Abstract
Purpose
An antitussive plant alkaloid, Noscapine HCl (Nos) displays anticancer activity and has a safe pharmacological profile in humans. The current study was aimed to investigate the in vitro and in vivo anti tumor activity of Nos to determine possible mechanisms of anti tumor activity for treatment of non-small cell lung cancer (NSCLC).
Methods
In vitro cytotoxicity of Nos was studied in H460 cells treated with different doses of Nos (10–160 μM) for 72 h and cell viability was determined using crystal violet assay. Apoptosis in H460 cells was evaluated by TUNEL assay after treatment of cells for 72 h with 30 and 40 μM doses of Nos. For in vivo studies, female athymic Nu/nu mice were xenografted with H460 tumors and on day 4 onwards Nos was administered orally at dose of 300, 450 and 550 mg/kg/day for 24 days. As a control, xenografted tumors were separately treated with Docetaxel (10 mg/kg i.v. bolus on day 5, 11, 17, 23). The tumor volumes were measured every five days. Expression of PARP, Bcl2, Bax, and caspase-3 families of proteins was measured by Western Blotting (WB), while TUNEL and Immunohistochemical methods were utilized to determine DNA fragmentation and cleaved caspase-3 levels respectively.
Results
Nos inhibited growth of H460 cells with the IC50 values of 34.7 ± 2.5 μM. Nos at 30 and 40 μM doses caused apoptosis as evidenced by nuclear condensation in treated H460 cells. Nos caused 49, 65 and 86% reduction in the xenografted tumor volumes at a dose of 300 (P < 0.05), 450 (P < 0.01), 550 mg/kg/day (P < 0.01), respectively, when compared to controls. Nos-dependent suppression of xenografted tumor growth involved up regulation of PARP, Bax, caspase-3 and repression of Bcl2 expression. An increase in Bax/Bcl2 ratio suggests involvement of a mitochondrial mediated apoptotic processes. Our studies revealed a non significant (P > 0.05) increase in Bax/Bcl2 ratio with Nos at a dose of 300 mg/kg/day, while a significant (P < 0.001) increase in Bax/Bcl2 ratio was observed with Nos doses of 450 and 550 mg/kg/day. Further, Nos caused elevated apoptosis in tumor xenografts as evidenced by enhanced expression of caspase-3 and positive TUNEL staining in regressed tumor tissues, thus suggesting induction of apoptosis by mitochondrial pathway.
Conclusion
Our studies suggest that potent antitumor activity of Nos against NSCLC cells. Oral administration of Nos showed significant reduction in tumor volume in human non-small cell lung tumor xenograft in nude mice in a dose dependant manner. Thus, Nos is a promising novel chemotherapeutic agent for the treatment of human lung cancer.
Keywords: Noscapine, Lung cancer, Xenograft, H460, Apoptosis
Introduction
Lung cancer is the leading cause of cancer-related death in the United States. Among lung cancer more than 80% of patients with lung cancer have non-small cell lung cancer (NSCLC), while the remaining 20% have small cell lung cancer [1, 2]. The treatment option in NSCLC includes surgery, radiation therapy, chemotherapy, laser therapy, and photodynamic therapy. In recent clinical trials, chemotherapy has been shown to decrease the chance for cancer recurrence and improve the chance for cure in patients who have undergone complete surgical removal of stage IB, II, or IIIA NSCLC [3, 4]. However, preliminary results of CALGB 9633 demonstrated statistically significant evidence that adjuvant chemotherapy with paclitaxel and carboplatin (PC) improved disease-free (DFS) and overall survival (OS) in resected stage IB NSCLC, but interin analysis demonstrated a p value for over all OS less than a prespecified stopping boundary [5]. Chemotherapy with either gemcitabine, taxanes or vinorelbine, together with a platinum drug is the First choice treatment in NSCLC. Even with recent advances in treatment, response and remission rates remain unacceptably low [6]. Although, the effectiveness of microtubule-interfering agents in cancer therapy has been validated by the use of taxanes and vinca alkaloids for the treatment of many types of cancers [7]. The clinical success of taxanes has been limited by the emergence of drug-resistance owing to the amplification of a membrane glycoprotein involved in efflux of the drug and toxicities such as leucocytopenias, diarrhea, alopecia, and peripheral neuropathies due to the blockage of axonal transport [8–10]. Furthermore, currently available antimicrotubule drugs have to be infused intravenously (i.v.) slowly over long periods of time in the clinic due to the use of cremophor as a vehicle in order to minimize the risk of hypersensitivity reaction [9]. This has prompted an ongoing worldwide search for microtubule-targeting compounds that display favorable toxicity profiles, have better therapeutic indices and improved pharmacological efficacy in the treatment of lung cancers.
Among the various antimicrotubule agents that perturb microtubule dynamics, noscapinoids constitute an emerging class of compounds receiving considerable attention [11–14]. Currently, Noscapine HCl (Nos) is in phase I/II clinical trials for the treatment of low grade non Hodgkin’s lymphoma or chronic lymphocytic leukemia refractory to chemotherapy and hematological malignancies. Microtubules are highly dynamic polymers that are crucial for accurate chromosome segregation during mitosis through formation of the bipolar mitotic spindle. To ensure faithful chromosome transmission, eukaryotic cells have evolved a surveillance mechanism called the spindle assembly checkpoint, which halts mitotic progression when the spindle has a defect or chromosomes are not properly attached by spindle microtubules [15]. It is not surprising that many chemical compounds that target microtubules can arrest cells at mitosis, this property attributed to the use of microtubule targeting agents in cancer chemotherapy. Nos attenuates microtubule dynamics just enough to activate the mitotic checkpoints to stop cell cycle and do not alter the steady state monomer/polymer ratio of tubulin [15]. Nos was found to inhibit cell proliferation in wide variety of cancer cells including many drug-resistant variants [16, 17] while evading normal cells. This unique property places Nos in a distinctive position over other available tubulin-binding agents that either over polymerize (taxanes) or depolymerize (vinca alkaloids) microtubules. Nos was found to be effective against malonoma [15], ovarian [16], lymphoma [18], human myelogenous leukemic cells [19], brain glioblastoma [20], and breast [21] cancers. Furthermore, Nos was also found to effectively inhibit the progression of melanoma [15], murine lymphoma [18], and human breast tumors [21] implanted in nude mice with little or no toxicity to the kidney, heart, liver, bone marrow, spleen, or small intestine and did not inhibit primary humoral immune responses in mice.
In conjunction with previous studies in other types of cancer [15–21] it is reasonable to explore the potential use of Nos for the treatment of lung cancer. Given the relatively favorable toxicity profile of Nos and the poor clinical outcome with current treatment options, it is important to test Nos as an anticancer agent for treatment of NSCLC. Noscapine binds stoichiometrically to tubulin and promotes microtubule polymerization, which causes growth arrest of tumor cells during mitosis. Moreover, Noscapine anticancer activity involves induction of apoptosis via mitochondrial pathways was demonstrated in various cancers [15, 18]. Therefore, the current study was aimed to investigate the in vitro and in vivo anti tumor activity of Nos and to evaluate mechanisms and efficacy against NSCLC cells. The H460 xenograft model was used to study in vivo antitumor activity after oral administration of Nos. In present investigations, the regressed tumor tissue specimens were additionally analyzed to determine density of apoptotic cells and expression of cleaved caspase-3 to evaluate molecular mechanisms involved in Nos anti-tumor activity. Nos induced apoptosis in H460 NSCLC cells in vitro and in vivo, in part by enhancing expression of apoptotic proteins such as PARP, Bax, and cleaved caspase-3. Comparing the effects of Nos on H460 cells both in culture and within in vivo xenograft tumors will result in a better understanding of its antitumor activity against NSCLC.
