Abstract
PURPOSE
SARC024 is a phase II clinical trial of the multikinase inhibitor regorafenib in specific sarcoma subtypes, including advanced osteosarcoma. We hypothesized that regorafenib would improve progression-free survival (PFS) in patients with sarcoma and report the results of the osteosarcoma cohort.
PATIENTS AND METHODS
This trial enrolled patients with progressive metastatic osteosarcoma with measurable disease by RECIST who had received at least one prior line of therapy. Patients were randomly assigned at a ratio of one to one to regorafenib or placebo. Crossover was allowed at time of disease progression. PFS was the primary end point of the study, which was powered to detect a difference of at least 3 months in median PFS.
RESULTS
Forty-two patients from 12 centers were enrolled between September 2014 and May 2018. Median age was 37 years (range, 18 to 76 years). Patients had received an average of 2.3 prior therapy regimens. Ten patients receiving placebo crossed over to active drug at time of progression. Study enrollment was stopped early, after a data safety monitoring committee review. Median PFS was significantly improved with regorafenib versus placebo: 3.6 months (95% CI, 2.0 to 7.6 months) versus 1.7 months (95% CI, 1.2 to 1.8 months), respectively (hazard ratio, 0.42; 95% CI, 0.21 to 0.85; P = .017). In the context of the crossover design, there was no statistically significant difference in overall survival. Fourteen (64%) of 22 patients initially randomly assigned to regorafenib experienced grade 3 to 4 events attributed to treatment, including one grade 4 colonic perforation.
CONCLUSION
The study met its primary end point, demonstrating activity of regorafenib in patients with progressive metastatic osteosarcoma. No new safety signals were observed. Regorafenib should be considered a treatment option for patients with relapsed metastatic osteosarcoma.
INTRODUCTION
Despite substantial efforts made over the past 30 years, outcomes for osteosarcoma have not changed significantly.1,2 Primary therapy typically consists of surgery and neoadjuvant and adjuvant chemotherapy with doxorubicin, cisplatin, and methotrexate, yielding cure rates of 65% to 70%.3 Patients who experience relapse with metastatic disease have limited options, often receiving additional cytotoxic therapy, such as ifosfamide or gemcitabine plus docetaxel, with expected 4-month progression-free survival (PFS) of only 12%.4-6
Activity of multikinase inhibitors in refractory osteosarcoma has been observed in recent trials.7-12 A small phase II trial of sorafenib suggested an improvement in PFS.7 Regorafenib, a multikinase inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, RET, KIT, platelet-derived growth factor receptor β and others, is a small-molecule inhibitor with a biochemical profile similar to that of sorafenib but is pharmacologically more potent.8 One patient with osteosarcoma who was treated in the phase I trial of regorafenib achieved a durable partial response (PR).8
The Sarcoma Alliance for Research Through Collaboration (SARC) conducted a trial of regorafenib in specific histologic subtypes of sarcoma. Patients eligible for SARC024 were enrolled in one of four histology-specific trial arms: Ewing sarcoma, alveolar or embryonal rhabdomyosarcoma, liposarcoma, and osteosarcoma. We have previously reported that regorafenib has activity in Ewing sarcoma and that no clear benefit was seen over placebo in liposarcoma; enrollment in the rhabdomyosarcoma cohort is ongoing.13,14 We now report the results of the randomized phase II cohort for patients with advanced or metastatic osteosarcoma.
PATIENTS AND METHODS
Patients
Eligible patients had a diagnosis of advanced or metastatic bone or extraskeletal osteosarcoma. Requirements at baseline included age 10 years or older, body surface area of 0.65 m2 or greater, WHO performance status of 0 to 2 (with prespecified maximum of 16 patients with WHO performance status of 2), at least one prior line of systemic therapy in the neoadjuvant, adjuvant, or metastatic setting, adequate organ function, ability to swallow oral medication, and measurable disease by RECIST (version 1.1),15 with evidence of progressive disease within 6 months of enrollment.
Patients previously treated with a small-molecule oral kinase inhibitor were excluded, as were patients with uncontrolled hypertension, clinically significant cardiac disease, nonhealing wound, Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or worse bleeding within 4 weeks, thromboembolic event within 6 months, ongoing CTCAE grade 2 or worse infection, or any malabsorption condition.
Study Design and Conduct
This study was performed after approval by institutional review boards at all sites in accordance with an assurance filed with and approved by the US Department of Health and Human Services. All patients provided written informed consent to participate in this study.
