Abstract
Aims
This meta-analysis provides summary odds ratio (OR) estimates for associations between treatment with (vs. without) renin–angiotensin system blockers and risk of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection and coronavirus disease 2019 (CoViD-19) severity (including case-fatality) in patients with hypertension, and in all patients (irrespective of hypertension).
Methods and results
PubMed, EMBASE, Web of Science, Google Scholar, medRxiv, and SSRN were searched (2 May 2020 to 12 August 2020) for non-randomized observational CoViD-19 studies. Event/patient numbers were extracted, comparing angiotensin-converting enzyme (ACE) inhibitor/angiotensin-receptor blocker (ARB) treatment (and each separately), to treatment with neither drug, for the outcomes: (i) likelihood of SARS-CoV-2 infection; (ii) CoViD-19 severity [including hospitalization, intensive therapy unit (ITU), ventilation]; (iii) case-fatality. The risk of bias was assessed (ROBINS-I). Random-effects meta-analysis estimates were pooled. Eighty-six studies including 459 755 patients (103 317 with hypertension), were analysed. In patients with hypertension, ACE inhibitor or ARB treatment was not associated with a greater likelihood of SARS-CoV-2 infection in 60 141 patients (OR 1.06, 95% CI 0.99–1.14), hospitalization in 5925 patients (OR 0.90, 0.62–1.31), ITU in 7218 patients (OR 1.06, 0.73–1.56), ventilation (or ITU/ventilation/death) in 13 163 patients (OR 0.91, 0.72–1.15) or case-fatality in 18 735 patients with 2893 deaths (OR 0.75, 0.61–0.92).
Conclusion
Angiotensin-converting enzyme inhibitors and ARBs appear safe in the context of SARS-CoV-2 infection and should not be discontinued.
PROSPERO registration number CRD42020186996.
Keywords: Angi, otensin-converting enzyme inhibitor, Angiotensin receptor blocker, COVID-19, Meta-analysis, Renin–angiotensin system, Severe acute respiratory syndrome coronavirus 2
Introduction
Concern has been raised about the safety of the renin–angiotensin system (RAS) blockers in relation to infection with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2).1–6 Specifically, it has been suggested that angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) might increase the risk of contracting SARS-CoV-2 infection and the severity of coronavirus disease 2019 (CoViD-19) in those who are infected.1–6 These concerns stemmed from the fact that SARS-CoV-2 causes infection by binding to ACE2 on the cell membrane and experimental evidence that RAS blockers up-regulate ACE2 in animal models.4,7,8 However, there are few data to support this finding in humans and the possible increase in plasma ACE2 in people treated with ACE inhibitors may be beneficial rather than harmful if circulating ACE2 binds SARS-CoV-2 and prevents it from binding to and entering cells.4,8,9 Indeed, there is an alternative hypothesis that down-regulation of ACE2 in cells infected with SARS-CoV-2 may lead to angiotensin II-induced injury.4,8,10 This is because ACE2 is the key enzyme involved in the degradation of angiotensin II, the cleavage of which is also thought to produce other cytoprotective angiotensin peptides.4,8–10 Consequently, rather than increasing the severity of CoViD-19, RAS blockers might reduce it and there is some experimental support for this hypothesis.4,8–11 It is clearly important to know which, if any, of these competing hypotheses is correct given the widespread use of ACE inhibitors and ARBs in patients with cardiovascular disease and with diabetes and nephropathy, who are already at high risk in relation to CoViD-19. Indeed, given their life-saving benefits in heart failure and after myocardial infarction, withholding ACE inhibitors and ARBs because of misplaced safety concerns could be harmful (and there is evidence that patients do deteriorate on discontinuing these treatments).4,8 Recently, several observational studies of rates of SARS-CoV-2 infection in patients treated and not treated with RAS blockers, along with the severity of CoViD-19 in these patients, have been published. However, many were small and individually did not provide a robust estimate of the risk of adverse outcomes associated with RAS blocker treatment. Indication bias, reflecting preferential use of these agents in sicker patients, for example in patients with heart failure, was often unaccounted for. Moreover, and largely overlooked, is the fact that ACE inhibitors and ARBs are pharmacologically distinct, with different effects on components of the RAS, which could influence their interaction with SARS-CoV-2.12–14 It is therefore important to understand whether the type of RAS blocker matters in relation to CoViD-19, especially as these drugs can generally be used interchangeably in clinical practice, and patients could be switched to the safer alternative. Consequently, we have undertaken a systematic review and meta-analysis of non-randomized, observational, studies to address these questions which are relevant to millions of patients with hypertension, diabetes, cardiovascular, and renal disease treated with RAS blockers worldwide.
Methods
Registration and guidelines
Registration
International Prospective Register of Systematic Reviews (PROSPERO) registration number CRD42020186996.15
Guidelines
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) (Supplementary material online, Appendix S1).16,17
Systematic review
Search strategy and selection criteria
We performed a systematic review of non-randomized observational studies (cohort, case–control, case series). Two researchers with prior training and experience in meta-analysis techniques independently performed searches. Studies were reviewed for inclusion by at least two independent reviewers. Any conflicts over inclusion were resolved by consensus. Databases searched include PubMed, EMBASE, Web of Science, and Google Scholar. We also searched the medRxiv and SSRN preprint servers in view of the rapid dissemination of many new studies related to CoViD-19 on these platforms. Our search started on 2 May 2020 and completed on 12 August 2020. We included publications published until 12 August 2020. We re-ran searches prior to the final analysis. We did not set any restrictions on language. Further hand searches were performed from references. We removed duplicates. Our search strategy (detailed in Supplementary material online, Appendix S2), in brief, included a combination of the following main search terms: ‘severe acute respiratory syndrome’ OR ‘SARS-CoV-2’ OR ‘coronavirus disease 2019’ OR ‘COVID-19’ OR ‘2019-nCoV’ OR ‘novel coronavirus’ AND ‘renin angiotensin system blockers’ OR ‘RAS blockers’ OR ‘angiotensin-converting enzyme inhibitors’ OR ‘ACE inhibitors’ OR ‘angiotensin-receptor blockers’ OR ‘angiotensin II receptor blocker’ OR ‘ARB’ AND ‘observational’ OR ‘cohort’ OR ‘case control’ OR ‘case series’.
Inclusion and exclusion criteria (populations)
We extracted data from two groups of patients (i) Patients with a history of hypertension (‘hypertension’) and (ii) All patients (including those with hypertension). We excluded studies that only had data on other selected groups of patients, for example, those with kidney transplantation, cancer, or diabetes, to minimize the risk of confounding by indication. Most of these studies included fewer than 500 patients.
