Abstract
Renal fibrosis is a critical event in the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD). Unfortunately, there are few options to target renal fibrosis in order to develop novel anti-fibrotic agents that could prevent CKD progression to ESRD. We evaluated the efficacy of a novel dual-acting molecule, DM509, in preventing renal fibrosis using the unilateral ureteral obstruction (UUO) renal fibrosis mouse model. DM509 acts simultaneously as a farnesoid X receptor agonist (FXRA) and a soluble epoxide hydrolase inhibitor (sEHi). In this study, groups of 8-12 weeks old C57BL/6J male mice went through either UUO or sham surgery (n=6/group). Mice were pre-treated with DM509 (10mg/kg/d) or vehicle administered in drinking water one day prior to the UUO surgery. Sham, vehicle and DM509 treatments continued until day 10 and blood and kidney tissue were collected for biochemical, histological, and gene expression analysis at the end of the treatment protocol. The UUO group exhibited kidney dysfunction with elevated blood urea nitrogen (BUN) compared to the sham group (63±7 vs. 34±6 mg/dL). DM509 treatment prevented renal dysfunction as evident from 36% lower BUN level in the DM509 treated UUO mice compared to UUO mice treated with vehicle. Vehicle treated UUO mice demonstrated renal fibrosis with elevated kidney hydroxyproline content (213±11 vs. 49±9 μg/mg protein) and kidney collagen positive area (13±2% vs. 1.1±0.1%) compared to the sham group. We found that DM509 treatment prevented renal fibrosis and DM509 treated mice had 34-66% lower levels of kidney hydroxyproline and collagen positive renal area compared to vehicle-treated UUO mice. In conclusion, our data provide evidence that the novel dual-acting FXRA and a sEHi, DM509, prevented renal dysfunction and renal fibrosis in UUO mouse model.
Keywords: soluble epoxide hydrolase inhibitor, farnesoid x receptor agonist, kidney fibrosis
Introduction.
Renal fibrosis is considered as critical pathophysiological event in the development and progression of chronic kidney disease (CKD). Progressive CKD results in end-stage renal disease (ESRD), which is the common clinical end point for all progressive renal diseases [3]. The common CKD etiologies and the consequent ESRD include diabetes, hypertension, glomerulonephritis, acute kidney injury, and chronic pyelonephritis. ESRD is a major burden to the health care system and a large percentage of the patients are inevitably placed on dialysis and ultimately require transplantation [3, 16]. The ESRD burden on health care is caused largely due to the lack of an effective anti-fibrotic agents that can target CKD.
Indeed, little success has been made over the past decade in developing agents or therapies that can prevent renal fibrosis to slow the progression of CKD to ESRD [23]. Currently, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are used to reduce proteinuria and slow CKD progression [6]. Nevertheless, these renin-angiotensin system drugs have incomplete efficacy in preventing renal fibrosis and CKD progression. Clinical trials targeting other mechanisms have failed, including the nuclear factor E2-related factor 2 inducer bardoxolone [4]. and the endothelin receptor blocker avosentan [17].
We recently developed a bifunctional molecule DM509, that concurrently acts as a farnesoid X receptor (FXR) agonist and soluble epoxide hydrolase inhibitor (sEHi) and demonstrated its marked anti-fibrotic action in liver disease models [10]. FXR is a ligand activated transcription factor and is a member of the nuclear receptor superfamily that is highly expressed in liver and kidney [19, 22]. Several recent studies demonstrated anti-fibrotic action for FXR agonists in liver and kidney disease models [7,16,21]. A strong renal anti-fibrotic action is also known for sEHi in several published studies [2,12,13,14]. Considering the promising anti-fibrotic action in liver disease and potential to treat kidney fibrosis, we determined anti-fibrotic action for the novel bifunctional FXR agonist and sEHi, DM509, in a mouse renal fibrosis model.
Materials and methods.
