Abstract
Enteric glial cells (EGC) modulate motility, maintain gut homeostasis, and contribute to neuroinflammation in intestinal diseases and motility disorders. Damage induces a reactive glial phenotype known as “gliosis”, but the molecular identity of the inducing mechanism and triggers of “enteric gliosis” are poorly understood. We tested the hypothesis that surgical trauma during intestinal surgery triggers ATP release that drives enteric gliosis and inflammation leading to impaired motility in postoperative ileus (POI). ATP activation of a p38‐dependent MAPK pathway triggers cytokine release and a gliosis phenotype in murine (and human) EGCs. Receptor antagonism and genetic depletion studies revealed P2X2 as the relevant ATP receptor and pharmacological screenings identified ambroxol as a novel P2X2 antagonist. Ambroxol prevented ATP‐induced enteric gliosis, inflammation, and protected against dysmotility, while abrogating enteric gliosis in human intestine exposed to surgical trauma. We identified a novel pathogenic P2X2‐dependent pathway of ATP‐induced enteric gliosis, inflammation and dysmotility in humans and mice. Interventions that block enteric glial P2X2 receptors during trauma may represent a novel therapy in treating POI and immune‐driven intestinal motility disorders.
Keywords: enteric nervous system, gut inflammation, motility disorders, postoperative ileus, purinergic signaling
Subject Categories: Digestive System, Immunology, Neuroscience
Enteric gliosis was shown to be part of an intestinal immune response upon abdominal surgery. ATP activates enteric glial cells via selective purinergic receptor signalling in mice and humans. Inhibition of this pathogenic pathway by the newly identified P2X2 antagonist ambroxol blocks ATP‐induced enteric gliosis and protects against postoperative ileus.
The paper explained
Problem
In various inflammation‐induced intestinal disorders, it has been shown that reactive enteric glia play a role in disease progression by contributing to inflammatory processes. However, less is known about the underlying pathogenic mechanism of EGC activation.
Results
Herein, we show that enteric gliosis occurs upon abdominal surgery and leads to postoperative ileus (POI), an inflammation‐based intestinal motility disorder. Activation of EGC in this process depends on ATP and selective purinergic signaling in EGCs. Within a comprehensive set of in vivo, ex vivo, and in vitro analyses in mice and human specimens, we found that ATP is released during abdominal surgery and activates purinergic P2X2 signaling that triggers gliosis in human and murine EGC. We further identified a novel P2X2 antagonist and P2X2 antagonism with ambroxol proved to ameliorate gliosis, reduce inflammatory responses, and improve clinical symptoms of POI.
Impact
We conclude that enteric gliosis and P2X‐purinergic receptors might be promising drug targets for therapeutic approaches in immune‐driven intestinal diseases.
Introduction
Enteric glial cells (EGCs) are a unique population of cells in the enteric nervous system (Furness, 2012) playing a pivotal role in the maintenance of gut homeostasis (Sharkey, 2015). They shape the immune environment through interactions with resident immune cells and other cell types (Brierley & Linden, 2014; Yoo & Mazmanian, 2017). In line with this, EGCs secrete neuroprotective (Abdo et al, 2010) and immune‐modulatory factors (Yoo & Mazmanian, 2017) and targeted ablation of glia (Rao et al, 2017) or inhibition (McClain et al, 2014) of glial signaling through connexin‐43 hemichannel communication between glia can disrupt motility. However, the neuroinflammatory effect of glial ablation is still unclear, as in some cases a fatal bowel inflammation was documented (Bush et al, 1998; Cornet et al, 2001; Aubé et al, 2006) while in a recent study, utilizing a new genetic mouse model, no immune‐modulatory effect was observed (Rao et al, 2017). In contrast to their immune‐modulatory role, several in vivo and in vitro studies by us and others provide evidence that murine or human EGCs can turn into reactive glia in an immune‐stimulated environment, e.g., under LPS presence (Rosenbaum et al, 2016; Liñán‐Rico et al, 2016), after viral protein HIV‐1 Tat (Esposito et al, 2017) or IL‐1 stimulation upon which EGCs release inflammatory mediators like cytokines, nitric oxide or reactive oxygen species (Stoffels et al, 2014; Brown et al, 2016; Liñán‐Rico et al, 2016; Rosenbaum et al, 2016). EGCs were also shown to interact with bacteria, and they can discriminate between beneficial and harmful bacteria (Turco et al, 2014).
Immune responses are often a consequence of tissue damage which leads to the release of intracellular molecules that act as danger‐associated molecular patterns (DAMP) and trigger innate immune processes (Yoo & Mazmanian, 2017). One prominent DAMP is ATP that is produced and utilized by all cell types (Idzko et al, 2014). In the healthy gut, ATP is involved in intestinal homeostasis, gastrointestinal motility, blood flow and synaptic transmission (Christofi, 2008). However, increased extracellular ATP concentrations resulting from tissue damage and trauma, excessive mechanical stimulation, shear stress in diseased blood vessels, cancer, inflammatory cells or a variety of acute or chronic diseases represent a pathogenic pro‐inflammatory mechanism contributing to symptomatology (Idzko et al, 2014; Di Virgilio et al, 2018).
ATP signaling is complex and is mediated by purinergic receptors to which ATP either binds directly or as an enzymatically metabolized form, e.g., ADP or adenosine (Galligan, 2008). Purinergic receptors are classified broadly into ionotropic P2X, metabotropic P2Y and P1 receptor families. ATP, or other nucleotides can variably activate P2X and P2Y while adenosine activates metabotropic P1 receptors (Galligan, 2008). Recent studies demonstrated the expression of purinergic receptors on EGCs and their role in the regulation of gastrointestinal motility (McClain et al, 2014), neuron‐to‐glia communication (Gulbransen & Sharkey, 2009) and neuronal survival (Brown et al, 2016). We have identified P1, P2X and P2Y purinergic receptors in primary human EGCs in primary culture networks and the molecular identity of the reactive hEGC phenotype was revealed by LPS induction (Liñán‐Rico et al, 2016). Recent progress in the field suggests that EGC may represent “a new frontier in neurogastroenterology and motility” (Ochoa‐Cortes et al, 2016).
Overall, EGCs modulate motility, maintain gut homeostasis, and contribute to neuroinflammation in intestinal diseases and motility disorders (Gulbransen & Christofi, 2018). The latter includes postoperative gastrointestinal dysfunction and postoperative ileus (POI), a common clinical complication observed upon abdominal surgery that is characterized by a transient impairment of gastrointestinal (GI) function after surgery. POI is associated with increased morbidity in patients, and despite implementation of enhanced recovery protocols for elective colorectal surgery (Hedrick et al, 2018), no good treatment option exists. POI remains a huge health care problem costing billions of dollars in extended hospitalizations (Iyer et al, 2009). POI is well known to originate from postoperative neuronal dysregulation and is based on an inflammation of the muscularis externa (ME) (Wehner et al, 2007). Recently, we demonstrated that this postoperative inflammation involves EGC reactivity (Stoffels et al, 2014), but the molecular identity of the induction and trigger mechanisms of EGC activation are not fully understood.
Herein, we tested the hypothesis that surgical manipulation and trauma triggers ATP release that drives enteric gliosis and intestinal inflammation leading to impairment of motility in POI. We accessed the relevance of reactive EGC in human bowel specimens and the well characterized mouse model of acute posttraumatic bowel inflammation resulting in POI. By transferring the discovered mechanistic insights to a clinically relevant treatment option of selective purinergic receptor antagonism with ambroxol, a newly identified P2X2 antagonist “drug”, we confirmed the potential therapeutic importance of ATP‐activated EGCs for inflammation‐induced POI that may be relevant to other motility disorders.
Results
Enteric glial cells respond to injury and inflammation and contribute to damage and regenerative processes (Grubišić & Gulbransen, 2017). Our investigation uncovered a purinergic pathway in reactive murine and human EGCs involved in the response to surgical trauma and inflammation.
ATP induction of a reactive EGC phenotype is dependent on a p38 MAPK signaling pathway
To evaluate enteric glia reactivity, we applied ATP, a trigger of purinergic signaling and an inflammatory mediator, to primary msEGC in culture. Our msEGC cultures were highly enriched in GFAP‐expressing cells (mean, 86 ± 2%, Fig EV1A) that also showed Sox10 and S100β immunoreactivity (Fig EV1B) representing the main EGC phenotype seen in vivo (Boesmans et al, 2015) and enriched glial marker expression (Appendix Fig S1A).
Figure EV1. ExATP induces gliosis in enteric glia cells.
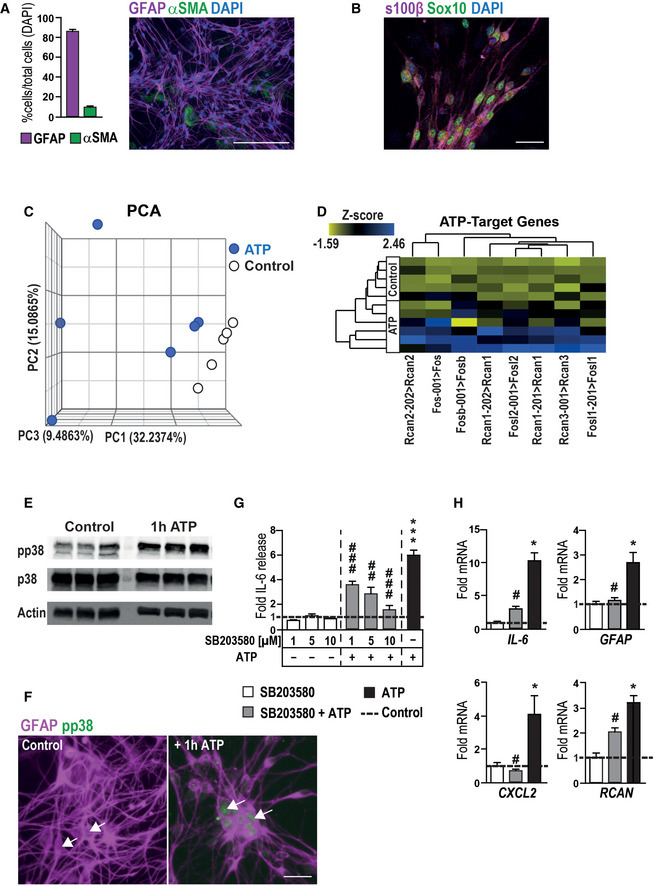
- Histological analysis of EGC culture purity by quantification of EGCs and fibroblasts in vitro. Representative immunofluorescence image shows GFAP (violet)‐positive EGCs and α smooth muscle actin (αSMA, green)‐positive fibroblasts with DAPI as counterstain. Scale bar 50 µm.
- Representative immunofluorescence image of s100β (violet)‐ and Sox10 (green)‐positive EGCs with DAPI as counterstain. Scale bar 10 µm.
- PCA plot of gene expression by ATP‐treated and untreated EGCs. Blue circles represent ATP‐treated EGC cultures, and white circles are matching controls; n = 5–6, respectively.
- Heat map of ATP‐target genes, showing a collection of known target genes of ATP signaling (n = 5–6, msEGCs).
- Representative Western blots of phospho‐p38‐MAPK (pp38) and p38‐MAPK (p38) in 1 h ATP‐treated EGCs. Actin was used as loading control (n = 3, msEGCs).
- Representative images of GFAP (violet)‐ and phospho‐p38‐MAPK (pp38, green)‐positive msEGCs with or without ATP treatment (100 µM) for 1 h. White arrows show pp38‐positive (ATP‐treated) or negative (untreated) EGCs. Scale bar is 10 µm.
- Effect of p38 inhibition on ATP‐induced IL‐6 release. Cells were treated with the p38‐MAPK inhibitor SB203580 (1, 5, 10 µM) alone or together with ATP (100 µM) for 24 h. ELISA measurement of IL‐6 in msEGCs supernatants (n = 7–22, msEGCs).
- Effect of p38 inhibition on ATP induced mRNA expression of gliosis markers in msEGCs. Cells were treated with SB203580 (10 µM) alone or together with ATP (100 µM) for 6 h (n = 4, msEGCs).
Data information: In (A), data are represented as percentage + SEM normalized to the total cell numbers, n = 8, msEGCs. In (G and H), data are represented as fold induction + SEM. Statistics were done in (G and H) by applying unpaired Student's t‐test and one‐way ANOVA with a subsequent Bonferroni test. * indicates significance to control, and # indicates significance to the ATP treatment with */# P < 0.05, ## P < 0.01, and ***/### P < 0.001.
Source data are available online for this figure.