Inclusion and exclusion criteria (outcomes)
We extracted data from studies which reported treatment (with either an ACE inhibitor or ARB or an ACE inhibitor and ARB, separately, where data were available) compared with neither of these treatments and the following outcomes (i) likelihood of a positive SARS-CoV-2 test; (ii) severity of CoViD-19; and (iii) case-fatality rate. For ‘severe’ CoViD-19 outcomes, in addition to case-fatality, we examined (i) admission to hospital; (ii) severe/critical illness; (iii) admission to an intensive therapy unit (ITU); (iv) mechanical ventilation; and (v) composites of ITU/ventilation/death, where reported. For the likelihood of a positive SARS-CoV-2 test outcome, we only included studies that had controls that had tested negative (i.e. we excluded studies reporting population controls who did not undergo testing for SARS-CoV-2 but were assumed to have a negative test).
Data analysis
Data collection
At least two individuals independently extracted data. Any disagreements were resolved by consensus. Information about study design, methodology, and baseline characteristics (age, sex, co-morbidities) was extracted. A standardized 2 × 2 table proforma was used to extract four key numbers: treated event, treated no event, control event, control no event. Where raw numbers were not directly available, authors of studies were contacted who then provided raw numbers (Mehta et al.18). Where required, calculations were performed with the assumption that no patients were taking both an ACE inhibitor and ARB at the same time (and if this assumption was made, it is indicated by a footnote in the respective tables/figures in the Results section).
Meta-analysis
We used the statistical software Stata/SE 15.0, using the Stata command ‘metan’ to perform a random-effects model (DerSimonian and Laird method) meta-analysis.19,20 We calculated the odds ratio (OR) (and 95% confidence intervals) and corresponding P-values with the χ2 test, for the odds of events occurring in the group on treatment with an ACE inhibitor or ARB vs. the group not on treatment with an ACE inhibitor or ARB. Forest plots were generated with a log-transformed OR X-axis. Heterogeneity was assessed with I2 with corresponding P-values. Studies with zero count events were excluded.
Analysis groups and subgroups
Two groups of patients were analysed: (i) patients with hypertension (the primary analysis) and (ii) all patients (irrespective of hypertension status). Our focus was on patients with hypertension to mitigate confounding by indication. We analysed outcomes by treatment with an ACE inhibitor or ARB, separately, where data were available.
Assessment of bias and small-study effects
For each study, two researchers independently performed a formal assessment for bias at the outcome level with the validated tool, ‘Risk Of Bias In Non-randomized Studies – of Interventions’ (ROBINS-I), as recommended by the Cochrane Collaboration.21–23 For analyses with ≥10 studies, we performed funnel plots and formally assessed for small-study effects (Egger’s test).24
Sensitivity analyses
A tipping-point analysis for all-cause mortality (as the most robust and unambiguous outcome) was performed using the largest study and highest OR from among those reported, to determine how many additional studies would be required to overturn the results of the meta-analysis. In an additional simulation, a study of the same size of the largest was added, with incrementally increasing ORs, to determine the effect size required to reverse the results. Sensitivity analyses were performed to identify differences between (i) peer-reviewed vs. preprint articles; and (ii) studies from different continents; across all outcomes.
Results
Results of systematic review search and summary of studies
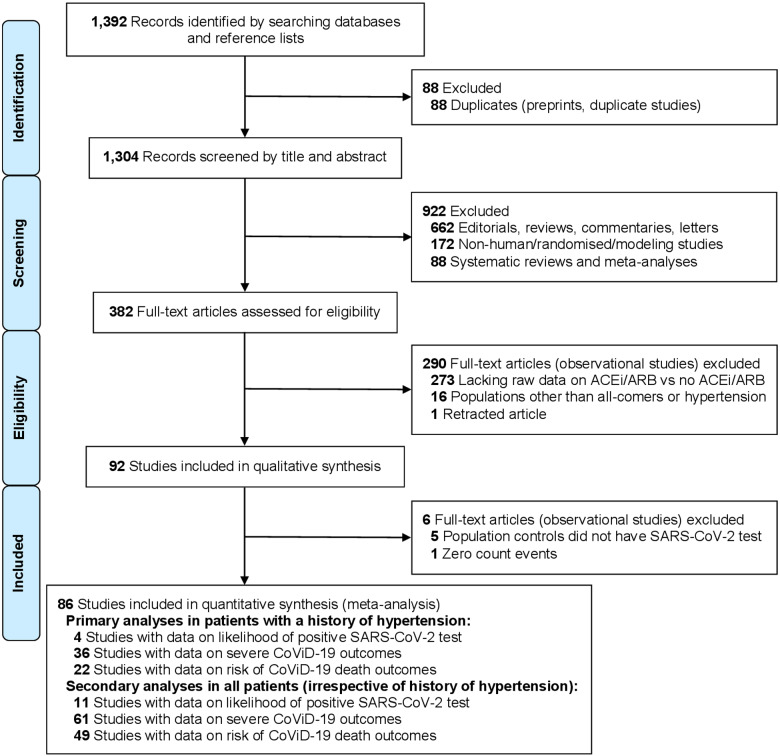
A total of 92 observational studies were included in our qualitative synthesis (up to 12 August 2020), of which 86 were used for quantitative synthesis (Figure 1). Of the studies included, 23 were from China (including at least 18 recruiting from the city of Wuhan or surrounding areas within the Hubei province), 18 from the United States of America (USA), 14 from Italy, 9 from Spain, 6 from each of France and South Korea, 5 from the United Kingdom (UK), 3 from Denmark, 2 from Turkey, and 1 from each of Australia, Belgium, Iran, Kuwait, and Switzerland; 1 was a multinational study (Supplementary material online, Table S1). Assessment of bias for each study was performed with the ROBINS-I tool, in relation to each outcome: the likelihood of positive SARS-CoV-2 test (Supplementary material online, Figure S1), the severity of CoViD-19 (Supplementary material online, Figure S2), and case-fatality rate (Supplementary material online, Figure S3).
Figure 1.