Animal experiments
Unilateral Ureteral Obstruction (UUO) Surgery
This study was approved and conducted according to guidelines of the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. The Biomedical Resource Center at the Medical College of Wisconsin housed animals with free access to water and food and a 12/12h light-dark cycle. Male C57Bl/6J mice (8-10 weeks old) were purchased from Jackson Laboratories, Bar Harbor, ME. Mice were administered 2.0% isoflurane to induce anesthesia prior to UUO surgery. UUO surgery was conducted by obstructing the left ureter proximal to the renal pelvis using a 6–0 silk tie [14,19]. Mice with sham surgery went through the same procedure as the UUO mice except that the ureter was not ligated.
Experimental Protocol
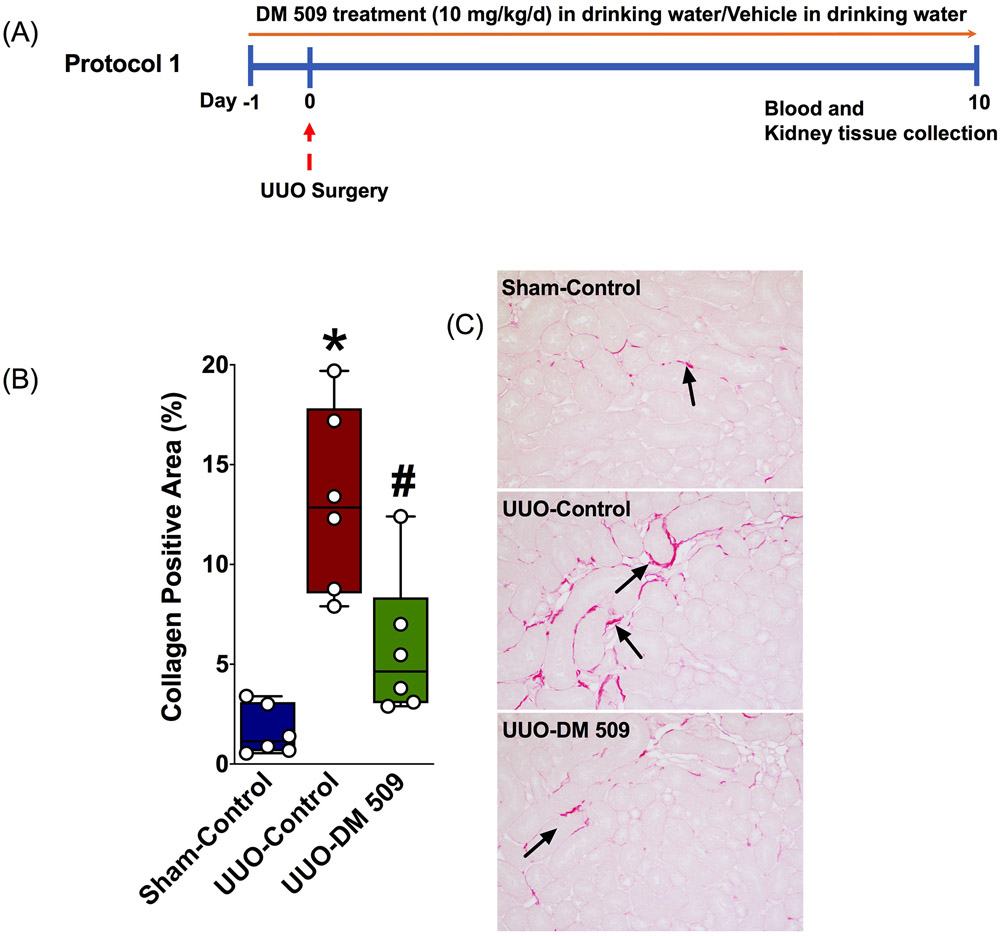
An experimental protocol was designed to investigate if our novel molecule DM509 can prevent the development of renal fibrosis. Mice went through UUO surgery 24 hours after the start of DM509 treatment (10mg/kg/d, p.o., n=6) or vehicle (n=6) and blood and kidney tissue were collected 10 days following UUO induction. A schematic of the experimental protocol is shown in Figure 1. DM509 drinking solution was prepared with a mixture of DMSO (0.05%) and cyclodextrin (0.5%). The vehicle was combination of DMSO (0.05%) and cyclodextrin (0.5%) given to mice in drinking water. At the end of the experimental protocol, mice were euthanized for blood and kidney tissue collection. The kidney tissue was processed for biochemical, histology, and gene expression analysis. Serum was separated from the blood and used for biochemical analysis of blood urea nitrogen (BUN).