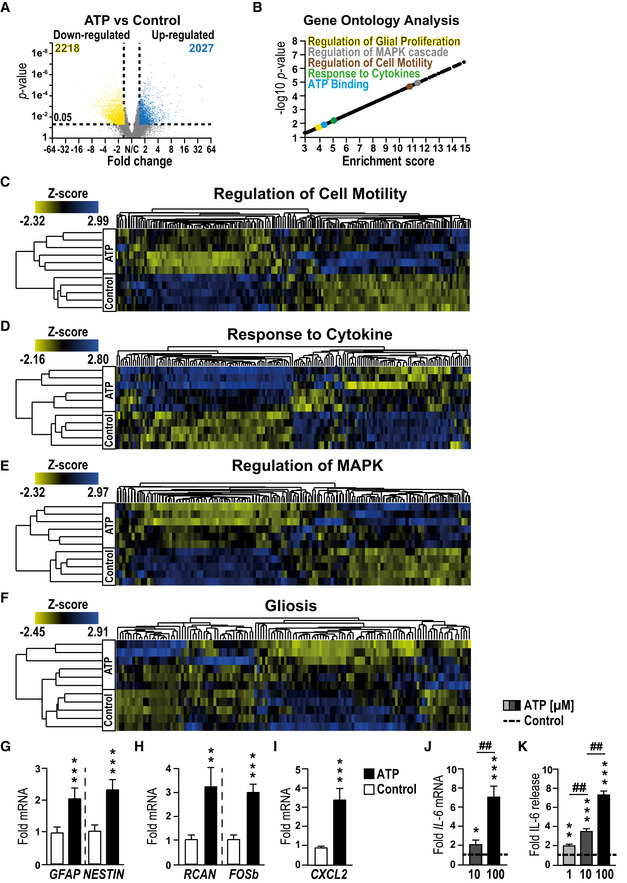
RNA‐Seq analysis of the glial transcriptome identified the unique gene dysregulation profile induced by ATP in msEGCs. We found profound changes in msEGC gene expression with 2,027 up‐regulated and 2,218 down‐regulated genes after ATP stimulation (fold change ≥ 1.5; P‐value: < 0.05, Fig 1A and principal component analysis shown in Fig EV1C). Therefore, ATP caused up‐regulation in 10% and down‐regulation in 11% of total glial transcriptome. Induction of genes, known to be expressed in direct response to ATP, including members of the regulator of calcineurin (RCAN) (Canellada et al, 2008) and FOS (Pacheco‐Pantoja et al, 2016) gene families were confirmed by both RNA‐Seq and qPCR (Figs 1H and EV1D). Gene ontology (GO) enrichment analyses demonstrated a general glial activation in ATP‐treated msEGCs showing enriched genes for “ATP binding” and “glial proliferation” (Fig 1B and Appendix Fig S1B). Importantly, challenge with ATP induced genes involved in the regulation of cell motility, cytokine response genes (Fig 1C and D and Dataset EV1) and the mitogen‐activated protein kinase (MAPK) pathways (Fig 1E and Dataset EV1) underlining the transition of msEGCs to an activated immune phenotype, also referred to as “gliosis”. The term gliosis is commonly used to describe reactive astrocytes, the CNS counterparts to EGCs. Transcriptionally, gliosis is characterized by the up‐regulation of a particular gene set, including, inflammatory response genes. To analyze the reactivity of the EGCs, we created a new GO term for gliosis based on all recent reports discussing CNS gliosis induced by inflammatory stimuli in vivo and in vitro (Zamanian et al, 2012; Hara et al, 2017; Liddelow et al, 2017; Fujita et al, 2018; Mathys et al, 2019; Rakers et al, 2019; Schirmer et al, 2019). Notably, we found that many gliosis‐related genes are also regulated in ATP‐activated msEGCs (Fig 1F, Appendix Fig S1C and Dataset EV1). Quantitative PCR confirmed the up‐regulation of key markers of gliosis including GFAP and NESTIN (Fig 1G) as well as inflammatory mediators like CXCL2 and IL‐6 (Fig 1I and J). The latter has been shown to be an important EGC‐derived cytokine released upon IL‐1β stimulation during surgical trauma (Stoffels et al, 2014). Our data confirmed a robust dose‐dependent and statistically significant increase in the levels of IL‐6, in both mRNA and protein (Fig 1J and K) upon ATP stimulation, indicating a prominent role of IL‐6 in activated EGCs, subsequently making it a reliable marker in enteric gliosis and a central part of our further investigations. To efficiently analyze and describe the glia transformation to a reactive phenotype, we chose six targets; NESTIN and GFAP, two structural glia genes; IL‐6 and CXCL2, two inflammatory mediators and FOSb and RCAN, two transcriptional targets of ATP signaling, as a reliable gliosis marker panel developed from our in silico‐based method to further evaluate purinergic enteric gliosis in subsequent studies.
Figure 1. ATP induces a gliosis in msEGCs.
-
AVolcano plot showing significantly regulated genes between control and ATP‐treated msEGCs.
-
BVisual representation of GO terms associated with enriched genes in ATP‐treated msEGCs compared to control.
-
C–FHeat maps of indicated GO terms in ATP‐treated msEGCs compared to control.
-
G–IqPCR analysis of indicated gliosis genes in ATP‐treated EGCs.
-
JqPCR analysis of IL‐6 in msEGCs that were treated for 6 h with ATP.
-
KIL‐6 protein levels in supernatants from msEGCs collected after 24 h treatment with ATP.
Data information: In (A), data are shown as fold change > 1.5, P‐value < 0.05; (n = 5 for untreated and n = 6 for ATP‐treated EGCs). In (G–K), data are shown as fold change + SEM. (G–I) n = 6–9, msEGCs. (J) n = 4–9, msEGCs. (K) n = 6–18, msEGCs. In (A–I): ATP concentration was 100 µM. In (J, K), ATP concentration was 1, 10, or 100 µM. Statistics were performed by applying unpaired Student's t‐test (G–K) and/or one‐way ANOVA with a subsequent Bonferroni test (J and K). In (A) a limma‐trend pipeline model and in (B) the Fishers exact test were performed. * indicates significance to control, and # indicates significance to ATP treatment with *P < 0.05, **/## P < 0.01 and ***P < 0.001.
Given that ATP treatment led to an activation of MAPK pathways (Fig 1E), we investigated the involvement of p38‐MAPK, an important molecular switch of inflammatory pathways and astrogliosis in the central nervous system (Roy Choudhury et al, 2014). ATP was shown to elevate phospho‐p38‐MAPK protein (Fig EV1E) which is strongly localized in the nucleus of GFAP‐positive msEGCs, absent in untreated msEGCs (Fig EV1F). Furthermore, ATP‐induced IL‐6 protein release was dose‐dependently suppressed using the p38‐MAPK‐inhibitor SB203580 (Fig EV1G); qPCR confirmed the transcriptional reduction of IL‐6 and other gliosis markers like GFAP, CXCL2, and RCAN (Fig EV1H).
Altogether, our data demonstrate that EGC gliosis can be triggered by ATP and induction of enteric gliosis depends on activation of the p38‐MAPK signaling pathway.
P2X receptors mediate the ATP‐triggered IL‐6 release from msEGC
ATP can be enzymatically dephosphorylated and is, together with its metabolites ADP and adenosine, able to signal via multiple purinergic receptors. Those receptors, broadly divided into the P2X, P2Y and P1 classes (Galligan, 2008), make ATP's signaling repertoire rather complex. Many of these receptor subtypes have been identified in enteric glia, although their role in normal or disease states remains unclear (Ochoa‐Cortes et al, 2016; Grubišić & Gulbransen, 2017; Gulbransen & Christofi, 2018).
As a starting point to pinpoint the purinergic receptor subtype(s) involved in enteric gliosis, we performed pharmacological screening with various agonists and antagonists of the purinergic signaling system. In our analysis, adenosine failed to stimulate IL‐6 release from msEGCs, suggesting that the P1 class is not involved in the ATP‐induced phenotype (Fig EV2A). Next, we tested the non‐selective P2‐class antagonist suramin that showed a blockade of ATP‐dependent IL‐6 release in a concentration‐dependent manner (Fig 2A). Similar results were observed with PPADS, another P2 antagonist (Fig EV2B). Additionally, the degradation resistant ATP isoform and P2 agonist ATPγS, dose‐dependently increased the IL‐6 release with comparable or even stronger efficacy than ATP itself (Fig 2B) and induced the expression of established gliosis marker genes (data not shown). These findings indicated that ATP, but not ADP, AMP, adenosine or inosine are likely involved in ATP‐induced EGC gliosis, thereby limiting the involved receptors to members of the P2 class.
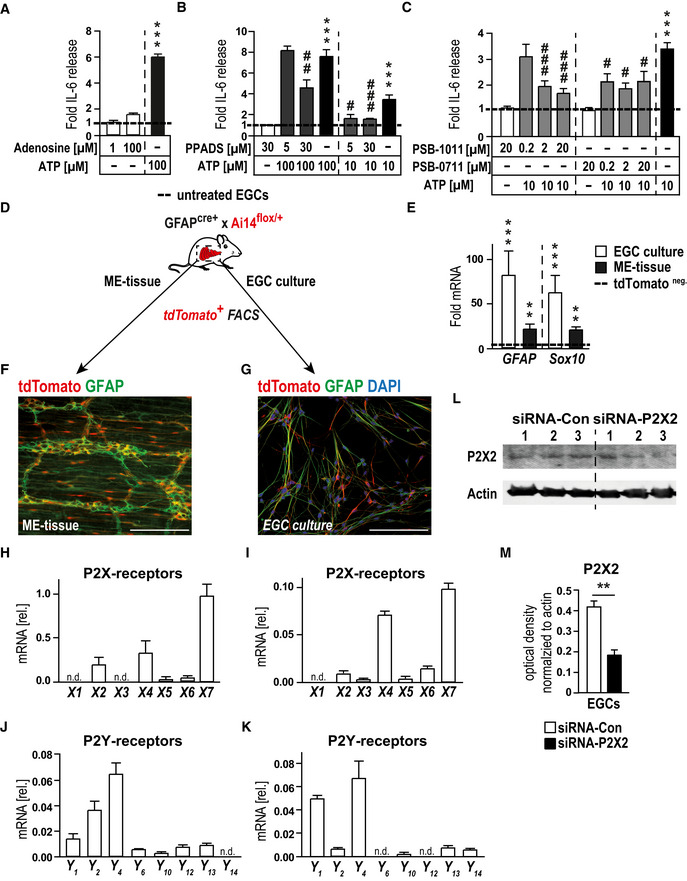
Figure EV2. ATP‐induced gliosis is mediated by p38‐MAPK and P2X2‐purinergic signaling.
-
AIL‐6 release measurement by ELISA of IL‐6 in msEGCs. Cells were treated adenosine (1 and 100 µM) or with ATP (100 µM) for 24 h; n = 14–16, msEGCs.
-
BProtein release measurement by ELISA of IL‐6 in msEGCs. Cells were treated with P2 antagonist PPADS (5, 30 µM) alone or together with ATP (10 or 100 µM) for 24 h; n = 11–12, msEGCs.
-
CProtein release measurement by ELISA of IL‐6 in msEGCs. Cells were treated with P2X2 antagonist PSB‐1011 (0.2, 2, 20 µM) or PSB‐0711 (0.2, 2, 20 µM) alone or together with ATP (10 µM) for 24 h; n = 9–13, msEGCs.
-
DSchematic overview of the isolation of msEGCs from small bowel muscularis externa of GFAPcre‐Ai14fl/wt mice: FACS‐sorted tdTomato+ msEGCs were either analyzed directly (ME‐tissue) or in cultured msEGCs before tdTomato‐FACS‐sorting and further analysis; n = 3–6.
-
EGene expression analysis by qPCR of GFAP and Sox10 in msEGC cultures (n = 10) and mouse ME tissue (n = 10).
-
F, GRepresentative images of co‐localization of GFAP (green) and tdTomato+ msEGC (red) in the ME and in cultured EGCs. Scale bars 50 µm.
-
H–KqPCR analysis of P2‐purinergic receptors in msEGCs isolated from ME (H, J; n = 3) or from cultured cells (I, K; n = 6), respectively.
-
L, MRepresentative Western blots of P2X2 in msEGCs transfected with siRNA‐control or siRNA‐P2X2 for 72 h together with an optical density measurement, see in M. Actin was used as loading control and normalization (n = 6, msEGCs).
Data information: In (A–C and E), data are represented as fold induction + SEM. In (H–K), data are represented as mean + SEM normalized to GAPDH expression. In (M), data are represented as optical density + SEM normalized to actin expression. Statistics were done by applying unpaired Student's t‐test in (A‐C, M and E) or both by unpaired Student's t‐test and one‐way ANOVA with a subsequent Bonferroni test in (B and C). * indicates significance to control, and # indicates significance to the ATP treatment with # P < 0.05, **/## P < 0.01, and ***/### P < 0.001.
Source data are available online for this figure.
Figure 2. ATP‐induced gliosis is mediated by p38‐MAPK and selective purinergic signaling.
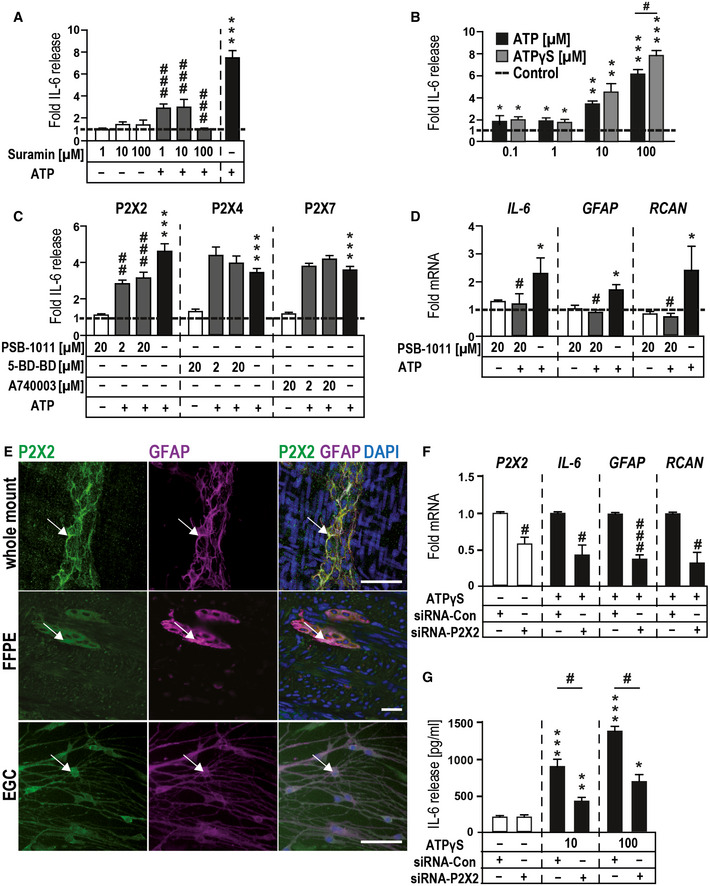
- Effect of P2 receptor antagonism on ATP‐induced IL‐6 release. Cells were treated with P2 antagonist suramin (1, 10, and 100 µM) alone or together with ATP (100 µM) for 24 h.
- ATP‐induced IL‐6 release in msEGCs measured by ELISA. Cells were treated with the indicated concentrations of ATP and ATPɣS for 24 h.
- Effects of P2X antagonists on ATP‐induced IL‐6 release. Cells were treated for 24 h alone or together with ATP (100 µM) in absence or presence of P2X2, P2X4, and P2X7 antagonists PSB‐1011, 5‐BD‐BD, and A740003, respectively.
- P2X2 antagonism of ATP induced mRNA expression of IL‐6, GFAP, and RCAN by qPCR in msEGCs. Cells were treated with the P2X2 antagonist PSB‐1011 (20 µM) alone or together with ATP (10 µM) for 6 h.
- Representative confocal images of P2X2 (green)‐ and GFAP (violet)‐positive msEGCs in vivo and in vitro. White arrows mark double‐positive (white) cells. Scale bar 50 µm.
- P2X2‐siRNA reduces P2X2‐mRNA and dampens the gliosis gene expression after ATPɣS (100 µM) treatment for 6 h.
- P2X2‐siRNA reduces IL‐6 release after ATPɣS treatment (10, 100 µM) for 6 h.