Study selection (PRISMA flow diagram). ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019; PRISMA, preferred reporting items for systematic reviews and meta-analyses; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
Likelihood of a positive SARS-COV-2 test
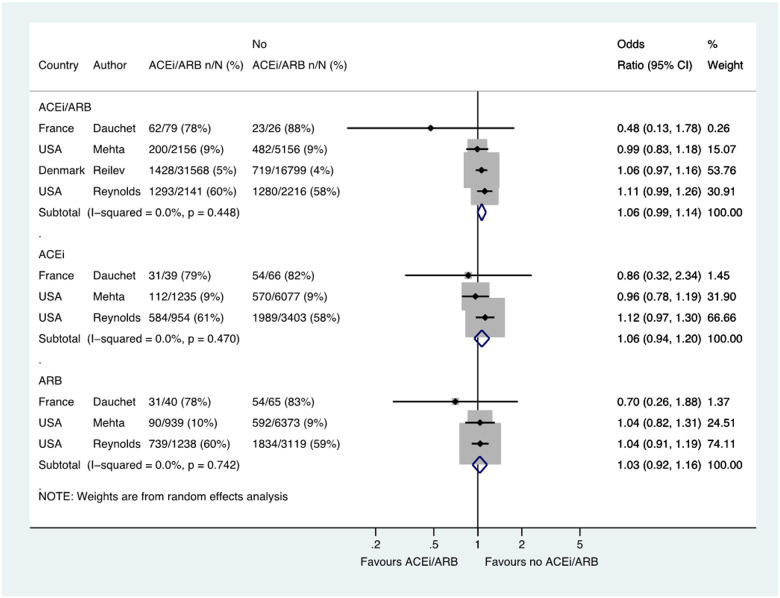
Across four studies, a total of 60 141 patients with hypertension had SARS-CoV-2 testing, of which 2983/35 944 (8.3%) tested positive in the group treated with an ACE inhibitor or ARB compared with 2504/24 197 (10.3%) in the group not treated with an ACE inhibitor or ARB group (OR 1.06, 95% CI 0.99–1.14) (Figure 2). Three of these studies with a total of 11 774 patients reported data for ACE inhibitor (OR 1.06, 0.94–1.20) and ARB groups (OR 1.03, 0.92–1.16) separately (Figure 2).
Figure 2.
Likelihood of positive SARS-CoV-2 test in patients with history of hypertension who were tested. Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; USA, United States of America. All studies were published in the year 2020. Dauchet (ACEi/ARB numbers manually calculated with assumption that no patients used ACEi and ARB at the same time). Mehta (includes previously unpublished data from authors).
Severe outcomes in patients with CoViD-19
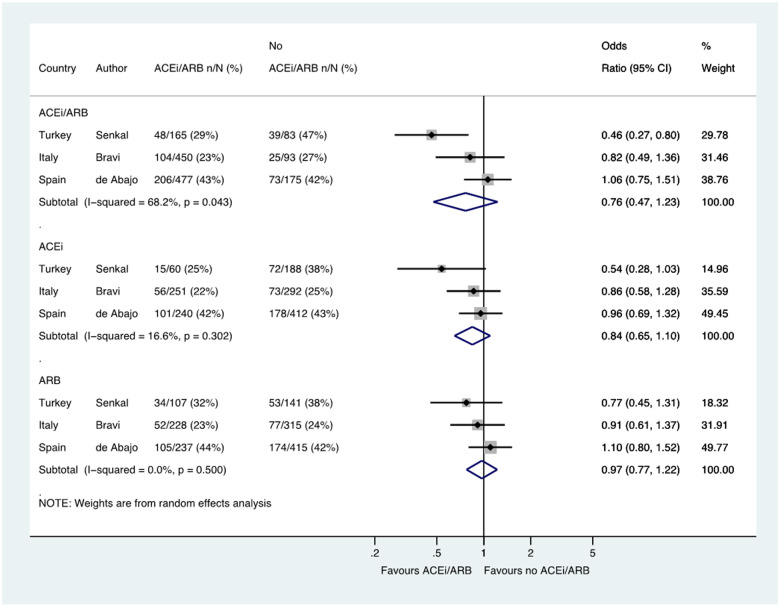
Hospital admission
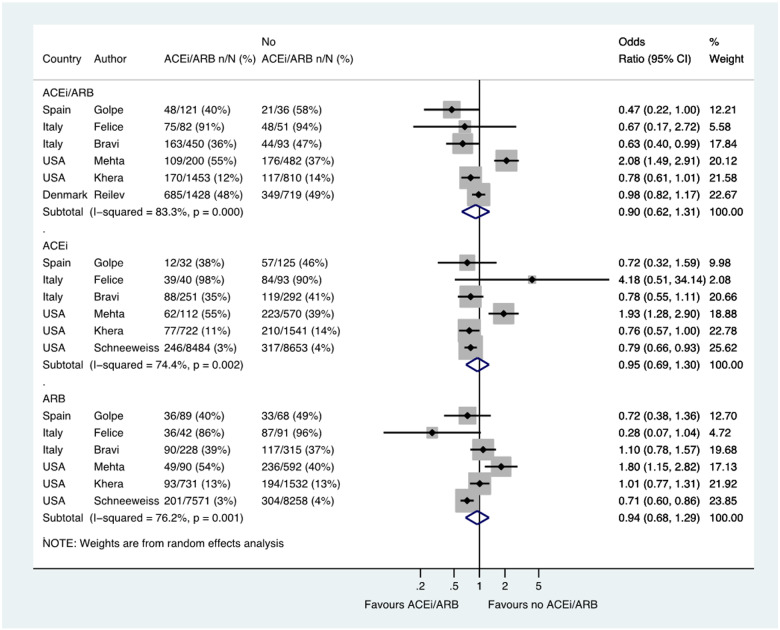
Six studies including a total of 5925 patients reported the likelihood of hospital admission among patients with hypertension, with admission occurred in 1250/3734 (33.5%) patients treated with an ACE inhibitor/ARB and in 755/2191 (34.5%) not treated with an ACE inhibitor/ARB (OR 0.90, 0.62–1.31) (Figure 3). Six studies reported ACE inhibitor and ARB data separately in 20 915 patients and 19 607 patients, respectively (OR 0.95, 0.69–1.30 and OR 0.94, 0.68–1.29, respectively) (Figure 3).
Figure 3.
Hospital admission in patients with history of hypertension who had CoViD-19. Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019; ITU, intensive therapy unit; USA, United States of America. All studies were published in the year 2020. Bravi (severe outcome = hospitalization (not ITU)) (raw numbers were back-calculated from %). Golpe (ACEi/ARB numbers manually calculated with assumption that no patients used ACEi and ARB at the same time). Mehta (includes previously unpublished data from authors).