Materials and methods
Noscapine HCl was purchased from Sigma Chemicals, St. Louis, MO, USA. Docetaxel was a gift from Aventis (Collegeville, PA, USA). ApoTag Red In Situ Apoptosis detection kitR was purchased from ChemiconR International, CA, USA. DeadEnd™ Colorimetric Apoptosis Detection System was purchased from Promega (Madison, WI). Antibodies against PARP, Bax, Bcl2 and caspase-3 were purchased from Cell Signaling Technology (Beverly, MA). All other chemicals were either reagent or tissue culture grade.
Cell lines
The human NSCLC cell lines H460 was obtained from American Type Culture Collection (Rockville, MD, USA). H460 cells were grown in RPMI medium (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS). The cell culture media contained antibiotic antimycotic solution of penicillin (5,000 U/ml), streptomycin (0.1 mg/ml), and neomycin (0.2 mg/ml). The cells were maintained at 37°C in the presence of 5% CO2 in air.
Animals
Female Nu/Nu mice (six weeks old form Harlan, Indianapolis, IN) were grouped and housed (n = 8 per cage) in sterile microisolator caging unit supplied with autoclaved Tek-Fresh bedding. The animals were kept under controlled conditions of 12:12 h light: dark cycle, 22 ± 2°C and 50 ± 15% relative humidity. The mice were fed (irradiated rodent chow Harlan Teklad) and autoclaved water ad libitum. The animals were housed at Florida A&M University in accordance with the standards of the Guide for the Care and Use of Laboratory Animals and the Association for Assessment and Accreditation of Laboratory Animal Care.
In vitro cytotoxicity studies
The H460 NSCLC cell lines was plated in 96-well micro titer plates, at a density of 1× 104 cells/well and allowed to incubate overnight and the cells were treated with various dilutions of Nos made in RPMI medium (10–160 μM) form Nos stock solution in DMSO. The cells were incubated for 72 h at 37 ± 0.2°C in a 5% CO2-jacketed incubator. The cell viability in each treatment was determined by crystal violet assay. The percentage of cell survival as a function of drug concentration was then plotted to determine the IC50 value (the drug concentration needed to prevent cell proliferation by 50%) [22, 23].
Induction of apoptosis in H460 cells by Nos
To detect apoptotic cells, the ApoTag Red In Situ Apoptosis detection kitR (ChemiconR International, CA, USA) was used. Cells were plated at a density of 1 × 106 cells/well in six-well plates and incubated overnight and cells were treated with Nos (30, 40 μM). After 72 h, cells were Fixed in 4% paraformaldehyde and mounted on slides using Shandon CytospinR (GMI, Inc. Minnesota, USA). Equilibration buffer was added to slides and incubated for 10 min followed by incubation in working strength TdT enzyme at 37°C for 1 h. The slides were incubated in stop/wash buffer for 10 min at room temperature. Working strength anti-digoxinenin conjugate (rhodamine) was added to each slide for 30-min incubation at room temperature. Slides were viewed using a Xuorescent microscope.
In vivo antitumor effect of Nos against H460 lung tumors
The adherent tumor cells were washed with PBS, harvested from confluent cultures by 5-min exposure to 0.25% trypsin and 0.02% EDTA solution in an incubator. Trypsinization was stopped with medium containing 10% FBS. The cells were centrifuged and the floating cells in the supernatant were discarded. The cell pellet was resuspended in medium containing 10% FBS and mixed thoroughly. Trypan blue staining was used to determine the number of viable cells. The resuspended cells were centrifuged and cell dilutions of 3 × 106 cells/100μl were prepared in RPMI medium. The 100 μl of cell suspension was injected subcutaneously into right flank area of each mouse. The protocol for in vivo experiments with nude mice was approved by the Animal Care and Use Committee, Florida A&M University, Tallahassee, FL. The mice were randomized into vehicle control and treatment groups of eight animals each when xenografts were palpable with a tumor size of 50 mm3. Four days after tumor implantation, Nos solution was prepared in phosphate buffer pH 3.5 and administrated by oral gavage at a dose of 300, 450 and 550 mg/kg daily for 28 days. Docetaxel (10 mg/kg i.v. bolus) administered on 5, 11, 17 and 23 days of the study and was used as positive control [22, 23]. The tumor dimensions were measured using a linear caliper and tumor volume was calculated using the equation V (mm3) = a × b2/2, where a is the largest diameter and b is the smallest diameter. The mice were fed with food and water ad libitum.
Western Blotting of tumor tissues
Tumor tissues harvested at 28 days post tumor implantation from control, Nos and docetaxel treated mice were cut into small pieces and homogenized in PBS. The homogenate was centrifuged at top speed for 10 min to sediment the tissue fragments. The proteins were extracted using RIPA buffer (50 mM Tris-HCL, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 (NP-40), 0.5% sodium deoxychlorate, and 0.1% sodium dodecyl sulfate) with pro-tease inhibitor (500 mM phenylmethylsulfonyl fluoride). Samples were vortexed, incubated on ice for 30 min, centrifuged again and the supernatants were stored at −80°C. For WB, equal amounts of supernatant protein (50 μg) from the control and different treatments were denatured by boiling for 5 min in SDS sample buffer, separated by 15% SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.5% Tween 20] and probed with PARP, Bax, Bcl2, and cleaved caspase-3 antibodies (1:500). Bound antibodies were revealed with HRP conjugated secondary antibodies (1:2000) using SuperSignal West pico chemiluminescent solution (Pierce, Rockford, IL). Beta actin protein was used as a loading control.
Immunohistochemistry and TUNEL assay of xenograft tumor tissues
Sections prepared from formalin-Fixed, paraffin-embedded lung tissues were used for IHC studies according to the protocol specified in the SignalStain™ Cleaved Caspase-3 [Asp 175] IHC kit (Cell Signaling, Beverly, MA). The section slides were washed in xylene and hydrated in different concentrations of alcohol. The slides were heated in sodium acetate solution at 95°C for 10 min for antigen retrieval. The slides were washed three times in PBS and incubated with the primary antibody against cleaved caspase-3 overnight at 4°C. Horseradish peroxidase-conjugated secondary antibody was applied to locate the primary antibody. The specimens were stained with Nova Red stain and counter-stained with hematoxylin. The presence of brown staining was considered a positive identification for activated caspase-3. The Olympus BX40 light microscope equipped with computer-controlled digital camera and imaging software (Q capture) was used to visualize the images on the slides.
For TUNEL assay, formalin-Fixed tumor tissues harvested 28 days after tumor implantation were embedded in paraffin and sectioned. DeadEnd™ Colorimetric Apoptosis Detection System (Promega, Madison, WI) was used to detect apoptosis in the tumor sections placed on slides according to the manufacturer’s protocol. Briefly, the equilibration buffer was added to slides and incubated for 10 min followed by 10-min incubation in 20 μg/ml proteinase K solution. The sections were washed in PBS and incubated with TdT enzyme at 37°C for 1 h in a humidified chamber for incorporation of biotinylated nucleotides at the 3′- OH ends of DNA. The slides were incubated in horseradish peroxidase-labeled streptavidin to bind the biotinylated nucleotides followed by detection with stable chromagen DAB. The images on the slides were visualized with an Olympus BX40 light microscope equipped with a computer-controlled digital camera (QImaging, Burnaby, BC, Canada) and Imaging software (Q capture). Three slides per group were stained and apoptotic cells were identified by dark brown cytoplasmic staining.
Statistical analysis
One-way ANOVA followed by Tukey’s Multiple Comparison Test was performed to determine the significance of differences among groups. Differences were considered significant in all experiments at P < 0.05. The statistical analysis was performed using GraphPad PRISM version 3.0 software (SanDiego, CA).