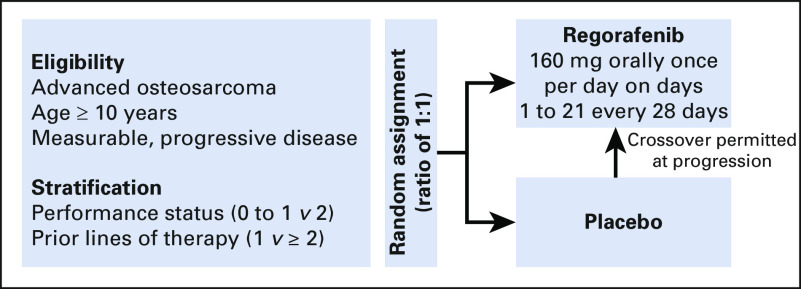
The osteosarcoma cohort of SARC024 was a multicenter randomized placebo-controlled double-blind phase II study with a primary end point of PFS. Patients were randomly assigned at a one-to-one ratio to receive either active drug (regorafenib) or placebo (Fig 1). Crossover to active drug was permitted at time of progression for patients receiving placebo.
FIG 1.
SARC024 study schema. Imaging occurred every 8 weeks for the first 32 weeks and then every 12 weeks.
Treatment
Patients received either placebo or regorafenib provided by Bayer at an initial dose of 160 mg (four 40-mg tablets) taken orally in the morning with a low-fat meal on days 1 to 21 of each 28-day cycle. Patients continued to receive study treatment until either RECIST (version 1.1) progression, more than 28 days elapsed since last dose of study drug, or patient- or physician-initiated discontinuation.
Dose Modification for Toxicities
Toxicities were evaluated and graded with CTCAE (version 4.03) on a weekly basis for the first 3 weeks, every other week for the following 12 weeks, and then every 4 weeks. Dose reductions were permitted for clinically significant grade 2 toxicities related to regorafenib at the discretion of the investigator and were required for clinically significant toxicities Grade 3 or worse. Two dose reductions, each by increments of 40 mg per day (to 120 and then to 80 mg), were permitted from the starting dose of regorafenib on the same schedule.
Response Assessment
Tumor assessments were performed by the investigators using RECIST (version 1.1). Baseline study scan was required within 28 days of cycle 1 day 1. Thereafter, tumor assessments were performed every 8 weeks for the first 32 weeks and then every 12 weeks, with a ± 7-day window of the anticipated scan date.
Statistical Analyses
The study was powered to detect a difference of at least 3 months in median PFS, which was considered a clinically meaningful improvement in outcome. As initially designed, 42 events were needed to detect a difference with 90% power and 5% one-sided significance level, with an expectation of 48 patients randomly assigned.
Secondary end points included incidence of CTCAE (version 4.03) adverse events (AEs), overall response rate per RECIST (version 1.1), time to tumor progression (TTP), PFS at 8 and 16 weeks, overall survival (OS), and PFS, OS, overall response rate, and TTP after crossover.
After the release of the REGOBONE (Regorafenib in Patients With Metastatic Bone Sarcomas) trial results,16 a study of similar design that showed a significant benefit of regorafenib in patients with osteosarcoma, an independent data safety and monitoring committee (DSMC) was convened because of concern regarding continuing enrollment in a placebo-controlled study. The DSMC recommended closing the study after enrollment of 42 of 48 planned participants and 31 of the required 42 PFS events.
All analyses were determined in the intention-to-treat population. Survival was estimated by the Kaplan-Meier method, and treatment groups were compared with a one-sided stratified log-rank test. A PFS prognostic factor analysis used a univariable Cox model, and significant factors were subsequently included in a multivariable Cox regression model (cutoff P < .05). For a predictive analysis of PFS, Cox models were generated with the investigated factor, treatment, and their interaction, with a significance value of P < .05. P values were not adjusted for multiple comparisons. Patients were stratified by WHO performance status (0 to 1 v 2) and by number of prior lines of therapy (one v two or more).