Severe/critical CoViD-19
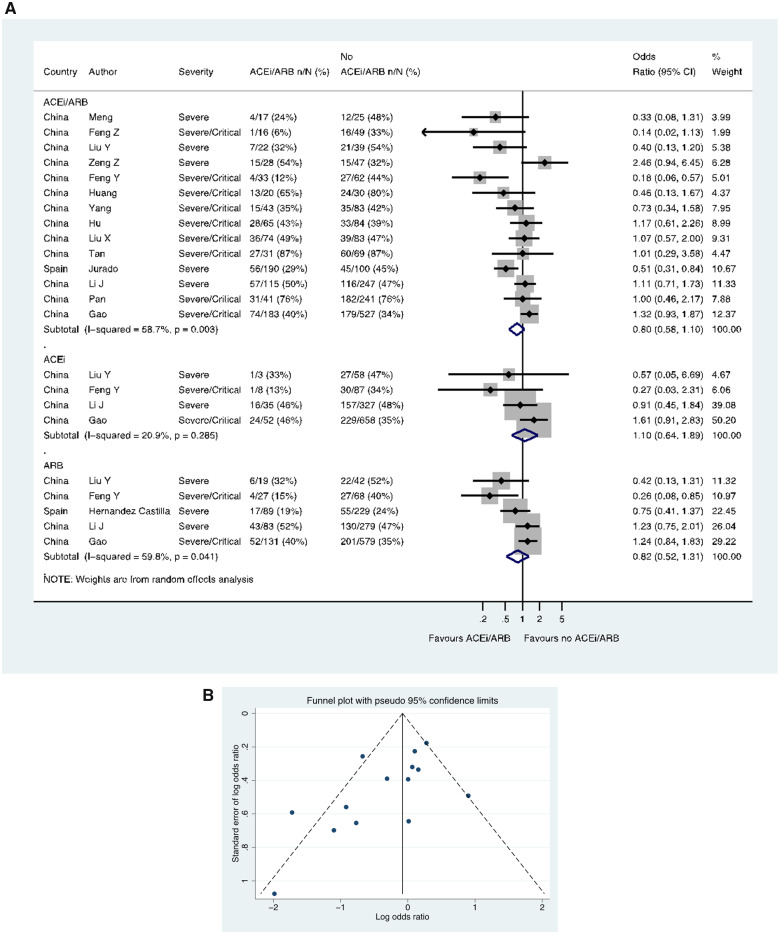
Fourteen studies (including 13 from China), including a total of 2564 patients with hypertension, reported severe/critical CoViD-19 (as distinct from ITU/ventilation/death), showing 368/878 (41.9%) events in the patients treated with an ACE inhibitor/ARB compared with 804/1686 (47.7%) events in the patients not treated with an ACE inhibitor/ARB (OR 0.80, 0.58–1.10) (Figure 4A). In these 14 studies with ACE inhibitor/ARB data, a funnel plot was performed to assess for publication bias (Figure 4B) and Egger’s test showed some evidence for the presence of small-study effects (estimated bias coefficient −1.861, standard error 0.811, P-value = 0.041). Four studies including 1228 patients reported ACE inhibitor data separately (OR 1.10, 0.64–1.89), whilst five studies including 1546 patients reported ARB data separately (OR 0.82, 0.52–1.31) (Figure 4A).
Figure 4.
Severe/critical CoViD-19 in patients with history of hypertension. (A) Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019. All studies were published in the year 2020. Definitions of severe and critical are detailed in Supplementary material online, Table S1 footnote. Liu Y (ACEi/ARB numbers manually calculated with assumption that no patients used ACEi and ARB at the same time). (B) Funnel plot. Egger’s test for small-study effects (14 studies): estimated bias coefficient −1.861, standard error 0.811, P-value = 0.041.
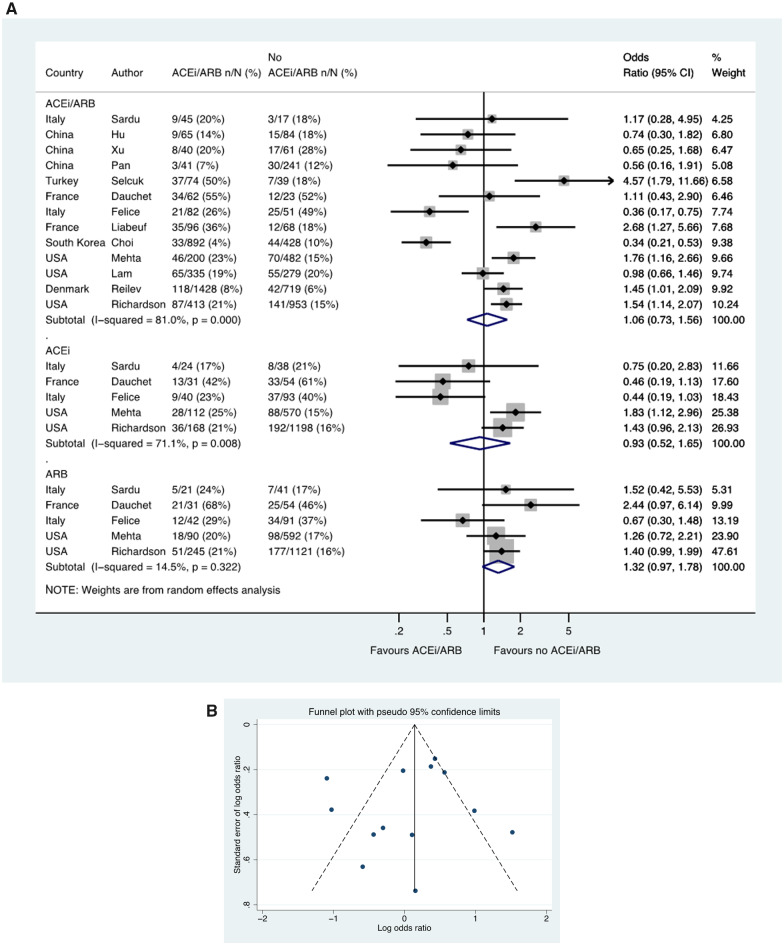
Admission to ITU
Thirteen studies including a total of 7218 patients with hypertension reported ITU admission, with a rate of 505/3773 (13.4%) in patients taking an ACE inhibitor/ARB compared to 473/3445 (13.7%) patients not taking an ACE inhibitor/ARB (OR 1.06, 0.73–1.56) (Figure 5A). In these 13 studies with ACE inhibitor/ARB data, a funnel plot was performed to assess for publication bias (Figure 5B) and Egger’s test showed weak evidence for the presence of small-study effects (estimated bias coefficient −0.872, standard error 1.469, P-value = 0.565). The OR in patients taking an ACE inhibitor (vs. no ACE inhibitor) was 0.93, 0.52–1.65 and for an ARB (compared with no ARB) was 1.32, 0.97–1.78 (Figure 5A).