Results
Inhibition of cell proliferation and induction of apoptosis by Nos in H460 cells
Nos inhibit proliferation of H460 cells in a dose-dependent manner (Fig. 1) with an IC50 value of 34.7 + 2.5 μM. The IC50 values of Nos against H460 cells are comparable to the cytotoxic effects observed with other cancers [15, 16, 18–21]. In situ DNA Nick end Labeling (TUNEL) was used to determine apoptosis in Nos-treated cells. Figure 2 shows H460 cells undergoing apoptosis following treatment with Nos 30 μM and 40 μM compared to untreated cells. The TUNEL staining was not observed with untreated cells (Fig. 2 A1, A2). Positive TUNEL staining (Fig. 2B1, B2, C1, C2) was observed in H460 cells after 72 h treatment. DAPI nuclear staining (Fig. 2A1, B1, C1) was used for identification of chromatin condensation, which is an important morphological characteristic of apoptosis. The cells that showed nuclear condensation also demonstrated positive TUNEL staining. Seventy-two hours of treatments with 30 and 40 μM doses of Nos resulted in, 32 and 57 % apoptotic cells, respectively.
Fig. 1.
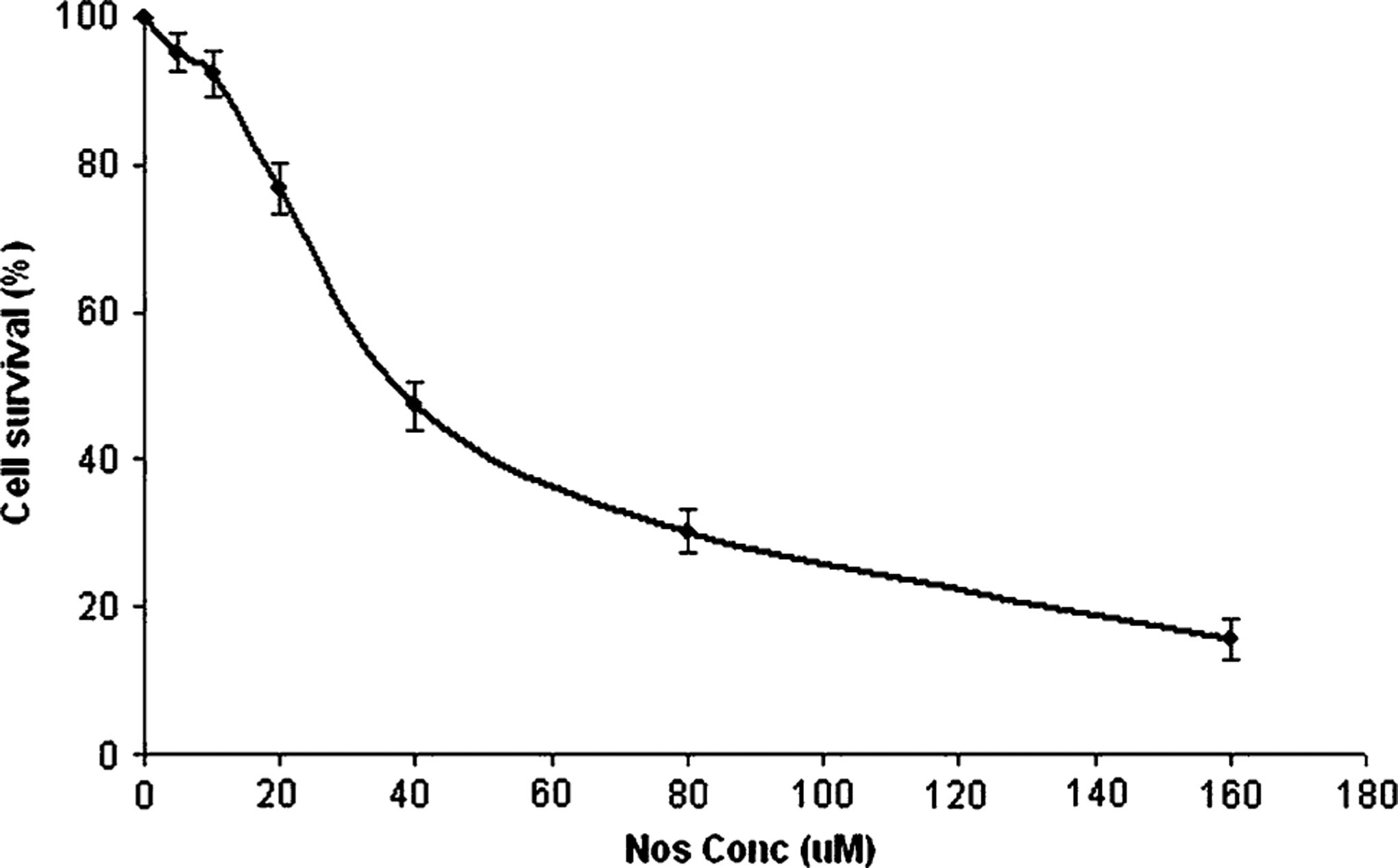
In vitro cytotoxicity profiles of Nos in H460 cells. Cells were treated with Nos and cytotoxicity was determined using the crystal violet assay as described in materials and methods. A plot of the percentage of cell survival versus Nos concentrations used for the determination of IC 50 values. Results are expressed as means ± SD for at least three separate determinations
Fig. 2.
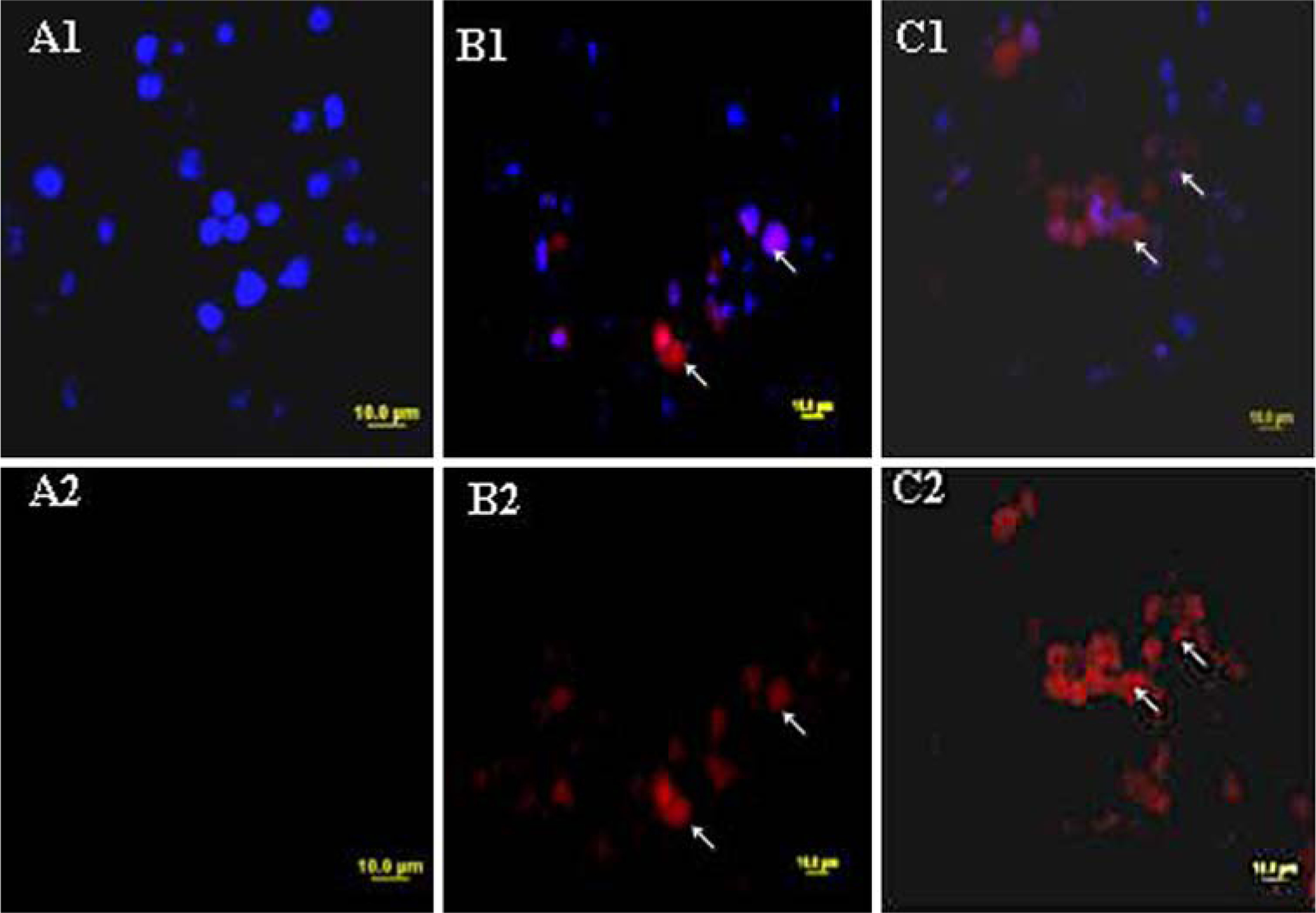
Fluorescence micrographs of H460 cells stained with rhodamine and DAPI for control cells (A1, A2), 72-h treatment with Nos 30 μM (B1, B2), and Nos 40 μM (C1, C2). DNA fragmentation indicated by positive staining and nuclear condensation indicated by DAPI nuclear staining. Micron bar 10 μm