Fig.1:
A schematic describing the experimental precool (A). Semi-quantitative measurement of collagen positive fibrotic kidney area in different experimental groups (B). A representative photomicrograph depicting collagen positive kidney fibrotic area (black arrows) in different experimental groups (C). *P vs. Sham-Control; #P vs. UUO-Control, N=5-7.
Biochemical Analysis
Kidney hydroxyproline levels were measured using a commercially available kit (Sigma Aldrich, USA). BUN was measured using a kit from Arbor Assay, USA and monocyte chemoattractant protein-1 (MCP-1) was measured using kit from R&D Systems, Inc., USA.
Gene Expression Analysis
Renal mRNA expression of several fibrotic [fibronectin, α- smooth muscle actin (α-SMA)] and inflammatory (TNF-α, IL1β, IL-6) were studied by Real Time-PCR (RT-PCR) analysis. RNeasy Mini Kit (QIAGEN, CA, USA) was used according to the manufacturer’s protocol and messenger RNA (mRNA) was prepared from each sample homogenate. The mRNA samples were quantified spectrophotometrically and 1μg of total RNA was reverse-transcribed to cDNA using iScript™ Select cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Gene expression was quantified by iScript One-Step RT-PCR Kit with SYBR green using the MyiQ™ Single Color Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Dissociation curve analysis was accomplished with iQ5 Optical System Software, Version 2.1 (Bio-Rad Laboratories, Hercules, CA, USA), and each amplified sample analysed for homogeneity. Denaturation was done at 95°C for 2 min followed by 40 cycles conducted at 95°C for 10s and at 60°C for 30s. All samples were run in triplicate and fold change in gene expression compared to controls determined by comparative threshold cycle (Ct) method. Target gene expression levels were determined by normalizing Ct values to two housekeeping genes. Statistical analyses were carried out using six samples from each experimental group and comparing to the control group.
Histopathology
Renal tissues were fixed in 10% formalin, sectioned a 4μm thickness, mounted on slides, and stained with Picrosirius Red (PSR) (Alfa Aesar, Tewksbury, MA) for histological examination at a 200x magnification using NIS Elements AR version 3.0 imaging software (Nikon instruments Inc., Melville, NY, USA). PSR stained kidney tissue sections was used to determine collagen-positive renal interstitial fibrosis levels. Histopathological changes were scored as published previously and scores presented as a percentage area-fraction relative to the total area analysed. Kidney interstitial fibrosis (PSR-collagen positive areas) scoring was performed in a blinded fashion by two observers.
Statistical Analysis
All data are expressed as mean ± S.E.M. GraphPad Prism® Version 4.0 software was utilized to conduct a one-way ANOVA followed by Tukey’s post-hoc test to establish statistical significance between groups (GraphPad Software Inc, La Jolla, CA, USA). Two-tailed unpaired Student’s t-test was applied to determine statistical significance between groups. A P <0.05 was deemed significant.
Results and discussion.
