observed high rates of refusals among participants at CCJ, particularly at the start of the investigation. One possibility that could have impacted rates of participation was that there is evidence to suggest that detained persons may fear losing personal privileges such as access to common areas or phone calls and risk financial consequences, including copays associated with accessing healthcare [18, 19]. Detained persons may want to avoid the disruption of being moved to isolation or another facility upon a positive test result, and some enrolled subjects reported that loss of access to the commissary was a major reason for refusal. Facility staff should consider noncoercive strategies to encourage testing uptake, such as health promotion and education. When possible, starting serial testing soon after a case is identified as well as planning for separation of persons who decline testing could prevent potential transmission from close contacts with unknown infection status. No differences in facility characteristics were observed in the 12 quarantined units.
This investigation was subject to several limitations. First, our investigation began as the numbers of new cases in the facility were decreasing and relied on PCR-based testing, which measures the current presence or absence of virus and cannot identify those who may have already cleared the infection [8]. The prevalence of disease could be underestimated, and the transmission potential may have been lower in tiers with recovered individuals. Also, symptom assessments and survey data are self-reported and dependent on a person’s ability to recall when symptoms occurred. Second, we observed high rates of refusals among detained persons, particularly at the start of the investigation, potentially resulting in underestimation of the burden of SARS-CoV-2 in this population.
Our findings highlight the utility of cohort-based approaches to testing, which can effectively identify presymptomatic and asymptomatic cases compared with symptom screening alone. Further, timely and effective measures to separate infected detained persons (ie, cohorting and isolation in single-occupancy cells) and staff can mitigate continued transmission of SARS-CoV-2 in detention facilities. Early testing of close contacts in quarantine, in conjunction with IPC and other mitigation measures, may slow transmission in correctional facilities and the surrounding community.
Notes
Authors’ contributions. R. D., K. C., P. K. M., P. A. A., J. E. T., I. G., and C. J. Z. provided overall leadership and guidance to the investigation. A. W., M. M. A., D. A. M., A. F. M., J. T. V., P. K. M., and C. J. Z. completed the investigation of cases and collected epidemiological data. R. D., K. C., K. A. F., A. W., R. S., M. K., K. K., M. T., A. M. B., J. E. T., P. A. A., S. R. B., C. C. M., R. L., J. G., B. J., and S. F. W. provided technical assistance and input in content areas, including epidemiological methods, data analysis and subject matter expertise. A. W., K. A. F., R. S., M. K., K. C., R. D., P. K. M., and C. J. Z. drafted and revised this manuscript. All authors reviewed, revised, and approved the final manuscript.
Acknowledgments. We acknowledge the contributions of Usha Samala and Massimo Pacilli from the Chicago Department of Public Health; Francois Owens and Frank Oliver from Cermak Health Services; Cook County Sheriff Thomas J. Dart, Brad Curry, Tarry Williams, Michael Miller, Peter Orris, and Matthew Burke from the Cook County Sherriff’s Office. We also acknowledge Ben Hallowell, Mariel Marlow, Megan Wallace, Amy Schumacher, Lauren Franco, Margaret Williams, Eric Manders, and Bert Kelly from the CDC’s COVID-19 Response Epi Task Force for their meaningful contribution supporting the investigation.
Disclaimer. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sawyer M. A country called prison: mass incarceration and the making of a new nation. Library J 2015; 140:98. [Google Scholar]
- 2. Wallace M, Hagan L, Curran KG, et al. COVID-19 in correctional and detention facilities—United States, February-April 2020. MMWR Morb Mortal Wkly Rep 2020; 69:587–90. [DOI] [PubMed] [Google Scholar]
- 3. Dumont DM, Brockmann B, Dickman S, Alexander N, Rich JD. Public health and the epidemic of incarceration. Annu Rev Public Health 2012; 33:325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin R. The challenge of preventing COVID-19 spread in correctional facilities. JAMA 2020; 323:1760–1. doi: 10.1001/jama.2020.5427 [DOI] [PubMed] [Google Scholar]
- 5. Hagan LM, Williams SP, Spaulding AC, et al. Mass testing for SARS-CoV-2 in 16 prisons and jails—six jurisdictions, United States, April-May 2020. MWR Morb Mortal Wkly Rep 2020; 69:1139–43. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6933a3.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook County Sherriff’s Office. Cook County Jail’s history. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6933a3.htm. Accessed 26 August 2020.
- 7.Cook County Sherriff’s Office. COVID-19 cases at CCDOC. Available at: https://www.cookcountysheriff.org/covid-19-cases-at-ccdoc/. Accessed 8 July 2020.
- 8. Zawitz C, Binder AM, Ghinai I, et al. Outbreak of COVID-19 in one of the largest jails in the United States—Chicago, IL, March 1–April 30, 2020. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.07.12.20148494v1. Accessed 26 August 2020. [Google Scholar]
- 9. Centers for Disease Control and Prevention. Interim guidance on management of coronavirus disease 2019 (COVID-19) in correctional and detention facilities. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/correction-detention/guidance-correctional-detention.html. Accessed 8 July 2020.
- 10. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Degli-Angeli E, Dragavon J, Huang ML, et al. Validation and verification of the Abbott RealTime SARS-CoV-2 assay analytical and clinical performance. J Clin Virol 2020; 129:104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. People with certain medical conditions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 26 August 2020.
- 15. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19); 2020 interim case definition, approved April 5, 2020. Available at: https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/. Accessed 26 August 2020.
- 16. Njuguna H, Wallace M, Simonson S, et al. Serial laboratory testing for SARS-CoV-2 infection among incarcerated and detained persons in a correctional and detention facility—Louisiana, April-May 2020. MMWR Morb Mortal Wkly Rep 2020; 69:836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spaulding AC, Perez SD, Seals RM, Hallman MA, Kavasery R, Weiss PS. Diversity of release patterns for jail detainees: implications for public health interventions. Am J Public Health 2011; 101(Suppl 1):S347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallace M, Marlow M, Simonson S, et al. Public health response to COVID-19 cases in correctional and detention facilities—Louisiana, March-April 2020. MMWR Morb Mortal Wkly Rep 2020; 69:594–8. [DOI] [PubMed] [Google Scholar]
- 19. Awofeso N. Prisoner healthcare co-payment policy: a cost-cutting measure that might threaten inmates’ health. Appl Health Econ Health Policy 2005; 4:159–64. [DOI] [PubMed] [Google Scholar]