Data information: In (A–D and F), data are shown as fold induction + SEM and (G) as IL‐6 pg/ml + SEM, (A): n = 10–15, msEGCs; (B): n = 3–15, msEGCs; (C): n = 8–17, msEGCs; (D): n = 4, msEGCs; (E): n = 3–5, msEGCs; (F): n = 3–5, msEGCs; (G): n = 3–5, msEGCs. Statistics in (A–D, F, G) were performed by applying unpaired Student's t‐test and/or one‐way ANOVA with a subsequent Bonferroni test. * indicates significance to control, and # indicates significance to ATP treatment with */# P < 0.05, **/## P < 0.01, and ***/### P < 0.001.
Next, a P2 receptor mRNA expression profile in msEGC was determined in cells isolated from GFAPcre x Ai14fl/wt mice, expressing tdTomato in all GFAP+ cells. Cells were either directly sorted upon ME digestion or sorted upon an intermediate cell culture period (Fig EV2D, F and G). Highly increased gene expression of GFAP and Sox10 in tdTomato+ compared to tdTomato− cells confirmed a successful enrichment of msEGC in both procedures (Fig EV2E). Comprehensive gene expression analyses of purinergic receptors in isolated EGC showed a distinct and comparable ex vivo and in vitro gene expression profile with three P2X receptor genes reaching the highest levels, exceeding not only P1 (Appendix Fig S2A and B), but also P2Y expression levels by several times. Accordingly, we directed our focus toward these P2X receptors expressed in enteric glia in the order P2X7 > P2X4 > P2X2 (Fig EV2H–K).
P2X2 receptors mediate the ATP‐triggered EGC gliosis
P2X7 has been shown to be prominently involved in inflammatory processes. However, neither blockade of P2X7 receptors (Fig 2C) nor its activation with selective agonists (Appendix Fig S2C) could influence IL‐6 release in EGCs. While we made the same observation with a P2X4 antagonist, P2X2 antagonism by PSB‐1011 (Baqi et al, 2011) significantly decreased the ATP‐triggered IL‐6 release by around 40% (Fig 2C) and demonstrated a dose‐dependent inhibitory effect (Fig EV2C). Another P2X2 antagonist (PSB‐0711) tested at a lower ATP concentration supported the findings of PSB‐1011 (Fig EV2C). PSB‐1011 treatment also reduced the gliosis‐triggered mRNA expression of IL‐6, GFAP, and RCAN (Fig 2D). The absence of cleaved‐caspase 3 in msEGCs and no changes in the MTT signal after PSB‐1011 treatment confirmed that the reduced IL‐6 release and gene expression was not due to apoptosis or reduced cell viability (Appendix Fig S2D and E). To reinforce the pharmacological data with a P2X2 antagonist, we were able to confirm a strong P2X2 immunoreactivity in msEGCs (Fig 2E) with a specific P2X2 signal (Appendix Fig S2F) and used a genetic approach with P2X2‐siRNA to block the response. The efficiency of the P2X2 knockdown was confirmed on mRNA (Fig 2F) and protein level (Fig EV2L and M) and it reduced ATP‐induced gliosis marker expression on mRNA (Fig 2F) and protein level (Fig 2G).
Together, these pharmacological and siRNA data prove that ATP activates a P2X2 receptor to trigger msEGC gliosis. P1, P2Y or any other highly expressed P2X receptors are not likely to be involved in ATP‐triggered EGC gliosis.
Surgical bowel manipulation in a mouse model of postoperative ileus induces ATP‐target gene expression and enteric gliosis
The next series of experiments were performed to investigate the role of ATP on EGC in an in vivo model of surgical intestinal manipulation (IM, Appendix Fig S3A) that induces enteric neuroinflammation in the ME and subsequently results in impaired gastrointestinal motility, clinically known as POI. Previous work of our group demonstrated that EGC are involved in POI pathogenesis (Stoffels et al, 2014). Hallmarks of POI are an increased IL‐6 release (Wehner et al, 2005), the infiltration of blood‐derived immune cells into the manipulated ME, activation of resident macrophages, and subsequent impairment of gastrointestinal (GI)‐transit (Appendix Fig S3B–D). To evaluate whether ATP is involved in the pathogenic mechanism of POI we first sought to measure ATP release in the peritoneal cavity in the lavage fluid to test whether it was elevated after IM. Here, we detected a time‐dependent increase of ATP release after IM (Fig 3A). Simultaneously, gene expression of the ATP degrading enzymes ENTPD2, ENTPD8 and CD73 decreased, indicating a shift in postoperative ATP metabolism that may favor higher extracellular ATP levels that can further activate EGCs (Fig EV3A). In the early phases of POI, immunohistochemistry showed FOSb+/Sox10+ cells in enteric ganglia (Fig EV3B) and strong induction of FOSb and RCAN gene expression by qPCR (Fig 3H) indicating active ATP signaling in EGC.
Figure 3. An ATP‐induced enteric gliosis in postoperative bowel inflammation.
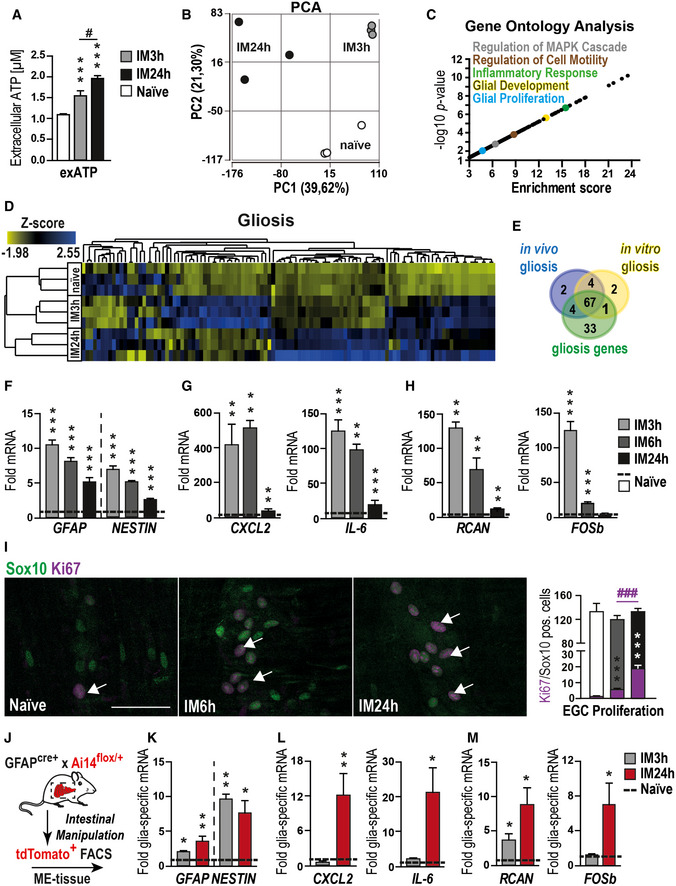
-
AATP measurement at the indicated postoperative time points in peritoneal lavages of mice that underwent intestinal manipulation (IM) or were naïve.
-
BPCA plot of gene expression from POI mice at different disease stages and naïve mice; n = 3 for each group.
-
CVisual representation of P‐value (−log10) against fold enrichment of GO terms associated with enriched genes in mice that underwent IM.
-
DHeat map of enriched genes connected with GO term gliosis in mice that underwent IM or in naïve animals.
-
EVenn diagram of gliosis genes expressed in vitro and in vivo.
-
F–HqPCR analysis of indicated gliosis marker in mice that underwent IM (n = 7).
-
IHistological analysis of EGC proliferation in vivo. Representative confocal images and quantification show Sox10 (green)‐ and Ki67 (violet)‐positive EGCs (white arrows) in the small bowel muscularis externa at naïve, IM6h and 24 h. Scale bar 50 µm.
-
JExperimental setup for glia‐specific RNA analysis. GFAP‐cre+ × Ai14‐floxed mice underwent IM, and small intestine ME was digested and sorted for tdTomato by FACS to provide glia‐specific RNA for qPCR measurements seen in (K‐M).
-
K–MqPCR analysis of indicated gliosis markers in td + glia cells from naïve mice and mice that underwent IM (n = 5–7, POI mice).
Data information: In (A), data are represented as mean + SEM; n = 7–12, POI mice. In (F–H and K–M), data are represented as fold induction + SEM. In (I), data are represented as mean of double‐positive cells per total Sox10‐positive cells + SEM; 9–20 whole mount specimens per conditions; n = 8, POI mice per group. Statistics were performed by applying unpaired Student's t‐test (A, C, F–I, K–M). In (C), the Fisher's exact test was performed. * indicates significance to naïve animals, and # indicates significance to the indicated time point with */# P < 0.05, **P < 0.01, and ***/### P < 0.001.
Figure EV3. Intestinal inflammation induces enteric gliosis.
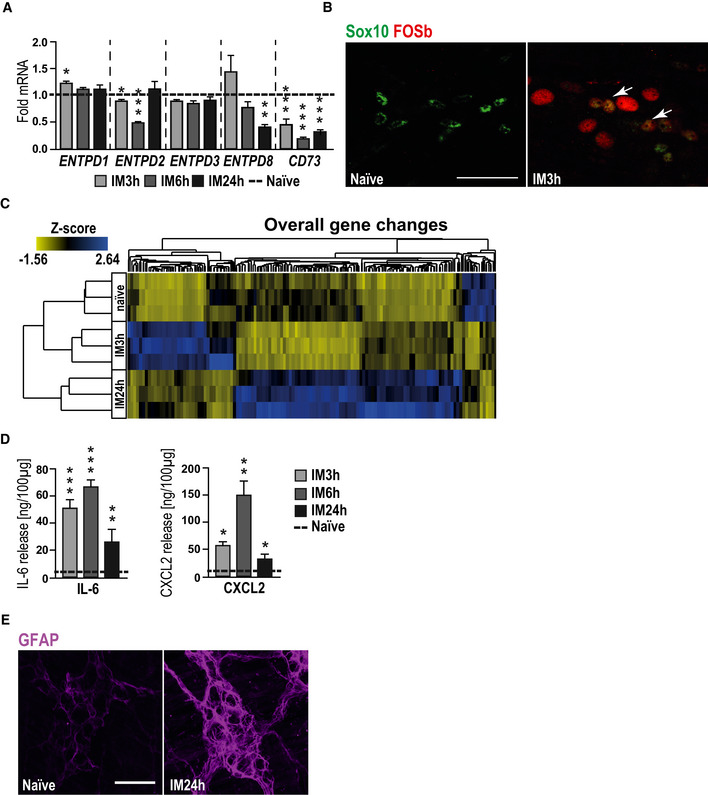
- Gene expression of ectonucleotidases in POI mice at indicated disease stages.
- Representative confocal images of the activation marker FOSb (red)‐ and Sox10 (green)‐positive EGCs (white arrows) in ganglia of intestinally manipulated mice (3 h after IM) or naïve mice. Scale bar 50 µm.
- Heat map of all significantly changed genes from POI mice at different disease stages and naïve mice; n = 3 for each group.
- Protein release analysis by ELISA of IL‐6 or CXCL2 in POI mice at indicated disease stages.
- Representative confocal images of gliosis marker GFAP (violet)‐positive EGCs in ganglia of intestinally manipulated mice (24 h after IM) or naïve mice. Scale bar 50 µm.
Data information: In (A), data are represented as fold change + SEM; n = 7, POI mice. In (D), data are represented as IL‐6/CXCL2 protein in 100 µg tissue + SEM; n = 7, POI mice. Statistics were done in (A, D) by applying unpaired Student's t‐test. * indicates significance to control with *P < 0.05, **P < 0.01, and ***P < 0.001.
Source data are available online for this figure.
RNA‐Seq analysis on murine ME specimens isolated 3 or 24 h after IM or from naïve animals showed substantial differences in the gene expression patterns between all tested groups (Figs 3B and EV3C and Dataset EV2). GO enrichment indicated significant regulation of gliosis‐associated genes 3 and 24 h after IM and showed similar activation patterns between the postoperative ME and ATP‐treated msEGC cultures with alterations in “MAPK”, “cell motility”, “inflammatory response signaling” and genes involved in “glial development and proliferation” (Fig 3C). Using the previous gliosis gene panel, we confirmed the induction of enteric gliosis during POI progression as demonstrated by gene up‐regulation (Fig 3D and Appendix Fig S3F). The strongest response occurred at IM24h. Moreover, a Venn diagram of the gliosis genes displays the similarity of regulated genes in vivo and in vitro with shared up and down‐regulated genes (Appendix Fig S3E), indicating a similar glial activation pattern (Fig 3E). This POI phenotype was confirmed by qPCR showing elevated levels of our established gliosis marker: GFAP, NESTIN, IL‐6, CXCL2, FOSb, and RCAN at 3 and 24 h after IM (Fig 3F–H) with a similar increase in IL‐6 and CXCL2 protein levels (Fig EV3D).
Next, we analyzed glial proliferation and morphology by immunohistochemistry, defining two hallmarks of gliosis (Buffo et al, 2008; Pekny & Pekna, 2016). During disease progression within 24 h the Ki67+/Sox10+ EGC numbers increased up to 10‐fold and the glial morphology gains a more complex “branchwood” in myenteric ganglia, revealing a postoperative change in the EGC phenotype (Figs 3I and EV3E). Furthermore, using once more the tdTomato+‐glia‐reporter mouse in our POI model, we were able to gain further insights into the glial expression profile after IM (Fig 3J). Interestingly, the expression analysis of gliosis markers in EGC showed a different pattern, as previously seen in whole ME tissue after IM. In line with the proliferation, the highest gene expression was detected at IM24h with impressive levels of up‐regulation in gliosis genes, including GFAP, NESTIN, IL‐6, CXCL2, RCAN, and FOSb mRNA (Fig 3K–M). These results provided us with an in vivo insight of enteric gliosis during acute inflammation demonstrating again the immune response of EGCs in POI, a postsurgical intestinal inflammatory disease.
P2X2 antagonism by ambroxol prevents enteric gliosis
Our next series of experiments tested whether P2X2 antagonism can attenuate or prevent enteric gliosis and improve motility in our mouse model of POI. While the P2X2 antagonists PSB‐1011 and PSB‐0711 were not applicable for in in vivo usage the prospect of future clinical application of a P2X2 antagonist drug to regulate enteric gliosis and protect against developing POI, prompted us to perform a drug library screening to identify a clinically feasible P2X2 antagonist. In this drug screening, ambroxol (Appendix Fig S4A) was identified, as it showed a significant inhibition of ATP‐induced calcium influx in 1321N1 astrocytoma cells transfected with the human P2X2 receptor (IC50: 5.69 ± 1.06 µM), but not in cells transfected with P2X1, P2X3, P2X4, or P2X7 receptors (Fig EV4A). Thus, ambroxol was characterized as a potent P2X2 receptor antagonist with selectivity for P2X2 vs the other P2X receptor subtypes.