DM509 treatment prevented kidney dysfunction in UUO model
In the present study we determined the ability of our novel dual acting molecule, DM509, to prevent kidney dysfunction in a mouse renal fibrosis model. We assessed renal dysfunction by measuring the BUN level. BUN is considered an important marker for kidney dysfunction. BUN measures urea in the blood and urea is the primary metabolite derived from dietary protein and tissue protein turnover. Urea is freely filtered at the glomerulus but not secreted, and it is reabsorbed by the renal tubules. In addition, as urine flow rates decrease, more urea is reabsorbed [1]. Therefore, BUN levels are inversely correlated with the decline of kidney function. The results of the current study demonstrate that there is kidney dysfunction with a 2-fold higher BUN level in the UUO mice compared to sham (63±7 vs. 34±6 mg/dL). Interestingly, DM509 prevented BUN level elevation and it was 36% lower in the DM509 treated mice compared to the mice treated with vehicle (40±8 mg/dL). These finding indicate that DM509 prevents kidney dysfunction in the UUO renal fibrosis model. It is important to note that sEH inhibitors and farnesoid receptor agonists are known to have beneficial effects on kidney function. The protective effects of sEH inhibitor have been reported in renal fibrosis [13,14] hypertensive kidney injury and diabetic kidney injury models [5,10]. Similar kidney protective effects are also reported for farnesoid receptor agonists in renal fibrosis [15], diabetic nephropathy [21] and renal ischemia reperfusion injury [7]. The present study demonstrates the novel finding that the dual acting molecule that simultaneously act as sEH inhibitor and farnesoid receptor agonist, DM509, prevents renal fibrosis progression in the UUO mouse model.
DM509 treatment prevented kidney fibrosis in UUO model
Renal fibrosis is the clinical hallmark for CKD and considered the most important predictor of long-term CKD prognosis [16]. Renal fibrosis progression is the result of an excess accumulation of connective tissue, primarily collagen. In the present study, we have used quantitative method to determine kidney collagen content by measuring tissue hydroxyproline levels. The spectrophotometric-based hydroxyproline assay is one of the few that allows for actual quantitation of collagen content. The assay is based on the fact that all collagens contain globular domains and share the common structural motif of triple helical segments. This triple helical structure is composed of three α-(polypeptide) chains and each chain consists of a repeating triplet amino acid sequence containing hydroxyproline. Hence, based on the absolute hydroxyproline quantitation, the collagen amount and concentration in tissues can be determined [8]. As such, in the present study, we measured kidney hydroxyproline content in the experimental groups. We found that the UUO mice developed marked kidney fibrosis with elevated kidney hydroxyproline content (213±11 vs. 49±9 μg/mg protein) and DM509 treatment prevented the elevation of hydroxyproline level by 34% (140±9 μg/mg protein). The effect of DM509 treatment on kidney fibrosis was further assessed using a semi-quantitative histological method. Our data found that DM509 treatment prevents kidney fibrosis by reducing collagen positive kidney fibrotic area in UUO mice. The vehicle treated UUO mice had higher collagen positive area compared to sham (13±2% vs. 1.1±0.1%), and DM509 reduced the collagen positive area by 66% (4.4±0.7%) (Figure 1).
Prevention of renal fibrosis has been demonstrated by sEH inhibitors in several experimental disease models including UUO renal fibrosis models [11,13,14]. Anti-fibrotic action for farnesoid x receptor agonists has also been reported in several disease models including UUO renal fibrosis model [15,21]. In the present study we provide novel evidence that the dual acting molecule that acts as a sEH inhibitor and farnesoid x receptor agonist, DM509, has excellent ability to prevent renal fibrosis in UUO mice model.