Figure 5.
ITU admission in patients with history of hypertension who had CoViD-19. (A) Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019; ITU, intensive therapy unit; USA, United States of America. All studies were published in the year 2020. Dauchet (ACEi/ARB numbers manually calculated with assumption that no patients used ACEi and ARB at the same time). Mehta (includes previously unpublished data from authors). (B) Funnel plot. Egger’s test for small-study effects (13 studies): estimated bias coefficient −0.872, standard error 1.469, P-value = 0.565.
Admission to ITU/death
Three studies including 1443 patients reported the composite outcome of admission to ITU or death which was reported in 358/1092 (32.8%) patients treated with a RAS blocker, compared with 137/351 (39.0%) patients not treated with these drugs (OR 0.76, 0.47–1.23) (Figure 6).
Figure 6.
Composite of ITU/death in patients with history of hypertension who had CoViD-19. Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019; ITU, intensive therapy unit. All studies were published in the year 2020. Bravi (very severe/lethal outcome = ITU/death) (raw numbers were back-calculated from %). de Abajo (ACEi/ARB numbers manually calculated with assumption that no patients used ACEi and ARB at the same time). Şenkal (severe outcome = hospitalization ≥14 days/ITU/death).
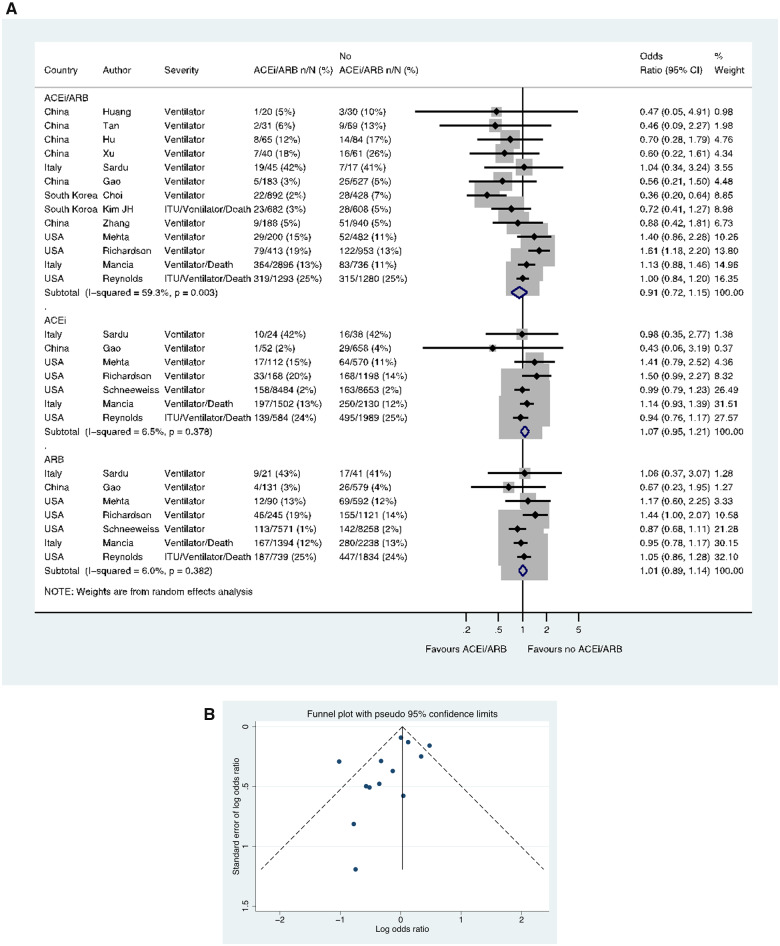
ITU/ventilation/death
Thirteen studies including a total of 13 163 patients with hypertension reported the use of mechanical ventilation (one reported a composite of ventilation/death and another ITU admission/ventilation/death). These outcomes occurred in 887/6948 (12.8%) patients taking an ACE inhibitor/ARB and in 753/6215 (12.1%) patients not taking an ACE inhibitor/ARB (OR 0.91, 0.72–1.15) (Figure 7A). In these 13 studies with ACE inhibitor/ARB data, a funnel plot was performed to assess for publication bias (Figure 7B) and Egger’s test showed weak evidence for the presence of small-study effects (estimated bias coefficient −1.141, standard error 0.670, P-value = 0.117). Seven studies reported data for ACE inhibitors and ARBs separately, in a total of 26 162 and 24 854 hypertension patients, respectively. There was no difference in this outcome for ACE inhibitor vs. no ACE inhibitor (OR 1.07, 0.95–1.21) or ARB vs. no ARB (OR 1.01, 0.89–1.14) (Figure 7A).
Figure 7.
Composite of mechanical ventilation/ITU/death in patients with history of hypertension who had CoViD-19. (A) Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019; ITU, intensive therapy unit; USA, United States of America. All studies were published in the year 2020. Kim JH (ITU/ventilator/death/sepsis). Mancia (ACEi/ARB numbers manually calculated with assumption that no patients used ACEi and ARB at the same time) (critical/fatal = ventilator/death). Mehta (includes previously unpublished data from authors). Reynolds (severe outcome = ITU/ventilator/death). (B) Funnel plot. Egger’s test for small-study effects (13 studies): estimated bias coefficient −1.141, standard error 0.670, P-value = 0.117.