Figure EV4. P2X2 signaling inhibition by ambroxol improves clinical symptoms in POI.
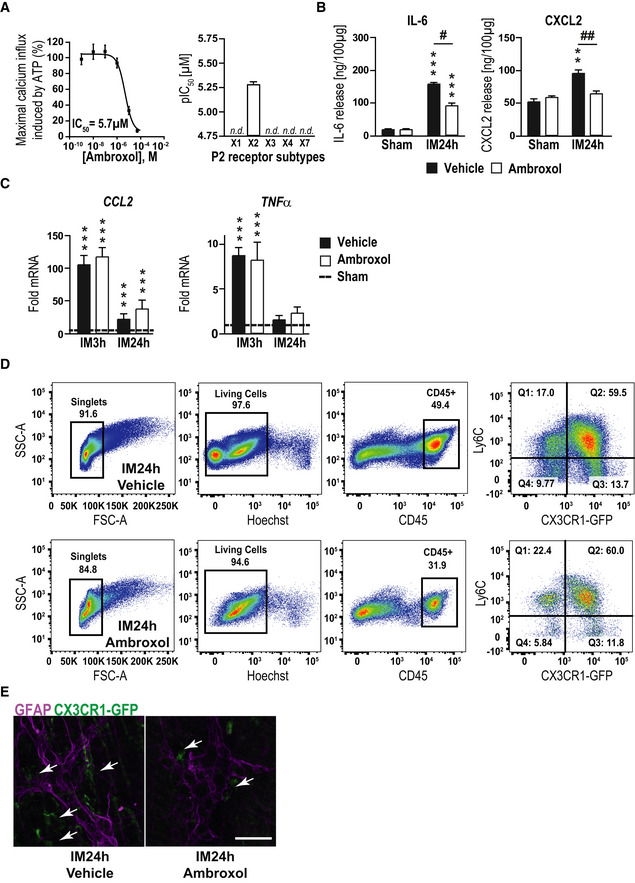
- Concentration‐dependent inhibition of ATP‐induced calcium influx in 1321N1‐astrocytoma cells with recombinant expression of the human P2X2 receptor. ATP was used in a concentration corresponding to its EC80 value (1 µM). An IC50 value for ambroxol of 5.69 ± 1.06 µM ± SEM was determined. Inhibitory potency of ambroxol at P2X receptor subtypes X1‐X7. At an initial test concentration of 20 µM, only the P2X2 receptor subtype was blocked by more than 50% indicating P2X2 receptor selectivity and no significant receptor inhibition was detected (n.d.) with other P2 receptor subtypes (n = 6).
- Protein release analysis by ELISA of IL‐6 and CXCL2 in POI mice treated with ambroxol or vehicle at 24 h.
- Gene expression analysis by qPCR of CCL2 and TNFα in POI mice treated with ambroxol or vehicle at IM3h and 24h; n = 6 POI mice.
- Representative FACS gating strategy of infiltrating cells in the ME of mice treated with ambroxol or vehicle. CD45, Ly6C, and CX3CR1 were used to distinguish resident macrophages (CD45+/Ly6C−/CX3CR1+), infiltrating monocytes (CD45+/Ly6C+/CX3CR1−), and infiltrated monocyte‐derived macrophages (CD45+/Ly6C+/CX3CR1+); n = 3–5 POI mice per group.
- Representative confocal images of GFAP (violet)‐positive EGCs and CX3CR1‐GFP‐positive macrophages (green, white arrows) around ganglia of intestinally manipulated mice treated with ambroxol or vehicle (24 h after IM). Scale bar 50 µm.
Data information: In (B), data are represented as IL‐6 or CXCL2 protein in 100 µg tissue + SEM; n = 6 POI mice. In (C), data are represented as fold change + SEM. Statistics were done in (B and C) by applying unpaired Student's t‐test and one‐way ANOVA with a subsequent Bonferroni test. * indicates significance to sham animals, and # indicates significance between vehicle and ambroxol treatment with # P < 0.05, **/## P < 0.01, and ***P < 0.001.
Source data are available online for this figure.
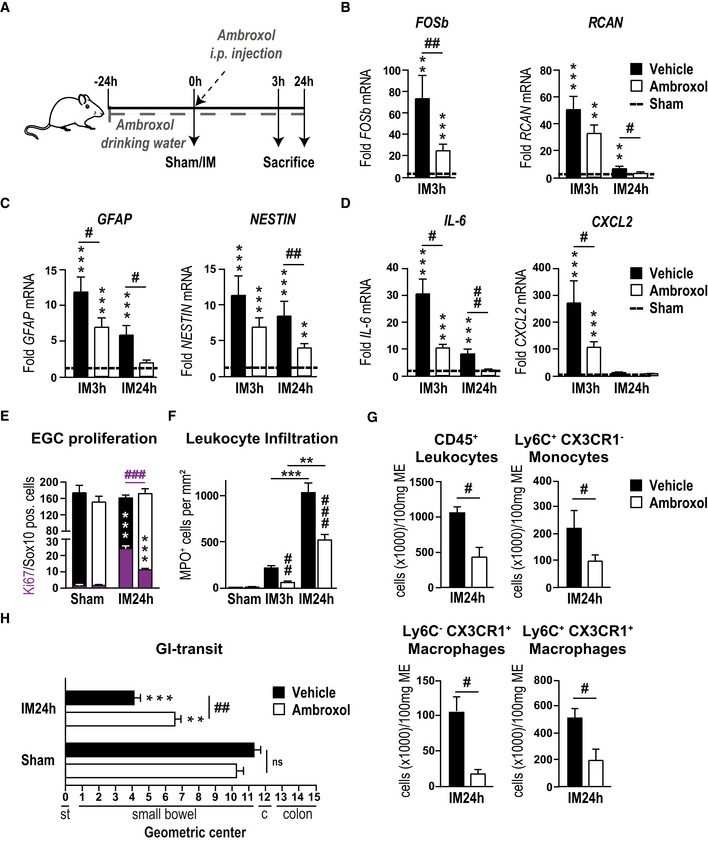
After confirming that ambroxol inhibited ATP‐triggered gliosis marker up‐regulation in msEGCs similar to the before used P2X2 antagonists without any apoptotic effects (Appendix Fig S4B–D), we tested ambroxol as a prophylactic treatment in the POI animal model (Fig 4A). Interestingly, we found a reduced postoperative ME gene expression of ATP‐gliosis targets FOSb and RCAN (Fig 4B) as well as GFAP and NESTIN (Fig 4C) 3 and 24 h after surgery. In line with these findings, postoperative levels of IL‐6 and CXCL2 (Figs 4D and EV4B) were also reduced. Simultaneously, other prototypical pro‐inflammatory markers in POI, such as CCL2 or TNF‐α, were not affected by ambroxol treatment (Fig EV4C). Antagonism with ambroxol had a discrete influence on pro‐inflammatory signaling pathways. To validate direct effects of the P2X2 antagonism on EGCs, we quantified glia proliferation at IM24h for both treatment groups. In comparison, ambroxol dampened the proliferation rate by almost 50%, indicating reduced glial activation during POI (Fig 4E). Finally, we examined a modulatory effect on immune cell infiltration by ambroxol and discovered that ambroxol significantly reduced the number of monocytes (CD45+/Ly6C+/CX3CR1−) and resident (CD45+/Ly6C−/CX3CR1+) or monocytes derived (CD45+/Ly6C+/CX3CR1+) macrophages (CD45+/Ly6C+/−/CX3CR1+) in the manipulated ME at 3 h (~ 70% reduction, MPO‐histology) and 24 h (~ 50% reduction, MPO‐histology and FACS; Figs 4F and G, and EV4D). In addition, histological analysis of the localization of CX3CR1+‐cells in context to EGCs showed fewer macrophages surrounding ganglia in the ambroxol‐treated group (Fig EV4E). Functionally, ambroxol led to a significant improvement in postoperative gastrointestinal transit time (geometric center ambroxol: 6.8 ± 0.4 vs vehicle: 4.1 ± 0.4, Fig 4H). These data indicated that P2X2 antagonism is an effective strategy to attenuate gliosis to improve clinical symptoms in the mouse POI model and makes ambroxol a relevant P2X2 antagonist drug candidate for future therapeutic approaches.
Figure 4. The novel P2X2 antagonist ambroxol prevents IL‐6 release from EGCs.
-
AAmbroxol treatment scheme. Mice were treated with ambroxol or vehicle and underwent a sham operation (laparotomy) or intestinal manipulation (IM). Small bowel muscularis externa (ME) was isolated and analyzed 3 or 24 h after surgery.
-
B–DPostoperative gene expression analyses of indicated gliosis marker in the ME.
-
EHistological analysis of EGC proliferation by quantification of Ki67 (violet)‐ and Sox10 ‐positive EGCs at IM24h. Mice were treated as seen in (A).
-
FHistological counting of infiltrating myeloperoxidase (MPO)‐positive leukocytes in the postoperative ME (n = 6–8).
-
GFACS analysis of infiltrating cells in the ME of mice treated with ambroxol or vehicle. CD45, Ly6C, and CX3CR1 were used to distinguish resident macrophages (CD45+/Ly6C−/CX3CR1+), infiltrating monocytes (CD45+/Ly6C+/CX3CR1−) and infiltrated monocyte‐derived macrophages (CD45+/Ly6C+/CX3CR1+).
-
HPostoperative in vivo GI transit measurement in mice treated with ambroxol or vehicle.
Data information: In (B–D), data are represented as fold change + SEM vs the sham groups (n = 6–8, POI mice per group). In (E), data are represented as mean of double‐positive cells per total Sox10‐positive cells + SEM; six whole mount specimens per conditions; n = 11 mice per IM group and n = 3 per sham group. In (F), data are presented as mean + SEM MPO+ cells/mm2 small intestine ME tissue. In (G), data are presented as cells per 100 mg ME tissue + SEM n = 3–5 POI mice per group. In (H), data are presented as mean + SEM; n = 12 mice per group. Statistics were performed by applying unpaired Student's t‐test and one‐way ANOVA with a subsequent Bonferroni test (B‐H). * indicates significance compared to sham animals, and # indicates significance between vehicle and ambroxol treatment with */# P < 0.05, **/## P < 0.01, and ***/### P < 0.001.
Human enteric gliosis is blocked by P2X2 antagonism
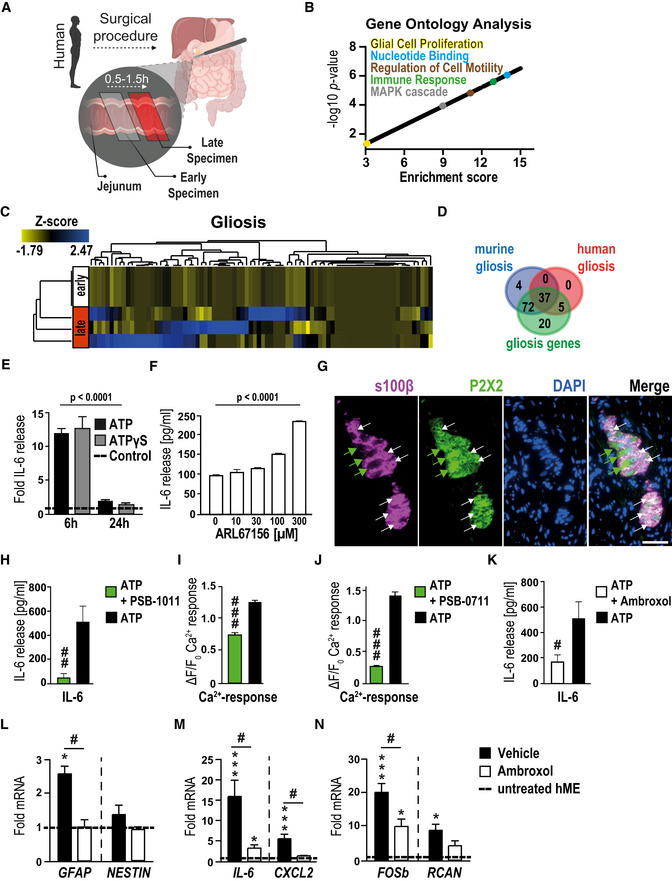
To determine whether findings in the mouse are translatable to human, we carried out additional experiments in human specimens from surgical patients who underwent pancreatectomy, a procedure in which intense IM is an unavoidable consequence, enabling the collection of small bowel specimen at two different intraoperative time points (Fig 5A). In this series of experiments, we analyzed the expression of gliosis markers and ATP‐dependent genes in ME samples of jejunum specimens. Similar to our previous analyses, we performed RNA‐Seq analysis in a limited set of patient ME samples to gain more insight into the glial activation status. The PCA showed a distinct difference between the early and late samples (Fig EV5A) and the consecutive GO analysis pointed to a similar activation pattern as previously shown in the murine system with enriched genes for “MAPK cascade”, “regulation of cell motility”, “immune response” and “glial proliferation” (Fig 5B, Dataset EV3). Consistently, the gliosis panel showed differential gene expression between late and early collected specimens, providing evidence for inflammation‐induced enteric gliosis during surgery (Fig 5C). A Venn diagram comparing the murine and the human enteric gliosis genes visualized 37 shared genes between these species (Fig 5D). To strengthen the RNA‐Seq data, we validated our gliosis marker panel in 13 more patients. Compared to the foremost collected ME specimens, specimens collected at the later time point showed a strong increase in IL‐6‐protein (Fig EV5B) and mRNA levels (Fig EV5C) in concordance with the induction of other gliosis markers (Fig EV5C).
Figure 5. ATP induces gliosis in hEGC.
-
ASchematic workflow on the collection and processing of surgical specimens collected during a pancreaticoduodenectomy. Samples were provided on ice directly from the operation room, and the ME was separated from lamina propria mucosae.
-
BVisual representation of P‐value (−log10) against fold enrichment of GO terms associated with enriched genes in patient specimen that underwent IM.
-
CHeat map of enriched genes connected with GO term gliosis in patient specimens that underwent IM at two time points: early and late; n = 3 human patients.
-
DVenn diagram of gliosis genes expressed in human (red) and murine (blue) ME tissue.
-
EIL‐6 protein release in hEGC cultures upon stimulation with ATP (200 µM) or ATPɣS (100 µM) after 6‐h and 24‐h treatment (n = 4–6, hEGCs).
-
FIL‐6 protein release in hEGC cultures upon stimulation with NTPDase inhibitor ARL67156 at indicated concentrations; n = 4–6, hEGCs.
-
GImmunofluorescence microscopy revealed P2X2 expression (green) in a majority of s100β+ (violet) hEGCs in intact myenteric ganglia of the human colon. White arrows mark double‐positive glia cells, and green arrows mark P2X2‐positive neurons. Scale bar, 50 µm.