H460 tumor xenograft regression in vivo
Figure 3a shows the tumor volume-time data profiles following Nos administration at a dose of 300, 450 and 550 mg/kg/day by gastric lavage and Docetaxel 10 mg/kg i.v. bolus in mice xenografted with H460 tumors compared to control. At 28 days post tumor implantation, the tumor volumes were found to be 2552 ± 310, 1102 ± 205, 846 ± 181, and 445 ± 96 mm3 (expressed as mean ± SEM) in control, Nos 300, 450 and 550 mg/kg/day treated mice, respectively (Fig. 3b). Oral administration of Nos at 300 mg/kg/day showed significant (P < 0.05) reduction in tumor volume in H460 xenografts. However, Nos administered at 450 and 550 mg/kg/day showed very significant (P < 0.01) reduction in tumor volume. At the end of the study period (28 days), there was 49, 65, 86, and 93% reduction in the tumor volume following Nos 300, 450, 550 mg/kg/day and docetaxel treated mice, respectively compared to control. We found that Nos treatment did not cause any apparent body weight loss in mice (Fig. 4). In contrast, although as indicated in Fig. 3a docetaxel therapy showed enhanced tumor regression (tumor volume of 233 ± 72 mm3, expressed as mean ± SEM), the mice in this group suffered a tremendous body weight loss (Fig. 4).
Fig. 3.
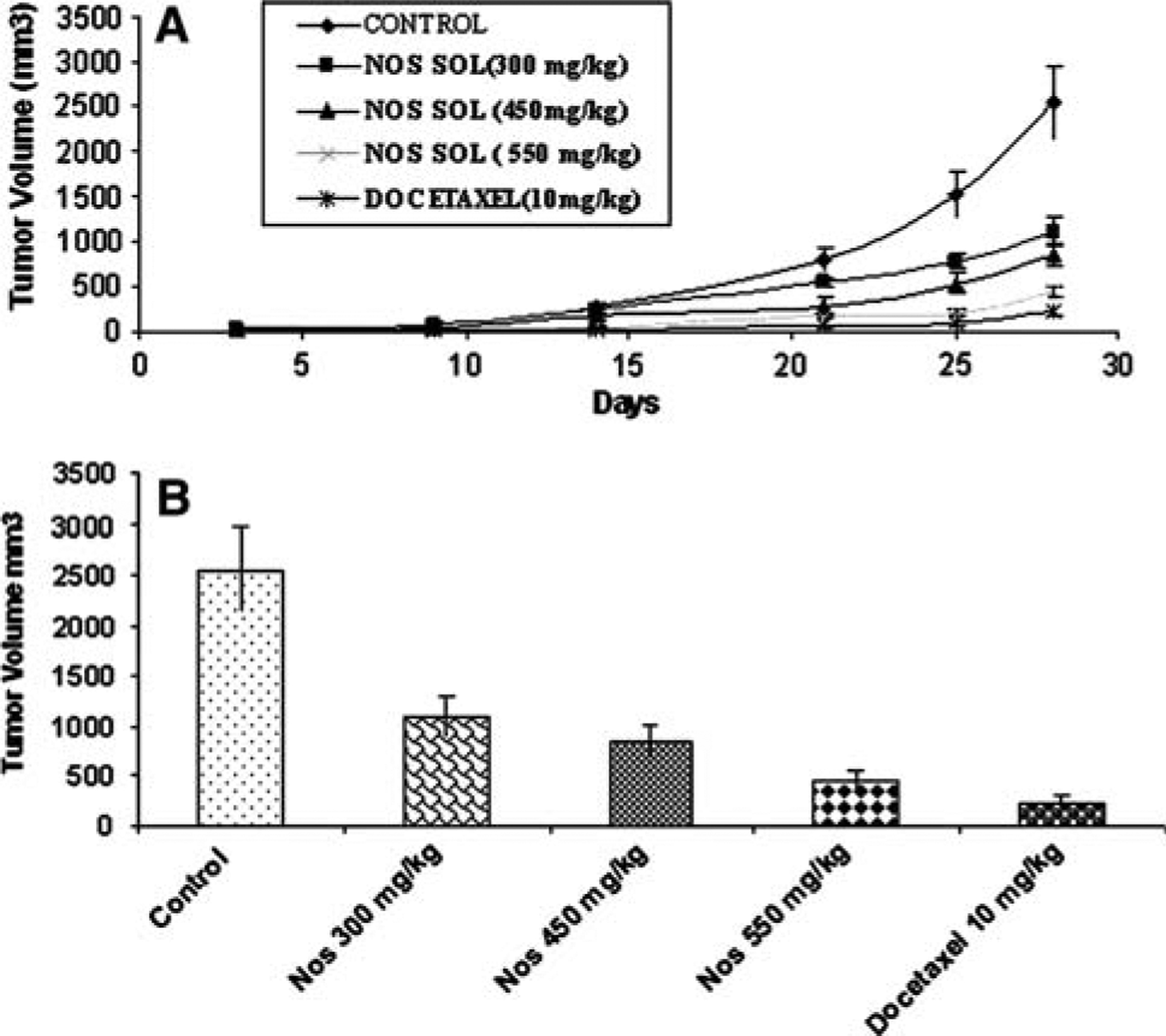
In vivo antitumor effect of different doses of Nos compared to untreated and Docetaxel treated (positive control) in H460 lung tumor Xenograft model. (a) progression profile of tumor growth kinetics and (b) tumor volume measurements on days 28 post-inoculation. (tumor volumes, mm3 ± SEM)
Fig. 4.