DM509 treatment prevented kidney expression of fibrotic and inflammatory genes in UUO model
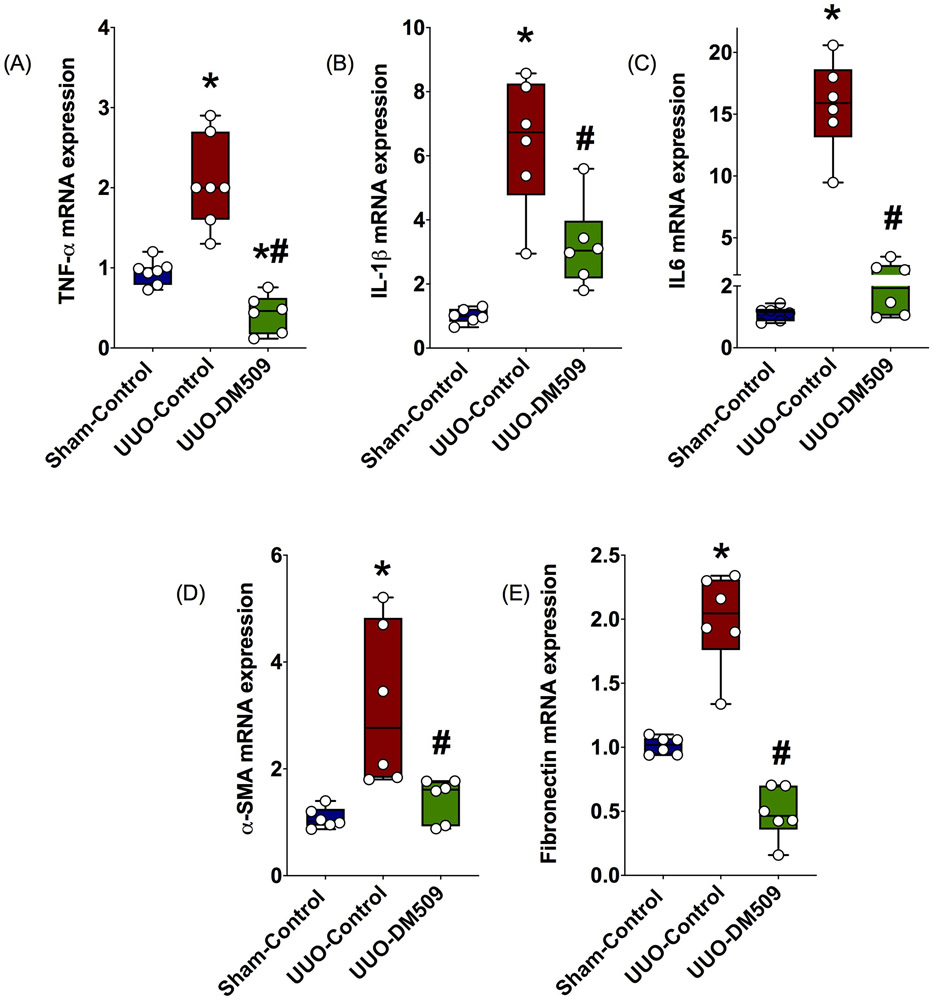
In the present study, we assessed kidney fibrotic mediators and inflammation in UUO mice treated with DM509. Fibrotic marker genes express several critical proteins that form the extracellular matrix in the kidney. It is important to note that kidney extracellular matrix formation is an important event in the kidney fibrotic process [16]. We found that the vehicle treated UUO mice had 2-3-fold higher kidney expression of α-smooth muscle actin (α-SMA) and fibronectin compared to sham, and DM509 treatment reduced expression of these fibrotic genes in the UUO mice by 40-60% (Figure 2). We also found that UUO mice had higher kidney expression of several pro-fibrotic inflammatory markers (TNF-α,IL1β, IL6) in the vehicle treated UUO mice compared to sham, and DM509 treatment prevented inflammatory gene expression in the UUO mice (Figure 2).
Fig. 2:
Kidney mRNA expressions of inflammatory marker genes in different experimental groups (A-C). Kidney mRNA expressions of α-smooth muscle actin (α-SMA) and fibronectin in different experimental groups. *P vs. Sham-Control; #P vs. UUO-Control, N=5-7.
These findings are consistent with previous studies demonstrating sEH inhibitors [13,14] and farnesoid x receptor agonists [7,15,21] prevent gene expression of fibrotic markers and pro-fibrotic genes in several kidney disease models.
Conclusion.
In the present study, we present exciting data on a novel molecule that simultaneously acts as a sEH inhibitor and farnesoid x receptor agonist, DM509. We provide convincing evidence that this novel dual-acting molecule, DM509, can prevent renal fibrosis development in the UUO renal fibrosis model. Overall, DM509 has potential to be developed as a novel kidney anti-fibrotic agent to treat CKD and prevent progression to ESRD and dialysis.
Contributor Information
A. Stavniichuk, Taras Shevchenko National University of Kyiv, ESC Institute of Biology and Medicine», Kyiv, Ukraine.
O. Savchuk, Taras Shevchenko National University of Kyiv, ESC Institute of Biology and Medicine», Kyiv, Ukraine.
Abdul Hye Khan, Department of Pharmacology & Toxicology, The Medical College of Wisconsin, Milwaukee, WI, USA.