-
HEffect of the P2X2 antagonism on ATP‐induced IL‐6 release. ELISA measurement of IL‐6 in hEGCs upon treatment with P2X2 antagonist PSB‐1011 (20 µM) alone or together with ATP (200 µM) treated for 24 h (n = 6, hEGCs).
-
IPSB‐1011 (20 μM) treatment inhibited ATP‐triggered calcium responses in HEK cells transfected with P2X2. Data are represented as ΔF/F0 + SEM; n = 102 HEK cells.
-
JThe P2X2 receptor antagonist PSB‐0711 (20 μM) nearly abolished the ATP‐triggered calcium response in HEK cells transfected with P2X2. n = 219 HEK cells.
-
KAmbroxol blocks ATP‐induced IL‐6 release in hEGCs; protein release measurement by ELISA of IL‐6 in hEGCs. Cells were treated with ATP (200 µM) alone or together with ambroxol (20 µM) for 24 h; n = 6, hEGCs.
-
L–NqPCR analysis of several gliosis and ATP‐target genes in the mechanically manipulated surgical specimens incubated in the presence or absence of ambroxol (20 µM) (n = 7, 4 human patients).
Data information: In (E, F, H, and K), data are represented as mean IL‐6 release + SEM. In (I and J), data are represented as ΔF/F0 + SEM. In (L–N), data are shown as fold induction + SEM. Statistics were performed by applying unpaired Student's t‐test and one‐way ANOVA with a subsequent Bonferroni test (E and F, H–N), and in (B), the Fisher's exact test was performed. * indicates significance compared to controls, and # indicates significance to ATP treatment or between vehicle and ambroxol with */# P < 0.05, ## P < 0.01, and ***/### P < 0.001.
Figure EV5. ExATP induces gliosis in human enteric glia.
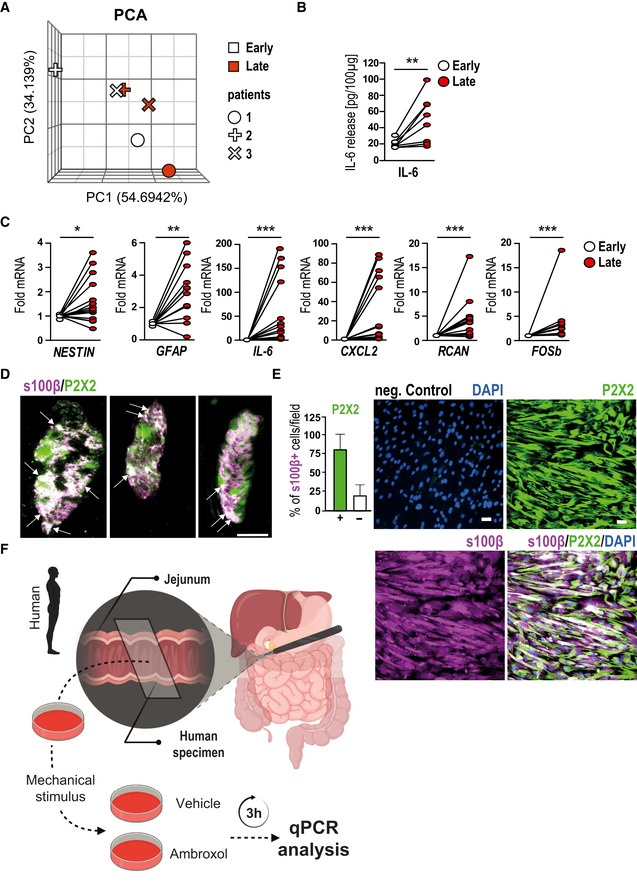
- PCA plot of gene expression from patient specimens at two different time points of the surgery; n = 3 for early and late specimens.
- IL‐6 protein measurement in human surgical specimens collected during a pancreaticoduodenectomy at an early and a late time point of surgery. Samples were provided on ice directly from the operation room, and muscularis externa (ME) was separated from the lamina propria mucosae; n = 9 human patients.
- Gene expression analyses of gliosis marker in human surgical specimens collected during a pancreaticoduodenectomy at an early and a late time point of surgery. The late specimens' mRNA level show an up‐regulation of gliosis genes.
- Immunofluorescence microscopy revealed P2X2 expression (green) in a majority of s100β+ (violet) hEGCs in intact myenteric ganglia of the human colon. White arrows mark double‐positive cells. Scale bar 50 µm.
- Immunofluorescence microscopy revealed P2X2 expression (green) in a majority of s100β+ (violet) hEGCs in culture. DAPI counterstained nuclei. Quantification of double‐positive cells showed that 75% of cultured hEGCs express P2X2 (marked with +). Scale bar 50 µm.
- Schematic workflow on the collection and processing of surgical specimens collected during a pancreaticoduodenectomy. Samples were provided directly from the operation room in oxygenated Krebs–Henseleit buffer and were mechanically activated ex vivo. Immediately after activation, specimens were incubated for 3 h with or without ambroxol (20 µM). Finally, ME was isolated and further processed for qPCR analysis.
Data information: In (B), data are represented as IL‐6 protein in 100 µg tissue; n = 9 human patients. In (C), data are represented as fold change; n = 13 human patients. In (E), data are represented as the percentage of P2X2+/s100β+ cells + SEM; n = 16, hEGCs. Statistics were done by applying unpaired Student's t‐test in (B, C). * indicates significance to control with *P < 0.05, **P < 0.01, and ***P < 0.001.
Source data are available online for this figure.
To determine the involvement of glia in inflammatory processes occurring after IM in patients, we subjected human EGCs, isolated and purified from single human myenteric ganglia dissociated from ME specimen (hEGC) to ATP and ATPγS in vitro stimulation according to our established protocols (Ochoa‐Cortes et al, 2016). Both treatments induced a more than 12‐fold increase in IL‐6 protein release after 6 h, which dropped to a still significant two‐fold induction after 24 h (Fig 5E). Interestingly, naïve hEGC exhibited a dose‐dependent increase in IL‐6 release upon stimulation with ARL67156, a selective ectonucleoside triphosphate diphosphohydrolase (ENTPDase) inhibitor (Fig 5F) indicating that ATP levels are tightly regulated by ENTPDases and that their inhibition creates a high‐enough endogenous ATP concentration to activate hEGC in vitro.
As purinergic activation in hEGC showed similarities to the mouse data, we also investigated human P2X2 signaling. Immunofluorescence microscopy revealed strong P2X2‐immunoreactivity in s100β+ hEGCs in myenteric ganglia in intact surgical tissues (Figs 5G and EV5D) and more than 80% of cultured hEGCs (Fig EV5E). Additionally, P2X2 antagonism by PSB‐1011 blocked the ATP‐triggered IL‐6 release in hEGCs (Fig 5H). The antagonistic specificity for the human system of our tested P2X2 inhibitors was also confirmed in HEK293‐P2X2 sniffer cells expressing a mutant human P2X2 receptor resistant to desensitization. This process leads to continuous elevation of free intracellular calcium levels upon ATP stimulus in a dose‐dependent manner (Appendix Fig S5A). ATP‐induced calcium levels were independently reduced by the P2X2 antagonists PSB‐1011 (Fig 5I) and PSB‐0711 (Fig 5J and Appendix Fig S5B) verifying that these antagonists are able to block responses mediated via hP2X2 in vitro. Based on the promising results of ambroxol as a therapeutic in mice, we utilized it further in an experimental approach in hEGC. Ambroxol treatment inhibited dose‐dependently ATP‐induced calcium influx in HEK293‐P2X2 sniffer cells (Appendix Fig S5C) and blocked ATP‐triggered IL‐6 release in hEGC (Fig 5K).
Together, these data confirm the relevance of ATP‐triggered P2X2 signaling in hEGC gliosis and identify ambroxol as a novel P2X2 antagonist in the human system. To finally confirm the role of ambroxol in human specimens, freshly isolated human full‐thickness jejunal samples, underwent an ex vivo mechanical alteration in the presence or absence of ambroxol (Fig EV5F). All relevant gliosis genes followed up in this study, were significantly down‐regulated in the ambroxol‐treated extracorporally manipulated jejunal ME (Fig 5L–N) indicating that ambroxol treatment is sufficient to attenuate trauma‐induced gliosis.
While future studies, particularly clinical trials, must prove if ambroxol also prevents surgery‐induced gliosis in patients, our ex vivo human data corroborate its role in dampening gliosis and ATP‐driven inflammatory processes and provide proof of concept for translatability of findings on P2X2 from mice to humans.
Discussion
In this study, we aimed to define and better understand the reactive glial phenotype of the enteric nervous system induced by ATP and clarifying its role, beneficial or adverse, concerning inflammation‐induced motility disorders, and in particular POI. In this regard, it has been previously shown by our group that activated EGCs contribute to the disease progression in POI (Stoffels et al, 2014). However, to this day, the significance of glial reactivity in the pathogenic mechanism remained unclear. We provide evidence to support the hypothesis that surgical manipulation and trauma triggers ATP release that drives enteric gliosis and intestinal inflammation leading to impaired motility and POI.
In general, reactive changes of glial cells are a hallmark of “gliosis” that is known to be induced in the CNS by numerous pathological conditions including traumatic (Andersson et al, 2011) or ischemic insults (Roy Choudhury et al, 2014) and in neurodegenerative diseases (Pekny & Pekna, 2014). As a predefined‐GO term for gliosis did not exist and in order to achieve a more integrative description of a reactive EGC phenotype, we created a non‐exclusive list of published genes regulated in reactive astrogliosis (Zamanian et al, 2012; Hara et al, 2017; Liddelow et al, 2017; Fujita et al, 2018; Mathys et al, 2019; Rakers et al, 2019; Schirmer et al, 2019). Based on this gene panel, we analyzed EGCs in vitro and in vivo and termed the glial activation “enteric gliosis”. We targeted ATP as a potential trigger mechanism of enteric gliosis. EGCs have been shown to respond to ATP in situ (Gulbransen & Sharkey, 2009; Boesmans et al, 2019) and in vitro (Gomes et al, 2009; Boesmans et al, 2013) and ATP is a potent trigger of innate immune responses. Increased release of ATP has been observed in multiple acute and chronic inflammatory diseases, including autoimmune diseases (Carta et al, 2015), sepsis (Csóka et al, 2015), sterile insults (Cauwels et al, 2014) and colitis (Grubišić et al, 2019). Thus, we hypothesized that increased ATP levels, which occur as a result of tissue damage to the intestine (Galligan, 2008) or extensive gut manipulation during the operation, might trigger an inflammatory response and activation of EGCs. Our comprehensive RNA‐Seq analysis confirmed profound transcriptional changes in EGCs upon direct ATP stimulation visualized by a separation of control and ATP‐treated EGCs in a PCA plot. The differences in the severity of the activation by purinergic stimuli most likely come from batch differences of primary cells. Moreover, we detected an EGC profile that is comparable to a reactive astrocyte, the CNS counterpart of EGCs (Grubišić & Gulbransen, 2017). In agreement with the up‐regulation of genes involved in MAPK signaling, the main switch in astrogliosis (Roy Choudhury et al, 2014), p38‐MAPK is also induced in ATP‐stimulated EGCs and its blockade completely abrogates enteric gliosis. Interestingly, another of our studies revealed the importance of p38‐MAPK (Wehner et al, 2009) in intestinal inflammation, although its role as a signaling pathway in enteric gliosis had not been investigated before. From these data, we conclude that ATP stimulation induces phenotypical changes in EGCs that are most precisely described by the term enteric gliosis.
So far, ATP's role in gliosis has not been investigated in detail, but previous studies had speculated on the possible involvement of purinergic receptors (Burda & Sofroniew, 2014). Herein, we identified P2X2, one of the highest expressed P2X receptors in murine and in human EGCs (Liñán‐Rico et al, 2016), as the purinergic receptor responsible for triggering enteric gliosis upon ATP stimulation. In humans and mice, we confirmed by immunohistochemistry, that hEGCs and glia in the intact human myenteric plexus strongly express the P2X2 receptor. While P1 receptors, in general, could be excluded from the list of involved receptors, other P2 receptors that have not been tested in our study either because of their low expression or due to a lack of selective antagonist/agonist could potentially be involved in ATP‐triggered gliosis. However, P2X7, the purinergic receptor with the highest expression in msEGCs and the third highest in hEGC (Liñán‐Rico et al, 2016) that is also expressed on virtually all immune cell types (Di Virgilio et al, 2017), is not involved in the ATP‐triggered reactive EGC phenotype.
In contrast, ATP signaling induces neuronal death in models of colitis, another intestinal inflammatory disease, by activating a complex involving P2X7 receptors, Pannexin‐1, Asc and caspases (Gulbransen et al, 2012). It appears that the glial P2X2 gliosis mechanism is unique to postsurgical inflammation. Interestingly, others have shown that chronic morphine induced constipation associated with intestinal inflammation involves a glial ATP‐connexin signaling pathway (Bhave et al, 2017).
In order to analyze the role of enteric gliosis in vivo and to access its value as a therapeutic target (Ochoa‐Cortes et al, 2016; Gulbransen & Christofi, 2018), we chose our established in vivo model of intestinal surgical manipulation (IM) that leads to development of POI (Wehner et al, 2009; Stoffels et al, 2014). Our comprehensive gliosis marker analysis, immune‐activation pathway analysis, morphological, proliferation and cytokine release analysis in response to ATP activation allowed us to conclude that abdominal surgery induces enteric gliosis. In line, detection of increased ATP levels in the peritoneal cavity, which is either actively or passively released following cellular damage (Carta et al, 2015), clearly indicate that ATP release is part of the postoperative inflammatory cascade (Cauwels et al, 2014) and can principally act as a DAMP and an inducer of gliosis. The simultaneous decrease in several nucleotide‐catabolizing ecto‐enzymes, including CD73 and ENTPD8, shifts the cellular metabolic machinery in favor of elevated extracellular levels of ATP in surgically manipulated bowel to reach levels that are sufficient to induce enteric gliosis in vivo via P2X2.