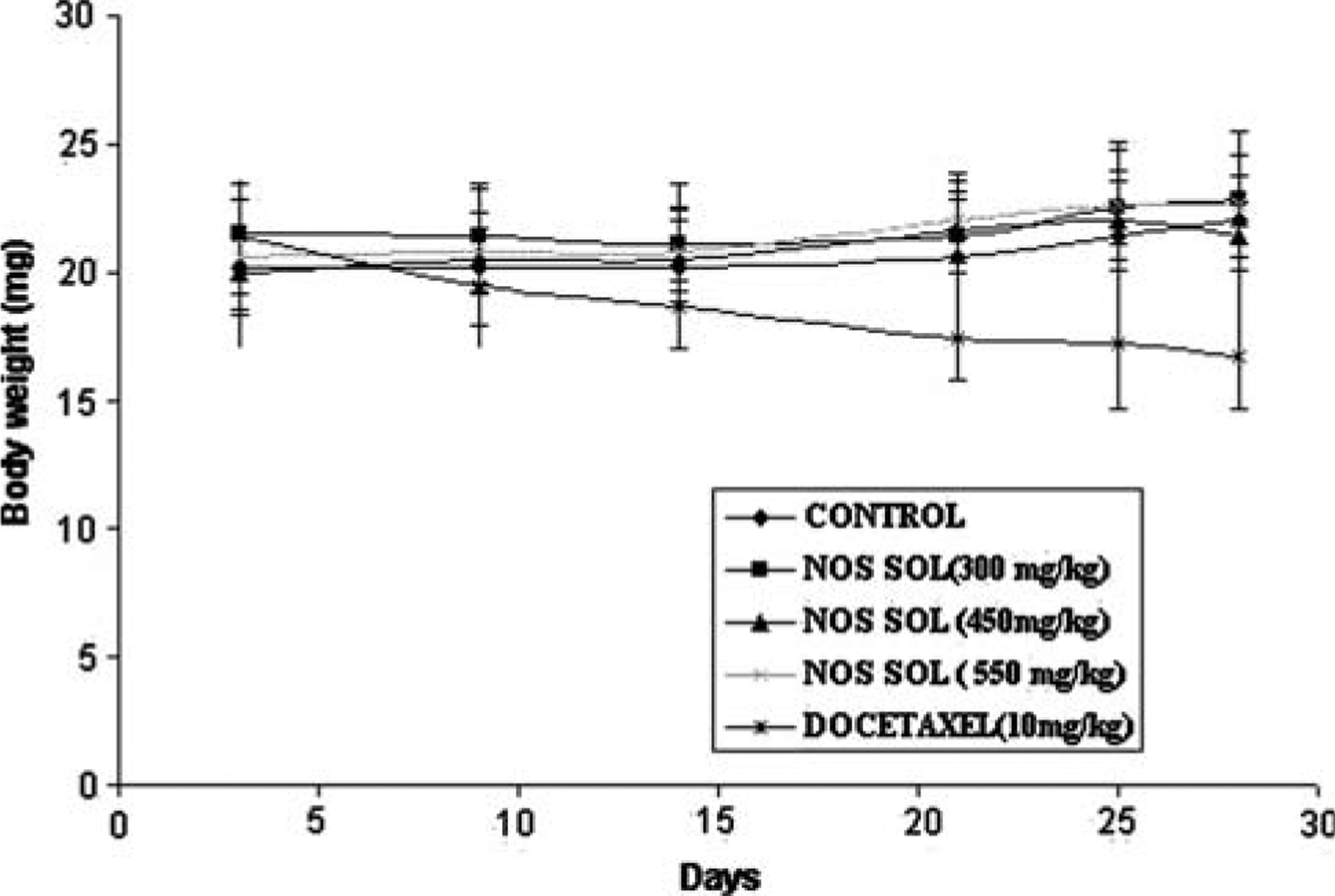
Measurement of body weight following different doses of Nos compared to untreated and Docetaxel treated (positive control) mice
Nos treatment is associated with up regulation of PARP, Bax, Caspase-3 and down regulation of Bcl2 protein expression
To further examine the role of apoptotic pathways in cyotoxicity induced by Nos, we assessed the activation of PARP, Bax, Caspase-3 and Bcl2 protein expression by WB analysis performed on regressed tumor tissues. Nos treatments significantly (P < 0.001) increased PARP, Bax, and Caspase-3 protein expression compared to control in H460 tumor tissues (Fig. 5a–d). Further, Nos treatments caused up regulation of PARP, Bax, and Caspase-3 protein expression that were was comparable with their levels in docetaxel treated tumors. Western blotting studies with H460 tumors indicated that there was significant (P < 0.001) down regulation of Bcl2 expression by Nos treatment compared to control (Fig. 5a, e). The Bax/Bcl2 ratio of 0.21 was observed in control tumors, while the Bax/Bcl2 ratio of 0.33, 0.68, and 0.97 were observed with Nos 300, 450, and 550 mg/kg treated tumors respectively. A nonsignificant (P > 0.05) increase in Bax/Bcl2 ratio was observed with Nos 300 mg/kg/day, while a significant (P < 0.001) increase in Bax/Bcl2 ratio was found with Nos 450 and 550 mg/kg/day (Fig. 5e).
Fig. 5.
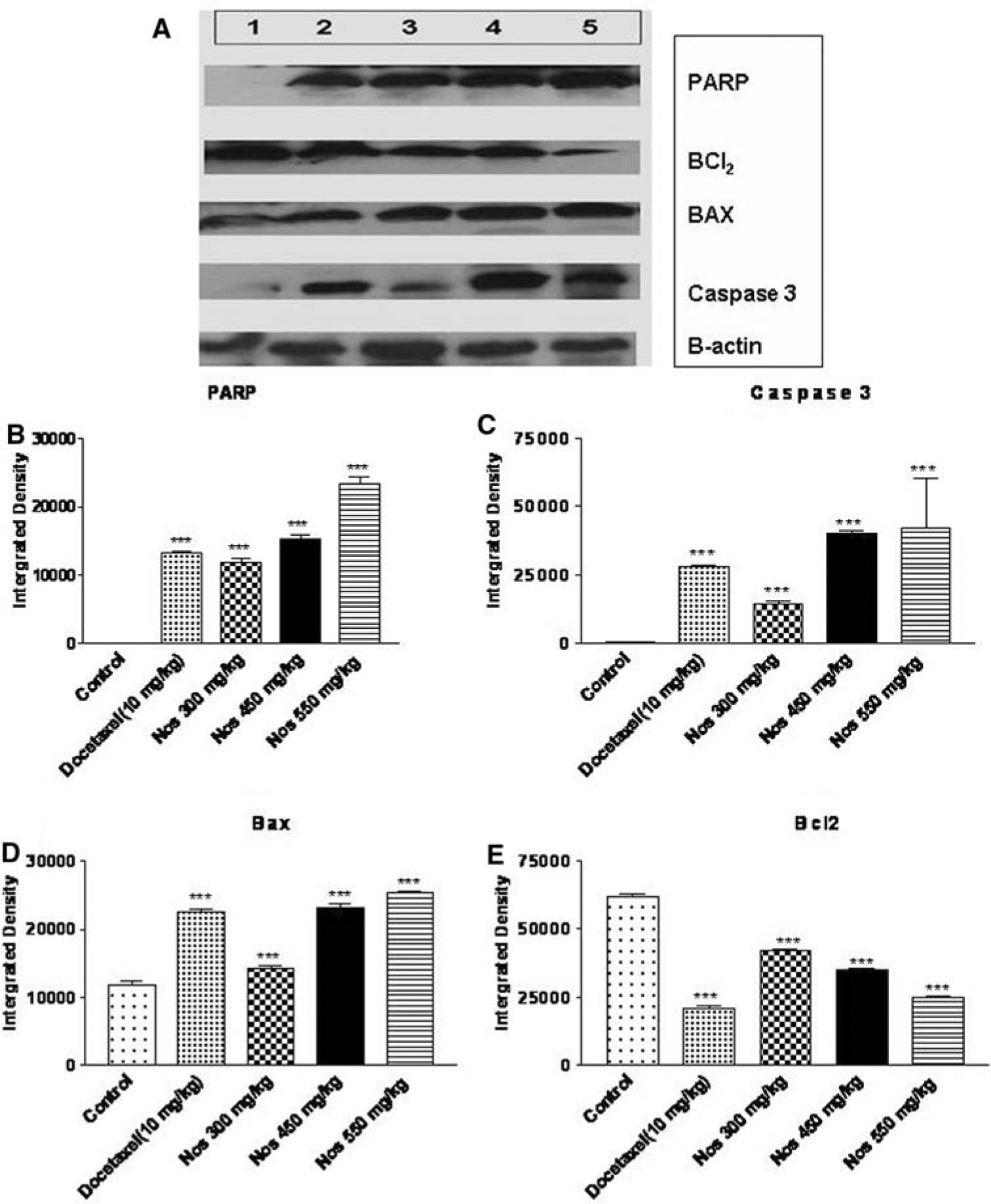
Western blotting of tumor tissue lysates to determine expressions apoptosis-related proteins. (a). The tumors were harvested 28 days post tumor implantation, lysates were prepared, and a 50 μg of protein was loaded in each lane. Lane 1 control, Lane 2 Docetaxel 10 mg/kg i.v. bolus, Lane 3 Nos 300 mg/kg/day, Lane 4 Nos 450 mg/kg/day and Lane 5 Nos 550 mg/kg/day. The densitometric analysis of the bands to determine level of expression of PARP (b), Caspase-3 (c), Bax (d), and Bcl2 (e) in xenograft human H40 lung tumors. The densitometric analysis of the bands was performed using the program ImageJ v1.33u. Error bars depict standard deviations ***P < 0.001 versus untreated control
Immunohistochemistry and TUNEL assay of xenograft tumor tissues
Expression of apoptotic cleaved caspase-3 was investigated in the control and treated tumors by Immunohistochemical analysis. Nos (300, 450, and 550 mg/kg/day) treated regressed tumors showed widespread staining of activated caspase-3 expression compared to controls (Fig. 6a–c), indicating that Nos induced apoptosis of H460 lung cancer cells in vivo. Nos-dependent apoptosis was further confirmed by staining the tumor sections harvested at the end of the study with TUNEL for detection of DNA fragmentation (Fig. 6d–f). Consistent with our in vitro data (Fig. 2), we observed increased TUNEL-positive cells in the regressed tumors of Nos-treated H460 xenografts (Fig. 6e, f). These results indicate that Nos causes tumor regression in part by inducing apoptosis.
