Summary
IFN-Is are crucial mediators of antiviral immunity and homeostatic immune system regulation. However, the source of IFN-I signaling under homeostatic conditions is unclear. We discovered that commensal microbes regulate the IFN-I response through induction of IFNβ by colonic DCs. Moreover, the mechanism by which a specific commensal microbe induces IFNβ was identified. OM-associated glycolipids of gut commensal microbes belonging to the Bacteroidetes phylum induce expression of IFNβ. Using Bacteroides fragilis and its OM-associated polysaccharide A, we determined that IFNβ expression was induced via TLR4-TRIF signaling. Antiviral activity of this purified microbial molecule against infection with either VSV or influenza was demonstrated to be dependent on the induction of IFNβ. In a murine VSV infection model, commensal-induced IFNβ regulated natural resistance to virus infection. Due to the physiological importance of IFN-Is, discovery of an IFNβ- inducing microbial molecule represents a potential approach for the treatment of some human diseases.
Introduction
The human body is colonized by trillions of microbial organisms collectively referred to as the commensal microbiota.(Erturk-Hasdemir and Kasper, 2013; Human Microbiome Project, 2012) With advances in culture-independent DNA sequencing technologies and bioinformatics, there has been a rapid increase in our knowledge of the diversity and dynamics of the microbiota over the past two decades.(Human Microbiome Project, 2012; Qin et al., 2010) Current estimates suggest that there are roughly as many bacterial cells as human cells in the body, the vast majority of which reside in the lower gastrointestinal (GI) tract, which is colonized by an estimated 1014 bacteria.(Qin et al., 2010; Savage, 1977; Sender et al., 2016; Turnbaugh et al., 2007) Despite these advances, relatively little is understood about the molecular mechanisms by which specific commensal microbes interact with their mammalian host. A growing body of research has revealed that commensal microbes are not just passive residents, but they play an important role in modulating vital host physiological systems, both locally and systemically.(Lin et al., 2014; Surana and Kasper, 2014) Understanding how microbes interact with their host is therefore not only biologically interesting, but it provides a platform for discovering potential therapeutic targets for a variety of human diseases.
One mechanism by which microbes can modulate the host immune system is through regulation of cytokine signaling. Type I interferons (IFN-Is) are a family of structurally similar cytokines, consisting primarily of two different classes of proteins, interferon-α (IFNα) and interferon-β (IFN-β), all of which signal through the common type I interferon receptor (IFNAR).(Theofilopoulos et al., 2005) IFN-I expression is regulated at the transcriptional level, and can be induced at high levels in all nucleated cells in response to sensing of pathogens by pattern recognition receptors (PRRs).(Ivashkiv and Donlin, 2014) Indeed, IFN-Is play a critical role in the response to the majority of virus infections through induction of a restrictive anti-viral state in cells, induction of apoptosis in infected cells, and regulation of immune cell subsets crucial to the antiviral response.(McNab et al., 2015; Muller et al., 1994; Stetson and Medzhitov, 2006; Yan and Chen, 2012) Several recent studies have demonstrated that IFN-Is are not just present during infection, but are expressed constitutively at low-levels and possess vital homeostatic functions.(Gough et al., 2012; Kole et al., 2013; Schaupp et al., 2020) Indeed, IFN-Is have the capacity to regulate the development or function of virtually every immune effector cell, contributing to the anti-inflammatory and anti-tumor responses.(Gough et al., 2012) Despite this importance, the mechanism by which IFN-Is are induced and regulated in the absence of infection has yet to be elucidated.
Due to the importance of IFN-Is to the health of the organism, it is of critical importance to gain a mechanistic understanding of how the constitutive IFN-I response is regulated in healthy individuals and might be disrupted leading to disease. We hypothesized that the commensal microbiota supplies PRR signaling to regulate the IFN-I response in the absence of infection. In this study, we discovered that the commensal microbiota regulates the IFN-I response both locally and systemically through induction of IFNβ by colonic lamina propria (LP) dendritic cells (DCs), enhancing resistance to virus infection. Moreover, the molecular mechanism by which a specific commensal microbe induces IFNβ was identified. Indeed, we found that outer membrane-associated glycolipids of human gut commensal microbes belonging to the Bacteroidetes phylum induce expression of IFNβ. Using Bacteroides fragilis (B. fragilis) as the paradigm, we determined that signaling to initiate IFNβ expression was through the toll-like receptor 4 (TLR4)-TIR-domain-containing adapter-inducing interferon-β (TRIF) pathway.
Results
The commensal microbiota regulates host ISG expression through IFNβ
To investigate the role of the commensal microbiota in the host IFN-I response, the effect of microbiota depletion on downstream interferon stimulated gene (ISG) expression in various murine tissues was analyzed. The vast majority of commensal microbes reside in the GI tract.(Savage, 1977; Turnbaugh et al., 2007) Analysis was therefore performed to determine effects of the microbiota on ISG expression in the intestinal immune compartment, in the mesenteric lymph nodes (mLN), and systemically, in the spleen. Age- and gender-matched wild type (WT) specific pathogen free (SPF) C57BL/6 mice were administered either vehicle control or a cocktail of broad-spectrum antibiotics (ABX) in the drinking water for 7 days to deplete the microbiota. The relative expression levels of a panel of ISGs were analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). A reduction in ISG expression was observed in both the mLN and spleen of WT ABX mice compared to WT SPF mice (Figures 1A–D, Tables S1–S2), demonstrating a role for microbiota regulation of the IFN-I response both locally and systemically.
Figure 1. Microbiota depletion reduces local and systemic ISG expression.
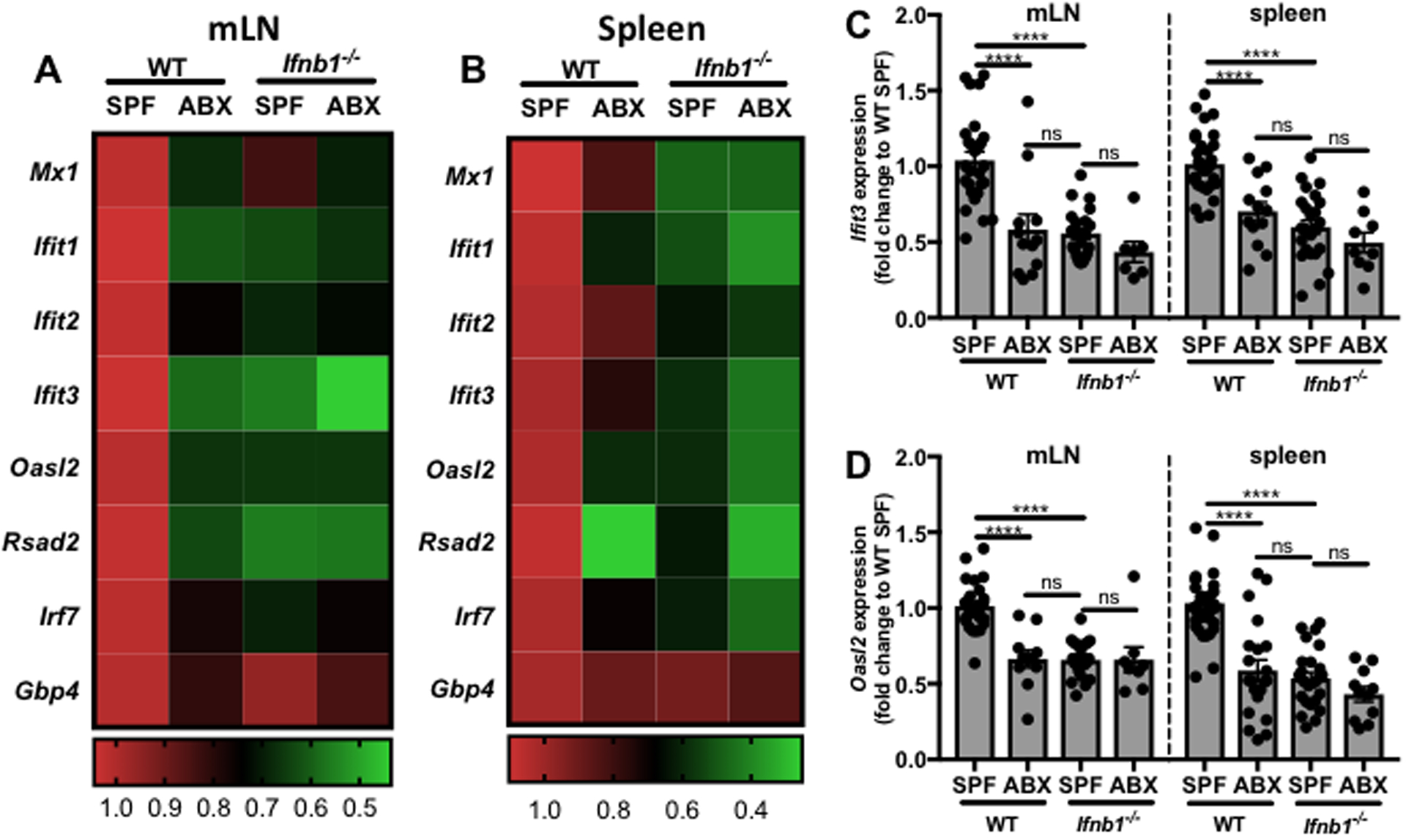
mLNs and spleens were harvested from WT or Ifnb1−/− SPF mice with and without broad-spectrum antibiotics (ABX) treatment. RNA was isolated from whole tissue samples followed by qRT-PCR to analyze ISG expression levels. Fold change gene expression in the mLN (A,C-D) or spleen (B,C-D) was calculated compared to WT SPF mice using the ΔΔCT method, with ActB as the reference gene and depicted as (A,B) a heat map of all ISGs tested or representative bar graphs of mean +/− SEM with each point representing one mouse for (C) Ifit3 and (D) Oasl2. One-way ANOVA statistical analysis followed by Tukey’s multiple comparisons test. Details of statistical analyses can be found in Tables S1–2. ns=not significant, ****p<0.0001.
Due to the potential for off-target effects of antibiotics, a second method of microbiota depletion was used to confirm a direct role of the commensal microbiota on the observed phenotype.(Morgun et al., 2015) Splenic and mLN ISG expression was analyzed in WT C57BL/6 germ free (GF) mice devoid of all microbes compared to WT C57BL/6 SPF mice. Consistent with the ABX mice, a reduction in mLN and splenic ISG expression was observed (Figures S1A–B, Tables S1–2).
Of the mammalian IFN-Is, IFNβ is unique with respect to its homeostatic functions and expression pattern.(Schreiber and Piehler, 2015) It was therefore hypothesized that the commensal microbiota induces the constitutive IFN-I response, as evidenced by ISG expression, specifically through regulation of IFNβ. ISG expression levels were compared by qRT-PCR between WT and IFNβ deficient (Ifnb1−/−) SPF mice. A significant reduction in ISG expression was observed in the mLN and spleen of Ifnb1−/− mice compared to WT mice (Figures 1A–D, Tables S1–2), confirming a role for IFNβ in the homeostatic IFN-I response. Interestingly, the magnitude of reduction was comparable to that observed upon microbiota depletion, with no significant difference between WT ABX and Ifnb1−/− SPF expression levels. To further test whether commensal-induced IFNβ is required for ISG expression, the microbiota of Ifnb1−/− mice was depleted by ABX administration. ABX treatment had no additional effect on mLN or splenic ISG expression in Ifnb1−/− mice (Figures 1A–D, Tables S1–2), demonstrating that commensal microbes regulate the IFN-I response specifically through IFNβ.
IFNβ is expressed by colonic dendritic cells
To investigate the source of commensal-induced IFNβ under homeostatic conditions, flow cytometric analysis was performed on tissues harvested from IFNβ-yellow fluorescent protein (YFP) reporter mice, in which cells activated to express IFNβ express YFP.(Scheu et al., 2008) By analyzing IFNβ-YFP expression in the colon LP, mLN, and spleen, IFNβ expression was found to be localized to the intestinal milieu, with significantly higher percentages of IFNβ-YFP+CD45+ leukocytes in the colon LP compared to the spleen or mLN (Figures 2A, Figure S2). The colon was chosen as a representative region of the GI tract because it is home to the greatest percentage of commensal organisms, both in terms of number and abundance.(Turnbaugh et al., 2007) Having identified the location of IFNβ expression, the cellular source of this cytokine was next investigated. Due to their role as sentinel cells in the immune system, dendritic cells (DCs) were hypothesized to be the main source of IFNβ under homeostatic conditions. It was found that out of the IFNβ-YFP+CD45+ cells in the colonic LP, the majority (~74%) expressed CD11c, a molecule expressed on the surface of DCs and commonly used as a DC marker (Figure 2B).(Singh-Jasuja et al., 2013) These findings demonstrate that colon LP DCs are a major source of IFNβ under homeostatic conditions.