Although the cellular source of ATP remains unknown, the immediate increase in the early phase of POI indicates that it is initially released from resident cells than from infiltrating leukocytes that extravasate into the ME in later stages of POI (Stein et al, 2018). These resident cells could be enteric neurons (Gomes et al, 2009) or resident macrophages (Riteau et al, 2012), as both lie in proximity to EGCs and contain large amounts of ATP that can be actively or passively released upon damage or during inflammation (Oliveira et al, 2014). Additionally, the reactive EGCs themselves are another likely local source of ATP release, and we have previously shown that LPS induction of primary hEGCs in culture elevates ATP release by several fold (Liñán‐Rico et al, 2016). Interestingly, we observed that fewer macrophages surround enteric ganglia upon ambroxol treatment in the POI model. However, it remained unclear if this is due to a general reduction in macrophages or due to a specific reduction in glial activation. Of interest, a recent story revealed direct effects of activated EGCs on macrophage function (Grubišić et al, 2020) substantiating our observation that gliosis is connected to monocyte/macrophage infiltration. Notably, we analyzed previous POI samples of CCR2−/− mice published by Stein et al (2018) to address the potential role of the infiltrating immune cells in ATP release and glial activation during the later time points in POI. Intriguingly, glial activation was reduced in CCR2−/− POI mice, indicating that the immune infiltrate maintains enteric gliosis after manifestation of POI and it is likely that this mechanism also depends on ATP release (Appendix Fig S4F). However, as CCR2−/− mice did not show improved motility 24 h after surgery but showed elevated—but reduced—gliosis marker expression, we interpret that the initial gliosis induced by resident cell activation alone is sufficient to trigger POI. Interestingly, CCR2−/− mice show disturbances in the resolution of POI at later time points (72 h) (Farro et al, 2017), supporting our hypothesis that the infiltrating monocytes contribute to the regenerative gliosis.
In humans, the same set of gliosis marker genes as in mice was time‐dependently up‐regulated in jejunal ME tissues of patients who underwent pancreaticoduodenectomy, a surgical procedure that unavoidably involves strong intestinal manipulation. After gut manipulation and trauma in patients, we detected a clear increase in gliosis markers in the late stages of the operation indicating that our murine data on enteric gliosis are translatable to humans. Therefore, we conclude that gliosis is a conserved cross‐species mechanism regulating the EGC inflammatory response. Moreover, hEGCs exhibited a comparable receptor expression profile and the same functional dependence on P2X2 as msEGC. Further evidence for a conserved enteric gliosis mechanism across different species originates from our recent in vitro studies in hEGCs describing an immune phenotype upon LPS and interferon‐γ signaling (Liñán‐Rico et al, 2016) similar to the one seen in msEGC in the present study.
Our findings lead to a new question: What is the purpose of this conserved gliosis; is the induction necessary for regenerative mechanisms or could an initial blockade lead to a less severe disease outcome? In the CNS, gliosis is targeted by therapeutic approaches in neurodegeneration (Colangelo et al, 2014) to minimize for example neuronal apoptosis (Livne‐Bar et al, 2016). In the ENS, there is no evident view on gliosis yet. The general glial activation/proliferation could serve as damage control and/or regeneration boost by assisting the ENS to regenerate faster after an inflammatory strike, but on the other side targeting EGCs in gastrointestinal immune‐driven diseases and motility disorders could also be a new and promising therapeutic approach (Gulbransen & Christofi, 2018). Incidentally, the strong up‐regulation of glial proliferation in manipulated mice indicated that EGC do participate in ENS regeneration in later stages of POI as already described in colitis‐induced chronic intestinal inflammation (Belkind‐Gerson et al, 2017), in addition to their immune‐modulatory role by, e.g., cytokine release. However, to clarify the potential regenerative role of ATP‐induced gliosis in POI, future studies need to focus on the regeneration stages of this disorder, i.e., 72 h and later after surgery. (Stein et al, 2018).
An internal compound library screening revealed that the antagonist potency of the oral drug ambroxol is comparable to the experimental organic P2X2 antagonist (Baqi et al, 2011) and is suitable for an in vivo investigation. Interestingly, ambroxol's immune‐modulatory effect is already documented in the clinical treatment of airway inflammation, but its mode of action is still unclear (Beeh et al, 2008). Indeed, perioperative ambroxol treatment reduced the postoperative gliosis marker increase and EGC proliferation. It also prevented infiltration of monocytes/macrophages and motility impairment in our POI model. The down‐regulation of ATP‐target genes FOSb and RCAN implied a direct antagonistic effect on purinergic activation by ambroxol. Importantly, ambroxol prevented induction of IL‐6 and CXCL2 but no other inflammatory genes, including TNFα and CCL2. This demonstrates that ambroxol does not function as a general anti‐inflammatory drug as previously speculated (Beeh et al, 2008) but instead selectively modulates ATP‐triggered pathways in EGCs. In support of ambroxol's immune‐modulatory action, a recent study using a P2X2/X3 antagonist (gefapixant), showed a down‐regulation of airway inflammation, the main target of ambroxol (Zhang et al, 2020), upon ambroxol treatment in a cough hypersensitivity syndrome model. Nevertheless, ambroxol is also known to affect potassium and calcium channels of neurons (Weiser, 2008) that modulate neuronal activity (Magalhaes et al, 2018). As motility is tightly regulated by these channels (Rao, 2020), we cannot exclude any enteric neuronal modulation by ambroxol in parallel to its impact on glial P2X2‐dependent signaling.
Given that the purinergic system is a prominent player in inflammation (Burnstock, 2020), selective P2X2 antagonism might be of particular therapeutic relevance in POI or perhaps other gut inflammatory or immune‐driven motility disorders. This is supported by animal studies which showed improved clinical symptoms in ambroxol‐treated mice in models of neurodegeneration (Migdalska‐Richards et al, 2016), neuropathic pain (Hama et al, 2010) and LPS‐induced acute lung injuries (Su et al, 2004). Notably, all these diseases lead to activated purinergic signaling (Burnstock, 2020). Finally, the effects of reduced enteric gliosis in humans were supported by ex vivo manipulation of jejunal samples from surgical patients demonstrating a direct inhibition of a mechanically induced gliosis‐related gene induction by ambroxol. In line with these findings, previous clinical trials done over 30 years ago with ambroxol also revealed an alleviated motility in the ambroxol‐treated group (Germouty & Jirou‐Najou, 1987) and ongoing clinical studies for Parkinson's Disease (NCT02941822; (Silveira et al, 2019 )) highlight the potential of ambroxol in treating a neurodegenerative disease with a connection to gut inflammation (Villumsen et al, 2019).
Although we used a broad panel of different techniques, we would like to mention that a final validation of our findings would require the use of a glial‐specific P2X2 knock out mouse. Furthermore, some of our analyses are based on whole ME tissue samples including multiple non‐glia cell types. Even though we validated most of these findings in enriched EGC cultures, a contribution of other cell types in vivo cannot completely be excluded. Nevertheless, future studies should aim to test the clinical efficacy of P2X2 antagonism for the treatment of POI in humans and immune‐driven inflammatory diseases, including motility disorders. Ambroxol or yet to be developed highly selective P2X2 antagonists with the implementation of the power of medicinal chemistry and congener drug development (Burnstock et al, 2017) are suggested to represent novel candidate drugs in the pipeline.
Subsumed, we provide evidence that ATP is able to induce a reactive EGC phenotype, increased inflammation and enteric gliosis in vivo in a P2X2 dependent manner. This mechanism proved to represent a pathogenic mechanism of POI, since ambroxol, a novel P2X2 antagonist, was shown to have efficacy in protecting against postoperative bowel inflammation and motility disturbances in mice and humans. Our “purinergic hypothesis of enteric gliosis in POI” is illustrated in Fig 6.
Figure 6. Purinergic mechanism of enteric gliosis in postoperative ileus.
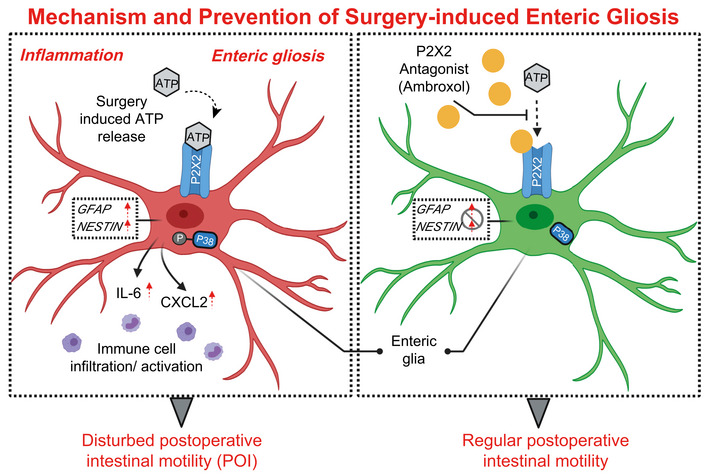
Gut surgical manipulation and trauma cause inflammation and increase ATP release that activates P2X2 receptors on enteric glia and induces a reactive glial phenotype termed “enteric gliosis”. In the context of inflammation, ATP activates a P2X2/p38 MAPK pathogenic signaling pathway associated with an increased expression in gliosis markers GFAP, NESTIN, and the release of cytokines like IL‐6 and CXCL2. Enteric gliosis exacerbates neuroinflammation, contributes to immune cell infiltration and that causes postoperative motility disturbances and POI. P2X2 antagonism by ambroxol prevents the ATP‐triggered enteric gliosis and protects against POI.
Materials and Methods
Murine EGC cultures
Primary enteric glia cell cultures were obtained by sacrificing C57BL/6 mice 8–16 weeks of age, extracting the small intestine and cleansing it with 20 ml of oxygenated Krebs–Henseleit buffer (126 mM NaCl; 2.5 mM KCl: 25 mM NaHCO3; 1.2 mM NaH2PO4; 1.2 mM MgCl2; 2.5 mM CaCl2, 100 IU/ml Pen, 100 IU/ml Strep and 2.5 μg/ml Amphotericin). The small bowel was cut in 3–5 cm long segments and kept in oxygenated ice‐cold Krebs–Henseleit buffer. Each segment was then drawn onto a sterile glass pipette and the ME was stripped with forceps to collect muscle tissue for further digestion steps. After centrifugation (300 g for 5 min), the tissue was incubated for 15 min in 5 ml DMEM containing Protease Type1 (0.25 mg/ml, Sigma‐Aldrich) and Collagenase A (1 mg/ml, Sigma‐Aldrich) in a water bath at 37°C, 150 rpm. The enzymatic digestion was stopped by adding 5 ml DMEM containing 10% FBS (Sigma‐Aldrich), centrifugation for 5 min at 300 g and resuspended in proliferation medium (neurobasal medium with 100 IU/Pen, 100 μg/ml Strep, 2.5 μg/ml Amphotericin [all Thermo Scientific], FGF and EGF [both 20 ng/ml, Immunotools]). Cells in proliferation media were kept at 37°C, 5% CO2 for 4 days to promote formation of enteric neurospheres. For experiments, enteric neurospheres were dissociated with trypsin (0.25%, Thermo Scientific) for 5 min at 37°C and distributed at 50% confluency on Poly‐Ornithin (Sigma‐Aldrich) coated six well plates in differentiation medium (neurobasal medium with 100 IU/Pen, 100 μg/ml Strep, 2.5 μg/ml Amphotericin, B27, N2 [all Thermo Scientific] and EGF [2 ng/ml, Immunotools]). After 7 days in differentiation medium, mature enteric glia cells were treated with ATP (0.1, 1, 10, 100 µM, Sigma), ATPɣS (0.1, 1, 10, 100 µM, Sigma), Adenosine (1, 100 µM, Sigma), PPADS (5, 30 µM, TOCRIS), Suramin (1, 10, 100 µM, TOCRIS), A740003 (2, 20 µM, TOCRIS), ambroxol (0.2, 2, 20 µM, TOCRIS), PSB‐0711 (2, 20 µM, TOCRIS), PSB‐1011 (0.2 2, 20 µM, TOCRIS), 5‐BDBD (2, 20 µM, TOCRIS) SB203580 (1, 5, 10 µM, TOCRIS) and further processed for RNA isolation or their conditioned medium used for ELISA or qPCR analysis.
For the siRNA approach, primary msEGCs were differentiated as mentioned above and transfected with a control‐siRNA (SIGMA) or P2X2‐siRNA (#4390771, Thermo Scientific) lipofectamine (Thermo Scientific) complex for 72 h according to the manufacturer's instructions. Afterward, the transfected cells were treated with ATPɣS (10, 100 µM, Sigma) and analyzed by qPCR and ELISA. For Western Blotting, primary msEGCs were lysed in RIPA buffer, centrifuged at maximum speed for 20 min and prepared with loading buffer (Bio‐Rad). All samples were processed with the Bio‐Rad Western Blot systems (any KD SDS‐gels, Trans‐Blot Turbo System) and incubated with the mentioned antibodies in Appendix Table S4 overnight at +4°C. Next, the blot was washed three times and incubated with secondary antibodies (Thermo Scientific) for 2 h and imaged with the Bio‐Rad ChemiDoc Imaging System.
Human surgical specimens
The human IRB‐protocol was approved by the ethics committee of the College of Medicine at The Ohio State University. Informed consent was obtained to procure viable human surgical tissue from colon or small bowel from patients with polyps undergoing a colectomy (sigmoid colon) or patients undergoing Roux‐en‐Y by‐pass surgery (jejunum) (Appendix Table S1). Human EGCs (hEGCs) in culture from 14 GI‐surgical specimens were used to study gene expression and IL‐6‐release in hEGCs. Human EGCs were also used for calcium imaging studies and P2X‐immunofluorescent labeling.
Collection of patient surgical specimens was also approved by the ethics committee of North‐Rhine‐Westphalia, Germany (Accession number: 266_14). Informed consent was obtained to procure human surgical tissue from the small bowel (jejunum) from patients undergoing a pancreatectomy at an early and a late time point during the surgical procedure (Appendix Table S2). Human samples were collected and used for RNA‐Seq and qPCR analysis.
Preparation of human EGC cultures
Tissue collection was performed by the surgeon and immersed immediately in ice‐cold oxygenated Krebs–Henseleit solution and promptly transported to the research facilities within 15 min in coordination with the Clinical Pathology Team (Liñán‐Rico et al, 2016). For isolating myenteric ganglia, tissue was pinned luminal side facing upward under a stereoscopic microscope and the mucosa, submucosa and most of the circular muscle were dissected away using scissors, and then flipped over to remove longitudinal muscle by dissection.