Fig. 6.
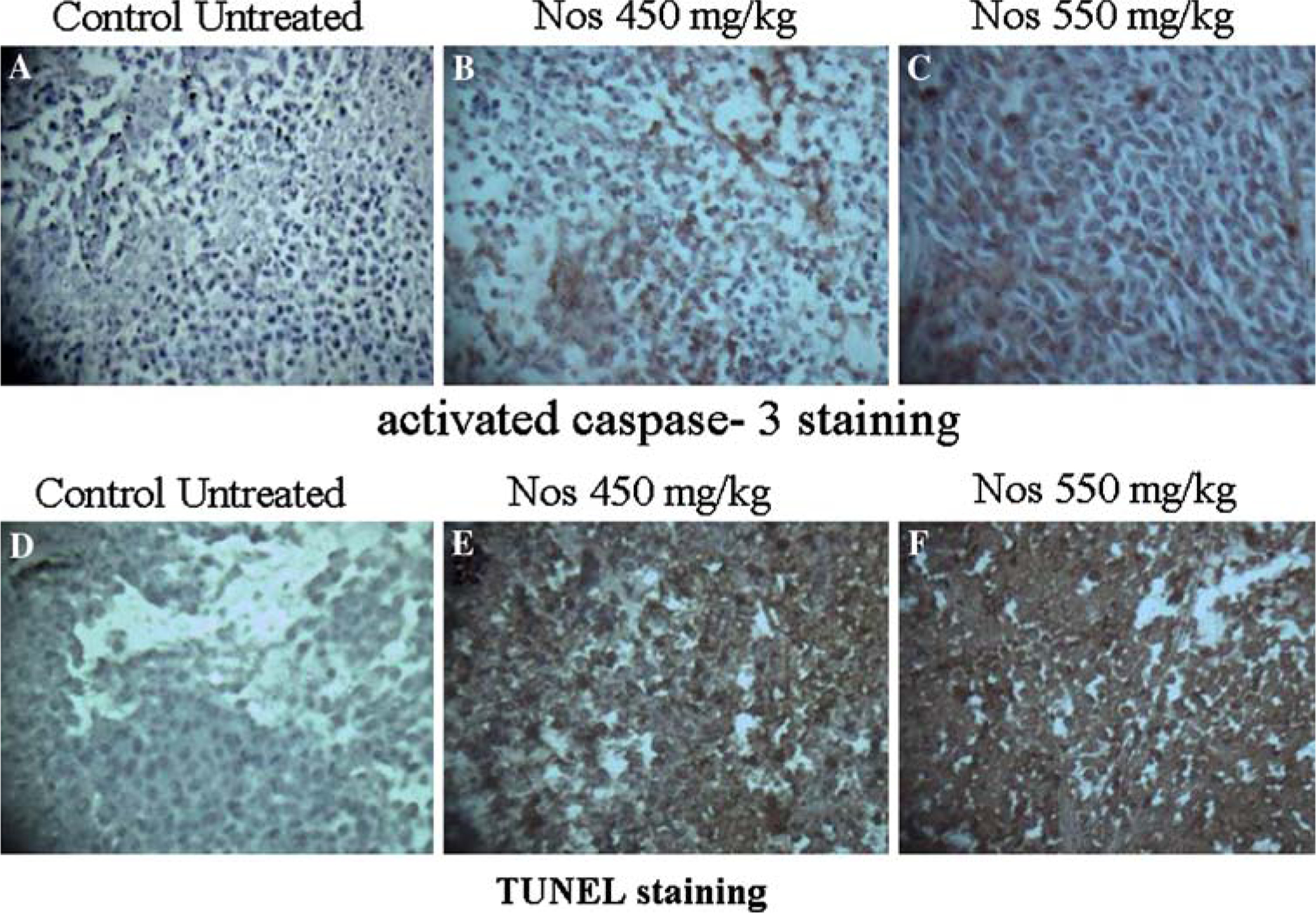
Nos inhibits tumor growth by triggering apoptosis. a–c, Activated (cleaved) caspase-3 was examined by immunohistochemical staining of paraffin-embedded tumor sections, from untreated control (a) and Nos-treated (b and c) mice. d–f Representative TUNEL stained micrographs of tumor sections from untreated control (d) and Nos-treated (e, f) mice
Discussion
Poor clinical outcome of the current treatment avenues has prompted the need for new therapeutic strategies for treatment of lung cancer. In fact, among anticancer agents, antimicrotubules constitute one of the most effective chemotherapeutic agents for treatment of lung cancers [7]. The antimicrotubule targeting agents are divided into two categories: microtubule-destabilizing agents such as the vinca alkaloids and colchicine, and microtubule stabilizing agents such as the taxanes (paclitaxel and docetaxel). Although, currently used antimicrotubule drugs are effective antitumor agents; their clinical utility is limited due to the development of drug resistance and associated severe side effects [8–10]. Moreover, many patients who have impressive initial response to these drugs have undergone relapse after treatment, because of development of drug resistance.
Nos is considered as safe antimicrotubule agent that alters microtubule dynamics without affecting the microtubule polymer mass [18]. Nos demonstrated antitumor activity both in vitro and in vivo in cancer cells that are resistant to the conventional antimicrotubule drugs. More importantly, Nos did not exhibit severe side effects that are commonly seen with many chemotherapeutic agents [8–10, 18]. Currently, Nos is in clinical trials (phase I/II) for the treatment of lymphoma and hematological malignancies. Many of these cancers cells lack mitotic checkpoint mechanism, which can remedied by the use of Nos without significantly impacting the microtubule morphology of non diving cells, owing to these and other advantages we evaluated Nos antitumor activity against NSCLC.
Based on these observations, we hypothesized that Nos is effective against NSCLC, where the refractory tumor cells lack mitotic checkpoint proteins. To our knowledge, this is the First study that demonstrated effectiveness of Nos against lung cancer. In this study, we demonstrated that Nos inhibits H460 cell proliferation in vitro and shows concentration-dependent inhibition of cell proliferation against model H460 NSCLC cell line (Fig. 1) with an IC50 value of 34.7 ± 2.5 μM. The inhibition of cell proliferation by Nos against H460 NSCLC cells was comparable with earlier studies with various types of cancers [15–21].