Figure 2. The commensal microbiota regulates IFNβ expression by dendritic cells in the colon LP.
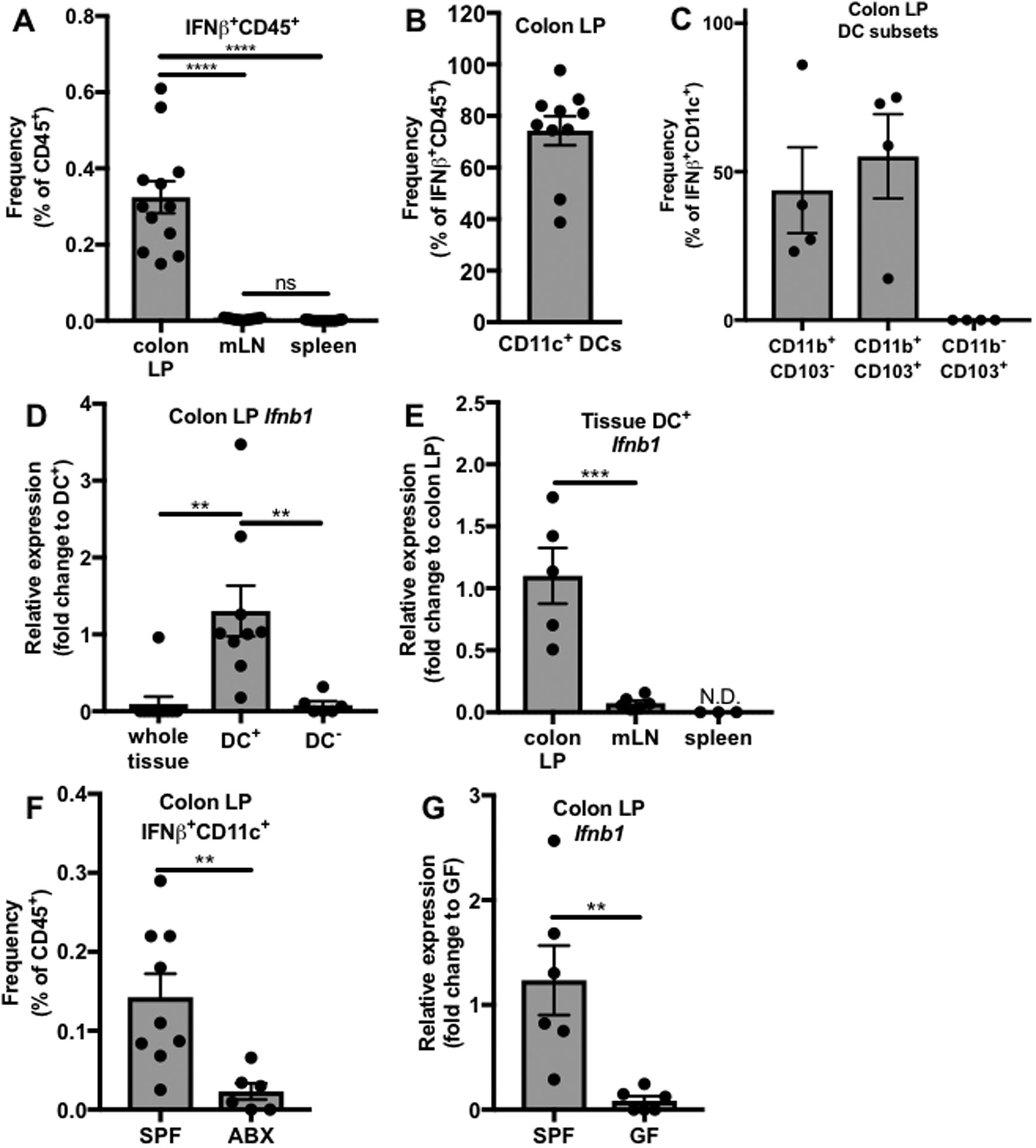
(A-C) Single cell suspensions were prepared from spleens, mLNs, and colon LP of SPF IFNβ-YFP reporter mice and analyzed by flow cytometry. (A) Frequency of IFNβ-YFP+ cells out of CD45+ cells (colon LP N=12, mLN N=13, spleen N=13). (B) Frequency of CD11c+ DCs out of total IFNβ-YFP+CD45+ cells in the colon LP (N=10). (C) Frequency of CD11b+CD103−, CD11b+CD103+, and CD11b−CD103+ DC subsets out of IFNβ-YFP+CD11c+ colonic LP dendritic cells. (D-E) DCs were isolated from single cell suspensions of different tissues from WT GF and WT SPF mice, yielding DC+ and DC− fractions, and Ifnb1 expression was analyzed by qRT-PCR. Relative expression of Ifnb1 in (D) the whole tissue (N=10), DC+ (N=9), and DC− (N=6) fractions of the colon LP or (E) the DC+ fraction of the colon LP (N=5), mLN (N=6), or spleen (N=3). (F) Flow cytometric analysis of frequency of colon LP IFNβ-YFP+CD11c+ cells out of CD45+ cells (ABX N=7, SPF N=10) in SPF or ABX treated IFNβ-YFP reporter mice. (G) qRT-PCR analysis of Ifnb1 expression in colon LP DC+ cells from WT SPF (N=6) or WT GF mice (N=6). (D-E,G) Fold change gene expression was calculated using the ΔΔCT method, with Actb as the reference gene, compared to the (D) DC+, (E) colon LP, or (G) SPF samples. Bars represent mean +/− SEM. Statistical analysis with (A,D,E) one-way ANOVA followed by Tukey’s multiple comparisons test and (F,G) unpaired t-test. N= number of mice, N.D.=not detected, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant.
As in other tissues, conventional DCs (cDCs) in the colon LP represent a heterogeneous population that can be classified into two subsets based on surface marker expression. cDC1 cells are CD103+CD11b− while cDC2 cells comprise both CD103−CD11b+ and CD103+CD11b+ cells.(Stagg, 2018) To determine which DCs express IFNβ, expression of CD103 and CD11b by the IFNβ-YFP+CD11c+CD45+ colonic dendritic cells was analyzed using flow cytometry. IFNβ expression was specific to the cDC2 subset of cells, including both CD103+CD11b+ and CD103−CD11b+ cells (Figure 2C).
To confirm these findings, a second experimental approach was used in which DCs were isolated from the colon LP, mLN, and spleens of WT SPF mice using a magnetic bead-based cell separation method. Expression of Ifnb1 was analyzed in the whole tissue single cell suspension as well as both the DC+ and DC− fractions of these tissues by qRT-PCR. There was no detectable Ifnb1 expression in the whole tissue or the DC− fraction of the majority of colon LP samples (Figure 2D) or in the mLN (Figure S3A) and spleen (Figure S3B). However, DCs in both the colon LP (Figure 2D–E) and the mLN (Figure 2E, Figure S3A) but not the spleen (Figure 2F, Figure S3B) expressed Ifnb1. Interestingly, DC expression of Ifnb1 is significantly higher in the colon LP than in the mLN (Figure 2E). These findings confirm that IFNβ is predominantly expressed by colon LP DCs.
To test the role of the microbiota in colonic DC IFNβ expression, two methods of microbiota depletion were employed. ABX were administered in the drinking water of IFNβ-YFP mice for 7 days, followed by flow cytometric analysis of colon LP cells. A significant reduction in the frequency of IFNβ-YFP+CD11c+ cells was observed in IFNβ-YFP mice after ABX treatment (Figure 2F). In addition, Ifnb1 transcript levels in colon LP DCs of WT GF mice were analyzed by qRT-PCR. A significant reduction in Ifnb1 expression in WT GF mice compared to WT SPF mice was observed (Figure 2G). These results confirm that the microbiota induces IFNβ expression by colon LP DCs
Having identified effects of commensal-induced IFNβ on ISG expression both locally and systemically, we investigated IFNβ levels in systemic circulation, through measurement of serum IFNβ levels using an enzyme-linked immunosorbent assay (ELISA). Consistent with reports that IFN-I expression is extremely low under homeostatic conditions, we were unable to detect IFNβ in the serum of WT SPF or ABX mice (Figure S3C).
B. fragilis induces expression of IFNβ by colon LP DCs
Of the trillions of commensal bacteria that inhabit the mammalian gut, one of the most prominent phyla represented is the Bacteroidetes, consisting of obligate anaerobic, Gram-negative rods. An estimated 25% of all colonic anaerobes belong to the genus Bacteroides within this phylum.(Salyers, 1984; Wexler, 2007) Recently an association was reported between several species of the genus Bacteroides, pDC numbers, and an IFN-I response gene signature.(Geva-Zatorsky et al., 2017) One of the Bacteroides sp. investigated in this study was B. fragilis, a common human colonic commensal bacterial species with previously demonstrated immunomodulatory properties.(An et al., 2014; Dasgupta et al., 2014; Mazmanian et al., 2005; Mazmanian et al., 2008) Due to its demonstrated ability to influence the immune system, as well as the association with pDCs and IFN-I, we hypothesized that B. fragilis regulates colonic DC IFNβ expression and thus regulates the IFN-I response.
GF mice were colonized with B. fragilis strain NCTC 9343 at 4 weeks of age, followed by isolation of colon LP DCs after 2 weeks and analysis of Ifnb1 expression. As a colonization control, GF mice were colonized with a different bacterial species with demonstrated immunomodulatory properties, Clostridium ramosum (C. ramosum), thus controlling for any non-specific effects due to bacterial colonization.(Geva-Zatorsky et al., 2017; Sefik et al., 2015) Colonization with B. fragilis significantly enhanced Ifnb1 expression in colon LP DCs, while C. ramosum colonization had no effect (Figure 3A). While B. fragilis predominantly colonizes the lower intestinal tract, it is possible that it might also regulate Ifnb1 expression in other parts of the intestine. We therefore also examined Ifnb1 expression by DCs isolated from the small intestine (SI) LP of B. fragilis monocolonized mice. In contrast to the colon, colonization with B. fragilis had no observable effect on SI LP DC Ifnb1 expression (Figure S4), demonstrating that regulation of DC IFNβ by this commensal organism is location specific.
Figure 3. B. fragilis PSA induces IFNβ in vitro and in vivo.