RESULTS
Patient Characteristics
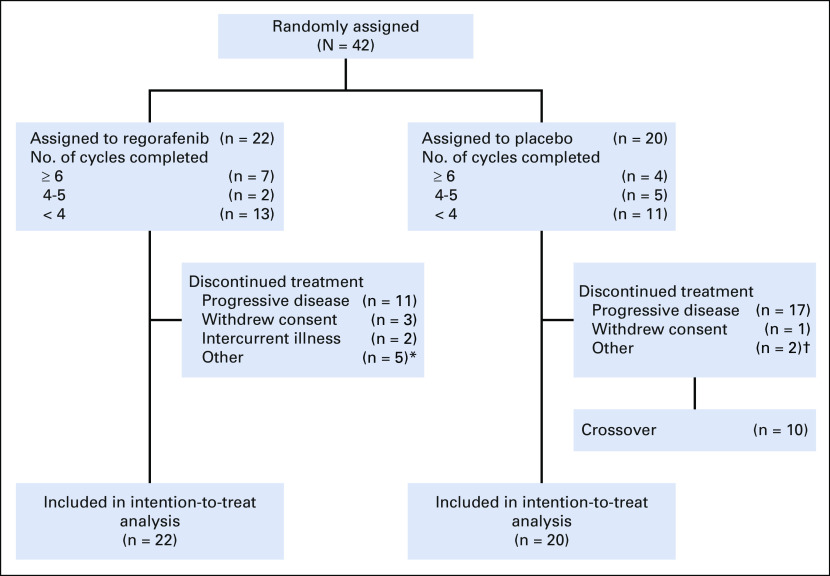
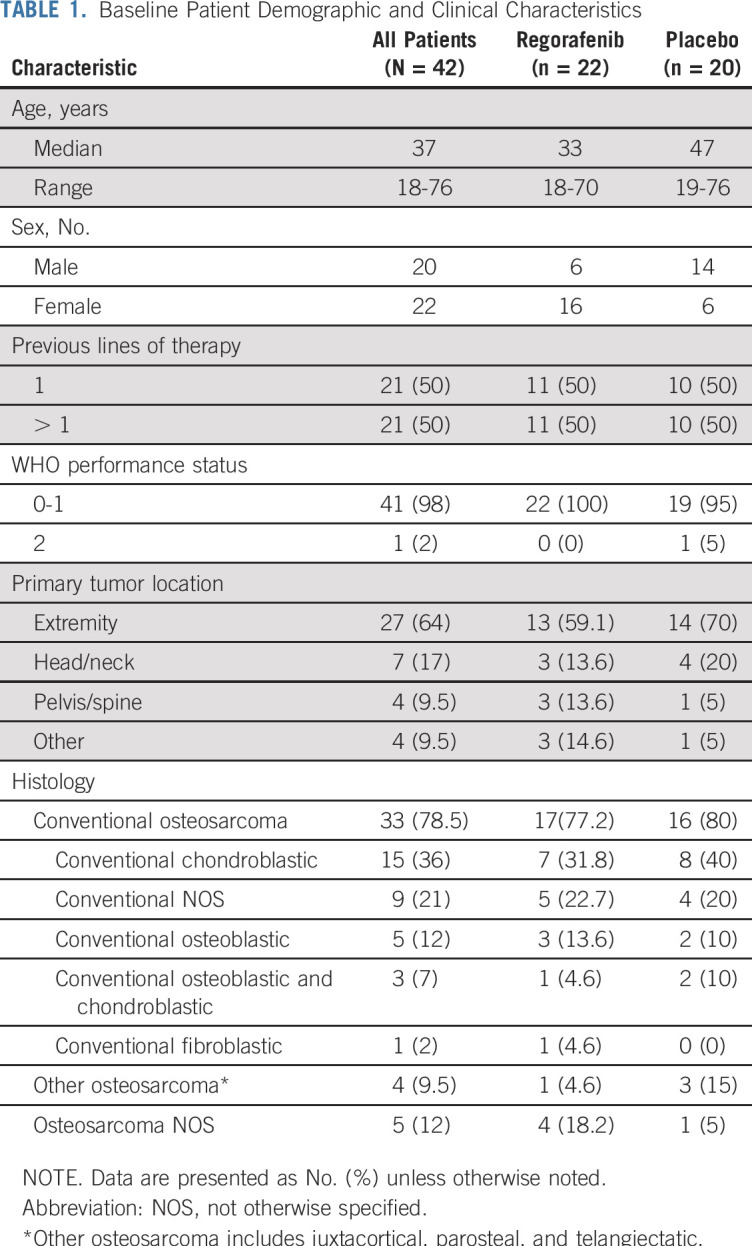
A total of 42 patients from 12 centers in the United States were enrolled between September 2014 and May 2018 (Fig 2). Baseline characteristics were balanced (Table 1), although patients randomly assigned to placebo were older and more commonly men. Median age was 37 years (range, 18 to 76 years). Patients had received an average of 2.3 prior therapy regimens (range, one to 16 regimens). Although history of radiotherapy was not part of required data, review of submitted pathology reports identified osteosarcoma arising within prior irradiation fields in five patients (breast cancer, n = 3; head and neck cancer, n = 1; soft tissue sarcoma, n = 1).
FIG 2.
CONSORT diagram. (*) Physician decision (n = 1), second cancer (n = 1), noncompliance (n = 1), and alternate therapy (n = 2). (†) Physician decision (n = 1) and financial reason (n = 1).
TABLE 1.