The cell proliferation inhibition by Nos was observed with other cancer cell types such as rat C6 glioma (IC50 = 250 μM), MCF-7 breast (IC50 = 42.3 μM), Renal 1983 bladder (IC50 = 39.1 μM), HeLa (IC50 = 25 μM), and thymocyte (IC50 = 10 μM) cells [20, 21]. In another investigation, Nos effectively inhibited the proliferation of both paclitaxel-sensitive and paclitaxel-resistant human ovarian carcinoma cells at IC50 values between 15 and 25 μM; supporting binding of Nos to tubulin at a site different from the paclitaxel-binding site [16]. Interestingly, the melanoma B16LS9 cells (IC50 = 50 μM) showed more sensitivity to the Nos treatment compared to primary melanocytes (IC50 = 200 μM) cells, which indicates that Nos is selectively potent on cancer cells compared to normal cells [15]. The antiproliferative activity of Nos varies with the type and sensitivity of cancer cells towards treatment.
To elucidate the mechanisms of cytotoxicity mediated by Nos in H460 cells, we evaluated the efficacy of Nos to induce apoptosis, following a 72 h treatment. The TUNEL assay results showed induction of apoptosis at 30 and 40 μM concentration of Nos compared to untreated cells (Fig. 2B1, B2, C1, C2). Typical morphological changes in late apoptotic cells include membrane blebbing, formation of apoptotic bodies, disruption of the cytoskeleton, hyper-condensation and fragmentation of the chromatin. Induction of apoptosis has been demonstrated following Nos treatment in various types of cancer cells like human ovarian carcinoma [16], mouse thymocytes E.G7-OVA and HeLa cells [21]. The human ovarian cells treated with 20 μM of Nos showed induction of apoptosis and the percentages of TUNEL-positive cells were increased with the time of treatment [16]. The percentages of TUNEL-positive cells were higher at 40 μM (57%) compared to 30 μM (32%), which demonstrates concentration dependant induction of apoptosis in H460 cells. The antimicrotubule agents, including Nos, are able to arrest mitosis in mammalian cells [15].
The Flow cytometric evaluation was used to study cell cycle arrest mechanism and to determine nature of cytotoxicity of Nos in various cancer cells [15, 21]. Landen et al. demonstrated that formation of abnormal nuclei and improperly aligned mitotic spindle in Murine B16LS9 melanoma cells following Nos treatment [15]. Also, Aneja et al. demonstrated that Nos (20 μM) induced cell cycle mitotic arrest and DNA degradation, an event reminiscent of apoptosis in HeLa cells [21]. The Nos cell cycle arrest mechanism studies against Human NSCLC cells using Flow cytometery are in progress in our lab to investigate the mitotic arrest mechanism.
After establishing the efficacy of Nos on H460 NSCLC in vitro, we designed in vivo experiments to test the efficacy of Nos against H460 NSCLC xenograft model in mice. We evaluated Nos in vivo antitumor efficacy at a different doses levels ranging from 150 to 550 mg/kg/day against H460 xenograft model in mice administered by oral gavage. Nos at a dose of 150 mg/kg/day showed negligible reduction in tumor volume (data not shown). However, the earlier reports have indicated that Nos was effective at similar doses: in regressing human breast tumors when administered 120 mg/kg/day intraperitoneally; and in regressing lymphoma tumors upon administration by oral gavage at a dose of 120 mg/kg/day [21]. In another investigation, oral administration of Nos at a dose of 300 mg/kg/day showed a significant regression of melanoma tumors compared to untreated animals [15]. Our studies indicate that the dose and route of administration is important to realize the efficacy of Nos in vivo. Therefore, we conducted studies to investigate the effect of Nos dose on the tumor regression. Our results demonstrated that Nos regressed the tumors in a dose dependant fashion following sequence 300 < 450 < 550 mg/kg/day (Fig 3a, b). Administration of Nos at 550 mg/kg (P < 0.01) through oral gavage showed higher reduction in tumor volume in H460 xenograft model compared to doses 450 (P < 0.01) and 300 mg/kg/day (P < 0.05) (Fig. 3a) reveals superior antitumor activity at higher doses. The dose dependant antitumor activity of Nos in xenograft model may be attributed to: (a) short plasma half life of Nos and its availability at the tumor site; and (b) extensive first-pass metabolism that reduces the oral bioavailability of Nos [24–26]. Our future studies will focus on improving the tumor targeting of Nos, so that it is effective at lower doses in vivo. There are very few reports available in literature on evaluation of Nos pharmacokinetics and absorption when given orally at various doses and a detailed systematic study is desirable.
The role of apoptosis was further investigated by determining expression of various apoptotic proteins such as PARP, caspase-3, Bax and Bcl2 in regressed tumors. We also determined the effect of Nos induced cleaved caspase activation on PARP cleavage, which is one of the downstream substrates of the caspase cascade and a reliable marker of apoptosis [27]. The WB blot analysis found a significant (P < 0.001) increase in expression of PARP and caspase-3 following Nos treatment at all doses (300, 450, 550 mg/kg) levels and suggesting the induction of apoptosis (Fig. 5a–c). Similarly, Nastaran et al showed that up regulation of PARP and Caspase-3 against human myelogenous leukemic cancer cells following Nos treatment [19]. It was previously reported that the induction of apoptosis by Nos in human myelogenous leukemic [19] and human colon cancer (p53-wt) cells [28] were mediated through mitochondria pathway and that increased Bax/Bcl2 ratio plays an important role in the induction of apoptosis. To check, if this holds true in case of H460 tumor tissues, the expression of Bcl2 family proteins such as Bax and Bcl2, following Nos treatment at different doses levels were determined. WB results showed a significant (P < 0.01) increase in expression of Bax levels and also significant (P < 0.01) down-regulation of Bcl2 protein accompanied with Bcl2 phosphorylation (Fig 5a, d, e) following Nos treatment at all doses levels. The protective effect of Bcl2 against apoptosis is lost by the phosphorylation of Bcl2 protein. The Nos 300, 450, 550 mg/kg treatment resulted in two, three and fivefold increase in the Bax/Bcl2 ratio compared to control demonstrating shifting of Bax/Bcl2 ratio plays an important role in Nos induced apoptosis. Furthermore, the increase in Bax/Bcl2 ratio indicates involvement of a mitochondrial mediated apoptotic process in H460 cells. Our findings related to the increase in Bax/Bcl2 ratio indicated similar line of evidence of involvement of mitochondrial pathway in Nos induced apoptosis reported with human myelogenous leukemic and human colon cancer cells [19, 28]. The up regulation of Bax and concomitant down-regulation of Bcl2 are pivotal in shifting the ratio of Bax/Bcl2 in the H460 cells and ultimately favor apoptosis. The WB results indicate involvement of a mitochondrial-mediated apoptotic process in antitumor activity of Nos against H460 NSCLC cells. The antitumor activity of Nos resulting from mitochondrial-mediated apoptotic process was also demonstrated against other cancer cell lines such as melanoma [15] ovarian [16], human myelogenous leukemic cells [19], brain glioblastoma [20], and breast [21] cancers. and human colon cancer cells [28].
To investigate the possible involvement of apoptosis in the antitumor effects of Nos, we studied caspase-3 expression and DNA fragmentation in tumor tissues obtained from control and treated mice by immunohistochemistry and TUNEL assay, respectively. We observed increased expression of cleaved caspase-3 (Fig. 6b, c) and increased number of apoptotic cells (Fig. 6e, f) in the Nos treated tumors compared to control tumor tissues (Fig. 6a, d). The studies conducted by Ye et al. with tumor xenograft of MCF-7 breast cell line and Renal 1983 bladder cell lines in mice following Nos treatment at 120 mg/kg/day demonstrated increased apoptotic activity in regressed tumor tissues [21]. Our in vivo results showed increased apoptotic activity and correlated very well with our in vitro results.
