Abstract
Background
Severe coronavirus-induced disease 2019 (COVID-19) leads to acute respiratory distress syndrome with an increased risk of venous thrombo-embolic events. To a much lesser extent, arterial thrombo-embolic events have also been reported in this setting.
Case summary
Here, we describe four different cases of COVID-19 infection with ischaemic arterial events, such as a myocardial infarction with high thrombus load, ischaemic stroke on spontaneous thrombosis of the aortic valve, floating thrombus with mesenteric, splenic and renal infarction, and acute limb ischaemia.
Discussion
Cardiovascular risk factors such as hypertension, obesity, and diabetes are comorbidities most frequently found in patients with a severe COVID-19 infection and are associated with a higher death rate. Our goal is to provide an overview of the clinical spectrum of ischaemic arterial events that may either reveal or complicate COVID-19. Several suspected pathophysiological mechanisms could explain the association between cardiovascular events and COVID-19 (role of systemic inflammatory response syndrome, endothelial dysfunction, activation of coagulation cascade leading to a hypercoagulability state, virus-induced secondary antiphospholipid syndrome). We need additional studies of larger size, to estimate the incidence of these arterial events and to assess the efficacy of anticoagulation therapy.
Keywords: COVID-19, Acute coronary syndrome, Stroke, Acute limb ischaemia, Case series
Learning points
Cardiovascular risk factors are comorbidities most frequently found in patients with a severe coronavirus-induced disease 2019 (COVID-19) infection and are associated with higher death rate.
Arterial thrombo-embolic event in COVID-19 infection may affect multiple territories such as myocardial infarction or ischaemic stroke and may either reveal or complicate COVID-19.
Endothelial dysfunction, sepsis-induced coagulopathy, virus-induced antiphospholipid syndrome, and systemic inflammatory response syndrome are the main pathophysiological mechanisms that could explain the association between arterial thrombo-embolic events and COVID-19.
Introduction
Adult patients with coronavirus-induced disease 2019 (COVID-19) can remain asymptomatic, present with fever and mild respiratory symptoms or show extensive pneumonia, which can lead to a life-threatening acute respiratory distress syndrome. Clinicians have already reported that infection with COVID-19 can be associated with various cardiac manifestations,1 venous thromboembolic events (VTEs),2,3 and sepsis-induced coagulopathy (SIC).4 The presence of cardiac involvement is associated with poor prognosis.5 COVID-19-associated arterial manifestations seem less frequent than VTE and their characteristics are still poorly known.
Here, we report four cases from our institution, encompassing the spectrum of arterial thrombo-embolic events in the context of COVID-19.
Timeline
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years) | 57 | 57 | 50 | 36 |
| Gender | Male | Male | Male | Male |
| Ethnicity | African | Caucasian | African | African |
| Cardiovascular risk factors | Hypertension, obesity | Type 2 diabetes, obesity | Obesity | None |
| First symptoms of COVID-19 |
March 15 Fever, cough, and asthenia |
March 16 Fever, cough, asthenia, and dyspnoea |
March 21 Fever, cough, asthenia, and dyspnoea |
March 27 Fever, cough, diarrhoea, ageusia, and dyspnoea |
| Diagnostic evidence for COVID-19 (diagnosis of COVID-19 infection was based on positivity of reverse transcription polymerase chain reaction (RT-PCR) or serology or computed tomography (CT) scan showing typical lesions) |
April 5 Pulmonary CT |
March 26 RT-PCR and pulmonary CT |
March 25 RT-PCR |
April 10 Pulmonary CT Serology |
| Arterial event |
April 5 Floating thrombus within the aortic arch: left subclavian artery thrombosis |
April 30 Intra-aortic thrombi: mesenteric, splenic, and renal infarction, ischaemia of the lower limbs |
March 25 Myocardial infarction: occlusion of the proximal left circumflex artery |
April 13 Spontaneous thrombosis of the aortic valve: occlusion of the M2 segment of the middle cerebral artery |
|
D-dimer (µg/L) Reference range (RR) < 500 |
NA | 1169 | 2804 | 1971 |
|
Fibrinogen (g/L) RR 2–4 |
6.3 | NA | 8.0 | 5.2 |
|
Activated partial thromboplastin time (s) RR < 1.2 s |
29.6 | NA | NA | 31.8 |
|
Prothrombin time (s) RR 10–13 s |
13.2 | 12.8 | 13.5 | 13.4 |
|
C-reactive protein (mg/L) RR <5 mg/l |
26 | 139 | 100 | 19 |
| Antiphospholipid serology | Lupus anticoagulant doubtful | Anti-cardiolipin IgM 21 | NA | Negative |
| Treatment | Thrombectomy and intravenous (IV) unfractionated heparin (UFH) | Low-dose aspirin and IV UFH | Dual antiplatelet therapy, thrombectomy and stent implantation, anti-GPIIb/IIIa and IV UFH | Thrombolysis, mechanical thrombectomy and IV UFH |
| Follow-up and outcome | Successful treatment, discharge at Day 5 | Critical limb ischaemia, planned revascularization surgery, discharge at Day 15 | Successful treatment, discharge at Day 4 | Successful thrombectomy, discharge at Day 17 |
Case presentation
Case 1
A non-smoker 57-year-old man, with a history of hypertension, was referred to the emergency care unit (ECU) of Henri Mondor University Hospital, in Créteil, France, on 5 April 2020, for syncope and severe pain in the left arm, suggestive of acute ischaemia and/or acute dissection. He had previously consulted his general practitioner for fever and asthenia, symptoms had spontaneously resolved.