Baseline Patient Demographic and Clinical Characteristics
Efficacy
At the time of analysis, a statistically significant and clinically meaningful difference in PFS was observed between regorafenib and placebo. Median PFS was 3.6 months (95% CI, 2.0 to 7.6 months) for regorafenib and 1.7 months (95% CI, 1.2 to 1.8 months) for placebo (hazard ratio, 0.42; 95% CI, 0.21 to 0.85; P = .017; Fig 3A). PFS at 8 weeks and 16 weeks was 79.0% and 44.4% for patients receiving regorafenib, respectively, compared with 25.0% (Fisher’s exact text P = .001) and 10.0% (Fisher’s exact test P = .027) for the placebo group.
FIG 3.
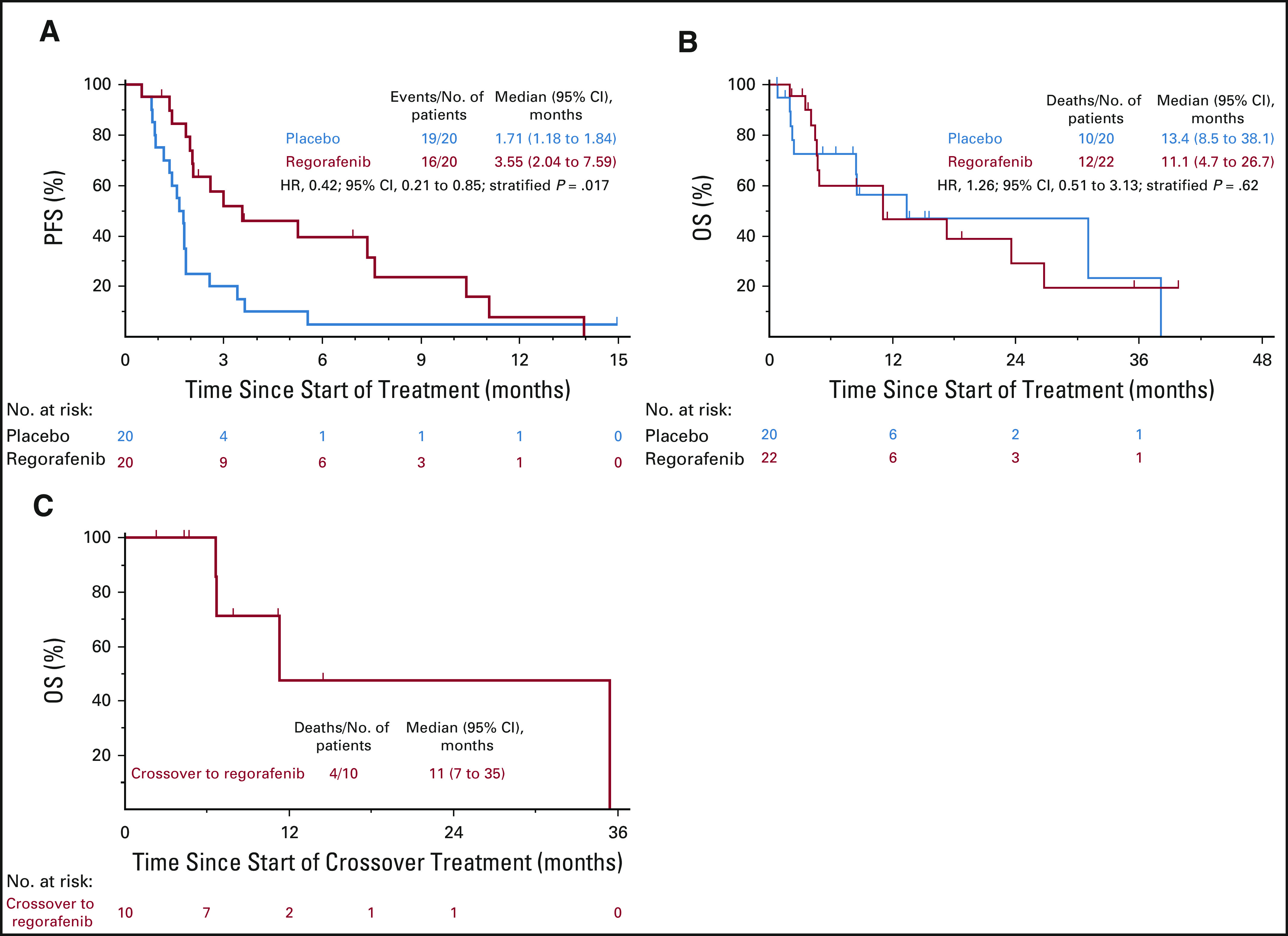
Kaplan-Meier estimates for (A) progression free survival (PFS), (B) overall survival (OS), and (C) OS after crossover from placebo to regorafenib. HR, hazard ratio.