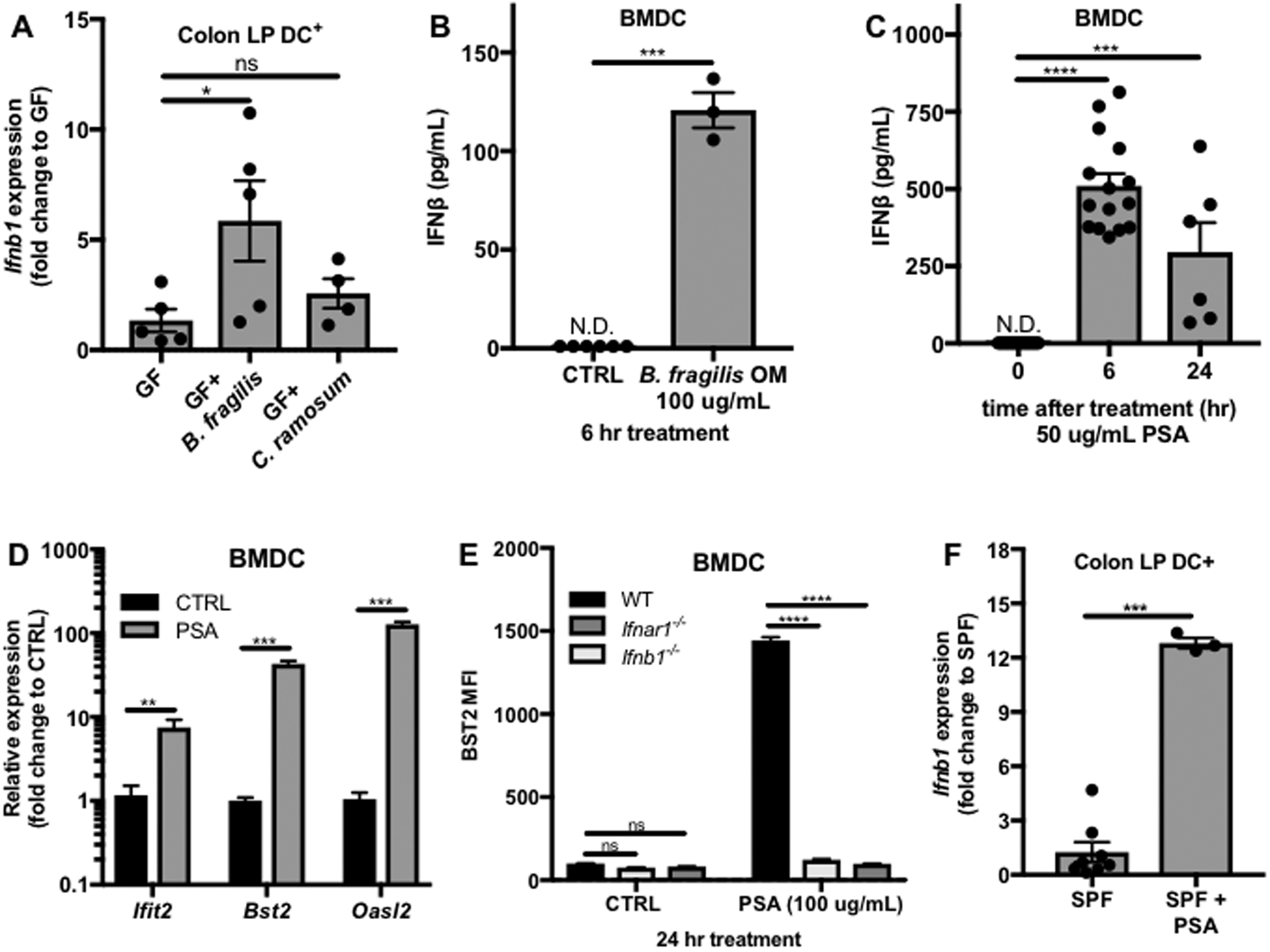
(A) WT GF mice were gavaged with vehicle (GF, N=5) or colonized at 4 weeks of age with B. fragilis (N=5) or C. ramosum (N=4). Two weeks post colonization, DCs were isolated from colon LP single cell suspensions, RNA was isolated, and Ifnb1 expression was analyzed by qRT-PCR. Fold change gene expression to GF was calculated using the ΔΔCT method with ActB as a reference gene. (B-E) BMDCs were cultured from WT, Ifnb1−/−, or Ifnar1−/− mice. (B) IFNβ levels in the supernatants of WT BMDCs treated with vehicle control (CTRL, N=6) or 100 ug/mL B. fragilis OM extract (N=3). (C) ELISA analysis of IFNβ levels in the supernatants of WT BMDCs treated with 50 ug/mL PSA for 0 (N=15), 6 (N=15), or 24 hrs (N=6). (D) RNA was isolated from WT BMDCs 24 hrs post treatment with vehicle control (CTRL, N=4) or 50 ug/mL PSA (N=3) and ISG expression was analyzed by qRT-PCR. Fold change gene expression to CTRL was calculated using the ΔΔCT method with Actb as the reference gene. (E) After 24 hrs of treatment with 100 ug/mL PSA, BST2 mean fluorescence intensity (MFI) gated on live CD11c+ cells was measured by flow cytometry (N=4 for each condition). (F) WT SPF mice were gavaged with 150 ug PSA and after 1.5 hrs Ifnb1 expression by colon LP DCs was analyzed by qRT-PCR (SPF, N=8; SPF+PSA, N=3). Fold change gene expression to SPF was calculated using the ΔΔCT method with Actb as the reference gene. Data represents average +/− SEM. Statistical analysis with (A,C) one-way ANOVA followed by Dunnett’s multiple comparisons test, (B,D,F) unpaired t-test and (E) Two-way ANOVA followed by Sidak’s multiple comparisons test to WT. N.D.= not detected, ns=not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
B. fragilis capsular polysaccharide A induces IFNβ expression
The outer membrane (OM) of Gram-negative bacteria, serves as the site of interaction between the bacterial cell and its environment and comprises several classes of potential immunomodulatory molecules.(Kasper and Seiler, 1975; May and Grabowicz, 2018) To test whether an OM component is responsible for IFNβ expression, an in vitro model was developed in which bone marrow-derived dendritic cells (BMDCs) isolated from WT mice were treated with OM complexes isolated from B. fragilis, followed by ELISA analysis of secreted IFNβ in the supernatants. B. fragilis OM complexes significantly induced IFNβ secretion by BMDCs (Figure 3B). Capsular polysaccharides can be attached to the OM through covalent linkage to lipid anchors, which are inserted into the OM lipid bilayer.(Cress et al., 2014) One capsular polysaccharide of B. fragilis, polysaccharide A (PSA) has been demonstrated to have immunomodulatory properties.(Dasgupta et al., 2014; Mazmanian et al., 2005; Mazmanian et al., 2008) To test whether PSA is an IFNβ-inducing component of the B. fragilis OM, BMDCs were treated with purified PSA and secretion of IFNβ in the supernatants was analyzed by ELISA. Indeed, PSA significantly induced IFNβ secretion by BMDCs (Figure 3C).
To confirm the ability of PSA to induce a productive IFN-I response through IFNβ, expression of downstream ISGs was analyzed by qRT-PCR in BMDCS treated for 24 hrs with PSA or vehicle control. An increase in ISG expression was observed in WT BMDCs treated with PSA (Figure 3D). Expression levels of the surface-expressed ISG, bone marrow stromal cell antigen 2 (BST2), were determined by flow cytometric analysis of mean fluorescence intensity (MFI) and also found to be increased in PSA-treated compared to vehicle control WT BMDCs (Figure 3E). Surface BST2 levels after PSA treatment were significantly reduced in IFNAR1 deficient (Ifnar1−/−) and Ifnb1−/− BMDCs, confirming that signaling of IFNβ through IFNAR1 is necessary for the observed increase in levels of a representative ISG by PSA. Together, these data demonstrate that PSA induces secretion of IFNβ and downstream ISG expression in vitro by BMDCs.
To confirm the relevance of these findings in vivo, Ifnb1 expression by colon LP DCs was analyzed in WT SPF mice administered 150 ug of PSA by oral gavage. A significant increase in Ifnb1 expression was observed 1.5 hrs after treatment (Figure 3F), suggesting that PSA is capable of signaling to colon LP DCs in vivo to induce Ifnb1 expression.
B. fragilis lipooligosaccharide signals through TLR4 to induce IFNβ
PSA has been previously demonstrated to signal via TLR2/1 heterodimers and dectin-1 on DCs to induce secretion of IL-10 by CD4+ T cells.(Dasgupta et al., 2014; Erturk-Hasdemir et al., 2019; Round and Mazmanian, 2010) The role of TLR2 and dectin-1 in PSA induction of IFNβ was therefore investigated by comparing the response of WT, dectin-1 (Clec7a−/−), and TLR2 (Tlr2−/−) deficient BMDCs to PSA using ELISA to detect IFNβ secreted in the supernatants. Much to our surprise, loss of dectin-1 or TLR2 had no effect on IFNβ secretion (Figure 4A), suggesting that PSA signals through a different and previously unidentified pathway to regulate IFNβ expression.
Figure 4. Bacteroides OM glycolipids signal through TLR4 to induce IFNβ.
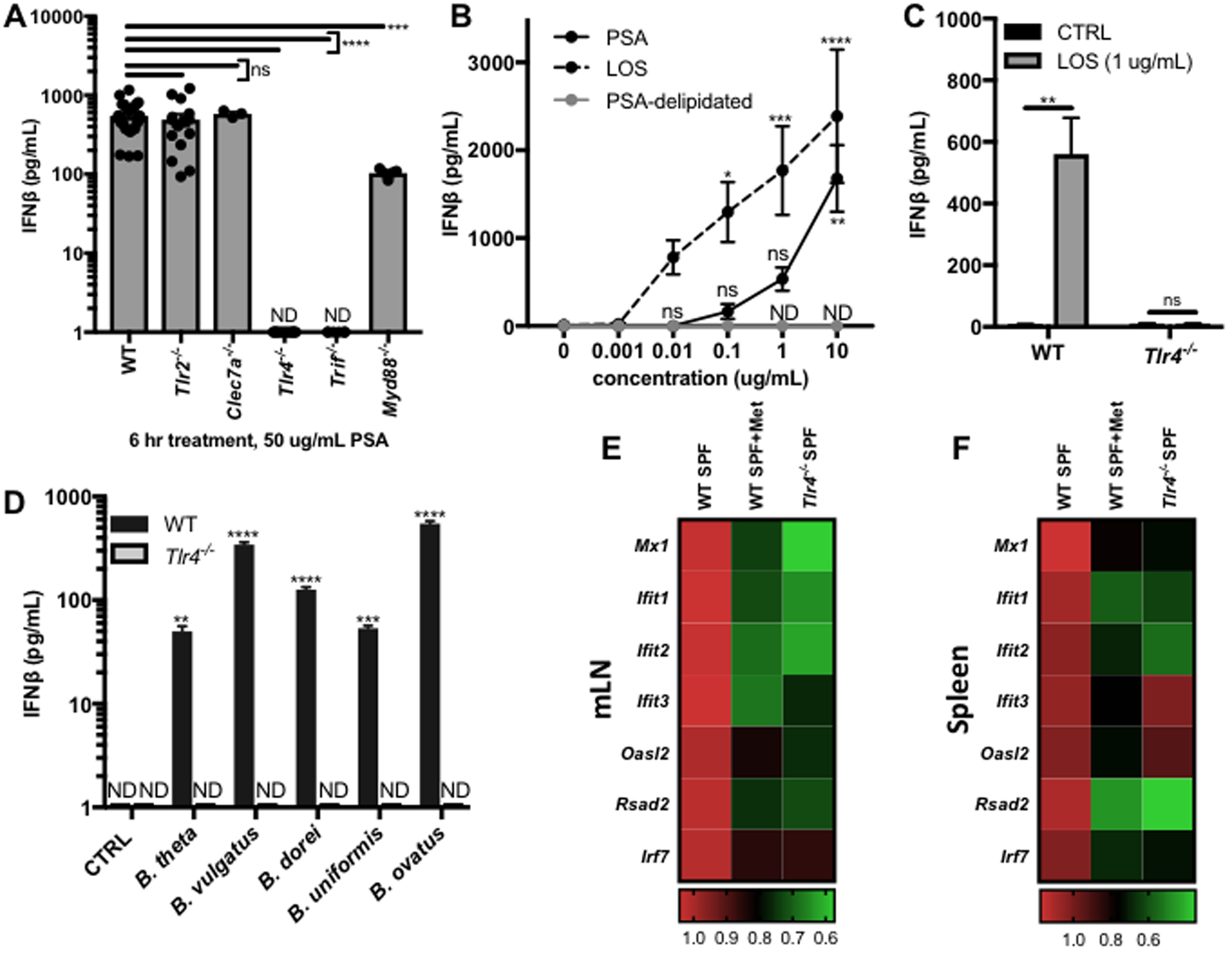
(A) BMDCs were differentiated from WT (N=27), Tlr2−/− (N=15), Clec7a−/− (N=3), Tlr4−/− (N=12), Trif−/− (N=6), or Myd88−/− (N=5) mice and treated with 50 ug/mL B. fragilis PSA for 6 hrs. IFNβ in the supernatants was measured by ELISA and normalized by subtracting vehicle control. (B) ELISA analysis of IFNβ in the supernatants of WT BMDCs treated for 6 hrs with a dose response of PSA (N=9), the LOS lipid domain of PSA (LOS, N=14), or delipidated PSA (PSA-delipidated, N=3, ND for all concentrations). ELISA analysis of IFNβ in the supernatants of WT or Tlr4−/− BMDCs treated for 6 hrs with (C) vehicle control (CTRL, N=8), 1 ug/mL LOS (N=8) or (D) 100 ug/mL Bacteroides OM extracts (N=3 per condition). (A-D) Data represents average +/− SEM. (E,F) RNA isolation and qRT-PCR analysis of ISG expression was performed on mLNs and spleens harvested from WT SPF mice, WT SPF mice after 1 week of metronidazole (Met) treatment, or from Tlr4−/− SPF mice. Fold change gene expression in the mLN (E) or spleen (F) was calculated compared to WT SPF mice using the ΔΔCT method, with ActB as the reference gene and depicted as a heat map. Statistical analysis with (A) one-way ANOVA followed by Dunnett’s multiple comparisons test to WT, (B,D) two-way ANOVA followed by Dunnett’s multiple comparisons test to 0 ug/mL CTRL, and (C) unpaired t-test. (E,F) Details of statistical analyses can be found in Tables S3–4. ND=not detected, ns=not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
The majority of IFN-I inducing PRRs sense viral nucleic acid, with the bacteria-sensing TLR2, dectin-1, and TLR4 pathways representing some of the few exceptions. TLR4 senses microbial glycolipids and of the IFN-Is predominantly induces IFNβ, consistent with the signaling observed by PSA.(Lu et al., 2008; Sheikh et al., 2014) TLR4 deficient (Tlr4−/−) BMDCs completely lost the ability to secrete IFNβ in response to PSA treatment (Figure 4A), confirming that PSA signals through TLR4 to induce IFNβ secretion.
