Abstract
Antibodies are now well established as therapeutics with many additional advantages over small molecules and peptides relative to their selectivity, bioavailability, half-life and effector function. Major classes of membrane-associated protein targets include G protein-coupled receptors (GPCRs) and ion channels that are linked to a wide range of disease indications across all therapeutic areas. This mini-review summarizes the antibody target landscape for both GPCRs and ion channels as well as current progress in the respective research and development pipelines with some example case studies highlighted from clinical studies, including those being evaluated for the treatment of symptoms in COVID-19 infection.
Keywords: GPCR, ion channel, complex membrane proteins, monoclonal antibody, nanobody, antibody targets, target landscape, erenumab, mogamulizumab, leronlimab, avdoralimab
Statement of Significance: This review of the GPCR and ion channel therapeutic antibody pipelines highlights the progress made in recent years with monoclonal antibodies now reaching late-stage clinical development and even attaining FDA approval as exemplified by erenumab and mogamulizumab.
INTRODUCTION
G protein-coupled receptors (GPCRs) and ion channels belong to complex membrane protein families that represent nearly half of the drug targets currently approved by the Food & Drug Administration (FDA) (34% and 13%, respectively) [1,2], including two GPCR monoclonal antibodies (mAbs) namely erenumab and mogamulizumab. Both of these drug target classes present significant therapeutic opportunities for a wide range of disease indications [3,4]. Historically, however, the success of antibody-based therapies has been limited due to the challenges encountered in the discovery process to identify functional antibodies against GPCRs and ion channels due to technical hurdles that have been described elsewhere in detail in recent reviews, such as the expression, generation of sufficient antigen in a biologically relevant format and a high level of purity [5–7]. Nevertheless, review of the respective global antibody pipelines demonstrates that significant achievements have been accomplished with GPCR-targeting antibodies over the past decade [6,8] enabled by a greater understanding of receptor biology, methods of antigen preparation, structural studies, assay miniaturization and sampling throughput. The structures of both these drug target classes are also beyond the remit of this mini-review but are described extensively elsewhere [6,9].
While several key learnings from GPCR antibody discovery have facilitated ion channel antibody discovery, this target class also presents its own unique challenges and, as such, the antibody pipeline here is still very much in an early stage [4]. Nevertheless, advances in antigen format and preparation, alternative host systems, improvements in electrophysiology methodology and throughput sampling are enabling greater activity and progress in the ion channel antibody discovery field [4,10].
The global antibody market is expected to grow to over US$210 billion by 2022 [11], and with a 22% approval success rate for all antibodies entering clinical study, as assessed for the period 2005–14 [12], antibody therapeutics are gaining further traction as a successful therapeutic class. This success has also been enabled by the different modalities afforded by targeting strategies and types of antibody-based therapy made accessible by next-generation modalities, e.g. bispecific and multi-specific antibodies, antibody–drug conjugates (ADCs), chimeric antigen receptor T-cells (CAR-T). Thus, further progress attained in the GPCR and ion channel therapeutic antibody field will yield further value to these drug target classes.
ANALYSIS OF ANTIBODY TARGET LANDSCAPE FOR GPCRS AND ION CHANNELS
An updated analysis of the antibody target landscape was undertaken in July 2020 based on information available in the public domain, including publications, company websites, conference presentations and posters, as well as commercially available databases and clinicaltrials.gov.
In total, there are now >270 GPCRs that have a strong disease rationale with a profile suitable for targeting with an antibody (Fig. 1A). The notable increase in the number of potential GPCR antibody targets in the past decade not only reflects an increased understanding of GPCR biology, but also the industry-wide interest in oncology and immuno-oncology. Approximately 20% of these targets have a strong level of validation (Phase 2 clinical study or beyond); i.e. it can reasonably be assumed that these GPCRs will have already met a number of deciding factors for target selection, such as demonstrating a role in disease, druggability, genetic validation, greater potential for selectivity as well as restriction of expression to the periphery by exclusion from the central nervous system due to the blood–brain barrier, in some instances will possess a peptide ligand and the potential to offer different mechanisms of action over small molecules and peptides by virtue of Fc effector function, T-cell engagement, antibody–drug conjugation or even CAR-T. The majority of opportunities are still found within the oncology, inflammatory/autoimmune and metabolic disease indications; however, other significant opportunities have also emerged in the therapeutic areas of respiratory disease, pain/neuropathy (including migraine), ophthalmology, cardiovascular, fibrosis and infectious disease with a modest number in genetic or rare diseases, Alzheimer’s disease/amyotrophic lateral sclerosis and, more recently, drug resistance to cetuximab in colorectal cancer. It should be noted that some GPCRs are implicated in multiple therapeutic areas, such as CCR5, which is a target of interest in infectious disease, oncology and immune-mediated disease.