Myenteric plexus tissue was cut and enzymatically dissociated as described elsewhere (Turco et al, 2014; Liñán‐Rico et al, 2016) with modifications as follows: Myenteric plexus tissue was minced into 0.1–0.2 cm2 pieces and dissociated in an enzyme solution (0.125 mg/ml Liberase, 0.5 μg/ml Amphotericin B) prepared in Dulbecco's modified Eagle's medium (DMEM)‐F12, for 60 min at 37°C with agitation. Ganglia were removed from the enzymatic solution by spinning down (twice), and re‐suspending in a mixture of DMEM‐F12, bovine serum albumin 0.1%, and DNase 50 μg/ml DNase (once). This solution, containing the ganglia, was transferred to a 100‐mm culture dish and isolated single ganglia free of smooth muscle or other tissue components were collected with a micropipette while visualized under a stereoscopic microscope and plated into wells of a 24‐well culture plate and kept in DMEM‐F12 (1:1) medium containing 10% fetal bovine serum (FBS) and a mixture of antibiotics (penicillin 100 U/ml, streptomycin 100 µg/ml, and amphotericin B 0.25 µg/ml) at 37°C in an atmosphere of 5% CO2 and 95% humidity.
After cells reach semi‐confluence after 3–4 weeks (P1), hEGCs were enriched and purified by eliminating/ separating fibroblasts, smooth muscle and other cells. EGC enrichment and purification was achieved by labeling the isolated cells with magnetic micro beads linked to anti‐specific antigen, D7‐Fib and passing them through a magnetic bead separation column following the manufacturer instructions (Miltenyi Biotec Inc, San Diego, CA). This purification protocol was performed twice (P2 and P3) to reach a cell enrichment of up to 10,000 fold, and 20,000 cells were plated on glass coverslips pre‐coated with 20 μg/ml laminin/P‐d‐Lys in 50 mm bottom glass #0 culture dishes for immunostaining and imaging or 12 well plates for IL‐6 release experiments. Cultured hEGCs were kept until confluent and harvested for additional experiments (4–10 days). On the day of the experiment, hEGCs were stimulated as indicated. Parallel to this, cells at each passage were split and seeded in plastic 25 mm2 culture flasks and used for study in passages 3–6.
Immunochemical Identification of glia in hEGC
To confirm the purity and identity of glial cells in the hEGC cultures, immunofluorescent labeling was done for glial markers (s100β, glial fibrillary acidic protein GFAP), for smooth muscle/epithelial actin and fibroblasts; hEGCs were fixed in 4% paraformaldehyde for 15 min at room temperature, rinsed three times with cold phosphate‐buffered saline (PBS) 0.1 M and placed at 4°C until further processing. Cells were treated with 0.5% Triton X, 10% normal donkey serum in PBS to permeabilize the cells and block nonspecific antibody binding for 30 min at room temperature. Primary antibodies were diluted in PBS‐0.1% Triton X, and 2% normal donkey serum, and were incubated with cells overnight (18–24 h) at 4°C. Next day preparations were rinsed three times in 0.1 M PBS/1 min and incubated 60 min at room temperature in secondary antibodies diluted in PBS‐0.1%, Triton X, and 2% normal donkey serum. Antibodies mentioned in Appendix Table S4 were used for analysis. Alexa Fluor 488 or 568 donkey anti‐mouse or anti‐rabbit secondary antibodies were used at a dilution of 1:400 (Cambridge, MA). Omission of primary antibodies was used to test for background staining of the secondary antibodies. Pre‐absorption of primary antisera with immunogenic peptides abolished immune‐reactivity. Data confirmed previous reports by Turco et al (2014) and are not shown except for illustrating that all cells express s100β immunoreactivity > 99% of cells.
Ex vivo human specimen experiments
Human surgical tissue for ex vivo experiments was collected from four patients undergoing a pancreatectomy. The study was approved by the Ethics Committee at University of Bonn. Human jejunum specimens were collected in ice‐cold oxygenated Krebs–Henseleit buffer during the surgical procedure and transported to the laboratory. Full‐thickness jejunum specimen were mechanically activated for 30 s and then incubated for 3 h with or without 20 µM ambroxol in oxygenated Krebs–Henseleit buffer. As baseline control a human ME sample was taken before the mechanical activation. After the incubation time, the jejunum specimens were dissected, and only mucosa‐free ME was used for further analysis.
For the ethics approval, the IRB‐protocol for human enteric glia isolation was approved by the ethics committee of the College of Medicine of the Ohio State University and the collection of patient material for the enteric glia analysis was approved by the ethics committee of North‐Rhine‐Westphalia, Germany (Accession Number: 266_14). Further, all experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report.
Cell lines
Human 1321N1 astrocytoma cells were loaded with a calcium‐chelating fluorescent dye (Molecular Devices), either fluo‐4 acetoxymethyl ester (fluo‐4 AM for cells transfected with P2X2, P2X4, or P2X7 receptor), or Calcium‐4 AM or Calcium‐5 AM for cells transfected with P2X1 or P2X3, respectively (Baqi et al, 2011).
HEK‐P2X2 sniffer cells were loaded with 2 μM fluo‐4/AM in a humidified incubator for 30 min and washed for 30 min prior to transferring to a perfusion chamber with oxygenated Krebs–Henseleit buffer on the stage of an upright Eclipse FNI Nikon scope equipped with a Andor iXon Ultra high speed camera for real‐time Ca2+ imaging. Elements software was used for data acquisition. Ambroxol was dissolved in dimethyl sulfoxide (DMSO) and added to the cells at a final concentration of 20 µM followed by stimulation with ATP at its respective EC80 concentration (P2X1 and P2X3 [100 nM], P2X2 and P2X4 [1 µM], P2X7 [1 mM]). The assay volume was 200 µl and the final DMSO concentration was 1%. ATP activation of the receptors led to increased calcium influx and consequently to increased fluorescence, which is blocked by treatment with antagonists.
ELISA
Release of IL‐6 and CXCL2 was measured in ME RIPA lysates isolated from small intestine segments at the indicated time points after IM. Release of IL‐6 in EGC cultures incubated with various treatments was measured at the indicated time points. All ELISAs were purchased from R&D Systems (Abingdon, England) and used according to the manufacturer's instructions. Values were normalized to tissue weights or untreated EGCs. Briefly, for animal tissue, the isolated ME (∼ 50 mg) was lysed with 1xRIPA buffer for 30 min, centrifuged for 30 min at maximum speed and the protein concentration determined with a BCA kit (Thermo Scientific). 100 µg of total protein was used to measure the release of IL‐6 or CXCL2 in duplicates. For EGCs, cells were treated with the indicated substances for 24 h, supernatant was collected, centrifuged at 5,000 g for 5 min and snap‐frozen in liquid nitrogen before processed for the IL‐6 ELISA.
RNA‐Seq
RNA samples were extracted using the RNeasy Mini Kit (Qiagen). RNA‐Seq libraries were prepared using the QuantSeq 3′ mRNA‐Seq Library Prep Kit (Lexogen) according to the manufacturer's instructions by the Genomics Core facility of the University Hospital Bonn. The RNA samples were prepared using the QuantSeq 3′ mRNA‐Seq Library Prep Kit for Illumina (Lexogen). The method has high strand specificity (>99.9%) and most sequences are generated from the last exon and the 3′ untranslated region. The method generates only one fragment per transcript and the number of reads mapped to a given gene is proportional to its expression. Fewer reads than in classical RNA‐seq methods are needed to determine unambiguous gene expression levels, allowing a high level of multiplexing. Library preparation involved reverse transcription of RNA with oligodT primers, followed by removal of RNA and second strand cDNA synthesis with random primers. The resulting fragments containing both linker fragments were PCR amplified with primers that also contain the Illumina adaptors and sample‐specific barcodes. All libraries were sequenced (single‐end 50 bp) on one lane of the Illumina Hiseq 2500. Only genes with an adjusted P‐value below 0.05 and a minimum fold change greater than 1.5 were considered to be differentially expressed between conditions.
Immunohistochemistry
Whole mount specimens were mechanically prepared by dissection of the (sub)mucosa, fixed in 4% paraformaldehyde/PBS for 30 min, permeabilized with 0.2% Triton X‐100/PBS for 15 min, blocked with 5% donkey serum/PBS for 1 h and incubated with primary IgGs mentioned in Appendix Table S4 at 4°C overnight. After three PBS washing steps, secondary antibodies (Dianova, anti‐rat IgG‐Cy2 1:800, anti‐guinea pig IgG‐Cy3, anti‐chicken IgY‐FITC and anti‐rabbit IgG‐FITC or ‐ Cy3 1:800 were incubated for 90 min. Specimen were mounted in Fluorogel‐Tris and imaged on a Leica confocal imaging system.
Primary cells were fixed in 4% paraformaldehyde/PBS for 30 min, permeabilized with 0.2% Triton X‐100/PBS for 15 min, blocked with 3% BSA/BPS for 1 h and incubated with primary IgGs mentioned in Appendix Table S4 at 4°C overnight.
After three PBS washing steps, secondary antibodies (Dianova, anti‐mouse IgG‐Cy2 1:800, anti‐guinea pig IgG‐FITC and anti‐rabbit IgG‐FITC or ‐ Cy3 1:800 were incubated for 60 min. Specimens were mounted in Fluorogel‐Tris and imaged using a Leica confocal imaging system.
Postoperative ileus mouse model
Postoperative ileus was induced by standardized intestinal manipulation as described previously.(Stoffels et al, 2014) Small bowel was eventrated after median laparotomy and gently rolled twice from oral to aboral using moist cotton swabs. After repositioning of the bowel, the laparotomy wound was closed by a two‐layer suture. Two different approaches for the ambroxol administration were used, according to Migdalska‐Richards et al (2016) animals received ambroxol (4 mM) or vehicle via drinking water starting 24 h before the surgery, until their sacrifice and according to Su et al (2004) animals received i.p. injections (45 mg/kg) shortly after surgery.
In vivo gastrointestinal transit
Gastrointestinal transit (GIT) was assessed by measuring intestinal distribution of orally administered fluorescently labeled dextran‐gavage 90 min after administration as described previously (Stoffels et al, 2014). The gastrointestinal tract was divided into 15 segments (stomach to colon). The geometric center (GC) of labeled dextran distribution was calculated as described previously. The stomach (st) correlates with a GC of 1, the small bowel correlates with a GC of 2–11, the cecum (c) correlates with a GC of 12 and the colon correlates with a GC of 13–15. GIT‐measurement was performed with sham and IM24h animals.
ATP measurement
ATP concentration was measured in lavage samples of naïve and POI mice at IM3h and IM24h with an ATP determination Kit (SIGMA‐Aldrich) according to the manufacturer's instructions.
MTT measurement
MTT signal was measured in EGCs after treating them with ambroxol, PSB1011 and PSB‐0711, (all 20 µM) with a MTT assay Kit (Abcam) according to the manufacturer's instructions.
MPO+‐cell infiltration
Jejunal mucosa‐free ME whole mount specimen were fixed in ethanol and stained with Hanker Yates reagent (Polyscience Europe, Germany) to identify myeloperoxidase expressing cells (MPO+). The mean number of MPO+ cells/mm2 for five random areas per animal was determined. MPO+ measurement was performed with naive animals, 3, 6 and 24 h after IM.
Quantitative PCR
Total RNA was extracted from ME specimens at indicated time points after IM using the RNeasy Mini Kit (Qiagen, Hilden, Germany) followed by deoxyribonuclease I treatment (Ambion, Austin, TX). Complementary DNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany). Expression of mRNA was quantified by real‐time RT‐PCR with TaqMan probes or primers shown in Appendix Table S3.
Quantitative polymerase chain reaction was performed with SYBR Green PCR Master Mix or TaqMan Gene Expression Master Mix (both Applied Biosystems, Darmstadt, Germany).
Fluorescence activated cell sorting
Fluorescence activated cell sorting (FACS) analysis was performed on isolated ME of the small bowel 24 h after intestinal manipulation treated with ambroxol or vehicle CX3CR1‐GFP+/− animals, respectively. Isolation of ME was achieved by sliding small bowel segments onto a glass rod, removing the outer muscularis circumferentially with moist cotton applicators and cutting the ME into fine pieces. ME was digested with a 0.1% collagenase type II (Worthington Biochemical, Lakewood, NJ, USA) enzyme mixture, diluted in HBSS, containing 0.1 mg/ml DNase I (La Roche, Germany), 2.4 mg/ml Dispase II (La Roche, Germany), 1 mg/ml BSA (Applichem), and 0.7 mg/ml trypsin inhibitor (Applichem) for 40 min in a 37°C shaking water bath. Afterward, single cell suspension was obtained using a 70 µm filter mesh. Cells were stained for 30 min at 4°C with the appropriate antibodies. For antibodies used in this study see Appendix Table S4. Flow cytometry analyses were performed on FACSCanto III (BD Biosciences), and data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Animals
Experiments were performed using 8–12‐week‐old mice kept in a pathogen‐free animal facility with standard rodent food and tap water ad libitum. C57/BL/6J, CX3CR1‐GFP+/− or GFAPcre × Ai14floxed mice were used for all experiments and were purchased from Jackson Laboratories directly or bred in our animal facility. All experiments were performed in accordance to the federal law for animal protection and approved by appropriate authorities of North‐Rhine‐Westphalia under the legal terms: 81‐02.04.2018.A344 and 81‐02.04.2016.A367.
Statistical analysis
Statistical analysis was performed with Prism V6.01 (GraphPad, San Diego, CA) using Student t‐test or one‐way ANOVA as indicated. In all Figs, P‐values are indicated as *P < 0.05, **P < 0.01 and ***P < 0.001 when compared to control or # P < 0.05, ## P < 0.01 and ### P < 0.001 when compared to indicated samples. All plots show mean + standard error of mean (SEM).
Experiments were repeated with more samples when the result was close to statistical significance, and sample sizes for animal studies were chosen following previously reported studies that have used the POI animal model, at least 6–10 independent mice per experimental setup. All animals were handled by standardized housing procedures and kept in exactly the same environmental conditions and were genotyped at 4 weeks of age and received a randomized number by which they were identified. Age‐ and sex‐matched animals were grouped randomly and used in the POI animal model. In each experimental set, all the control or experimental mice were treated with the same procedure and manipulation. For the experimental setup with ambroxol, the treatment of mice was blinded, and all mice were randomly caged. The analysis was performed on the treatment groups without knowing what group was treated with ambroxol. By this, we avoided any group or genotype‐specific effects due to timing of experiments or handling of animals.
Author contributions
RS, PL, AM, ML, IK, BS, CS, IG, EM, and EV‐H performed research. RS, FLC, CEM, and SW analyzed data. RS, JCK, FLC, and SW prepared and revised the manuscript. RS, CEM, FLC, and SW designed the research. YB, CS, and CEM produced the P2X2‐specific antagonists. T.G. organized the patient material.