TLR4 signaling proceeds through two different signaling adapter molecules, myeloid differentiation primary response protein (MyD88) and TRIF. MyD88 canonically activates the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) to induce proinflammatory gene expression while TRIF activates interferon regulatory factor 3 (IRF3) to mediate IFNβ expression.(Maeshima and Fernandez, 2013) Consistent with these well-described signaling events, PSA induction of IFNβ was entirely dependent on TRIF, as evidenced by the lack of increased IFNβ secretion in TRIF deficient (Trif−/−) BMDCs in response to PSA (Figure 4A). A significant decrease in IFNβ secretion was also observed in PSA-treated MyD88 deficient (Myd88−/−) BMDCs (Figure 4A), potentially due to regulatory effects that MyD88 expression has on BMDC function.
The canonical TLR4 ligand is LPS, the primary component of the OMs of Gram-negative bacteria.(Maeshima and Fernandez, 2013) In contrast to the archetypal Gram-negative bacterial species Escherichia coli (E. coli), the OM of B. fragilis lacks LPS but instead consists of a lipooligosaccharide (LOS), comprising a lipid A domain linked to a short oligosaccharide.(Lindberg et al., 1990) We recently identified that PSA contains a glycolipid portion, which structurally resembles B. fragilis LOS, and now believe that the polysaccharide domain of PSA is covalently linked to LOS as a means of attachment to the OM.(Erturk-Hasdemir et al., 2019) The lipid A component of the LOS of Bacteroides has structural and biological features which distinguish it from the much studied lipid A of E. coli (Erturk-Hasdemir et al., 2019; Kasper, 1976). It was therefore hypothesized that the LOS portion of PSA is responsible for induction of IFNβ. Consistent with this hypothesis, purified B. fragilis LOS was observed to induce IFNβ in a dose-dependent manner through TLR4 (Figure 4B–C). In contrast, PSA that was delipidated using acid hydrolysis followed by chloroform/methanol extraction to remove the lipid induced no detectable levels of IFNβ (Figure 4B), confirming that the LOS domain of PSA is responsible for IFNβ signaling.
IFNβ induction is a shared function of Bacteroides sp.
LOS-like molecules are not unique to B. fragilis, and multiple bacterial species of the genus Bacteroides have been demonstrated to have LPS or LOS molecules consisting of glycolipids that are structurally similar to B. fragilis LOS and distinct from classical E. coli LPS.(Cullen et al., 2015; Kasper, 1976; Vatanen et al., 2016) We therefore hypothesized that induction of IFNβ by Bacteroides LOS/LPS represents a broader mechanism by which commensal microbes are capable of influencing the host immune system. OM complexes were isolated from several different species of Bacteroides to test their effect on cytokine secretion by BMDCs. OMs extracted from all of the Bacteroides sp. tested induced IFNβ by BMDCs in a TLR4-dependent manner (Figure 4D), suggesting that the LPS/LOS species of each of these Bacteroides is indeed capable of signaling through TLR4 to induce IFNβ.
To address the role of Bacteroides sp. and TLR4 signaling in regulation of the IFN-I response in vivo, ISG expression was compared in WT SPF mice, Tlr4−/− SPF mice, and WT SPF mice 7 days post treatment with metronidazole, a bactericidal antibiotic which specifically targets anaerobic bacteria, including Bacteroides sp.(Edwards, 1979; Salyers, 1984) WT mice treated with metronidazole alone and Tlr4−/− SPF mice both exhibited significant decreases in ISG expression in the mLN (Figure 4E, Table S3) and spleen (Figure 4F, Table S4). These findings confirm the potential for anaerobic commensal bacteria, of which Bacteroides are one of the most dominant taxa, to regulate the host IFN-I response through TLR4 signaling.
Commensal-induced IFNβ enhances resistance to VSV infection
It was recently reported that constitutive, low-level induction of ISG expression by the commensal microbiota is required to prime macrophages to respond robustly to subsequent respiratory virus infection.(Abt et al., 2012) It was therefore hypothesized that commensal-induced IFNβ is functionally important for the priming of the antiviral response and resistance to virus infection. A murine vesicular stomatitis virus (VSV) infection model, which causes meningoencephalitis and fatal paralytic disease, was used to investigate the role of commensal-induced IFNβ. To test the contribution of IFNβ to protection in this disease model, cohoused Ifnb1+/+ and Ifnb1−/− littermates were infected by subcutaneous injection into the footpad with 106 plaque-forming units (PFU) of VSV strain Indiana. Ifnb1−/− mice exhibited increased susceptibility to disease based on all parameters tracked, with increased daily and cumulative disease scores (Figures 5A and 5D), incidence of disease (Figure 5B), and reduced survival (Figure 5C). These findings demonstrate a strong role for IFNβ in protection against subcutaneous VSV infection.
Figure 5. IFNβ is required for protection from murine VSV infection.
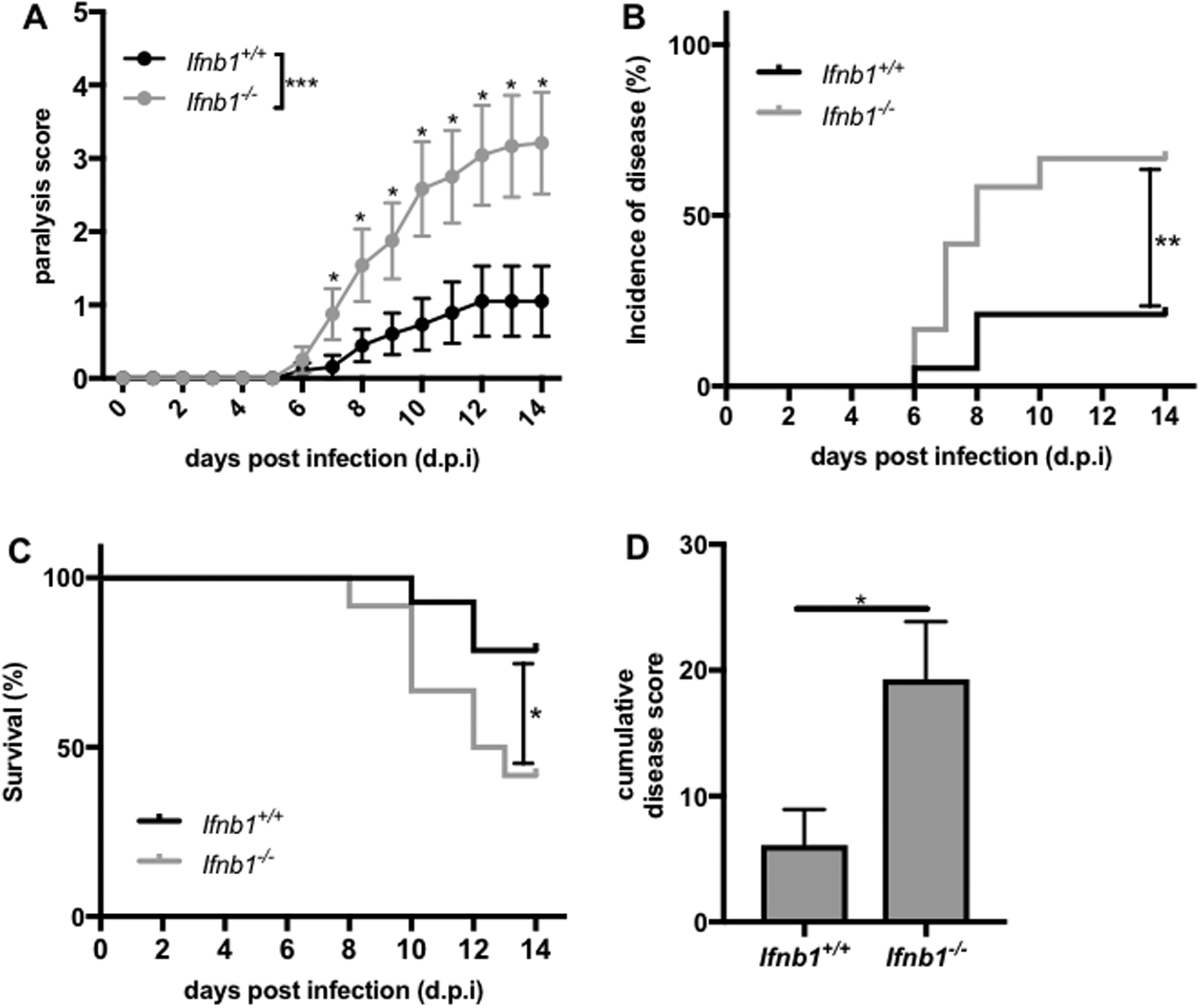
Littermate Ifnb1+/+ (N=19) or Ifnb1−/− (N=12) mice were infected with 106 PFU of VSV strain Indiana by subcutaneous injection into the footpad. Mice were monitored daily for (A) paralysis score, (B) percentage of mice with paralysis (incidence of disease), and (C) survival. (D) The sum of the daily disease scores for each mouse for the duration of the experiment (cumulative disease score) was calculated 14 d.p.i. Statistical analysis with (A) linear regression analysis and unpaired t-test (for each day), (B,C) log-rank test, and (D) unpaired t-test. *p<0.05, **p<0.01, ***p<0.001.
VSV infection itself induces IFNβ expression through PRR signaling, which likely contributes to ISG expression after infection and antiviral immunity.(Kato et al., 2005) Therefore, the increased susceptibility observed in the Ifnb1−/− mice likely reflects virus infection-induced IFNβ in addition to any contributions of priming levels of IFNβ induced by commensal microbes prior to infection. To investigate the effects of priming of the antiviral response by constitutive, microbiota-induced IFNβ, an IFNβ neutralizing antibody (anti-IFNβ) was used to selectively inhibit IFNβ prior to infection.(Sheehan et al., 2015). WT mice were administered two doses of anti-IFNβ or mouse IgG2a isotype control antibody by IP injection, 72 hrs and 24 hrs prior to infection with 106 PFU of VSV. Anti-IFNβ treated mice had increased severity of disease (Figure S5) compared to isotype control treated mice. These data reveal that blockade of IFNβ prior to infection enhances susceptibility to subsequent VSV infection, supporting a role for commensal-induced, homeostatic IFNβ.
To further investigate the impact of the commensal microbiota on VSV infection, WT mice were administered ABX for 7 days prior to infection, stopping on the day of infection. Increased susceptibility to disease was observed in WT ABX mice compared to WT SPF mice, with significantly increased paralysis scores (Figures 6A,G), incidence of disease (Figure 6B), and reduced survival (Figure 6C). These data reveal a novel role for the commensal microbiota in natural resistance to VSV infection. Ifnb1−/− mice were also subjected to 7 days of ABX treatment prior to infection with VSV Indiana. Interestingly, ABX treatment had no effect on the susceptibility of Ifnb1−/− mice to VSV infection (Figures 6D–G). In the absence of IFNβ, depletion of the microbiota therefore does not alter the response to VSV, demonstrating that the microbiota exerts its protective effect specifically through IFNβ.