Figure 1.
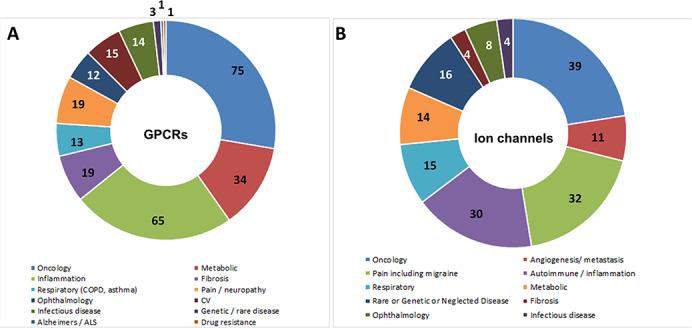
The GPCR and ion channel antibody target landscape. Therapeutic areas in which GPCRs and ion channels have been identified as suitable targets for antibody-based molecules are shown. (A) In total, there are now >270 GPCRs that have a strong disease rationale with a profile suitable for targeting with an antibody. The therapeutic areas of oncology, metabolic disease, inflammation, fibrosis and pain/nephropathy present the highest number of potential targeting opportunities; however, there are now increasing opportunities in respiratory diseases, ophthalmology, cardiovascular indications and infectious disease. The number of potential GPCR targets in each of these therapeutic areas is indicated. (B) In total, there are now >170 ion channels with a similar level of rationale suitable for targeting with an antibody. The number of potential ion channel targets in each of these therapeutic areas is indicated. Some membrane protein targets are implicated in more than one disease indication (such as Kv1.3 and CCR5). COPD, chronic obstructive pulmonary disease; CV, cardiovascular; ALS, amyotrophic lateral sclerosis.
The ion channel landscape presents over 170 antibody targeting opportunities with similar levels of validated biology (Fig. 1B) and has not changed significantly over the past few years. Here, the main opportunities can be found in the oncology, pain (including migraine) and autoimmune/inflammation therapeutic areas, although other significant targeting opportunities are presented by metabolic, respiratory disease genetic/rare or neglected disease indications and a modest number in fibrosis, ophthalmology and infectious disease. The role of ion channels in infectious diseases is also being explored further and an area of emerging biology [13]. In fact, many viruses encode their own ion channels, which emphasize the importance of ionic balance during viral infection [14]. Voltage-gated Ca2+ channel 1.2 (Cav1.2) has been suggested to act as an entry channel in Influenza A infection [15]. Ironically, based on current literature and recent epidemiological evidence, smoking status appears to provide a protective role against COVID-19 infection, suggesting that the nicotinic acetylcholine receptor could also present a potential therapeutic target [16]. Several other ion channels have also been identified as potential targets for infectious disease indications in a recent review [13], including the calcium two-pore channel 2 for SARS-CoV-2 [17].
ANALYSIS OF THE GPCR-ANTIBODY PIPELINE
An updated analysis of the GPCR-antibody pipeline was conducted in parallel with the GPCR-target landscape. It is striking that this pipeline has sufficiently matured with successful strategies employed such that next-generation modalities are now reaching the clinical phase of development, including nanobodies, i-bodies, ADCs, bispecifics and CAR-T (Table 1). Currently, there are only two GPCR-mAbs approved by the FDA, namely erenumab, a CGRP-R antagonist, for the treatment of migraine, and mogamulizumab, a CCR4 binder with enhanced antibody-dependent cellular cytotoxicity (ADCC), approved in the USA and the European Union for Sezary syndrome and mycosis fungoides (cutaneous T-cell lymphomas) and approved in Japan for adult T-cell leukemia–lymphoma and peripheral T-cell lymphoma. There are encouraging signs that a third GPCR-targeting mAb is anticipated to attain FDA approval this year, which is leronlimab (targeting CCR5) and is described in one of the case studies below.
Table 1.