At the time of data cutoff, 20 (48%) of 42 patients were alive. Median follow-up among living patients was 7.4 months. Median OS was 11.1 months (95% CI, 4.7 to 26.7 months) for patients initially randomly assigned to regorafenib and 13.4 months (95% CI, 8.5 to 38.1 months) for patients initially randomly assigned to placebo (hazard ratio, 1.26; 95% CI, 0.51 to 3.13; P = .62; Fig 3B). Ten patients receiving placebo crossed over to active drug at time of progression (Fig 3C). Three patients were receiving blinded treatment at the time of study closure; all three were immediately unblinded and were shown to have been receiving active drug.
Three patients (13.6%) randomly assigned to regorafenib achieved PRs per RECIST (version 1.1). These patients had received one, three, and five lines of prior therapy, respectively. There were no objective responses in the placebo group.
No prognostic or predictive factors for PFS were identified. Tumor location, histology, age, prior lines of therapy, and TTP during prior line of therapy were not significantly associated with PFS. Although male sex was associated with worse PFS as a single variable, the association was no longer significant after taking into account the effect of treatment, because there were more men randomly assigned to placebo.
Toxicity
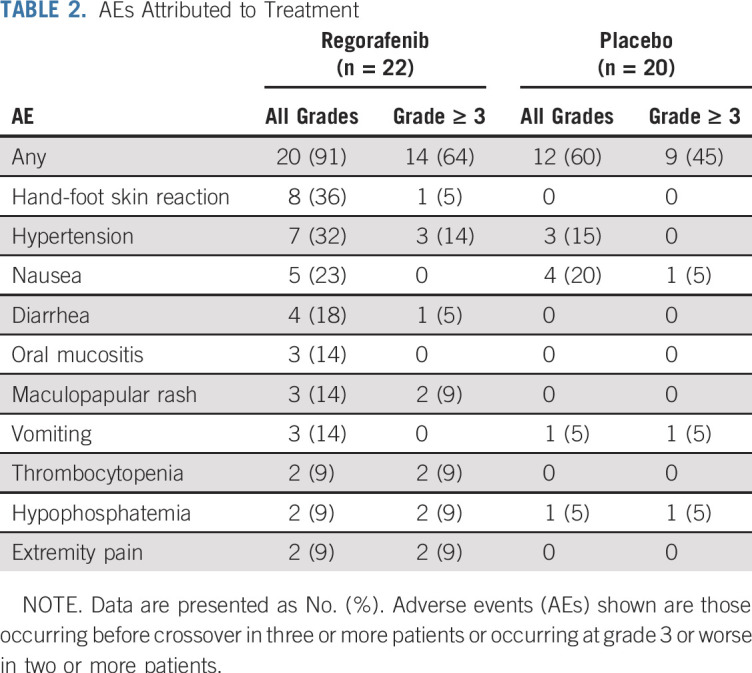
Treatment-emergent AEs were comparable to those reported in prior studies of regorafenib. Table 2 lists all treatment-emergent AEs deemed at least possibly related to study drug occurring in three or more patients or occurring at grade 3 or worse in two or more patients. AEs were more frequent in the regorafenib arm than in the placebo arm. Patients receiving regorafenib experienced hand-foot skin reactions (grade 1 to 2 in seven patients [32%] and grade 3 in one patient [5%] compared with no patients receiving placebo) and GI disorders (grade 1 to 2 in 11 patients [50%] compared with grade 1 to 3 in five patients [25%] receiving placebo). Grade 3 hypertension occurred in three patients (14%) assigned to regorafenib, with an additional four patients (18%) experiencing grade 2 hypertension; three patients (15%) receiving placebo experienced grade 1 to 2 hypertension. There was one grade 3 pneumothorax and one grade 4 colonic perforation attributed to study drug; both of these events occurred in patients who achieved PRs. These two patients only completed two cycles at the 160-mg dose because of AEs but continued to receive treatment at either the 120- or 80-mg dose level. There were no grade 5 events.
TABLE 2.
AEs Attributed to Treatment
During initial blinded treatment, 20 patients required dose interruption (13 assigned to regorafenib, seven assigned to placebo). Dose was reduced for twelve patients assigned to regorafenib and one assigned to placebo. Median dose at end of blinded treatment was 120 mg (range, 80 to 160 mg) for regorafenib and 160 mg (range, 40 to 160 mg) for placebo. Median number of cycles of regorafenib completed at full dose (160 mg) was two (range, zero to 12 cycles).