Figure 6. Microbiota-induced IFNβ enhances resistance to VSV infection.
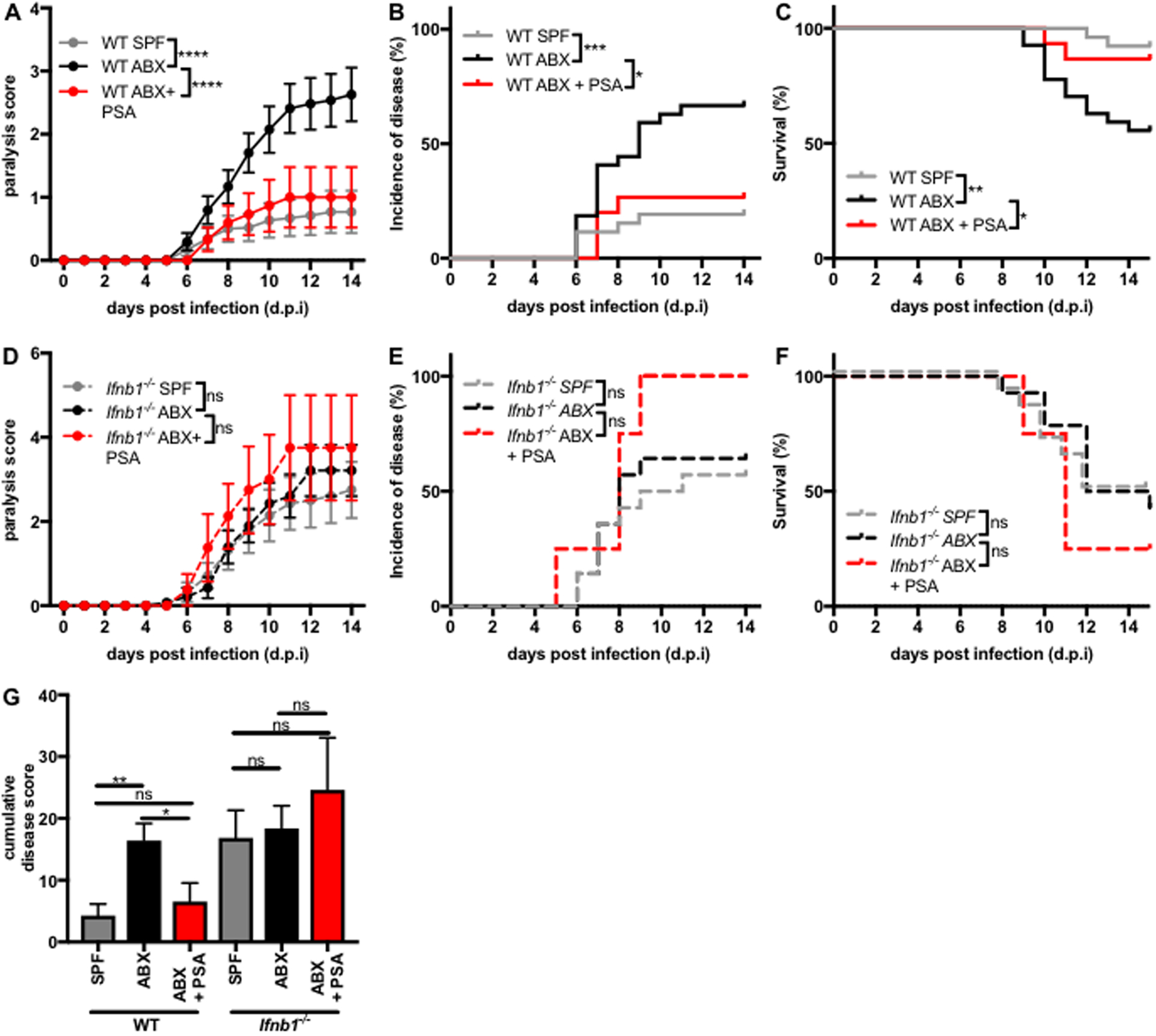
WT or Ifnb1−/− SPF mice were treated with vehicle control or ABX for 7 days prior to infection as well as vehicle or 75 ug PSA daily starting 4 days before until the day of infection with 106 PFU VSV strain Indiana. Mice were monitored daily for (A,D) paralysis score, (B,E) percentage of mice with paralysis (incidence of disease), and (C,F) survival. (G) The sum of the daily disease scores for each mouse (cumulative disease score) was calculated 14 d.p.i. Statistical analysis with (A,D) linear regression analysis, (B,C,E,F) log-rank test, and (G) one-way ANOVA followed by Tukey’s multiple comparisons test. WT SPF N=26, WT ABX N=27, WT ABX+PSA N=15, Ifnb1−/− SPF N=14, Ifnb1−/− ABX N=14, Ifnb1−/− ABX+PSA N=4. ns=not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
B. fragilis PSA possesses IFNβ-mediated antiviral activity
To further investigate the antiviral activity of commensal-induced IFNβ, we sought to identify whether signaling of a purified IFNβ-inducing commensal microbial molecule, B. fragilis PSA, is sufficient to protect against virus infection. While LOS and the LOS domain of PSA are responsible for IFNβ induction, whole PSA was used in this system due to its enhanced solubility. An in vitro virus infection model was developed in which BMDCs were treated with a dose response of PSA for 24 hrs prior to infection with a green fluorescent protein (GFP) expressing strain of VSV Indiana (VSV-GFP) at a multiplicity of infection (MOI) of 1, followed by flow cytometric analysis of the percentage of infected (GFP+) live cells 24 hrs post infection (h.p.i.) (Figure S6A).(Dalton and Rose, 2001) Priming with PSA inhibited virus infection in a dose-dependent manner, resulting not only in a lower percentage of infected cells (Figure 7A) but also increased cell viability (Figure 7C). To investigate the mechanism of antiviral activity, infection with VSV-GFP was compared in WT, Ifnb1−/−, or Tlr4−/− BMDCs that were primed with PSA for 24 hrs. Protection by PSA was completely lost in the absence of TLR4 or IFNβ signaling (Figure 7E), confirming that the observed antiviral function of PSA is indeed mediated by IFNβ signaling.
Figure 7. B. fragilis PSA reduces virus infection of BMDCs by signaling through TLR4 to induce IFNβ.
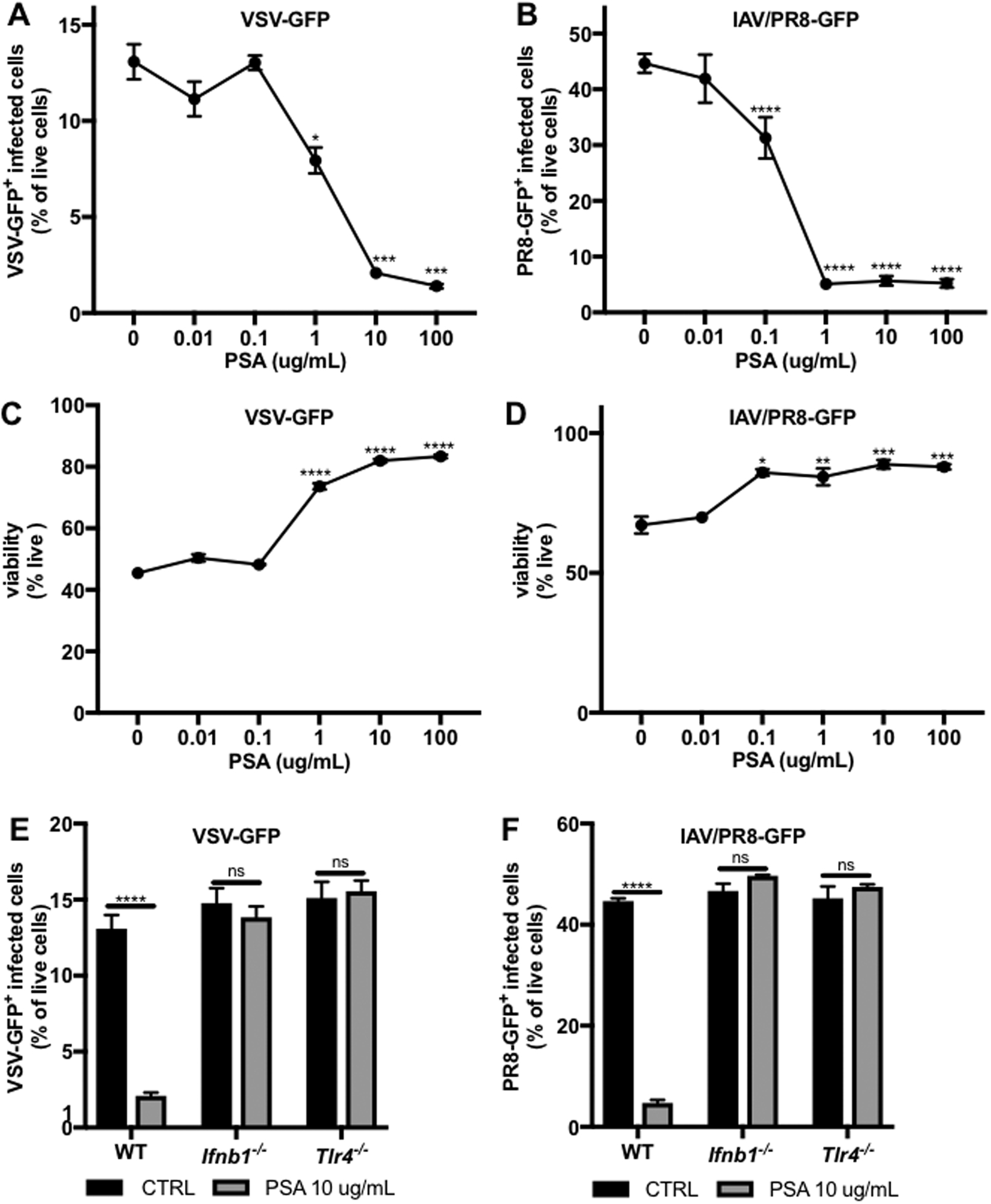
BMDCs were infected with (A,C,E) VSV-GFP or (B,D,F) IAV/PR8-GFP (both MOI=1). GFP+ virus-infected cells were analyzed by flow cytometry 24 h.p.i. in WT, Ifnb1−/−, or Tlr4−/− BMDCs primed for 24 hours prior to infection with (A-D) a dose response of PSA or (E-F) 10 ug/mL PSA. Data represents mean +/− SEM. Statistical analysis with unpaired t-test to 0 ug/mL CTRL. VSV-GFP N=3–6 samples per condition. IAV/PR8-GFP N=3–12 samples per condition. ns=not significant, *p<0.05. **p<0.01, ***p<0.001, ****p<0.0001.
To identify whether the observed in vitro protective effects of PSA are limited to VSV infection, a second virus infection model was developed, using the murine adapted influenza A virus strain PR8 (IAV/PR8). WT, Ifnb1−/−, or Tlr4−/− BMDCs were primed with PSA followed by infection with a GFP-expressing IAV/PR8 strain (PR8-GFP) at an MOI of 1, followed by flow cytometric analysis 24 h.p.i. (Figure S6B).(Manicassamy et al., 2010) Priming of WT BMDCs with PSA inhibited subsequent IAV infection, with a significant reduction in the percentage of infected cells (Figure 7B) and increase in cell viability (Figure 7D). This antiviral effect was completely abrogated in the absence of IFNβ or TLR4 (Figure 7F), revealing that signaling of PSA through the same pathway protects cells from infection with two different viruses. These results establish a novel in vitro broad-spectrum antiviral function of B. fragilis PSA.
To investigate the in vivo antiviral activity of PSA, WT or Ifnb1−/− ABX mice were administered PSA daily by oral gavage starting 4 days prior to and continuing to the day of infection with VSV. PSA treatment significantly reduced disease severity in WT ABX mice, with decreased daily and cumulative paralysis scores (Figures 6A,G), incidence of disease (Figure 6B), and enhanced survival (Figure 6C), to levels statistically indistinguishable from WT SPF mice. In Ifnb1−/− ABX mice, PSA treatment had no observable effect on disease severity (Figures 6D–G), supporting the hypothesis that the protective effects of PSA are mediated through IFNβ. These findings demonstrate that not only is commensal induction of IFNβ necessary for protection against VSV infection, but treatment with a single IFNβ-inducing commensal microbial molecule is sufficient to restore the protective effects of the whole microbiota in this model.