Examples of next-generation antibody modalities for GPCRs and ion channels
| Target class | Modality | Organization | Indication | Stage |
|---|---|---|---|---|
| GPCRs | ||||
| FZD10 | ADC (90Y) | Oncotherapy Science | Synovial sarcoma | Phase 1 |
| LGR5 × EGFR | BS (with ADCC) | Merus | Metastatic CRC | Phase 1 |
| SSTR2 × CD3 | BS | Xencor | NET/GIST | Phase 1 |
| GPRC5D × CD3 | BS (duobody) | Genmab/Janssen | Myeloma | Phase 1 |
| CCR7 | ADC | Novartis | CLL | Phase 1 |
| GPR20 | ADC | Daiichi Sankyo | GIST | Phase 1 |
| CXCR4 | Alternative scaffold—iBody | AdAlta | Fibrosis | Phase 1 |
| CCR4 | Diabody immunotoxin | MGH/Harvard University | Cancer | Preclinical |
| CXCR4 | ADC | John Hopkins | AML | Preclinical |
| LGR5 | ADC (89Zr) | Texas Therapeutics Institute | CRC (and immunoPET) | Preclinical |
| PAC1 × CGRP-R | BS | Amgen | Migraine | Preclinical |
| CXCR5 × CD3 | BS | Max-Delbrück-Center of Molecular Medicine | Cancer | Preclinical |
| Calcitonin R | ADC | Monash | GBM | Preclinical |
| GLP-1R × GCG | BS | Calibr | Metabolic diseases | Preclinical |
| SSTR4 | Peptide–Ab conjugate | Peptide Logic | Pain | Preclinical |
| EMR1 | CAR-T | Humanigen | Eosinophilic leukemia | Preclinical |
| GnRH | CAR-T | Protheragen | Ovarian, prostate, pancreatic cancer | Preclinical |
| GPRC5D | CAR-T | MSK/Juno Therapeutics | Myeloma | Preclinical |
| CB1 × AT1 | BS | Ichan School of Medicine | Liver fibrosis | Discovery |
| CCR5 | Biparatopic | Scripps Research Institute | HIV | Discovery |
| CCR5 × HIV envelope | BS/TS | Scripps Research Institute | HIV | Discovery |
| GABAB × TfR | BS | Denali | Alzheimer’s disease | Discovery |
| Ion channels | ||||
| P2X7 | Nanobody | Univ Med Centre Hamburg | Autoimmune | Preclinical |
| nfP2X7 | CAR-T | Biosceptre | Hematological malignancies | Discovery |
| Solid tumors | Discovery | |||
| Kv10.1 | Nanobody–TRAIL fusion | Max Planck Institute | Cancer | Discovery |
| Kv2.1 | Nanobody | Institut Pasteur Tunis | Cancer | Discovery |
| TRPV4 | i-Body | AdAlta | Fibrosis | Discovery |
| Kv1.3 | Knot-Body | Maxion Therapeutics | Autoimmune | Discovery |
| Nav1.7 | Ab–GPTx1 peptide conjugate | Amgen | Pain | Discovery |
| Ab–JzTx-V peptide conjugate | Amgen | Pain | Discovery | |
These encompass a wide variety of bispecific formats, ADCs, CAR-T and antibodies with an engineered activity, such as peptide–antibody conjugates. BS, bispecific; TS, trispecific; Ab, antibody; CRC, colorectal cancer; NET, neuroendocrine tumor; GIST, gastrointestinal stromal tumor; CLL, chronic lymphocytic leukemia; AML, adult acute myeloid leukemia; ImmunoPET, Immuno-positron emission tomography; GBM, glioblastoma.
It is evident that there is significant interest and sustained activity in directing mAbs to the GPCR drug class as evidenced by the total number of programs in the research and development (R&D) pipeline and the success of more mAbs attaining advanced clinical development over the past decade (Fig. 2A). The analysis of the pipeline trend reveals that there are over 240 active programs targeting >80 GPCRs, with 59 mAbs in clinical development, as well as seven post-approval Phase 4 studies. A remarkable increase in preclinical studies and research discovery activities over the past 10 years is also observed. As with all other previous analyses, some GPCRs are implicated in more than one disease; thus, several mAbs are in evaluation for more than one disease and therefore at multiple stages of development, such as mogamulizumab, leronlimab and erenumab. It is also noteworthy that these mAbs are the only GPCR-targeting therapeutic antibodies to transition successfully to Phase 3 clinical studies, so far.
Figure 2.