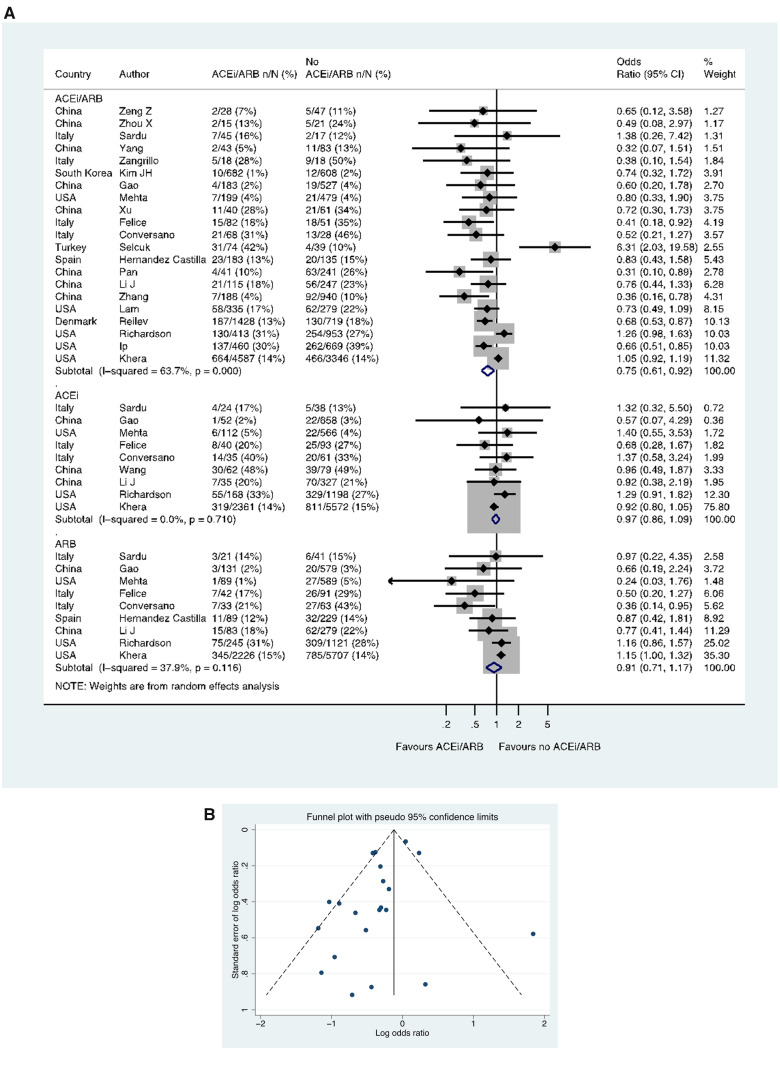
Case-fatality in patients with CoViD-19
Twenty-two studies including a total of 18 876 patients with hypertension reported case-fatality. In 21 studies including 18 735 patients, of those taking an ACE inhibitor/ARB, 1348/9227 (14.6%) died, compared with 1545/9508 (16.2%) patients not taking an ACE inhibitor/ARB (OR 0.75, 0.61–0.92) (Figure 8A). In these 21 studies with ACE inhibitor/ARB data, a funnel plot was performed to assess for publication bias (Figure 8B), and Egger’s test showed weak evidence for the presence of small-study effects (estimated bias coefficient −0.964, standard error 0.495, P-value = 0.066). Nine studies reported data on the use of ACE inhibitors and ARBs separately in 11 481 and 11 658 patients respectively, with the OR for death in those receiving an ACE inhibitor (vs. no ACE inhibitor) of 0.97, 0.86–1.09 and for an ARB (vs. no ARB) of 0.91, 0.71–1.17 (Figure 8A). Four additional studies reported case-fatality in patients with hypertension but were excluded from quantitative analysis, as they had zero count events in the ACE inhibitor/ARB groups.25–28
Figure 8.
Case-fatality rate in patients with history of hypertension who had CoViD-19. (A) Random effects meta-analysis. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CoViD-19, coronavirus disease 2019; USA, United States of America. All studies were published in the year 2020. Mehta (includes previously unpublished data from authors). Studies with zero count events were excluded. (B) Funnel plot. Egger’s test for small-study effects (21 studies): estimated bias coefficient −0.964, standard error 0.495, P-value = 0.066. Studies with zero count events were excluded.
Sensitivity analyses
Tipping-point analysis
For the outcome of case-fatality in patients with hypertension, a sensitivity tipping-analysis, using the largest study (7933 patients with 1130 deaths) and highest OR (6.3) from among those reported, showed that an additional nine studies would be required to tip this result (to an OR of 1.52, 1.06–2.18) (Supplementary material online, Figure S4A,B); conversely, adding a study of the same size as the largest reported and using an extreme OR (1030 vs. 100 deaths among 7933 patients, OR 13.56) did not tip the results (OR 0.83, 0.50–1.37) (Supplementary material online, Figure S4C).
Peer-reviewed vs. pre-print articles
Sensitivity analyses did not identify any differences between peer-reviewed vs. preprint articles in patients with hypertension, for the outcomes of the likelihood of a positive SARS-CoV-2 test (OR 1.07, 0.96–1.19 vs. 0.94, 0.54–1.65, respectively) (Supplementary material online, Figure S5A), hospitalization (OR 0.84, 0.36–1.97 vs. 0.89, 0.72–1.10 respectively) (Supplementary material online, Figure S5B), severe/critical CoViD-19 (OR 0.85, 0.61–1.17 vs. 0.78, 0.28–2.18, respectively) (Supplementary material online, Figure S5C), admission to ITU (OR 1.19, 0.80–1.76 vs. 0.80, 0.28–2.34, respectively) (Supplementary material online, Figure S5D) or the composite of ITU/ventilation/death (OR 1.09, 0.91–1.31 vs. 0.51, 0.26–1.01, respectively) (Supplementary material online, Figure S5E). Comparing peer-reviewed vs. preprint articles in patients with hypertension, for the outcome of case-fatality, the OR was 0.70, 0.50–0.99 vs. 0.79, 0.61–1.02, respectively (Supplementary material online, Figure S5F).
By continent
Sensitivity analyses did not identify any intercontinental differences for the outcomes of the likelihood of a positive SARS-CoV-2 test (OR 0.94, 0.54–1.65 in Europe vs. 1.07, 0.96–1.19 in North America) or hospitalization (OR 0.74, 0.51–1.08 in Europe vs. 1.27, 0.49–3.31 in North America) (Supplementary material online, Figure S6A,B). Although there were some intercontinental differences for the outcomes of severe/critical CoViD-19 (OR 0.85, 0.62–1.18 in Asia vs. 0.51, 0.31––0.84 in Europe), admission to ITU (OR 0.82, 0.32–2.07 in Asia vs. 1.14, 0.58–2.21 in Europe vs. 1.39, 1.01–1.92 in North America), composite of ITU/ventilation death (OR 0.59, 0.44–0.78 in Asia vs. 1.13, 0.88–1.44 in Europe vs. 1.28, 0.91–1.80 in North America), or case-fatality rate (OR 0.68, 0.42–1.11 in Asia vs. 0.66, 0.54–0.82 in Europe vs. 0.90, 0.70–1.17 in North America), some of these analyses only had ≤ 3 studies representing each continent (Supplementary material online, Figures S6C–F).