Upon admission, his temperature was 36.2°C, blood pressure measured at the right arm was 150/85 mmHg, and heart rate was 84 b.p.m. Body mass index (BMI) 30.3 kg/m2. Oxygen saturation was 98% (room air), respiratory rate was normal. There was a left upper-limb motor deficit (grade 1/5) and an abolition of the pulses of the left upper limb. Lung auscultation was unremarkable.
The computed tomography (CT) angiography (CTA) scan of the thoracic aorta showed left subclavian artery thrombosis and ulcerated plaques with floating thrombus within the aortic arch (Figure 1). Chest CT revealed the presence of ground-glass opacities in both lungs with mild extension, typical of COVID-19. Laboratory results on admission showed elevated C-reactive protein (CRP) 26.0 mg/L [reference range (RR) < 5 mg/L]. The electrocardiogram (EKG) was normal.
Figure 1.

Computed tomography scan showing floating thrombus of the thoracic aorta.
An arteriography with thrombectomy was performed and the patient was later treated with intravenous (IV) unfractionated heparin (UFH).
Transthoracic echography (TTE) was normal. There was a prolongation of the activated partial thromboplastin time and the search for lupus anticoagulant (LA) was inconclusive. The outcome was favourable after 4 days of IV UFH, the patient was discharged on April 10 on vitamin K antagonist (VKA) therapy.
Case 2
A 57-year-old man, with a previous history of uncomplicated type 2 diabetes, was admitted to the ECU on 26 March 2020, for dyspnoea and fever.
Upon admission, his temperature was 37.2°C, blood pressure was 132/87 mmHg, and heart rate was 107 b.p.m. BMI 27.8 kg/m2. Oxygen saturation was 86% (room air) and respiratory rate was 20/per min. Lung auscultation was normal.
Laboratory results showed: elevated CRP (52.4 mg/L) and lymphocytopenia. Results for the reverse transcription polymerase chain reaction (RT-PCR) for COVID-19 were positive. Chest CT showed ground-glass opacities and peripheral condensations in both lungs with severe extension (over 50%), typical of COVID-19.
On Day 1, the patient was provided with oxygen therapy at 4 L/min, anti-viral treatment with hydroxychloroquine and antibiotic therapy, given the suspicion of a concomitant bacterial infection, and a prophylactic dose of low-molecular-weight heparin (LMWH). On Day 2, respiratory parameters declined and oxygen therapy was increased at 9 L/min. I.V Tocilizumab was administered off-label at 800 mg, allowing stabilization of the respiratory state. On April 30, the patient complained of a pain in the right leg, associated with a cyanotic appearance. The arterial Doppler ultrasound revealed features of acute ischaemia of the right leg, with occlusion of the right superficial femoral artery. The CTA of the abdominal aorta and lower limbs showed multiple intra-aortic thrombi, occlusion of the distal superior mesenteric artery, splenic and renal ischaemic lesions, occlusion of the right superficial femoral artery at the canal of Hunter and occlusion of the left supra-articular popliteal artery (Figure 2). EKG was normal.
Figure 2.

Computed tomography scan showing left subclavian artery occlusion.