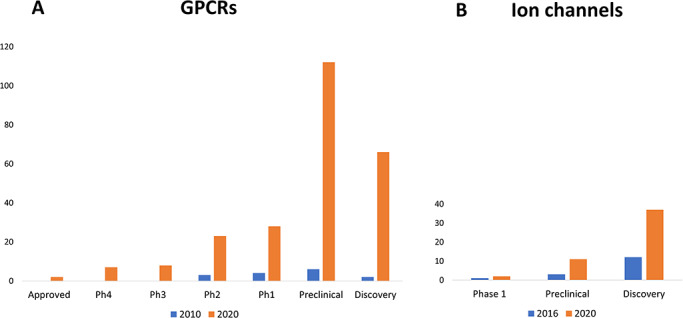
R&D pipeline trends. (A) GPCRs: in 2010, there were 15 programs that targeted 10 different GPCR targets. In 2020, at the time of this analysis, there were over 240 active programs addressing >80 GPCR targets. This includes 59 mAbs in clinical development. Some antibodies are being evaluated for more than one disease indication and therefore can be at multiple stages of development. The extent of clinical success and number of programs in early discovery and development over the past decade is striking with greatest activity at the preclinical stage. (B) Ion channels: there are two antibody programs in clinical development (Ph1) targeting nfP2X7 for basal cell carcinoma (Biosceptre) and Orai1 for the treatment of atopic dermatitis (Daiichi Sankyo). Some ion channel targets have more than one program for different therapeutic indications (e.g. Kv1.3, P2X7 and CACNA2D1). Compared with 2016, there is a notable increase (~3-fold) in preclinical research and discovery activities in 2020. Ph1, Phase 1; Ph2, Phase 2; Ph3, Phase 3; Ph4, Phase 4 post-marketing studies.
The diversity of GPCR-antibody pipeline targets is depicted in Figure 3A, which also demonstrates that the GPCR pipeline continues to evolve with increasing clinical viability, including several preclinical examples of CAR-T entities targeting adhesion GPCRs, such as GPRC5D and EMR1. In fact, over two-thirds of all active programs are directed to Family A GPCRs, including the chemokine receptors that historically are the most well studied and generally tend to be more stable than other GPCRs, making them an attractive prospect. Family B GPCRs continue to represent the next most frequent group of active programs, and these encompass both de novo-generated mAbs and engineered entities, e.g. where a pharmacologically active peptide sequence has been linked to an IgG molecule, such as glutazumab (GMA-102), which is in Phase 2 studies for the treatment of both Type 2 diabetes and obesity.
Figure 3.
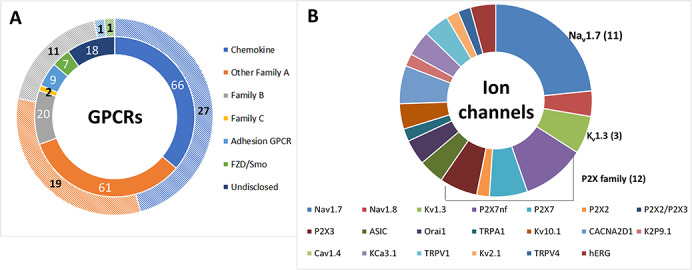
The composition of the R&D pipeline. (A) Antibodies are now in development targeting all the major classes of non-olfactory GPCRs, where two-thirds of the GPCR-antibody pipeline are directed to Family A GPCRs (half of which are chemokine receptors). A diverse range of mechanisms of action have been harnessed, including antagonist, agonist, ADCC, bispecific (e.g. T-cell redirection, biparatopic or bispecific for two different antigens), ADCs, antibody–peptide fusions, incorporation into CAR-T. The GPCR-mAb field has matured sufficiently for further granularity to be depicted, such that the outer hatched colored doughnut ring represents antibodies progressing through clinical development, whereas the inner ring, using the same color scheme but with no hatching, shows the numbers of antibodies in preclinical and discovery research. (B) The range of ion channel targets under investigation remains similar to the past couple of years with 50 active programs directed to 20 different ion channels. In addition, a few trends can be observed, such as an increase in activity focused on Nav1.7. Kv1.3 and the P2X family is also frequent targets of interest. For both GPCR and ion channel pipelines, some antibodies are being evaluated for more than one disease indication and therefore may be at multiple stages of development. FZD, frizzled; Smo, smoothened.
CASE STUDY: LERONLIMAB (CCR5) IN COVID-19 INFECTION
CytoDyn Inc. are developing leronlimab, a humanized IgG4 that targets CCR5, for the treatment of human immunodeficiency virus (HIV) infection and a number of other indications including oncology [18], but more a recent development is the proposal to use leronlimab as a potential treatment for COVID-19 patients [19]. This is due to the ability of the mAb to suppress T-regulatory cells that inhibit anti-viral T-cell responses, as well as repolarization of lung macrophage activity and lowering plasma viral load [19], thereby enhancing the immune response. There is also the possibility that leronlimab could be combined with other retroviral therapies currently in use for the treatment of COVID-19 disease, such as remdesivir. Details outlining the discovery and characterization of leronlimab (previously known as PRO140) have been described elsewhere [6,20]. At the end of July 2020, CytoDyn submitted an application in the UK for approval of leronlimab for HIV and COVID-19; in August 2020, CytoDyn submitted a request for Emergency Use Authorization to the FDA for the treatment of mild to moderate COVID-19 based on data from the Phase 2 study (NCT04343651); furthermore, given the urgency of therapies for the treatment of COVID-19, CytoDyn are seeking Fast Track Approval from the Medicines & Healthcare Products Regulatory Agency (MHRA) in the UK [21]. Other Phase 2 clinical studies in severe-to-critical patients are ongoing (NCT04347239) with Phase 3 studies also planned.