Wojciech K. Jankiewicz, Department of Pharmacology & Toxicology, The Medical College of Wisconsin, Milwaukee, WI, USA..
John D. Imig, Department of Pharmacology & Toxicology, The Medical College of Wisconsin, Milwaukee, WI, USA.
Daniel Merk, Institute of Pharmaceutical Chemistry, Goethe-University of Frankfurt, Frankfurt am Main, Germany.
References (Scopus)
- 1.Berl T, Schrier RW. Disorders of water metabolism 6th ed. Schrier RW, editor. Renal and electrolyte disorders. Philadelphia: Lippincott Williams and Wilkins; 2002. p. 1–63. [Google Scholar]
- 2.Chiang CW, Lee HT, Tarng DC, Kuo KL, Cheng LC, Lee TS. Genetic deletion of soluble epoxide hydrolase attenuates inflammation and fibrosis in experimental obstructive nephropathy. Mediators Inflamm. 2015;693260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Gilbertson DT, et al. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin. J. Am. Soc. Nephrol 2009;4(Suppl1):S5–S11. [DOI] [PubMed] [Google Scholar]
- 4.De Zeeuw D, Akizawa T, Audhya P. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med 2013;369(26):2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmarakby AA, Faulkner J, Pye C, Rouch K, Alhashim A, Maddipati KR, Baban B. Role of haem oxygenase in the renoprotective effects of soluble epoxide hydrolase inhibition in diabetic spontaneously hypertensive rats. Clin Sci (Lond). 2013;125(7):349–59. [DOI] [PubMed] [Google Scholar]
- 6.Fried LF, Emanuele N, Zhang JH. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med 2013;369(20):1892–1903. [DOI] [PubMed] [Google Scholar]
- 7.Gai Z, Chu L, Xu Z, Song X, Sun D, Kullak-Ublick GA. Farnesoid X receptor activation protects the kidney from ischemia-reperfusion damage. Sci Rep. 2017;29;7(1):9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewitson TD, Smith ER, Samuel CS. Qualitative and quantitative analysis of fibrosis in the kidney. Nephrology (Carlton). 2014;19(11):721–6. [DOI] [PubMed] [Google Scholar]
- 9.Hye Khan MA, Schmidt J, Stavniichuk A, Imig JD, Merk D. A dual farnesoid X receptor/soluble epoxide hydrolase modulator treats non-alcoholic steatohepatitis in mice. Biochem Pharmacol. 2019;166:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imig JD. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol Ther. 2018;192:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;56(4):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92(1):101–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Imig JD, Yang J, et al. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol. 2014;307(8):F971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Yoon SP, Toews ML, et al. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308(2):F131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Ghoshal S, Sojoodi M, Arora G, Masia R, Erstad DJ, Ferriera DS, Li Y, Wang G, Lanuti M, Caravan P, Or YS, Jiang LJ, Tanabe KK, Fuchs BC. The farnesoid X receptor agonist EDP-305 reduces interstitial renal fibrosis in a mouse model of unilateral ureteral obstruction. FASEB J. 2019;33(6):7103–7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. [DOI] [PubMed] [Google Scholar]
- 17.Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol 2010;21(3):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Hye Khan MA, Levick SP, Lee KS, Hammock BD, Imig JD. Novel Omega-3 Fatty Acid Epoxygenase Metabolite Reduces Kidney Fibrosis. Int J Mol Sci 2016;17(5). pii: E751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh JM, Yu CT, Tang K, et al. (2006). The expression profiles of nuclear receptors in the developing and adult kidney. Mol Endocrinol. 2006;20(12):3412–3420. [DOI] [PubMed] [Google Scholar]
- 20.Verbeke L, Mannaerts I, Schierwagen R, et al. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H, et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59(11):2916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong J, Yang HC, Fogo AB. A perspective on chronic kidney disease progression. Am. J. Physiol. Renal Physiol 2017;312(3):F375–F384. [DOI] [PMC free article] [PubMed] [Google Scholar]