The patient was given low-dose aspirin and IV UFH. Thrombectomy or thrombolysis could not be performed, as his respiratory state did not allow general anaesthesia.
TTE showed physiological mitral regurgitation. Immunoglobulin (IgM) M anti-cardiolipin antibodies (aCls) were slightly positive at 21 Units Isotype M Phospholipid (UMPL, RR <20 UMPL), IgG aCl were negative, as well as anti-β2GP1 antibodies.
The patient’s ischaemic manifestations improved under anticoagulation first by IV UFH and then VKA, repeat investigations by CTA and new assessment of aCl were scheduled a month later to discuss a revascularization surgery. The patient was transferred to a rehabilitation unit on April 10.
Case 3
A 50-year-old man with a history of obesity presented to the ECU for an ongoing chest pain for 3 h. He had been suffering from a flu-like syndrome for the past 4 days and intermittent chest pain for 48 h. Clinical examination revealed fever (38.1°C) and low oxygen saturation (90%) without acute respiratory failure. Initial EKG showed lateral ST-elevation. The patient was treated with dual antiplatelet and anticoagulant therapies and immediately transferred for coronary angiogram. The coronary angiogram revealed an acute occlusion of the proximal left circumflex artery with a high thrombus load, successfully treated through thrombectomy and followed by stent implantation (Figure 3). Given the high thrombus load, treatment with anti-GPIIb/IIIa antibody and UFH was added for 24 and 48 h, respectively. After treatment, the chest pain and ST-segment deviation resolved but dyspnoea sustained, with an oxygen requirement of 5 L/min. Lung auscultation showed bilateral crackles. TTE showed a normal ejection fraction without increase in left ventricular filling pressure. Results from the RT-PCR for COVID-19 came back positive. Evolution was good without any dedicated treatment for COVID-19 and the patient was transferred to a cardiac rehabilitation unit on Day 4.
Figure 3.

Computed tomography scan of floating thrombus (left arrow) with splenic (right arrow) and renal infarction.
Case 4
A 36-year-old man presented to the ECU with intermittent chest pain and dyspnoea for the last 24 h. He described an influenza-like illness with cough and ageusia for the past 2 weeks. His wife had previously been tested positive for COVID-19. Clinical examination revealed fever (37.9°C) and a 100% oxygen saturation. Lung auscultation showed bilateral crackles. EKG was normal. Laboratory results showed a high sensitive troponin of 402 ng/L (RR < 14 ng/L) and a CRP of 19 mg/L.
A CT scan revealed areas of ground-glass opacities, suggestive of COVID-19. TTE showed a normal left ejection fraction but with an antero-apical hypokinesia. Coronary angiogram was normal. The cardiac magnetic resonance imaging was inconclusive for a myocarditis associated with COVID-19. He was given a prophylactic dose of LMWH.
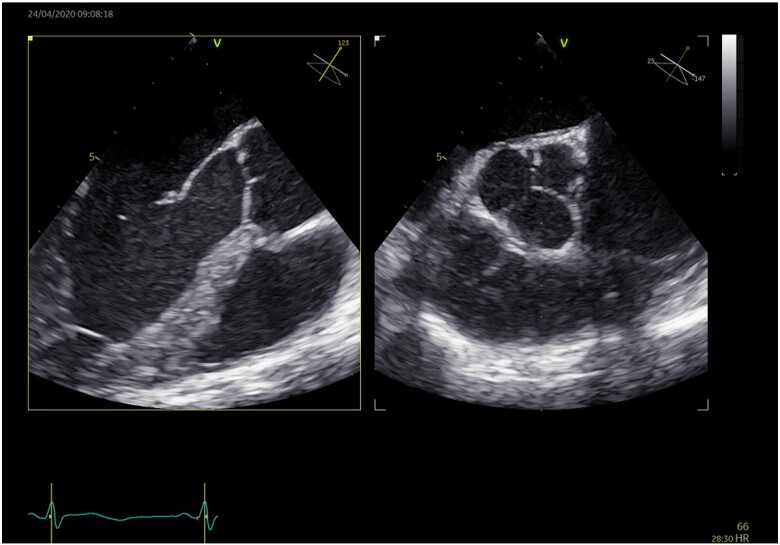
Thirty-six hours later, neurological defect including motor aphasia, confusion and right hemiparesis with an NIHSS of 13 appeared. A brain CT scan showed an acute occlusion of the M2 segment of the middle cerebral artery (Figure 3). The patient was treated with thrombolysis and mechanical thrombectomy with complete recovery. The evolution was favourable without recurrence or bleeding. Regarding the aetiology of this stroke, there was no cardiovascular risk factor. TTE was normal, except for a suspicion of patent foramen ovale, without evidence for a pulmonary embolism. Duplex ultrasound found no evidence of deep-vein thrombosis, ruling out the diagnosis of paradoxical embolism. The RT-PCR for COVID-19 was negative, but the clinic and CT scan were sufficient to confirm the infection. Besides COVID-19, there was no other infection (negatives blood cultures). LA was weakly positive. Transoesophageal echocardiography (TOE) was performed and showed vegetation on the aortic valve, suggestive of infective endocarditis, valvular fibroelastoma, or spontaneous thrombosis of the aortic valve (Figure 4). At Day 13, bacterial serology and blood cultures remained negative in the absence of any previous antibiotic therapy. The patient was given IV UFH. A check of TOE at 7 days from the start of heparin shows a complete regression of the thrombus. In the end, the patient was found seropositive for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As such, the most probable scenario was a thrombosis of the aortic valve, following a COVID-19 infection.