CASE STUDY: AVDORALIMAB (C5AR1) IN COVID-19 INFECTION
Innate Pharma are developing avdoralimab (IPH5401), a fully human Fc-silent IgG1, for the treatment of solid tumors, hepatocarcinoma and non small-cell lung carcinoma where the mAb is currently in Phase 2 clinical study. The mAb mechanism of action blocks the C5a ligand–C5aR1 pathway, thereby ameliorating effects of the complement cascade in tumor biology by reducing the migration and activation of neutrophils and myeloid-derived suppressor cells into the tumor microenvironment, as well as enabling antitumor activities of effector cells, such as T-cells and natural killer cells. Preclinical studies suggest that avdoralimab may be applied as a monotherapy or in combination with other anticancer therapies, such as checkpoint inhibitors. The C5a ligand also mediates pro-inflammatory conditions observed in the acute respiratory disease syndrome associated with COVID-19 infection via cytokine release [22]. Thus, this antagonist mAb has the potential to block C5aR1, thereby reducing the inflammatory response in the lungs. A Phase 2 clinical study is currently evaluating the therapeutic effect of avdoralimab in COVID-19 patients with severe pneumonia (NCT04371367), which has been supported by a recent exploratory translational study (EXPLORE COVID-19) confirming the C5a ligand–C5aR1 pathway is activated in patients who progress to severe COVID-19 infection [23]. Moreover, the authors also reported that avdoralimab prevented C5a-mediated human myeloid cell recruitment and activation as well as inhibition of acute lung injury in human C5aR1 knock-in mice.
CASE STUDY: CX3CR1 NANOBODY
An example of an alternative antibody-based format successfully targeting a GPCR is provided by BI655088, which is a bivalent nanobody (with an albumin-binding VHH domain for extended half-life) in Phase 1 clinical development. BI655088 antagonizes CX3CR1, a chemokine receptor expressed on dendritic cells, memory effector T-cells, monocytes and macrophages. The CX3CR1–fractalkine axis is implicated in atherosclerosis and inflammatory diseases, such as chronic kidney disease, modulating monocyte adhesion activity. Its attraction as an antibody therapeutic target is underlined by the requirement for small molecules requiring subcutaneous injections at high plasma concentrations (thereby rendering them unsuitable for therapeutic application). BI655088 inhibits fractalkine-mediated signaling through CX3CR1 and ameliorates the progression of atherosclerosis in the apoE knockout mouse disease model [24]. Nanobodies (VHHs) were engineered into a bivalent format in order to enhance binding avidity and potency, as well as linked to an albumin-binding VHH for half-life extension without deleteriously affecting the efficacy of the nanobody construct. In vivo proof-of-concept was demonstrated in a human CX3CR1 knock-in mouse created to provide an atherosclerosis model for further in vivo pharmacological evaluation. The model was validated by confirming the presence of CX3CR1 in atherosclerotic plaques. After two 30 mg/kg doses, serum levels of BI655088 and fractalkine were determined by immunoassay and free receptor levels assessed by flow cytometry to confirm target engagement and a 30 mg/kg (twice a week) dose of BI655088 administered over a course of 6 weeks produced a significant decrease in atherosclerotic plaque formation (62%) in the descending aorta [24]. This in vivo data confer the utility of BI655088 which is currently listed in Phase 1 clinical development for chronic kidney disease (NCT02696616).