DISCUSSION
The SARC024 study demonstrates benefit of regorafenib in patients with relapsed metastatic osteosarcoma, with a doubling of median PFS compared with placebo. Regorafenib represents one of the few agents tested in a randomized setting showing a PFS advantage over placebo. Data from a similarly designed study of regorafenib in osteosarcoma (REGOBONE) are consistent with the findings from our study.16 Patients in the REGOBONE study were treated with regorafenib (n = 26) or placebo (n = 12), with an imbalance in patient sex but otherwise well-matched groups. The 8-week nonprogression rate was 65% for regorafenib and 0% for placebo, with two PRs with regorafenib, for a response rate of 8%. Median PFS was 3.8 months with regorafenib versus 1 month for placebo.
The release of the REGOBONE data prompted the decision to conduct an unplanned interim analysis of the SARC024 osteosarcoma cohort. An independent DSMC recommended stopping our study early based on this interim analysis, conducted after enrolling 42 of 48 planned participants. Although only 31 of the planned 42 PFS events were recorded before stopping enrollment, the superiority of regorafenib over placebo has held up with further follow-up.
A number of chemotherapeutic agents or combinations have been tested in relapsed or refractory metastatic osteosarcoma, with mixed evidence of benefit. Gemcitabine-based therapy seems to have some activity in metastatic osteosarcoma,5 whereas trabectedin resulted in no responses in a phase II trial.17 A summary of seven negative phase II trials from the Children’s Oncology Group (COG) helped establish a natural history of unresectable osteosarcoma with event-free survival of 12% at 4 months.4 The placebo arms of this trial and the REGOBONE study demonstrate similar rapid progression: 10% 16-week PFS in SARC024 and 0% 12-week PFS in REGOBONE.16 The COG publication suggests that an agent resulting in 4-month PFS greater than 30% demonstrates sufficient efficacy to warrant additional study; SARC024 establishes a 44% 16-week PFS with regorafenib.
SARC024 began enrolling before the publication of the COG summary experience. The population in our trial was substantially older than patients included in COG trials and included a high proportion of patients with chondroblastic subtype and several patients with radiation-associated osteosarcoma, both characteristics known to be associated with worse outcome; it is unknown if COG survival estimates are applicable to this older population.18,19 Unfortunately, despite eligibility permitting enrollment down to age 10 years, no patients younger than age 18 years enrolled. The reason for this is likely multifactorial, but a reluctance to participate in a placebo-controlled trial on the part of both pediatric providers and parents seems to have been a barrier to adolescent enrollment. With close follow-up as required in this trial, the placebo-controlled randomized design did not negatively affect OS.
A growing body of evidence suggests multitarget tyrosine kinase inhibitors have efficacy in osteosarcoma. A case series of 15 patients treated with pazopanib reported one PR (7%) and median PFS of 6 months.9 A single-arm phase II trial of sorafenib achieved 4-month PFS of 46%, median PFS of 4 months, and 8% PR rate (three of 35 patients).7 A similarly designed trial of apatinib achieved 4-month PFS of 57%, median PFS of 4.5 months, and an astounding 43% PR rate (16 of 37 patients).10 An ongoing study of lenvatinib in osteosarcoma reported initial results at ASCO 2018, with 4-month PFS of 33%, median PFS of 3.4 months, and 8% PR rate (two of 26 patients).11 Most recently, an open-label phase II trial of cabozantinib reported 6-month PFS of 33%, median PFS of 6.2 months, and 12% PR rate (five of 42 patients).12
Combination strategies integrating kinase inhibitors such as regorafenib are worthy of further study. Previous studies incorporated mammalian target of rapamycin pathway inhibition in an attempt to improve responses to early-generation multikinase inhibitors. A two-stage single-arm phase II trial of sorafenib and everolimus reported 6-month PFS of 45%, median PFS of 5 months, and 5% PR rate (two of 38 patients).20 Although this combination achieved a commendable PFS rate, the low response rate and high toxicity rate suggest alternative strategies should be pursued.