Supplementary analyses of all patients (irrespective of history of hypertension)
In our supplementary analyses, examination of all patients, irrespective of history of hypertension, generally showed worse outcomes in individuals treated with a RAS blocker, compared to those not treated with these drugs (Supplementary material online, Results (Quantitative Analyses), Figures S7–S14). Several of these studies also suggested an increased likelihood of admission to hospital in patients with a positive test for SARS-CoV-2. We believe that this finding likely reflects confounding, and this view is supported by studies in which cases and controls were matched (or adjusted analyses were performed). For example, in a report from Spain, which included 1139 cases and 11 390 matched population controls, neither treatment with an ACE inhibitor or an ARB was associated with a higher risk of hospital admission.29 In our analysis of more than 60 000 patients overall (irrespective of history of hypertension), with over 7000 deaths, case-fatality was higher in patients treated with a RAS blocker, but there was a strong likelihood of confounding.
Discussion
Patients with hypertension, cardiovascular disease, diabetes, and chronic kidney disease have particularly poor outcomes if infected by SARS-CoV-2.1–6 The possibility that the many millions of such patients treated with a RAS blocker might be at additional risk is therefore of great concern, both during the current pandemic and potential future waves of CoViD-19. Because of the large number of studies and patients included in the current meta-analysis, we provide the most robust answers to date to the two principal questions raised in relation to treatment with RAS blockers (i.e. whether these drugs increase the risk of acquiring infection with SARS-CoV-2 and whether RAS blockers increase the risk of more serious CoViD-19). The large dataset created also enabled us to examine the two major types of RAS blocker (i.e. ACE inhibitors and ARBs) separately, another important question given their distinct pharmacological properties, the potential differential effect on ACE2, and the possibility of patients switching to a safer alternative.
Given the diverse nature of the studies available, we focused on the 103 317 patients with hypertension, to mitigate biases such as confounding by indication. Of course, even among patients with hypertension, there is likely to be a preference for RAS blockers in individuals with concomitant coronary heart disease, heart failure, and diabetic kidney disease. Despite this, in our analyses, treatment with a RAS blocker was not associated with a higher risk of acquiring SARS-CoV-2 infection or of the most severe outcomes, including death (Supplementary material online, Central Figure).
Susceptibility to SARS-CoV-2 infection
Specifically, neither treatment with an ACE inhibitor nor an ARB was associated with a greater likelihood of SARS-CoV-2 infection and our supplementary analysis of all patients, irrespective of history of hypertension, gave consistent findings (Supplementary material online, Results (Quantitative Analyses)). Eight additional relevant and large studies reported the likelihood of a positive test for SARS-CoV-2 but were not included in our meta-analysis because they included population controls who did not undergo testing for SARS-CoV-2—the rationale for this was to minimize heterogeneity and selection bias between studies—none of these studies showed an increased risk of SARS-CoV-2 infection in patients treated with RAS blockers (Supplementary material online, Results (Qualitative Analyses)). Collectively, these data make it very unlikely that either type of RAS blocker increases the risk of infection with SARS-CoV-2 and refutes the suggestion that this may be a particular concern with ACE inhibitors.12
Severity of CoViD-19 and case-fatality rate
We also examined the severity of CoViD-19, including admission to ITU, use of mechanical ventilation, and death. The risk of ITU admission was not higher in patients treated with a RAS blocker and this was also true for the likelihood of ventilation (or ventilation/death), with 978 and 1640 cases for these analyses, respectively. Similarly, the case-fatality rate was not higher among patients treated with a RAS blocker, with 2893 deaths in total. These findings are reassuring, despite the high probability of residual confounding in these analyses.30 Further support for this interpretation, comes from the Chinese studies we included which reported an additional category of severe SARS-CoV-2-related pneumonia/CoViD-19 (independently of whether patients were admitted to ITU or required ventilation), reflecting local guidelines for the diagnosis and treatment of new coronavirus pneumonia.31 Some of the Chinese studies showed larger effects, as indicated by Egger’s test. Additional supportive findings are detailed in our Supplementary material online, Results (Quantitative Analyses). Collectively, these studies showed no increase in the risk of severe CoViD-19 in relation to treatment with a RAS blocker (or with an ACE inhibitor or ARB, separately).
Study limitations and strengths
We did not have individual patient data and could not adjust for the difference between patients treated and not treated with RAS blockers, especially in comorbidity. It is possible that our findings are subject to publication bias, although there are few registered studies not reported and it would require a very large study with very negative outcomes to overturn these findings (as shown in the sensitivity analysis), especially given the neutrality of our findings despite the likelihood of unfavourable residual confounding. Although we focused on patients with a history of hypertension, we did not have information about control of blood pressure and did not consider dose of ACE inhibitor or ARB or other drugs that patients may have received. In this respect, the control groups were heterogeneous as our comparison was with the absence of a RAS blocker, rather than specific alternative antihypertensive drugs, which may introduce further confounding by indication. Another limitation was the heterogeneity among the studies included, although we addressed this to some extent by investigating the two well-defined outcomes of a SARS-CoV-2 positive test and death, although the threshold for ITU admission and ventilation probably varies from institution to institution. Clearly, our conclusions must also be tempered by the observational nature of these data, which may be affected by ‘collider bias’, sometimes referred to as sample selection bias due to non-random sampling.32 There are many ongoing randomized studies of ACE inhibitor and ARB use in patients with CoViD-19 (Supplementary material online, Table S2). The first randomized trial to report, BRACE-CORONA, found no difference in the number of days alive and out of hospital in those continuing vs. suspending ACE inhibitor or ARB treatment for 30 days.33 These findings provide further reassurance on the safety of continuing these treatments in the context of CoViD-19. In addition to these limitations our meta-analysis has some strengths, including the number of studies and patients included, and the acquisition of unpublished data allowing us to specifically investigate patients with hypertension, going some way towards addressing the biases confounding interpretation of non-randomized analyses of outcomes related to treatment.
Conclusion
In conclusion, our meta-analysis provides reassurance for physicians and patients that each of ACE inhibitors and ARBs are safe in the context of SARS-CoV-2 infection and should not be discontinued.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Funding
The British Heart Foundation (Centre of Research Excellence Grant RE/18/6/34217 to N.S., M.C.P., P.S.J., and J.J.V.M.).