ANALYSIS OF THE ION CHANNEL-ANTIBODY PIPELINE
Similarly, an updated analysis of the ion channel-antibody pipeline was conducted in parallel with that for the ion channel target landscape. In comparison with the progression of GPCR-mAbs, it is clear that the ion channel-mAb pipeline is still early stage (Fig. 2B); only two ion channel-targeting antibodies have attained early clinical development (Phase 1) over the 4-year period since 2016, namely BIL010t (developed by Biosceptre), a polyclonal antibody that targets nonfunctional P2X7 (nfP2X7), for the treatment of basal cell carcinoma and DS-2741 (developed by Daiichi Sankyo), a mAb that targets Orai1, for the treatment of atopic dermatitis. Figure 3B reveals that the most frequent targets of interest include Nav1.7, which shows an increase in the number of active publicly disclosed programs compared with 2019 [4] and includes the engineering approach reported by Amgen, whereby the peptide toxins, GpTx-1 and JzTx-V, are conjugated to an IgG scaffold [25,26], the P2X family and Kv1.3 (including a KnotBody being developed by Maxion Therapeutics). CAR-T modalities are also being pursued at the discovery stage by Biosceptre for the treatment of hematological malignancies and solid tumors, both of which target nfP2X7 (Table 1).
CASE STUDY: ORAI1 MAB DS-2741
DS-2741 is a humanized Fc-silent IgG1 that targets Orai1, the pore-forming subunit of the calcium release-activated calcium (CRAC) channel, and is in development at Daiichi Sankyo for the treatment of atopic dermatitis. It is a specific blocker of Orai1, thereby neutralizing the activity of CRAC by downregulating aberrant calcium entry that would otherwise activate pro-inflammatory mediators [27]. CRAC activation has been implicated in a range of autoimmune and allergic diseases including atopic dermatitis. Specifically, CRAC has been shown to play a role in T-cell activation and other immune cells, such as mast cells [28]. DS-2741 demonstrated suppression of CRAC-mediated human and mouse T-cell activation and mast cell degranulation in human Orai1 knock-in mice, as well as ablating house dust mite antigen-induced dermatitis in a human Orai1 knock-in mouse disease model [27]. In January 2020, a Phase 1 study (NCT04211415) was initiated to evaluate single ascending dose and multiple doses the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of DS-2741 after subcutaneous injection in healthy Japanese male subjects, as well as a single-dose study to assess the PK, safety, PD and efficacy of DS-2741 after subcutaneous injection in Japanese subjects with moderate-to-severe atopic dermatitis.
EMERGING TRENDS
Activity in the oncology therapeutic area has increased dramatically, perhaps not surprising given the current focus on immuno-oncology therapeutics and the ability to harness Fc effector functions, such as ADCC, or T-cell redirection via bispecific/multi-specific modalities that use a CD3 engaging entity for T-cell mediated killing or other tumor-targeting modalities, such as ADCs and CAR-T. The same considerations and challenges will apply as for other bispecifics and ADCs that target non-GPCRs, i.e. biology driven, and not be target class specific. At least 28 GPCR-mAbs have now attained Phase 2 level of clinical development with a similar number in Phase 1 studies. A notable level of activity can be observed for both preclinical development and discovery of GPCR-mAbs, which bodes well for developing the global antibody pipeline. A recent report outlined a high-throughput functional screening strategy that has the exciting potential to expedite the GPCR-mAb discovery timeline by combining glycophosphatidylinositol-anchored antibody cell display with β-arrestin recruitment-based cell sorting [29]. The next milestone for ion channel mAbs will be further progression into preclinical development and to secure first-in-human studies. Next-generation modalities are now progressing particularly alternative scaffolds or formats, such as nanobodies and i-bodies, and ADCs (which are also in development for targeting adhesion GPCRs and orphan GPCRs) with examples listed in Table 1.
DISCUSSION AND CONCLUSIONS
Antibodies are well established as therapeutics that can hold several advantages over small molecules and peptides, such as improved PK, reduced dosing, peripheral restriction, as previously detailed elsewhere [4,6,30] and are suitable for treating chronic conditions, such as cancer, diabetes and rheumatoid arthritis. The majority of naked monospecific mAbs are antagonist in nature (whereas only a small proportion of GPCR antibody targets require an agonist mode of action). More recently, allosteric modulators have been described and these may be a more suitable and finessed therapeutic approach for metabolic targets, such as GLP-1R, GCG and PTH-1R, and pain targets, such as Nav1.7.
Growth and a higher success rate in the GPCR-antibody pipeline has increased considerably compared with 2010 [30] as evidenced by far greater numbers of programs reaching preclinical development and beyond. It is anticipated that by the end of 2020, there will be three approved mAbs that target GPCRs with several other therapeutic antibodies in late-stage clinical development. It is exciting to observe the exploration of next-generation modalities encompassing alternative scaffolds, bispecifics, ADCs and even CAR-T. Emerging biology is identifying and validating further new targets for study, as well as additional physiological or disease-related roles, for example, as has been demonstrated for CCR5.