Discussion
We have described a novel mechanism of immunomodulation by the commensal microbiota through IFNβ-mediated regulation of the homeostatic IFN-I response. Furthermore, we identified the mechanism by which a specific species of commensal bacteria regulates this response, signaling of B. fragilis glycolipids through TLR4 to induce expression of IFNβ by colon LP DCs. Importantly, this commensal-mediated regulation of IFNβ and the IFN-I response was found to play a critical role in maintaining health of the host by enhancing resistance to virus infection.
Our findings are consistent with previous reports of commensal regulation of the IFN-I response, which identified that the microbiota induces constitutive IFN-I production and basal levels of ISG expression.(Abt et al., 2012; Schaupp et al., 2020) However, our work builds on the existing literature by identifying which IFN-I family cytokine member is required for regulation of homeostatic ISG expression, a specific cellular source of commensal-induced IFNβ, and the molecular mechanism of IFNβ induction by a commensal microbial species. Interestingly, among the IFN-I family members, IFNβ has been demonstrated to possess unique functionality, with increased antiproliferative and regulatory function. IFN-I signaling can result in several different signaling outcomes all mediated through the same receptor. Importantly, the many different biological outcomes, whether pro- or anti-inflammatory, could be either beneficial or detrimental to the host depending on the context. The observed specific induction of IFNβ by the microbiota suggests controlled expression of the distinct IFN-Is to favor homeostatic expression only of the more regulatory variant, IFNβ.
Intestinal DCs have previously been established to carry commensal antigen to the mLN, where they are restricted from entering into systemic immune circulation, and thus remain in the intestinal immune compartment.(Macpherson and Uhr, 2004) Our results are consistent with a model in which IFNβ is expressed in response to detection of microbial antigens by colon LP DCs, which then remain in the intestinal LP or traffic to the mLN. Despite this tight locational restriction of IFNβ expression, we observed systemic decreases in ISG expression in both Ifnb1−/− and ABX mice as well as functional effects of commensal induced-IFNβ during virus infection at an extra-intestinal site. It is possible that IFNβ secreted in the intestinal environment enters the systemic circulation to mediate these effects. IFNβ is extremely potent, capable of inducing ISG expression with a half-maximal effective concentration (EC50) of 0.2 pM.(Schreiber and Piehler, 2015) While there were no detectable levels of IFNβ in the serum of WT SPF mice, it is possible that a minute quantity of IFNβ might enter the circulation from the intestinal immune compartment and induce systemic effects on ISG expression. Alternatively, secretion of IFNβ by intestinal DCs might induce gene expression changes locally, in neighboring cells, which traffic to distal body sites. Further investigation will be required to determine which of these mechanisms accounts for systemic ISG expression under homeostatic conditions.
We discovered that the glycolipids of B. fragilis, PSA and LOS, signal through TLR4 to induce expression of IFNβ by DCs. Canonically, activation of TLR4 by bacterial LPS signals not only through TRIF to induce IFN-I signaling, but also through MyD88 to induce proinflammatory mediators.(Maeshima and Fernandez, 2013) Through activation of a robust and uncontrolled immune response, LPS exposure can cause severe systemic inflammation, leading to potentially lethal septic shock.(Bohannon et al., 2013; Molinaro et al., 2015) As a result of its endotoxicity, the clinical applications of LPS as an immunomodulator have been limited. However, most research on LPS has focused on E. coli.(Maeshima and Fernandez, 2013) Using numerous assays including proinflammatory cytokine secretion, the Limulus amoebocyte lysate (LAL) assay, rabbit pyrogenicity test, chick embryo lethality, and the Shwartzman reaction, B. fragilis LOS has previously been demonstrated to have low endotoxic activity, suggesting it is a less inflammatory LPS species.(Erturk-Hasdemir et al., 2019; Kasper, 1976; Sveen et al., 1977) This difference in activity can be attributed to several structural differences between the two molecules. Whereas E. coli LPS is hexa-acylated, B. fragilis LOS represents a mixture of penta-, tetra-, and tri-acylated molecules.(Weintraub et al., 1989) In addition, the acyl-chains of B. fragilis LOS are longer, with C15-C17 fatty acid chains compared to C12-C14 fatty acids used by E. coli LPS.(Raetz et al., 2007; Weintraub et al., 1989) Finally, the diglucosamine backbone of B. fragilis LOS is mono- or non-phosphorylated compared to the bis-phosphorylated lipid A of E. coli.(Molinaro et al., 2015; Weintraub et al., 1989) The number and length of the acyl-chains as well as the phosphorylation state of the disaccharide backbone in LPS are critical factors affecting the interaction with TLR4 and the downstream function of these molecules.(Maeshima and Fernandez, 2013) In this way, B. fragilis glycolipids might be able to induce the protective beneficial effects of IFNβ without the consequences of uncontrolled, pathologic inflammation.
Our findings suggest that induction of IFNβ through TLR4 signaling is not limited to B. fragilis but is a shared function of an entire class of commensal microbial molecules, Bacteroides LPS/LOS. Indeed, OM extracts from all of the Bacteroides sp. tested were able to induce IFNβ. OMs comprise numerous bacterial molecules, all of which have the potential to interact with immune cells and influence their response.(Kasper and Seiler, 1975; May and Grabowicz, 2018) Isolation of LPS/LOS from each of these bacterial species will therefore be required to further assess their IFNβ-inducing capabilities. Importantly, the genus Bacteroides makes up a large proportion of the human GI microbiome and is widespread in prevalence across the human population.(Salyers, 1984; Wexler, 2007) Indeed, Bacteroides LOS/LPS species might be the most abundant microbial molecules in the GI tract. As such, it is plausible that Bacteroides-induced IFNβ represents a ubiquitous mechanism by which commensal microbes communicate with their host to regulate the immune system and other physiological processes, ultimately contributing to human health.
Low-level induction of ISG expression under homeostatic conditions primes cells of the immune system so that they can respond effectively to subsequent virus infection and ultimately reduces severity of disease.(Abt et al., 2012) It is therefore plausible that the observed antiviral effect of commensal-induced IFNβ is mediated through priming of constitutive expression of ISGs, thus arming the immune system to respond immediately and robustly to virus infection. Due to the important role of IFN-I signaling and ISG expression in the context of the majority of mammalian virus infections, such a finding might represent a broad-spectrum, and therefore universally important, antiviral activity of the commensal microbiota.(van den Broek et al., 1995) In addition to antiviral immunity, IFN-Is have been described to influence numerous other vital aspects of host physiology, including immune tolerance, antitumor immunity, cytokine signaling regulation, bone homeostasis, and hematopoiesis.(Gough et al., 2012) It is therefore possible that the commensal microbiota influences each of these processes as well, through the ability to regulate IFNβ. Future studies will be required to determine the importance of commensal-induced IFNβ in each of these physiological processes and the potential therapeutic use of IFNβ-inducing commensal microbial molecules, such as PSA, in a wide array of human diseases. Current IFN-I based therapies rely on the administration of non-physiologic amounts of exogenous IFN-Is, which can have unwanted side effects.(Gottberg et al., 2000; Sleijfer et al., 2005) Delivery of an IFNβ-inducing microbial molecule thus represents a novel IFN-I-based therapeutic approach, which could enhance the IFN-I response while still being subjected to homeostatic regulatory mechanisms, reducing the potential for undesired side effects.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Dennis Kasper (dennis_kasper@hms.harvard.edu).
Materials Availability
This study did not generate new unique reagents
Data and Code Availability
This study did not generate new datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Details of mouse strains used in this study can be found in the Key Resources Table. Conventional SPF mice were housed under SPF conditions in the Harvard Medical School mouse vivarium. All genetically deficient mice and their respective controls were gender- and age-matched (typically 5–10 weeks) and both males and females were used in this study. Mice were assigned randomly to experimental groups. Germ free C57BL/6 mice were originally purchased from the National Gnotobiotic Rodent Resource Center of the University of North Carolina at Chapel Hill, and were bred and maintained in sterile vinyl isolators in the animal facility at Harvard Medical School. Sterility was checked weekly by aerobic and anaerobic microbial culture. For monocolonization studies, experimental manipulation of mice was performed in autoclaved cages in which animals received autoclaved food and water. All experiments on animals were approved by the Harvard Medical Area Standing Committee on Animals (animal protocol number IS00000187–3).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Pacific blue anti-mouse CD45 antibody | Biolegend | 103126, clone 30-F11 |
| PE anti-mouse CX3CR1 | Biolegend | 149005, clone SA011F11 |
| PerCP-Cy5.5 anti-mouse CD11b | Biolegend | 101228, clone M1/70 |
| PE-Cy7 anti-mouse CD11c | Biolegend | 117318, clone N418 |
| APC anti-mouse CD103 | Biolegend | 121413, clone 2E7 |
| Fixable Viability Dye eFluor780 | eBioscience | 65-0865-14 |
| purified anti-mouse IFNβ | Leinco Technologies | I-1182, clone HDβ−4A7 |
| Mouse IgG2a isotype control antibody | Leinco Technologies | I-118, clone C1.18.4 |
| FITC anti-mouse BST2 (CD317, PDCA-1) | Biolegend | 127007, clone 927 |
| Bacterial and Virus Strains | ||
| Bacteroides fragilis | Kasper laboratory (Erturk-Hasdemir et al., 2019; Mazmanian et al., 2008) | strain NCTC 9343 |
| Bacteroides thetaiotaomicron | ATCC | 29741 |
| Bacteroides vulgatus | ATCC | 8482 |
| Bacteroides dorei | DSMZ-German Collection of Microorganisms and Cell Cultures | 17855 |
| Bacteroides uniformis | ATCC | 8492 |
| Bacteroides ovatus | ATCC | 8483 |
| Clostridium ramosum | Kasper laboratory | strain A031 |
| Vesicular stomatitis virus (VSV) | Dr. Sean Whelan | strain Indiana |
| GFP-expressing Vesicular stomatitis virus strain Indiana (VSV-GFP) | Dr. Jack Rose | Dalton and Rose, 2001 |
| GFP-expressing Influenza A virus strain PR8 (IAV/PR8-GFP) | Dr. Adolfo Garcia Sastre | Manicassamy et al., 2010 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Vancomycin hydrochloride | Alfa Aesar | J62790-06 |
| Gentamicin sulfate | Sigma-Aldrich | G1914-25G |
| Ampicillin | MP Biomedicals | 0219014680 |
| Metronidazole | Alfa Aesar | H60258-22 |
| Amphotericin B | Amresco, Inc. | 97061-608 |
| dithiothreitol (DTT) | Sigma-Aldrich | DTT-RO |
| collagenase type IV | Sigma-Aldrich | C4-28-100MG |
| Paraformaldehyde solution 4% in PBS | sc-281692 | Santa Cruz Biotechnology |
| GM-CSF Recombinant Mouse Protein | ThermoFisher Scientific | PMC2013 |
| ACK Red Blood Cell Lysing Buffer | Gibco | A1049201 |
| Brucella 5% SB HEMIN VIT K1 plates | BBL, Becton, Dickinson and Company | 6217793 |
| Dehydrated Brain Heart Infusion Broth | BBL, Becton, Dickinson and Company | 211059 |
| Critical Commercial Assays | ||
| RNeasy mini kit | Qiagen | 74106 |
| Quantitect Reverse Transcription Kit | Qiagen | 205313 |
| RT2 SYBR Green ROX qPCR Mastermix | Qiagen | 330523 |
| Pan-DC Microbeads, mouse | Miltenyi Biotec | 130-092-465 |
| VeriKine-HS Mouse Interferon Beta Serum ELISA Kit | pbl Assay Science | 42410-1 |
| VeriKine Mouse Interferon Beta ELISA Kit | pbl Assay Science | 42400-2 |
| Experimental Models: Organisms/Strains | ||
| WT SPF mice | Jackson Laboratory | C57BL/6J, stock# 000664 |
| WT GF mice | GF C57BL/6 origin from the University of North Carolina, colony housed in Kasper lab animal facility | C57BL/6J |
| Ifnar1−/− mice | Jackson Laboratory | B6.129S2-Ifnar1tm1Agt/Mmjax, stock number 010830 |
| Tlr4−/− mice | Jackson Laboratory | B6.B10ScN-Tlr4lps-del/JthJ, stock number 007227 |
| Myd88−/− mice | Jackson Laboratory | B6.129P2(SJL)-Myd88tm1.1Defr/J, stock number 009088 |
| Trif−/− mice | Jackson Laboratory | C57BL/6J-Ticam1Lps2/J, stock number 005037 |
| IFNβ-YFP mice | Jackson Laboratory | B6.129-Ifnb1tm1Lky/J, stock number 010818 |
| Ifnb1−/− mice | created from ES cell clone 12782A-D4, generated by Regeneron Pharmaceuticals, Inc. and obtained from the KOMP Repository (www.komp.org) | Ifnb1tm1(komp)vlcg |
| Oligonucleotides | ||
| See Table S5 for qRT-PCR primers | N/A | N/A |
Bacteria
The bacteria strains used in this study are described in the Key Resources Table. All bacteria used in this study were grown on Brucella blood agar plates or in liquid culture in rich Brain Heart Infusion Broth (BD) supplemented with Hemin (0.01%), vitamin K (0.1 mg/mL), glucose (0.5%), Monopotassium phosphate (0.5%), and L-cysteine (0.1%) in an anaerobic chamber (Coy Industries) at 37° Celsius (C).