Immune checkpoint inhibitors have shown little activity in metastatic or recurrent osteosarcoma, with pembrolizumab showing only one responder of 22 patients treated in the SARC028 trial and nivolumab plus ipilimumab showing one of nine patients with a radiologic response in the Alliance cooperative group trial.21,22 Admittedly, to date, RECIST responses are rare, even with agents considered active in advanced osteosarcoma, and event-free survival or PFS is recommended as a clinically relevant end point in osteosarcoma trials.4 Six of 22 patients in SARC028 achieved stable disease, resulting in 12-week PFS of 32%, suggesting activity worth further evaluation of immune checkpoint inhibitors in osteosarcoma.21
Recent reports have revealed significant synergy in multiple cancer types when combining checkpoint inhibitors with multikinase inhibitors, particularly inhibitors such as regorafenib with significant affinity for vascular endothelial growth factor receptors.23-25 A number of trials are beginning to examine such combinations in bone and soft tissue sarcomas. With the SARC024 results reported here and those of SARC028 reported previously,21 we propose future evaluation of the combination of regorafenib with checkpoint inhibition. Although skin and GI toxicities may be overlapping, the mechanisms for the toxicities differ significantly between these drug classes. Reports thus far have indicated that most treatment-related AEs are manageable.23,25,26
No new safety concerns with regorafenib treatment were identified during SARC024, and AEs were generally manageable with dose reduction. During initial blinded treatment, 13 (59%) of 22 patients assigned to regorafenib required dose interruption, which is similar to reports in other diseases (31% to 97%).27-30 Notably, median dose at end of blinded treatment was 120 mg. Although it is possible that initial dose-intensity of regorafenib contributes to efficacy, pharmacokinetic and pharmacodynamic assessments reported in the initial phase I study suggest minimal differences between the 120- and 160-mg doses.8 Furthermore, the Regorafenib Dose Optimization Study in metastatic colorectal cancer established that a dose-escalation strategy starting at 80 mg is superior to one starting at 160 mg.31 Given the efficacy of regorafenib seen in SARC024 and REGOBONE despite the frequency of dose reductions or interruptions, consideration should be given to treatment at a starting dose of 80 or 120 mg in future studies.
In conclusion, regorafenib is active in advanced osteosarcoma, resulting in clinically meaningful improvement in PFS with tolerable AEs. Future studies of combination regimens that include regorafenib are a natural next step in the development of novel therapeutics for patients with osteosarcoma.
ACKNOWLEDGMENT
We thank the patients who volunteered for this study, their families who supported them, and the clinical trial study staff at each site who conducted the study.
Footnotes
Presented at the Annual Meeting of the Connective Tissue Oncology Society, Rome, Italy, November 14-17, 2018.
Supported in part by Bayer HealthCare Pharmaceuticals (Berlin, Germany), which also provided regorafenib.
Listen to the podcast by Dr Van Tine at ascopubs.org/jco/podcasts
SARC024 was conducted under a research agreement between Bayer HealthCare Pharmaceuticals (Berlin, Germany) and the Sarcoma Alliance for Research Through Collaboration (Ann Arbor, MI). Bayer HealthCare Pharmaceuticals did not participate in data collection. Cancer Research and Biostatistics (Seattle, WA) statisticians independently conducted the analyses. The lead author made the decision to publish, and the article was submitted to Bayer HealthCare Pharmaceuticals for comment.
Clinical trial information: NCT02048371.
AUTHOR CONTRIBUTIONS
Conception and design: Lara E. Davis, Christopher W. Ryan, Sant Chawla, Denise K. Reinke, Richard F. Riedel, Steven Attia, Robert G. Maki
Administrative support: Denise K. Reinke
Provision of study material or patients: Lara E. Davis, Christopher W. Ryan, Kristen N. Ganjoo, Mark Agulnik, Michael B. Livingston, Damon Reed, Denise K. Reinke
Collection and assembly of data: Lara E. Davis, Vanessa Bolejack, Christopher W. Ryan, Kristen N. Ganjoo, Elizabeth T. Loggers, Sant Chawla, Mark Agulnik, Vicky Keedy, Daniel Rushing, Scott Okuno, Denise K. Reinke, Richard F. Riedel, Steven Attia, Leo Mascarenhas, Robert G. Maki
Data analysis and interpretation: Lara E. Davis, Vanessa Bolejack, Christopher W. Ryan, Kristen N. Ganjoo, Elizabeth T. Loggers, Sant Chawla, Mark Agulnik, Michael B. Livingston, Damon Reed, Vicky Keedy, Scott Okuno, Richard F. Riedel, Steven Attia, Leo Mascarenhas, Robert G. Maki
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Lara E. Davis
Consulting or Advisory Role: Immune Design, Loxo, Epizyme
Christopher W. Ryan