In conclusion, our data provide compelling evidence that Nos is effective against H460 NSCLC cells as well as tumor xenografts by inducing apoptosis. We demonstrated that Nos is an effective cytotoxic agent against H460 NSCLC cells and showed antitumor activity via mitochondrial-mediated apoptotic process. Nos was also able to significantly reduce the tumor growth in H460 xenograft model. While, the currently available chemotherapeutic agents are associated with drug resistance and debilitating toxic side effects, Nos provides promise as an effective anticancer agent with significantly lower toxicity on normal cells. The current investigation data argue strongly that Nos may be promising chemotherapeutic agents for the treatment of human lung cancer. However, it will be necessary to continue investigations of its antitumor activity on other lung cancer cells. The current investigation also indicates that the delivery and targeting of Nos is critical to realizing its full chemotherapeutic potential.
Acknowledgments
We thank Dr. Akash Gunjan, Assistant Professor, College of Medicine Dept. of Biomedical Sciences, Florida State University, Tallahassee, FL for availing X ray Wlm processor facility and Dr. Rakesh, post doctoral researcher, College of Medicine Dept. of Biomedical Sciences, Florida State University, for helping us in conducting part of molecular work.
References
- 1.Wakelee H, Belani CP (2005) Optimizing First-Line treatment options for patients with advanced NSCLC. Oncologist 10:1–10 [DOI] [PubMed] [Google Scholar]
- 2.Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR (2004) American Society of Clinical Oncology Treatment of Unresectable Non-Small-Cell Lung Cancer Guideline: Update 2003. J Clin Oncol 22(2):330–353 [DOI] [PubMed] [Google Scholar]
- 3.The International Adjuvant Lung Cancer Trial Collaborative Group (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med 350:351–360 [DOI] [PubMed] [Google Scholar]
- 4.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, Fry W, Bethune D, Ayoub J, Ding K, Seymour L, Graham B, Tsao MS, Gandara D, Kesler K, Demmy T, Shepherd F (2005) Vinorelbine plus cisplatin vs. observation in resected non-small cell lung cancer. N Engl J Med 352:2589–2597 [DOI] [PubMed] [Google Scholar]
- 5.Strauss GM, Herndon J, Maddaus MA, Johnstone DW, Johnson EA, Watson DM, Sugarbaker DJ, Schilsky RL, Green MR (2004) Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in stage IB non-small cell lung cancer: report of Cancer and Leukemia Group B protocol 9633. J Clin Oncol, 22(14S):621s, Abstract #7019 [Google Scholar]
- 6.Socinski MA, Stinchcombe TE (2007) Duration of First-line chemotherapy in advanced non–small-cell lung cancer: less is more in the era of effective subsequent therapies. J Clin Oncol 25(33):5155–5157 [DOI] [PubMed] [Google Scholar]
- 7.Jordan MA (2002) Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2:1–17 [DOI] [PubMed] [Google Scholar]
- 8.Fleming S, Lucas F, Schofield MA (2001) Therapeutic area review of oncology products and players. Expert Opin Emerg Drugs 6:317–329 [DOI] [PubMed] [Google Scholar]
- 9.Van Zuylen L, Verweij J, Sparreboom A (2001) Role of formulation vehicles in taxane pharmacology. Invest New Drugs 19:125–141 [DOI] [PubMed] [Google Scholar]
- 10.Markman M (2003) Managing taxane toxicities. Support Care Cancer 11:144–147 [DOI] [PubMed] [Google Scholar]
- 11.Anderson JT, Ting AE, Boozer S, Brunden KR, Crumrine C, Danzig J, Dent T, Faga L, Harrington JJ, Hodnick WF, Murphy SM, Pawlowski G, Perry R, Raber A, Rundlett SE, Stricker-Krongrad A, Wang J, Bennani YL (2005) Identification of novel and improved antimitotic agents derived from noscapine. J Med Chem 48:7096–7098 [DOI] [PubMed] [Google Scholar]
- 12.Anderson JT, Ting AE, Boozer S, Brunden KR, Danzig J, Dent T, Harrington JJ, Murphy SM, Perry R, Raber A, Rundlett SE, Wang J, Wang N, Bennani YL (2005) Discovery of S-phase arresting agents derived from noscapine. J Med Chem 48:2756–2758 [DOI] [PubMed] [Google Scholar]
- 13.Aneja R, Zhou J, Vangapandu SN, Zhou B, Chandra R, Joshi HC (2006) Drug-resistant T-lymphoid tumors undergo apoptosis selectively in response to an antimicrotubule agent, EM011. Blood 107:2486–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D, Joshi HC (2003) Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol 63:799–807 [DOI] [PubMed] [Google Scholar]
- 15.Landen JW, Lang R, McMahon SJ, Rusan NM, Yvon AM, Adams AW, Sorcinelli MD, Campbell R, Bonaccorsi P, Ansel JC, Archer DR, Wadsworth P, Armstrong CA, Joshi HC (2002) Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res 62:4109–4114 [PubMed] [Google Scholar]
- 16.Zhou J, Gupta K, Yao J, Ye K, Panda D, Giannakakou P, Joshi HC (2002) Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J Biol Chem 277:39777–39785 [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Liu M, Luthra R, Jones J, Aneja R, Chandra R, Tekmal RR, Joshi HC (2005) EM012, a microtubule-interfering agent, inhibits the progression of multidrug-resistant human ovarian cancer both in cultured cells and in athymic nude mice. Cancer Chemother Pharmacol 55:461–465 [DOI] [PubMed] [Google Scholar]
- 18.Ke Y, Ye K, Grossniklaus HE, Archer DR, Joshi HC, Kapp JA (2000) Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother 49:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidari N, Goliaei B, Moghaddam PR, Rahbar-Roshandel N, Mahmoudian M (2007) Apoptotic pathway induced by noscapine in human myelogenous leukemic cells. Anticancer Drugs 18(10):1139–1147 [DOI] [PubMed] [Google Scholar]
- 20.Landen JW, Hau V, Wang M, Davis T, Ciliax B, Wainer BH, Van Meir EG, Glass JD, Joshi HC, Archer DR (2004) Noscapine crosses the blood–brain barrier and inhibits glioblastoma growth. Clin Cancer Res 10:5187–5201 [DOI] [PubMed] [Google Scholar]
- 21.Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC (1998) Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA 95:1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaik MS, Chatterjee A, Singh M (2004) Effect of a selective cyclooxygenase (COX)-2 inhibitor, nimesulide on the growth of lung tumors and their expression of COX-2 and peroxisome proliferator-activated receptor-γ. Clin Cancer Res 10:1521–1529 [DOI] [PubMed] [Google Scholar]
- 23.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Ichite N, Singh M (2007) 15-Deoxy-Delta12,14-prostaglandin J2 enhances docetaxel antitumor activity against A549 and H460 non-small-cell lung cancer cell lines and xenograft tumors. Anticancer Drugs 18(1):65–78 [DOI] [PubMed] [Google Scholar]
- 24.Karlsson MO, Dahlstrom B, Eckernas SA, Johansson M, Alm AT (1990) Pharmacokinetics of oral noscapine. Eur J Clin Pharmacol 39:275–279 [DOI] [PubMed] [Google Scholar]
- 25.Aneja R, Dhiman N, Idnani J, Awasthi A, Arora SK, Chandra R, Joshi HC (2007) Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin-binding anticancer agent Cancer Chemother Pharmacol 60:831–839 [DOI] [PubMed] [Google Scholar]
- 26.Dahlstrom B, Mellstrand T, Lofdahl CG, Johansson M (1982) Pharmacokinetic properties of noscapine. Eur J Clin Pharmacol 22:535–539 [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG (1993) SpeciWc proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis Cancer Res 53:3976–3985 [PubMed] [Google Scholar]
- 28.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC (2007) p53 and p21 Determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res 67:3862–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]