METHOD DETAILS
Antibiotics Treatment
For studies involving depletion of the microbiota, SPF mice of the indicated strains were treated with a broad-spectrum antibiotics cocktail for 7 days. Vancomycin hydrochloride (0.5 g/L, Alfa Aesar), gentamicin sulfate (0.5 g/L, Sigma-Aldrich), and ampicillin (1 g/L, MP Biomedicals) were administered in the drinking water. Metronidazole (8 g/L, Alfa Aesar) and amphotericin B (0.1 g/L, Amresco Inc) were administered in 200 uL by OG once daily. Fecal samples were collected and plated under aerobic and anaerobic conditions to confirm microbiota depletion. For studies involving treatment with metronidazole alone, Metronidazole (0.5g/L, Alfa Aesar) was administered in the drinking water for 7 days.
qRT-PCR gene expression analysis
RNA was isolated from whole tissues or the DC+ and DC− fractions of spleens, mLNs, and colons following manufacturer’s instructions with the RNeasy mini kit (Qiagen). cDNA was prepared with the Quantitect Reverse Transcription Kit (Qiagen). qRT-PCR was performed on a QuantStudio7 Flex or Pro Real-Time PCR system (Applied Biosystems) with RT2 SYBR Green ROX qPCR Mastermix (Qiagen). Amplification was achieved using an initial cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Gene expression was normalized using Actb as a reference gene. The forward (f) and reverse (r) primers used to detect expression of the corresponding murine genes are described in Table S5.
Preparation of tissue single-cell suspensions
Single-cell suspensions of the colonic lamina propria were prepared as previously described.(Dasgupta et al., 2014) Briefly, the tissue was cut transversely into approximately 2 cm pieces and then longitudinally to expose the lumen. To extract epithelial cells, tissue pieces were incubated in 1mM dithiothreitol (Sigma) in PBS for 10 minutes, washed, and incubated in 30mM Ethylenediaminetetraacetic acid (EDTA) three times for 8 minutes each. To digest the tissue, colon pieces were treated with RPMI medium supplemented with 5% fetal bovine serum (FBS) and 1 mg/mL collagenase type IV for 45 minutes at 37°C in an atmosphere of 5% carbon dioxide (CO2) and then passed through a 70 μM filter. For single-cell suspensions of mLNs and spleens, tissues were harvested, treated with 1mg/mL collagenase type IV in RPMI for 30 minutes at 37°C in an atmosphere of 5% CO2, and passed through a 70 μm filter. Splenic samples were treated with red blood cell lysis buffer (Gibco).
Dendritic cell isolation
Single-cell suspensions of the indicated tissues were prepared as described. DC positive and negative fractions were isolated with mouse pan-DC microbeads (Miltenyi), following the manufacturer’s protocol.
Monocolonization of GF mice
GF WT C57BL/6 mice were administered B. fragilis strain NCTC 9343 or C. ramosum strain AO31 by oral gavage [200 uL, approximately 108-109 colony-forming units (CFU) per mouse]. Mice were housed in autoclaved cages and maintained on a diet of autoclaved food and water for 2 weeks and then euthanized for analysis.
IFNβ ELISA analysis
For serum analysis, WT SPF or antibiotics treated mice were euthanized and blood was collected into 1.1 ml Z-Gel Micro Tubes (Sarstedt). Blood was left at room temperature for 30 minutes, centrifuged to remove coagulated cells [12,000 revolutions per minute (RPM), 10 minutes], and levels of IFNβ in the serum were quantified with the High Sensitivity Mouse IFN Beta ELISA kit (PBL Assay Science) according to the manufacturer’s protocol. For cell culture supernatant analysis, cell free supernatants were collected from BMDC cultures and IFNβ levels were quantified with the Mouse IFN Beta ELISA kit (PBL Assay Science) according to the manufacturer’s protocol.
BMDC culture
DCs were derived from bone marrow of the indicated mice as previously described.(Helft et al., 2015) Briefly, bone marrow was collected from femurs, treated with red blood cell lysis buffer (Gibco), and cultured in 10 cm tissue culture (TC)-treated dishes at a density of 10×106 cells in 10 mL per plate in complete RPMI medium (cRPMI: RPMI 1640 supplemented with 10 mM HEPES, 10% fetal bovine serum, 2 mM L-glutamine, 50 units/mL penicillin, 50 μg/mL streptomycin, and 50 μM 2-mercaptoethanol) with 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). Cells were cultured for 6 days at 37°C in an atmosphere of 5% CO2 with replenishment of medium on day 3. On day 6 the non-adherent and loosely adherent cells were harvested and plated at a density of 106 cells/mL in 200 uL per well of a 96-well round-bottom TC-treated cell culture plate for use in the indicated experimental procedures.
Bacteroides OM extraction
OM complexes were isolated from the indicated Bacteroides sp. as previously described.(Kasper and Seiler, 1975) Briefly, mid-log phase bacteria were pelleted, washed twice in 0.15 M sodium chloride (NaCl) and resuspended in a buffer of 0.15 M NaCl, 0.05 M sodium phosphate, and 0.01 M EDTA. The resuspended organisms were incubated for 30 minutes at 60°C then subjected to mild shearing pressure by passing through a 25-gauge hypodermic needle. Cells were pelleted from the suspension and the supernatants were harvested and subjected to two rounds of centrifugation at 33,000 RPM for 2 hrs at 4° C to pellet the OM extracts.
B. fragilis glycolipid and polysaccharide purification
PSA was purified from B. fragilis mutant strain Δ44 and LOS was purified from an acapsular B. fragilis mutant strain by phenol-water extraction as previously described.(Tzianabos et al., 1992) To obtain the purified delipidated polysaccharide domain of PSA, PSA was subjected to acid hydrolysis with 2% acetic acid at 90 °C for 2 h. The sample was neutralized the lipid was removed using chloroform/methanol extraction.
Flow cytometric analysis
The antibodies used for flow cytometric analysis are described in the Key Resources Table. Single-cell suspensions were stained with a suitable combination of fluorochrome-conjugated antibodies and fixable viability dye. Cells were fixed in 2% paraformaldehyde in PBS, and examined with an LSR II flow cytometer (BD). The data were analyzed with FlowJo software.
Mouse VSV infection
Mice were anesthetized by IP injection of ketamine and xylazine. 1×106 PFU of VSV strain Indiana was delivered in 10 uL per mouse by subcutaneous injection into the footpad as previously described.(Fensterl et al., 2014) Body weight and clinical disease score were monitored daily for 14 days following infection. VSV-infected mice exhibit an ascending paralytic disease that was scored as follows: 0 = no symptoms, 1= tail paralysis, 2= hind-limb weakness, 2.5= paralysis of one hind limb, 3= paralysis of both hind limbs, 3.5= paralysis of both hind limbs and forelimb weakness, 4=forelimb and hind-limb paralysis, 5= moribund). Mice with a body condition score ≤ 2 or a disease score ≥ 4 were humanely euthanized.
in vitro virus infections
On day 6 of culture, GM-CSF BMDCs were plated at a density of 106 cells/mL in 200 uL of cRPMI medium per well of a 96-well round-bottom TC-treated cell culture plate with 20 ng/mL GM-CSF and the indicated treatments for 24 hrs, washed, and resuspended in cRPMI medium containing either IAV/PR8-GFP or VSV-GFP at an MOI of 1. Cells were harvested for flow cytometric analysis 24 hrs post infection.
IFNβ neutralization
Mice were administered two doses of 250 ug IFNβ neutralizing antibody (anti-IFNβ, clone HDβ−4A7; Leinco Technologies) or Mouse IgG2a isotype control antibody (Leinco Technologies) by IP injection, 72 hrs and 24 hrs prior to infection with 106 PFU of VSV strain Indiana.
QUANTIFICATION AND STATISTICAL ANALYSIS
Information regarding statistical details and methods for each experiment can be found in the figure legends. Data is presented as mean +/− SEM unless otherwise indicated. Statistical analysis was performed using GraphPad Prism 7.0.
Supplementary Material
Figure S2. Dendritic cell IFNβ-YFP representative flow cytometric data. Related to Figure 2. Single cell suspensions were prepared from the colon LP of SPF IFNβ-YFP reporter mice and analyzed by flow cytometry. Representative dot plots and gating strategy to characterize IFNβ-YFP+ cells.
Figure S1 Microbiota depletion reduces local and systemic ISG expression. Related to Figure 1. mLNs and spleens were harvested from age- and gender-matched WT SPF or GF mice. RNA was isolated from whole tissue samples followed by qRT-PCR to analyze ISG expression levels. Fold change gene expression in the mLN (A) or spleen (B) was calculated compared to WT SPF mice using the ΔΔCT method, with ActB as the reference gene. Bar graphs represent mean +/− SEM. Statistical analysis for each gene with unpaired t-test. Details of the statistical analysis can be found in tables S1 and S2. ns=not significant, *p<0.05, **p<0.01, ***p<0.001.
Figure S3. Analysis of IFNβ in the mLN, spleen, and serum. Related to Figure 2. (A-B) Single cell suspensions were prepared from mLNs and spleens harvested from WT SPF mice. Dendritic cell isolation was performed, yielding dendritic cell positive (DC+) and dendritic cell negative (DC−) fractions. Ifnb1 expression was analyzed by qRT-PCR and relative expression of Ifnb1 was normalized to Actb. (A) Relative expression of Ifnb1 in mLN whole tissue (N=3), DC+ (N=5), and DC− (N=3) samples. (B) Relative expression of Ifnb1 in spleen whole tissue (N=5), DC+ (N=3), and DC− (N=3) samples. (C) ELISA analysis of IFNβ in the serum of WT SPF (N=5) or antibiotics treated mice (SPF+ABX, N=5). Bars represent mean +/− SEM. Statistical analysis with (A) one-way ANOVA followed by Tukey’s multiple comparisons test. N.D.=not detected, ns=not significant.
Figure S4. Ifnb1 expression by SI LP DCs of B. fragilis monocolonized mice. Related to Figure 3. (A) WT germ free mice were gavaged with vehicle (GF, N=3) or colonized at 4 weeks of age with B. fragilis (N=3). Two weeks post colonization, dendritic cells were isolated from SI LP single cell suspensions, RNA was isolated, and Ifnb1 expression was analyzed by qRT-PCR. Fold change gene expression to GF was calculated using the ΔΔCT method with ActB as a reference gene. Statistical analysis with unpaired t-test. ns= not significant.
Figure S5 Inhibition of IFNβ prior to infection increases susceptibility to vesicular stomatitis virus infection. Related to Figure 5. Age- and gender-matched WT SPF mice were treated with 250 ug anti-IFNβ (N=11) or mouse IgG2a isotype control antibody (N=11) by intraperitoneal injection 72 and 24 hours prior to subcutaneous infection with 106 PFU VSV strain Indiana. Mice were monitored daily for (A) paralysis score and (C) percentage of mice with paralysis (incidence of disease). (C) The sum of the daily disease scores for each mouse for the duration of the experiment (cumulative disease score) was calculated 14 d.p.i. Statistical analysis with (A) linear regression analysis, (B) unpaired t-test, and (C) log-rank test. ns=not significant, *p<0.05, ****p<0.0001.