While the ion channel-antibody pipeline is gradually gaining traction with two antibodies now in clinical development, the status of the ion channel-antibody pipeline is still evolving and reminiscent of the GPCR-mAb pipeline a decade ago. Ion channels are acknowledged as important therapeutic targets, but they remain a challenge for drug discovery. There are still few functional ion channel mAbs described in the literature; nevertheless, it is gratifying to see a novel first-in-class ion channel-targeting antibody reach the clinic (namely DS-2741). Despite the challenge, there is a continued focus on Nav1.7 (and to a lesser extent Nav1.8), although one Nav1.7 targeting mAb has progressed to preclinical development, and this was identified by manipulation of a bacterial scaffold protein for antigen generation (Visterra acquired by Otsuka in 2018); many of the other mAbs in the discovery stage appear to have been generated via engineering functionality into CDR regions, for example, the KnotBody (Maxion Therapeutics) or peptide toxin–IgG conjugates (Amgen). Antibodies directed to the ligand-gated P2X family and Kv1.3 continue to dominate the pipeline that, for the former at least, most likely reflects the large size of the extracellular region presented for epitope targeting.
Ongoing momentum in the structural field will enable deeper knowledge of the biology involved with both drug class and assist targeting strategies, for example, structure-based-guided discovery of a single-domain antibody agonist directed to the apelin receptor [31] and the co-crystallization structure of Nav1.7 in complex with accessory proteins, voltage-sensing domain toxins and pore-binding toxins [32]. The solutions to technical challenges associated with expression and screening continue to be finessed and will no doubt facilitate isolation of further functional antibodies in the future. While not covered by this article, mention should be made regarding transporters, another class of complex membrane protein targets that present similar technical hurdles as GPCR and ion channels; at least a couple of mAbs have reached preclinical development that are directed to aquaporin-4 (Keio University) and glutamate 4 or SLC2A4 (Integral Molecular). Thus, the future prospects for antibody therapeutic development directed to these membrane target classes continues to hold promise.
Conflict of interest statement. C.J.H. has provided or is currently providing consulting services as an independent consultant in the fields of GPCR and ion channel antibodies to Abilita Bio Inc., DJS Antibodies Ltd, Kyowa Kirin Pharmaceutical Research, Heptares Therapeutics Ltd, TetraGenetics Inc., Twist Biopharma and xCella Biosciences Inc. and is a shareholder of Heptares Therapeutics Ltd.
REFERENCES
- 1. Hauser, AS, Attwood, MM, Rask-Andersen, M et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 2017; 16: 829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vincent D Shaw Stockbroking Company Report. 2015. Jun 9. Bionomics Limited (BNO) A paradigm shift in ion channel drug development. http://www.bionomics.com.au/upload/investors/analystcoverage/Shaw%20Research%20Bionomics%20Limited%20(BNO)%20%20A%20Paradigm%20Shift%20in%20Ion%20Channel%20....pdf (4 January 2018, last accessed).
- 3. Heng, BC, Aubel, D, Fussenegger, M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol Adv 2013; 31: 1676–94. [DOI] [PubMed] [Google Scholar]
- 4. Hutchings, CJ, Colussi, P, Clarke, TG. Ion channels as therapeutic antibody targets. MAbs 2019; 11: 265–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douthwaite, JA, Finch, DK, Mustelin, T et al. Development of therapeutic antibodies to G protein-coupled receptors and ion channels: opportunities, challenges and their therapeutic potential in respiratory diseases. Pharmacol Ther 2017; 169: 113–23. [DOI] [PubMed] [Google Scholar]
- 6. Hutchings, CJ, Koglin, M, Olson, WC et al. Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat Rev Drug Discov 2017; 16: 787–810. [DOI] [PubMed] [Google Scholar]
- 7. Dodd, R, Schofield, DJ, Wilkinson, T et al. Generating therapeutic monoclonal antibodies to complex multi-spanning membrane targets: overcoming the antigen challenge and enabling discovery strategies. Methods 2020. doi: 10.1016/j.ymeth.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 8. Hutchings, CJ. A review of antibody-based therapeutics targeting G protein-coupled receptors: an update. Expert Opin Biol Ther 2020; 20: 925–35. [DOI] [PubMed] [Google Scholar]
- 9. Wilkinson, TC, Gardener, MJ, Williams, WA. Discovery of functional antibodies targeting ion channels. J Biomol Screen 2015; 20: 454–6. [DOI] [PubMed] [Google Scholar]
- 10. Colley, CS, England, E, Linley, JE et al. Screening strategies for the discovery of ion channel monoclonal antibodies. Curr Protoc Pharmacol 2018; 82: e44. [DOI] [PubMed] [Google Scholar]
- 11. Global Monoclonal Antibodies (mAbs) Market Report 2020. https://www.businesswire.com/news/home/20191211005627/en/Global-Monoclonal-Antibodies-mAbs-Market-Report-2020 (July 2020, last accessed).