Conflict of interest: M.M.Y.L.'s employer, the University of Glasgow, has received grant support from Boehringer Ingelheim. N.S. has consulted for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant funding from Boehringer Ingelheim. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent. M.V. is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL1TR002541), receives research grant support from Amgen and Boehringer Ingelheim, serves on advisory boards for Amgen, American Regent, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa, and participates on clinical endpoint committees for studies sponsored by Galmed, Novartis, and the NIH. M.C.P. has received research funding from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, Pharmacosmos, and served on Consultancy and End point committees for Boehringer Ingelheim, Novartis, AstraZeneca, Novo Nordisk, Abbvie, Bayer, Takeda, Cardiorentis, Pharmacosmos. P.S.J.'s employer, the University of Glasgow, has been paid by AstraZeneca and Novartis for his work in clinical trials, by Novartis and AstraZeneca for speaker fees and advisory board fees, and by Boehringer Ingelheim for a grant. J.J.V.M.'s employer, the University of Glasgow, has been paid by Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, Theracos for his work on clinical trials, consulting and other activities, and by Abbott, Hikma, Sun Pharmaceuticals, and Servier for personal lecture fees. All other coauthors have no relevant disclosures.
Supplementary Material
Contributor Information
Matthew M Y Lee, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Kieran F Docherty, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Naveed Sattar, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Neil Mehta, Department of Medicine, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland Clinic, Cleveland, OH, USA.
Ankur Kalra, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Section of Cardiovascular Research, Heart, Vascular and Thoracic Department, Cleveland Clinic Akron General, Akron, OH, USA.
Amy S Nowacki, Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA.
Scott D Solomon, Cardiovascular Division, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA, USA.
Muthiah Vaduganathan, Cardiovascular Division, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA, USA.
Mark C Petrie, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Pardeep S Jhund, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
John J V McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
References
- 1. Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020;27:taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 2020;323:1769–1770. [DOI] [PubMed] [Google Scholar]
- 3. Perico L, Benigni A, Remuzzi G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron 2020;144:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin – angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 2020;41:1801–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomson G. COVID-19: social distancing, ACE 2 receptors, protease inhibitors and beyond? Int J Clin Pract 2020;74:e13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020;525:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sama IE, Ravera A, Santema BT, Goor H. V, Maaten J. T, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, Veldhuisen D. V, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui C-C, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paz Ocaranza M, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, Lavandero S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol 2020;17:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Froldi G. What could be the better choice between ACE inhibitors and AT1R antagonists in coronavirus disease 2019 (COVID-19) patients? J Med Virol 2020;92:2302–2303. [DOI] [PubMed] [Google Scholar]
- 13. Kovarik JJ, Kopecky C, Antlanger M, Domenig O, Kaltenecker CC, Werzowa J, Hecking M, Mahr S, Grömmer M, Wallner C, Aumayr K, Kain R, Zuckermann A, Poglitsch M, Säemann MD. Effects of angiotensin-converting-enzyme inhibitor therapy on the regulation of the plasma and cardiac tissue renin-angiotensin system in heart transplant patients. J Heart Lung Transplant 2017;36:355–365. [DOI] [PubMed] [Google Scholar]
- 14. Kovarik JJ, Antlanger M, Domenig O, Kaltenecker CC, Hecking M, Haidinger M, Werzowa J, Kopecky C, Säemann MD. Molecular regulation of the renin-angiotensin system in haemodialysis patients. Nephrol Dial Transplant 2015;30:115–123. [DOI] [PubMed] [Google Scholar]
- 15. National Institute for Health Research (NIHR) Centre for Reviews and Dissemination University of York. International Prospective Register of Systematic Reviews (PROSPERO). 2020. https://www.crd.york.ac.uk/prospero/ (19 September 2020).
- 16. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18. Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, Milinovich A, Svensson LG, Jehi L, Young JB, Chung MK. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:e201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 20. Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J 2008;8:3–28. [Google Scholar]
- 21. Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE Guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 2019;111:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cochrane Bias Methods Group, Cochrane Non-Randomised Studies Methods Group. Risk of bias tools. 2020. https://www.riskofbias.info/ (19 September 2020).
- 24. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, Gao H, Liu L, Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 2020;9:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Z, Cao J, Yao Y, Jin X, Luo Z, Xue Y, Zhu C, Song Y, Wang Y, Zou Y, Qian J, Yu K, Gong H, Ge J. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med 2020;8:430–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan N-D, Qiu Y, Xing X-B, Ghosh S, Chen M-H, Mao R. Associations between angiotensin converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology 2020;159:1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu J, Zhang X, Zhang X, Zhao H, Lian J, Hao S, Jia H, Yang M, Lu Y, Xiang D, Cai H, Zhang S, Gu J, Ye C, Yu G, Jin C, Zheng L, Yang Y, Sheng J. COVID-19 is more severe in patients with hypertension; ACEI/ARB treatment does not influence clinical severity and outcome. J Infect 2020;81:979–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Abajo FJ, Rodríguez-Martín S, Lerma V, Mejía-Abril G, Aguilar M, García-Luque A, Laredo L, Laosa O, Centeno-Soto GA, Ángeles Gálvez M, Puerro M, González-Rojano E, Pedraza L, de Pablo I, Abad-Santos F, Rodríguez-Mañas L, Gil M, Tobías A, Rodríguez-Miguel A, Rodríguez-Puyol D, Barreira-Hernandez D, Zubiaur P, Santos-Molina E, Pintos-Sánchez E, Navares-Gómez M, Aparicio RM, García-Rosado V, Gutiérrez-Ortega C, Pérez C, Ascaso A, Elvira C, MED-ACE2-COVID19 Study Group. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodilla E, Saura A, Jiménez I, Mendizábal A, Pineda-Cantero A, Lorenzo-Hernández E, Fidalgo-Montero MDP, López-Cuervo JF, Gil-Sánchez R, Rabadán-Pejenaute E, Abella-Vázquez L, Giner-Galvañ V, Solís-Marquínez MN, Boixeda R, Peña-Fernández A. D L, Carrasco-Sánchez FJ, González-Moraleja J, Torres-Peña JD, Guisado-Espartero ME, Escobar-Sevilla J, Guzmán-García M, Martín-Escalante MD, Martínez-González ÁL, Casas-Rojo JM, Gómez-Huelgas R. Association of hypertension with all-cause mortality among hospitalized patients with COVID-19. J Clin Med 2020;9:3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Health Commission & National Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin Med J 2020;133:1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, Sharp GC, Sterne JPalmer TM, Davey Smith G, Tilling K, Zuccolo L, Davies NM, Hemani G. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 2020;11:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. European Society of Cardiology (ESC) website. https://www.escardio.org/The-ESC/Press-Office/Press-releases/LOPES (19 September 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.
Funding
The British Heart Foundation (Centre of Research Excellence Grant RE/18/6/34217 to N.S., M.C.P., P.S.J., and J.J.V.M.).