Figure S6. BMDC virus infection representative flow cytometric data. Related to Figure 7. BMDCs were infected with (A) VSV-GFP or (B) IAV/PR8-GFP (both MOI=1). Infection was analyzed by flow cytometry 24 h.p.i. Cells were first gated on viability, followed by analysis of GFP+ virus-infected cells.
Acknowledgements
The authors would like to thank Barbara Reinap and Jason Daugherty for purification of B. fragilis PSA and LOS, Dr. Adolfo Garcia Sastre for PR8-GFP, Dr. Jack Rose for VSV-GFP, and Dr. Sean Whelan for WT VSV Indiana used in animal studies. This work was supported by DOD award W81XWH1910625 (D.L.K), the Howard Hughes Medical Institute (A.I.), and partially supported by NIH/NIAID R42AI120269 (A.I.).
Footnotes
Declaration of Interests
Two patent applications have been filed by Harvard University with relation to this work. D.L.K. and K.L.S. are listed as inventors on both.
References
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. (2012). Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, and Kasper DL (2014). Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL, and Sherwood ER (2013). The immunobiology of toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock 40, 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress BF, Englaender JA, He W, Kasper D, Linhardt RJ, and Koffas MA (2014). Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev 38, 660–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, and Goodman AL (2015). Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KP, and Rose JK (2001). Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology 279, 414–421. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, and Kasper DL (2014). Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 15, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DI (1979). Mechanism of antimicrobial action of metronidazole. J Antimicrob Chemother 5, 499–502. [DOI] [PubMed] [Google Scholar]
- Erturk-Hasdemir D, and Kasper DL (2013). Resident commensals shaping immunity. Curr Opin Immunol 25, 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk-Hasdemir D, Oh SF, Okan NA, Stefanetti G, Gazzaniga FS, Seeberger PH, Plevy SE, and Kasper DL (2019). Symbionts exploit complex signaling to educate the immune system. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V, Wetzel JL, and Sen GC (2014). Interferon-induced protein Ifit2 protects mice from infection of the peripheral nervous system by vesicular stomatitis virus. J Virol 88, 10303–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, et al. (2017). Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 168, 928–943 e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottberg K, Gardulf A, and Fredrikson S (2000). Interferon-beta treatment for patients with multiple sclerosis: the patients’ perceptions of the side-effects. Mult Scler 6, 349–354. [DOI] [PubMed] [Google Scholar]
- Gough DJ, Messina NL, Clarke CJ, Johnstone RW, and Levy DE (2012). Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, and Reis e Sousa C (2015). GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 42, 1197–1211. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project, C. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv LB, and Donlin LT (2014). Regulation of type I interferon responses. Nat Rev Immunol 14, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper DL (1976). Chemical and biological characterization of the lipopolysaccharide of Bacteroides fragilis subspecies fragilis. J Infect Dis 134, 59–66. [DOI] [PubMed] [Google Scholar]
- Kasper DL, and Seiler MW (1975). Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis 132, 440–450. [DOI] [PubMed] [Google Scholar]
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, et al. (2005). Cell type-specific involvement of RIG-I in antiviral response. Immunity 23, 19–28. [DOI] [PubMed] [Google Scholar]
- Kole A, He J, Rivollier A, Silveira DD, Kitamura K, Maloy KJ, and Kelsall BL (2013). Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis. J Immunol 191, 2771–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Chang CJ, Lu CC, Martel J, Ojcius DM, Ko YF, Young JD, and Lai HC (2014). Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed J 37, 259–268. [DOI] [PubMed] [Google Scholar]
- Lindberg AA, Weintraub A, Zahringer U, and Rietschel ET (1990). Structure-activity relationships in lipopolysaccharides of Bacteroides fragilis. Rev Infect Dis 12 Suppl 2, S133–141. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, and Ohashi PS (2008). LPS/TLR4 signal transduction pathway. Cytokine 42, 145–151. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, and Uhr T (2004). Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665. [DOI] [PubMed] [Google Scholar]
- Maeshima N, and Fernandez RC (2013). Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell Infect Microbiol 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, and Garcia-Sastre A (2010). Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A 107, 11531–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May KL, and Grabowicz M (2018). The bacterial outer membrane is an evolving antibiotic barrier. Proc Natl Acad Sci U S A 115, 8852–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, and Kasper DL (2005). An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, and Kasper DL (2008). A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625. [DOI] [PubMed] [Google Scholar]
- McNab F, Mayer-Barber K, Sher A, Wack A, and O’Garra A (2015). Type I interferons in infectious disease. Nat Rev Immunol 15, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro A, Holst O, Di Lorenzo F, Callaghan M, Nurisso A, D’Errico G, Zamyatina A, Peri F, Berisio R, Jerala R, et al. (2015). Chemistry of lipid A: at the heart of innate immunity. Chemistry (Easton) 21, 500–519. [DOI] [PubMed] [Google Scholar]
- Morgun A, Dzutsev A, Dong X, Greer RL, Sexton DJ, Ravel J, Schuster M, Hsiao W, Matzinger P, and Shulzhenko N (2015). Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut 64, 1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, and Aguet M (1994). Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Reynolds CM, Trent MS, and Bishop RE (2007). Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem 76, 295–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, and Mazmanian SK (2010). Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA (1984). Bacteroides of the human lower intestinal tract. Annu Rev Microbiol 38, 293–313. [DOI] [PubMed] [Google Scholar]
- Savage DC (1977). MICROBIAL ECOLOGY OF THE GASTROINTESTINAL TRACT. Annu Rev Microbiol 31, 107–133. [DOI] [PubMed] [Google Scholar]
- Schaupp L, Muth S, Rogell L, Kofoed-Branzk M, Melchior F, Lienenklaus S, Ganal-Vonarburg SC, Klein M, Guendel F, Hain T, et al. (2020). Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 181, 1080–1096 e1019. [DOI] [PubMed] [Google Scholar]
- Scheu S, Dresing P, and Locksley RM (2008). Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proc Natl Acad Sci U S A 105, 20416–20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G, and Piehler J (2015). The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol 36, 139–149. [DOI] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. (2015). MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, and Milo R (2016). Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 164, 337–340. [DOI] [PubMed] [Google Scholar]
- Sheehan KC, Lazear HM, Diamond MS, and Schreiber RD (2015). Selective Blockade of Interferon-alpha and -beta Reveals Their Non-Redundant Functions in a Mouse Model of West Nile Virus Infection. PLoS One 10, e0128636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh F, Dickensheets H, Gamero AM, Vogel SN, and Donnelly RP (2014). An essential role for IFN-beta in the induction of IFN-stimulated gene expression by LPS in macrophages. J Leukoc Biol 96, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Jasuja H, Thiolat A, Ribon M, Boissier MC, Bessis N, Rammensee HG, and Decker P (2013). The mouse dendritic cell marker CD11c is down-regulated upon cell activation through Toll-like receptor triggering. Immunobiology 218, 28–39. [DOI] [PubMed] [Google Scholar]
- Sleijfer S, Bannink M, Van Gool AR, Kruit WH, and Stoter G (2005). Side effects of interferon-alpha therapy. Pharm World Sci 27, 423–431. [DOI] [PubMed] [Google Scholar]
- Stagg AJ (2018). Intestinal Dendritic Cells in Health and Gut Inflammation. Front Immunol 9, 2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, and Medzhitov R (2006). Type I interferons in host defense. Immunity 25, 373–381. [DOI] [PubMed] [Google Scholar]
- Surana NK, and Kasper DL (2014). Deciphering the tete-a-tete between the microbiota and the immune system. J Clin Invest 124, 4197–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveen K, Hofstad T, and Milner KC (1977). Lethality for mice and chick embryos, pyrogenicity in rabbits and ability to gelate lysate from amoebocytes of Limulus polyphemus by lipopolysaccharides from Bacteroides, Fusobacterium and Veillonella. Acta Pathol Microbiol Scand B 85B, 388–396. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, and Kono DH (2005). Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 23, 307–336. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, and Gordon JI (2007). The human microbiome project. Nature 449, 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Pantosti A, Baumann H, Brisson JR, Jennings HJ, and Kasper DL (1992). The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem 267, 18230–18235. [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Zinkernagel RM, and Aguet M (1995). Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev 148, 5–18. [DOI] [PubMed] [Google Scholar]
- Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hamalainen AM, et al. (2016). Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 165, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A, Zahringer U, Wollenweber HW, Seydel U, and Rietschel ET (1989). Structural characterization of the lipid A component of Bacteroides fragilis strain NCTC 9343 lipopolysaccharide. Eur J Biochem 183, 425–431. [DOI] [PubMed] [Google Scholar]
- Wexler HM (2007). Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20, 593–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, and Chen ZJ (2012). Intrinsic antiviral immunity. Nat Immunol 13, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2. Dendritic cell IFNβ-YFP representative flow cytometric data. Related to Figure 2. Single cell suspensions were prepared from the colon LP of SPF IFNβ-YFP reporter mice and analyzed by flow cytometry. Representative dot plots and gating strategy to characterize IFNβ-YFP+ cells.
Figure S1 Microbiota depletion reduces local and systemic ISG expression. Related to Figure 1. mLNs and spleens were harvested from age- and gender-matched WT SPF or GF mice. RNA was isolated from whole tissue samples followed by qRT-PCR to analyze ISG expression levels. Fold change gene expression in the mLN (A) or spleen (B) was calculated compared to WT SPF mice using the ΔΔCT method, with ActB as the reference gene. Bar graphs represent mean +/− SEM. Statistical analysis for each gene with unpaired t-test. Details of the statistical analysis can be found in tables S1 and S2. ns=not significant, *p<0.05, **p<0.01, ***p<0.001.
Figure S3. Analysis of IFNβ in the mLN, spleen, and serum. Related to Figure 2. (A-B) Single cell suspensions were prepared from mLNs and spleens harvested from WT SPF mice. Dendritic cell isolation was performed, yielding dendritic cell positive (DC+) and dendritic cell negative (DC−) fractions. Ifnb1 expression was analyzed by qRT-PCR and relative expression of Ifnb1 was normalized to Actb. (A) Relative expression of Ifnb1 in mLN whole tissue (N=3), DC+ (N=5), and DC− (N=3) samples. (B) Relative expression of Ifnb1 in spleen whole tissue (N=5), DC+ (N=3), and DC− (N=3) samples. (C) ELISA analysis of IFNβ in the serum of WT SPF (N=5) or antibiotics treated mice (SPF+ABX, N=5). Bars represent mean +/− SEM. Statistical analysis with (A) one-way ANOVA followed by Tukey’s multiple comparisons test. N.D.=not detected, ns=not significant.
Figure S4. Ifnb1 expression by SI LP DCs of B. fragilis monocolonized mice. Related to Figure 3. (A) WT germ free mice were gavaged with vehicle (GF, N=3) or colonized at 4 weeks of age with B. fragilis (N=3). Two weeks post colonization, dendritic cells were isolated from SI LP single cell suspensions, RNA was isolated, and Ifnb1 expression was analyzed by qRT-PCR. Fold change gene expression to GF was calculated using the ΔΔCT method with ActB as a reference gene. Statistical analysis with unpaired t-test. ns= not significant.
Figure S5 Inhibition of IFNβ prior to infection increases susceptibility to vesicular stomatitis virus infection. Related to Figure 5. Age- and gender-matched WT SPF mice were treated with 250 ug anti-IFNβ (N=11) or mouse IgG2a isotype control antibody (N=11) by intraperitoneal injection 72 and 24 hours prior to subcutaneous infection with 106 PFU VSV strain Indiana. Mice were monitored daily for (A) paralysis score and (C) percentage of mice with paralysis (incidence of disease). (C) The sum of the daily disease scores for each mouse for the duration of the experiment (cumulative disease score) was calculated 14 d.p.i. Statistical analysis with (A) linear regression analysis, (B) unpaired t-test, and (C) log-rank test. ns=not significant, *p<0.05, ****p<0.0001.
Figure S6. BMDC virus infection representative flow cytometric data. Related to Figure 7. BMDCs were infected with (A) VSV-GFP or (B) IAV/PR8-GFP (both MOI=1). Infection was analyzed by flow cytometry 24 h.p.i. Cells were first gated on viability, followed by analysis of GFP+ virus-infected cells.
Data Availability Statement
This study did not generate new datasets or code.