Figure 4.
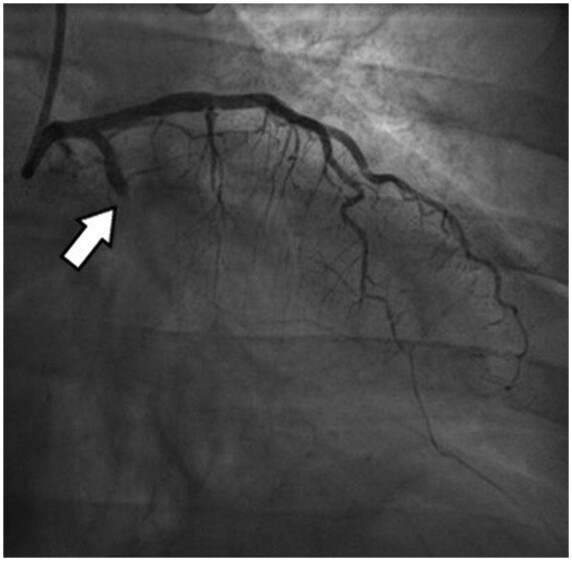
Coronary angiogram, the arrow revealing an acute occlusion of the proximal left circumflex artery.
Figure 5.

Coronary angiogram, the arrowrevealing an acute occlusion of the proximal left circumflex artery with a high thrombus load.
Figure 6.

Computed tomography angiography scan of the cerebral arteries, the arrow showing occlusion of the M2 segment of the middle cerebral artery.
Figure 7.
Two-dimensional transoesophageal echocardiography, the arrow showing vegetation appended to a cups of the aortic valve before anticoagulant treatment.
Discussion
There is a high prevalence of cardiovascular risk factors among symptomatic adults patients admitted to hospital for COVID-19.6,7 These comorbidities are associated with a high risk of developing a severe and life-threatening form of the infection.5–8 Whereas an increased incidence of VTE in the context of COVID-19 has been recognized and reported,2,3 data upon COVID-19-associated arterial manifestations remain scarce.
A previous study in China showed that the incidence of stroke among hospitalized patients with Covid-19 was approximately 5%. Patients with stroke had more often biological markers of coagulopathy and a more severe form of the disease with poorer prognosis.9 Similarly, the study by Klok et al.3 found 3.7% of arterial thrombotic events among 184 patients with COVID-19 in intensive care unit (ICU).