- 12. Kaplon, H, Reichert, JM. Antibodies to watch in 2019. MAbs 2019; 11: 219–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charlton, FW, Pearson, HM, Hover, S et al. Ion channels as therapeutic targets for viral infections: further discoveries and future perspectives. Viruses 2020; 12: 844–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Royle, J, Dobson, SJ, Müller, M et al. Emerging roles of viroporins encoded by DNA viruses: novel targets for antivirals? Viruses 2015; 7: 5375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujioka, Y, Nishide, S, Ose, T et al. A sialylated voltage-dependent Ca2+ channel binds hemagglutinin and mediates influenza a virus entry into mammalian cells. Cell Host Microbe 2018; 23: 809–18. [DOI] [PubMed] [Google Scholar]
- 16. Changeux, JP, Amoura, Z, Rey, FA et al. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. C R Biol 2020; 343: 33–9. [DOI] [PubMed] [Google Scholar]
- 17. Ou, Liu, Y, Lei, X et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020; 11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplon, H, Muralidharan, M, Schneider, Z et al. Antibodies to watch in 2020. MAbs 2020. doi: 10.1080/19420862.2019.1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patterson, BK, Seethamraju, H, Dhody, K et al. Disruption of the CCL5/RANTES-CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID-19. medRxiv 2020. doi: 10.1101/2020.05.02.20084673. [DOI] [Google Scholar]
- 20. Olson, WC, Rabut, GE, Nagashima, KA et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol 1999; 73: 4145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. CytoDyn Requests “Fast Track Approval” for COVID-19 Patients from U.K.’s Regulatory Agency MHRA Based on its Top-line Report Showing Statistically Significant Endpoint, NEWS2 (p<0.023) and Notable Safety Results. https://www.cytodyn.com/newsroom/press-releases/detail/462/cytodyn-requests-fast-track-approval-for-covid-19 (19 August 2020, last accessed).
- 22. Risitano, AM, Mastellos, DC, Huber-Lang, M et al. Complement as a target in COVID-19? Nat. Rev Immunol 2020; 20: 343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carvelli, J, Demaria, O, Vély, F et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Low, S, Wu, Jerath, K et al. VHH antibody targeting the chemokine receptor CX3CR1 inhibits progression of atherosclerosis. MAbs 2020. doi: 10.1080/19420862.2019.1709322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biswas, K, Nixey, TE, Murray, JK et al. Engineering antibody reactivity for efficient derivitization to generate Na(V)1.7 inhibitory GpTx-1 peptide-antibody conjugates. ACS Chem Biol 2017; 12: 2427–35. [DOI] [PubMed] [Google Scholar]
- 26. Murray, JK, Wu, B, Tegley, CM et al. Engineering Na(V)1.7 inhibitory JzTx-V with a potency and basicity profile suitable for antibody conjugation to enhance pharmacokinetics. ACS Chem Biol 2019; 14: 806–18. [DOI] [PubMed] [Google Scholar]
- 27. Aki, A, Tanaka, K, Nagaoka, N et al. Anti-ORAI1 antibody DS-2741a, a specific CRAC channel blocker, shows ideal therapeutic profiles for allergic disease via suppression of aberrant T-cell and mast cell activation. FASEB BioAdv 2020; 2: 478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Capite, J, Parekh, AB. CRAC channels and Ca2+ signaling in mast cells. Immunol Rev 2009; 231: 45–58. [DOI] [PubMed] [Google Scholar]
- 29. Ren, H, Li, J, Zhang, N et al. Function-based high-throughput screening for antibody antagonists and agonists against G protein-coupled receptors. Commun Biol 2020. doi: 10.1038/s42003-020-0867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutchings, CJ, Koglin, M, Marshall, FH. Therapeutic antibodies directed at G protein-coupled receptors. MAbs 2010; 2: 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma, Y, Ding, Y, Song, X et al. Structure-guided drug discovery of a single-domain antibody agonist against human apelin receptor. Sci Adv 2020. doi: 10.1126/sciadv.aax7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen, H, Liu, D, Wu, K et al. Structures of human Na(v)1.7 channel in complex with auxiliary subunits and animal toxins. Science 2019; 363: 1303–8. [DOI] [PubMed] [Google Scholar]