Conflict of interest
SW and JCK receive royalties from Wolter Kluwer for contribution to the postoperative ileus section of the UpToDate library. All other authors declare no competing interests.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Source Data for Expanded View
Review Process File
Acknowledgements
The authors thank the Next Generation Sequencing Core Facility and the Institute for Genomic Statistics and Bioinformatics of the University Clinics Bonn for supporting the RNA‐Seq analysis. The authors thank the Flow Cytometry Core Facility of the University Clinics Bonn for supporting the isolation of tdTomato+ EGCs. The sniffer cells (HEK mixed clone 228) were a gift from Dr. Terrance Egan, Saint Louis University to Fievos L. Christofi, The Ohio State University. The gpSox10 antibody was a kind gift of Professor Wegner, University of Erlangen. NIDDKNIHR01DK113943 to Dr. Fievos L. Christofi, an NCI Cost shared resource grant P30LA16058 for the molecular core facility in the College of Medicine, The Ohio State University. This publication was supported by a personnel grant of the German research council (DFG) to SW (WE4204/3‐1), BonnNI (Q‐611.0754), and the ImmunoSensation2 Cluster of Excellence (EXC 2151–390873048).
EMBO Mol Med (2021) 13: e12724.
Data availability
All RNA‐seq data have been submitted to the GEO database. The datasets produced in this study are available in the following databases: RNA‐Seq: mRNA from EGC and ME tissue in GSE134943 accessible here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE134943) and RNA‐Seq data from mRNA of ME tissue from patients in GSE149181 accessible here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE149181; other data are available in the Expanded View Datasets or the Appendix Tables. The software tools used for this study include Partek Flow, available from https://www.partek.com/partek‐flow/#features; Venn Diagramm Software, available from http://bioinformatics.psb.ugent.be/webtools/Venn/; and Gene Set Enrichment Analysis, available from https://www.partek.com/partek‐flow/#features.
References
- Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche J‐P, Vanden Berghe P, Neunlist M, Lardeux B (2010) Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J 24: 1082–1094 [DOI] [PubMed] [Google Scholar]
- Andersson HC, Anderson MF, Porritt MJ, Nodin C, Blomstrand F, Nilsson M (2011) Trauma‐induced reactive gliosis is reduced after treatment with octanol and carbenoxolone. Neurol Res 33: 614–624 [DOI] [PubMed] [Google Scholar]
- Aubé A‐C, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M (2006) Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55: 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqi Y, Hausmann R, Rosefort C, Rettinger J, Schmalzing G, Müller CE (2011) Discovery of potent competitive antagonists and positive modulators of the P2X2 receptor. J Med Chem 54: 817–830 [DOI] [PubMed] [Google Scholar]
- Beeh KM, Beier J, Esperester A, Paul LD (2008) Antiinflammatory properties of ambroxol. Eur J Med Res 13: 557–562 [PubMed] [Google Scholar]
- Belkind‐Gerson J, Graham HK, Reynolds J, Hotta R, Nagy N, Cheng L, Kamionek M, Shi HN, Aherne CM, Goldstein AM (2017) Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci Rep 7: 2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, Akbarali HI (2017) Connexin‐purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J 31: 2649–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesmans W, Cirillo C, van den Abbeel V, van den Haute C, Depoortere I, Tack J, Vanden Berghe P (2013) Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25: e151–e160 [DOI] [PubMed] [Google Scholar]
- Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V (2015) Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63: 229–241 [DOI] [PubMed] [Google Scholar]
- Boesmans W, Hao MM, Fung C, Li Z, van den Haute C, Tack J, Pachnis V, Vanden Berghe P (2019) Structurally defined signaling in neuro‐glia units in the enteric nervous system. Glia 67: 1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Linden DR (2014) Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 11: 611–627 [DOI] [PubMed] [Google Scholar]
- Brown IAM, McClain JL, Watson RE, Patel BA, Gulbransen BD (2016) Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin‐43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn A‐P, Mori T, Götz M (2008) Origin and progeny of reactive gliosis: asource of multipotent cells in the injured brain. Proc Natl Acad Sci USA 105: 3581–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Jacobson KA, Christofi FL (2017) Purinergic drug targets for gastrointestinal disorders. Curr Opin Pharmacol 37: 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G (2020) Introduction to purinergic signaling. Methods Mol Biol 2041: 1–15 [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV (1998) Fulminant jejuno‐ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201 [DOI] [PubMed] [Google Scholar]
- Canellada A, Ramirez BG, Minami T, Redondo JM, Cano E (2008) Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1‐4 and cyclooxygenase‐2 as NFAT target genes. Glia 56: 709–722 [DOI] [PubMed] [Google Scholar]
- Carta S, Penco F, Lavieri R, Martini A, Dinarello CA, Gattorno M, Rubartelli A (2015) Cell stress increases ATP release in NLRP3 inflammasome‐mediated autoinflammatory diseases, resulting in cytokine imbalance. Proc Natl Acad Sci USA 112: 2835–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P (2014) Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis 5: e1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL (2008) Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal 4: 213–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M (2014) Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci Lett 565: 59–64 [DOI] [PubMed] [Google Scholar]
- Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS (2001) Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA 98: 13306–13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csóka B, Németh ZH, Törő G, Idzko M, Zech A, Koscsó B, Spolarics Z, Antonioli L, Cseri K, Erdélyi K et al (2015) Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J 29: 3626–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 Receptor in Infection and Inflammation. Immunity 47: 15–31 [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Sarti AC, Falzoni S, de Marchi E, Adinolfi E (2018) Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18: 601–618 [DOI] [PubMed] [Google Scholar]
- Esposito G, Capoccia E, Gigli S, Pesce M, Bruzzese E, D'Alessandro A, Cirillo C, Di Cerbo A, Cuomo R, Seguella L et al (2017) HIV‐1 Tat‐induced diarrhea evokes an enteric glia‐dependent neuroinflammatory response in the central nervous system. Sci Rep 7: 7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farro G, Stakenborg M, Gomez‐Pinilla PJ, Labeeuw E, Goverse G, Di Giovangiulio M, Stakenborg N, Meroni E, D'Errico F, Elkrim Y et al (2017) CCR2‐dependent monocyte‐derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut 66: 2098–2109 [DOI] [PubMed] [Google Scholar]
- Fujita A, Yamaguchi H, Yamasaki R, Cui Y, Matsuoka Y, Yamada K‐I, Kira J‐I (2018) Connexin 30 deficiency attenuates A2 astrocyte responses and induces severe neurodegeneration in a 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine hydrochloride Parkinson's disease animal model. J Neuroinflammation 15: 227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294 [DOI] [PubMed] [Google Scholar]
- Galligan JJ (2008) Purinergic signaling in the gastrointestinal tract. Purinergic Signal 4: 195–196 [Google Scholar]
- Germouty J, Jirou‐Najou JL (1987) Clinical efficacy of ambroxol in the treatment of bronchial stasis. Clinical trial in 120 patients at two different doses. Respiration 51(Suppl 1): 37–41 [DOI] [PubMed] [Google Scholar]
- Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Vanden Berghe P (2009) ATP‐dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil 21: 870.e62 [DOI] [PubMed] [Google Scholar]
- Grubišić V, Gulbransen BD (2017) Enteric glia: the most alimentary of all glia. J Physiol (Lond ) 595: 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubišić V, Perez‐Medina AL, Fried DE, Sevigny J, Robson SC, Galligan JJ, Gulbransen BD (2019) NTPDase1 and ‐2 are expressed by distinct cellular compartments in the mouse colon and differentially impact colonic physiology and function after DSS‐colitis. Am J Physiol Gastrointest Liver Physiol 317): G314–G332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubišić V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E, Ajijola OA, Watson RE, Poole DP, Robson SC et al (2020) Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep 32: 108100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA (2009) Purinergic neuron‐to‐glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358 [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM et al (2012) Activation of neuronal P2X7 receptor‐pannexin‐1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Christofi FL (2018) Are we close to targeting enteric glia in gastrointestinal diseases and motility disorders? Gastroenterology 155: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama AT, Plum AW, Sagen J (2010) Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol Biochem Behav 97: 249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y et al (2017) Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin‐N‐cadherin pathway after spinal cord injury. Nat Med 23: 818–828 [DOI] [PubMed] [Google Scholar]
- Hedrick TL, McEvoy MD, Mythen MMG, Bergamaschi R, Gupta R, Holubar SD, Senagore AJ, Gan TJ, Shaw AD, Thacker JKM et al (2018) American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on postoperative gastrointestinal dysfunction within an enhanced recovery pathway for elective colorectal surgery. Anesth Analg 126: 1896–1907 [DOI] [PubMed] [Google Scholar]
- Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509: 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Saunders WB, Stemkowski S (2009) Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm 15: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W‐S, Peterson TC et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liñán‐Rico A, Turco F, Ochoa‐Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel‐Rasoul M, Fadda P, Grants I, Whitaker E et al (2016) Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm Bowel Dis 22: 1812–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne‐Bar I, Lam S, Chan D, Guo X, Askar I, Nahirnyj A, Flanagan JG, Sivak JM (2016) Pharmacologic inhibition of reactive gliosis blocks TNF‐α‐mediated neuronal apoptosis. Cell Death Dis 7: e2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes J, Gegg ME, Migdalska‐Richards A, Schapira AH (2018) Effects of ambroxol on the autophagy‐lysosome pathway and mitochondria in primary cortical neurons. Sci Rep 8: 1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Davila‐Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X et al (2019) Single‐cell transcriptomic analysis of Alzheimer's disease. Nature 570: 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain J, Grubišić V, Fried D, Gomez‐Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD (2014) Ca2+ responses in enteric glia are mediated by connexin‐43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalska‐Richards A, Daly L, Bezard E, Schapira AHV (2016) Ambroxol effects in glucocerebrosidase and α‐synuclein transgenic mice. Ann Neurol 80: 766–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa‐Cortes F, Turco F, Linan‐Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL (2016) Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis 22: 433–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira S, López‐Muñoz A, Candel S, Pelegrín P, Calado Â, Mulero V (2014) ATP modulates acute inflammation in vivo through dual oxidase 1‐derived H2O2 production and NF‐κB activation. J Immunol 192: 5710–5719 [DOI] [PubMed] [Google Scholar]
- Pacheco‐Pantoja EL, Dillon JP, Wilson PJM, Fraser WD, Gallagher JA (2016) c‐Fos induction by gut hormones and extracellular ATP in osteoblastic‐like cell lines. Purinergic Signal 12: 647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94: 1077–1098 [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M (2016) Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 1862: 483–491 [DOI] [PubMed] [Google Scholar]
- Rakers C, Schleif M, Blank N, Matušková H, Ulas T, Händler K, Torres SV, Schumacher T, Tai K, Schultze JL et al (2019) Stroke target identification guided by astrocyte transcriptome analysis. Glia 67: 619–633 [DOI] [PubMed] [Google Scholar]
- Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G (2017) Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153: 1068–1081.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M (2020) An increasingly complex view of intestinal motility. Nat Rev Gastroenterol Hepatol 17: 72–73 [DOI] [PubMed] [Google Scholar]
- Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I (2012) ATP release and purinergic signaling: a common pathway for particle‐mediated inflammasome activation. Cell Death Dis 3: e403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum C, Schick MA, Wollborn J, Heider A, Scholz C‐J, Cecil A, Niesler B, Hirrlinger J, Walles H, Metzger M (2016) Activation of myenteric glia during acute inflammation in vitro and in vivo . PLoS One 11: e0151335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury G, Ryou M‐G, Poteet E, Wen Y, He R, Sun F, Yuan F, Jin K, Yang S‐H (2014) Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke. Brain Res 1551: 45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D, Vistnes S, Stockley JH, Young A, Steindel M et al (2019) Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573: 75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA (2015) Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest 125: 918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira CRA, MacKinley J, Coleman K, Li Z, Finger E, Bartha R, Morrow SA, Wells J, Borrie M, Tirona RG et al (2019) Ambroxol as a novel disease‐modifying treatment for Parkinson's disease dementia: protocol for a single‐centre, randomized, double‐blind, placebo‐controlled trial. BMC Neurol 19: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K, Lysson M, Schumak B, Vilz T, Specht S, Heesemann J, Roers A, Kalff JC, Wehner S (2018) Leukocyte‐derived interleukin‐10 aggravates postoperative ileus. Front Immunol 9: 2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels B, Hupa KJ, Snoek SA, van Bree S, Stein K, Schwandt T, Vilz TO, Lysson M, Veer CV’t, Kummer MP et al (2014) Postoperative ileus involves interleukin‐1 receptor signaling in enteric glia. Gastroenterology 146: 176–187.e1 [DOI] [PubMed] [Google Scholar]
- Su X, Wang L, Song Y, Bai C (2004) Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med 30: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco F, Sarnelli G, Cirillo C, Palumbo I, de Giorgi F, D'Alessandro A, Cammarota M, Giuliano M, Cuomo R (2014) Enteroglial‐derived S100B protein integrates bacteria‐induced Toll‐like receptor signalling in human enteric glial cells. Gut 63: 105–115 [DOI] [PubMed] [Google Scholar]
- Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T (2019) Inflammatory bowel disease increases the risk of Parkinson's disease: a Danish nationwide cohort study 1977–2014. Gut 68: 18–24 [DOI] [PubMed] [Google Scholar]
- Wehner S, Schwarz NT, Hundsdoerfer R, Hierholzer C, Tweardy DJ, Billiar TR, Bauer AJ, Kalff JC (2005) Induction of IL‐6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery 137: 436–446 [DOI] [PubMed] [Google Scholar]
- Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, Kalff JC (2007) Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner S, Straesser S, Vilz TO, Pantelis D, Sielecki T, La Cruz VF, de la Cruz VF, Hirner A, Kalff JC (2009) Inhibition of p38 mitogen‐activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology 136: 619–629 [DOI] [PubMed] [Google Scholar]
- Weiser T (2008) Ambroxol: a CNS drug? CNS Neurosci Ther 14: 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BB, Mazmanian SK (2017) The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 46: 910–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang S, Yu L, Xu X, Qiu Z (2020) The role of ATP in cough hypersensitivity syndrome: new targets for treatment. J Thorac Dis 12: 2781–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Source Data for Expanded View
Review Process File
Data Availability Statement
All RNA‐seq data have been submitted to the GEO database. The datasets produced in this study are available in the following databases: RNA‐Seq: mRNA from EGC and ME tissue in GSE134943 accessible here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE134943) and RNA‐Seq data from mRNA of ME tissue from patients in GSE149181 accessible here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE149181; other data are available in the Expanded View Datasets or the Appendix Tables. The software tools used for this study include Partek Flow, available from https://www.partek.com/partek‐flow/#features; Venn Diagramm Software, available from http://bioinformatics.psb.ugent.be/webtools/Venn/; and Gene Set Enrichment Analysis, available from https://www.partek.com/partek‐flow/#features.