Colleagues from Argenteuil, France, recently published a series of seven patients with severe arterial thrombotic events associated with COVID-19. The majority of these were lower limb ischaemia or floating thrombus of the aorta.10
There are several suspected pathophysiological mechanisms to explain the association between cardiovascular events and COVID-19 including:
Endothelial dysfunction: the receptor for viral adhesion is an angiotensin-converting enzyme 2 receptor on endothelial cells,11 with viral replication causing inflammatory cell infiltration, endothelial cell apoptosis, and microvascular prothrombotic effects.12
Activation of coagulation cascade leading to a hypercoagulability state called SIC,13 a precursor state to disseminated intravascular coagulation, associated with elevated prothrombin time, elevated D-dimer, and thrombocytopenia, but without hypofibrinogenemia. It is related to an infection-induced systemic inflammatory response with endothelial dysfunction and microthrombosis with organ failure and usually no bleeding.12 SIC in patients with COVID-19 is associated with an increased risk of death.13
Virus-induced secondary antiphospholipid syndrome has been described in COVID-19 infection,4 although a transient positivity of aCl and/or LA is known in various viral infections.14 These antibodies are usually considered as non-pathogenic and reflect only B cell activation, but their role in promoting venous or arterial thrombotic events during COVID-19 cannot be ruled out at this stage.15
Role of systemic inflammatory response syndrome. Significant inflammation is present in patients with severe COVID-19 infection, based on elevated levels of tumour necrosis factor-α, interleukins (ILs), including IL-1 and IL-6, increased CRP, erythrocyte sedimentation rate and fibrinogen at presentation. These biological disturbances are higher in ICU patients than non-ICU patients. Those cytokines can initiates coagulation activation and thrombin generation via tissue factor expression and suppression of endogenous anticoagulant pathways.12
In order to reduce the incidence of VTE and arterial thrombosis, we need additional studies of larger size, to estimate the incidence of these arterial events and to assess the efficacy of prophylactic or full anticoagulation therapy, as the study by Tang could suggest.16 Another perspective of antithrombotic therapy could be Tissue plasminogen activator treatment.17 The use of active treatments on endothelium such as renin–angiotensin system inhibitors or statin is being considered. Immunosuppressive treatments have shown promising results, dexamethasone reduce deaths by one-third in ventilated patient and by one-fifth in other patients receiving oxygen only.18 Finally, IL blocade seem to be effective in retrospective cohort studies,19 results of randomized trials and impact on thrombotic complications are still pending.
Our study presents an overview of the different arterial thrombo-embolic events encountered in COVID-19. To our surprise, as described in Case 1 and 3, the arterial thrombo-embolic event may reveal the COVID-19 infection. This was also reported in the study of Kashi et al.,10 where arterial ischaemia revealed COVID-19 infection for three out of seven patients.
Unfortunately, in our work, not all cases were proven by RT-PCR, but clinical presentation and CT scan were typical for Patient 1 and SARS-CoV-2 serology was finally positive for Patient 4. CT scan sensitivity is probably overestimated but seems correct in a high prevalence of COVID-19 area like Ile de France.20
Conclusion
Cardiovascular risk factors are comorbidities most frequently found in patients with a severe COVID-19 infection and are associated with a higher death rate. In this report, we have described the clinical spectrum of various ischaemic arterial manifestations associated with COVID-19 infection. Similar to the occurrence of VTE, ischaemic arterial events appear to be more frequently associated during the course of COVID-19 and may also reveal COVID-19. Given the seriousness of these complications, clinicians will have to pay particular attention to any signs suggestive of stroke, myocardial infarction or another sign of peripheral artery disease, for as long as this virus circulates.
Lead author biography
Dr Guillet Henri graduated University School of Medicine of Paris Est Créteil in 2012 and began his medical training at the Henri Mondor University Hospital. He then continued his practical training in the field of internal medicine, haematology, and vascular medicine and is currently working in the Sickle Cell Disease centre in Henri Mondor University Hospital.

Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Figure 8.
Two-dimensional transoesophageal echocardiography showing regression of vegetation appended to a cups of the aortic valve after anticoagulant treatment.
Supplementary Material
References
- 1. Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E. et al. The variety of cardiovascular presentations of COVID-19. Circulation 2020;141:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danzi GB, Loffi M, Galeazzi G, Gherbesi E.. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020;41:1858–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W. et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med 2020;382:E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin C, Zhou L, Hu Z, Yang S, Zhang S, Chen M. et al. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan. China. Stroke 2020;51:2219–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kashi M, Jacquin A, Dakhil B, Zaimi R, Mahé E, Tella E. et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res 2020;192:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou P, Yang X, Wang X, Hu B, Zhang L, Zhang W. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connors JM, Levy JH.. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020;135:2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asherson RA, Cervera R.. Antiphospholipid antibodies and infections. Ann Rheum Dis 2003;62:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Connell NT, Battinelli EM, Connors JM.. Coagulopathy of COVID-19 and antiphospholipid antibodies. J Thromb Haemost 2020;10.1111/jth.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z.. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA. et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 2020;18:1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
- 19. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F. et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim H, Hong H, Yoon SH.. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis Hyungjin. Radiology 2020;296:E145–E155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.