Consulting or Advisory Role: Eisai, Exelixis, Genentech/Roche, Novartis, Pfizer
Research Funding: Argos Therapeutics (Inst), Bristol-Myers Squibb (Inst), CytRx (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Exelixis (Inst), Genentech (Inst), GlaxoSmithKline/Novartis (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), MabVax (Inst), Merck (Inst), Morphotek (Inst), Threshold Pharmaceuticals (Inst), TRACON Pharma (Inst), Pfizer (Inst)
Kristen N. Ganjoo
Consulting or Advisory Role: Daiichi Sankyo, Novartis, Immune Design, Janssen, Eli Lilly
Elizabeth T. Loggers
Research Funding: Daiichi Sankyo (Inst), Epizyme (Inst), Bayer HealthCare Pharmaceuticals (Inst)
Sant Chawla
Honoraria: Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, Sarcoma Alliance for Research Through Collaboration, Janssen
Consulting or Advisory Role: Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, Sarcoma Alliance for Research Through Collaboration, Janssen,
Speakers’ Bureau: Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, Sarcoma Alliance for Research Through Collaboration, Janssen
Research Funding: Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, Sarcoma Alliance for Research Through Collaboration, Janssen
Mark Agulnik
Consulting or Advisory Role: Novartis, Eli Lilly, Immune Design, Bayer HealthCare Pharmaceuticals
Speakers’ Bureau: Janssen, Eisai, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals
Damon Reed
Consulting or Advisory Role: Loxo, Shire, Epizyme
Vicky Keedy
Consulting or Advisory Role: Karyopharm Therapeutics
Research Funding: Medpacto (Inst), Plexxikon (Inst), Roche (Inst), Daiichi Sankyo (Inst), Eli Lilly (Inst), BioMed Valley Discoveries (Inst), Immune Design (Inst), GlaxoSmithKline (Inst), TRACON Pharma (Inst), Advenchen Laboratories (Inst)
Daniel Rushing
Honoraria: Bayer HealthCare Pharmaceuticals, Eisai, Eli Lilly
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Eisai, Eli Lilly
Denise K. Reinke
Research Funding: Sarcoma Alliance for Research Through Collaboration (Inst)
Richard F. Riedel
Employment: Biotech Prosthetic Orthotics of North Carolina (I), Limbguard (I)
Leadership: Biotech Prosthetic Orthotics of North Carolina (I), Limbguard (I)
Stock and Other Ownership Interests: Biotech Prosthetic Orthotics of North Carolina (I), Limbguard (I)
Consulting or Advisory Role: GlaxoSmithKline, Novartis, Merck, Eli Lilly, Eisai, Daiichi Sankyo, Loxo, Ignyta, Bayer HealthCare Pharmaceuticals, NanoCarrier
Research Funding: Novartis (Inst), Eisai (Inst), TRACON Pharma (Inst), Threshold Pharmaceuticals (Inst), Bayer HealthCare Pharmaceuticals (Inst), Agios (Inst), Karyopharm Therapeutics (Inst), Immune Design (Inst), Merck (Inst), AADi (Inst), Plexxikon (Inst), Arog (Inst), Eli Lilly (Inst), Daiichi Sankyo (Inst), Astex Pharmaceuticals (Inst), Ignyta (Inst), Roche/Genentech (Inst), NanoCarrier (Inst)
Patents, Royalties, Other Intellectual Property: PandoNet/Limbguard (I)
Travel, Accommodations, Expenses: Janssen, EMD Serono, Daiichi Sankyo, Ignyta, NanoCarrier
Steven Attia
Research Funding: AB Science (Inst), TRACON Pharma (Inst), CytRx (Inst), Bayer HealthCare Pharmaceuticals (Inst), Novartis (Inst), Daiichi Sankyo (Inst), Eli Lilly (Inst), Immune Design (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda Pharmaceuticals (Inst), Incyte (Inst), Springworks (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst)
Travel, Accommodations, Expenses: Immune Design
Leo Mascarenhas
Honoraria: Bayer HealthCare Pharmaceuticals
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Eli Lilly (Inst)
Speakers’ Bureau: Bayer HealthCare Pharmaceuticals
Research Funding: AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals, AstraZeneca/MedImmune, Eli Lilly
Robert G. Maki
Honoraria: Eli Lilly, Springer, Wiley
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Eli Lilly/ImClone, Sarcoma Alliance for Research Through Collaboration, AADi, Karyopharm Therapeutics, Arcus Ventures, American Board of Internal Medicine, Deciphera, Foundation Medicine, Janssen Scientific Affairs, TRACON Pharma
Research Funding: TRACON Pharma (Inst), Eli Lilly (Inst), Regeneron (Inst), Immunocore (Inst), Daiichi Sankyo (Inst), Presage Biosciences (Inst), Xencor (Inst), Pfizer (Inst), FORMA Therapeutics (Inst), Deciphera (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate: I write and edit chapters for their online text book; Springer and Wiley: books for contributions I have made to the published texts
Travel, Accommodations, Expenses: TRACON Pharma, Bayer HealthCare Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity N Engl J Med 3141600–16061986 [DOI] [PubMed] [Google Scholar]
- 2.Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial J Clin Oncol 332279–22872015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelan JS, Davis LE.Osteosarcoma, chondrosarcoma, and chordoma J Clin Oncol 36188–1932018 [DOI] [PubMed] [Google Scholar]
- 4.Lagmay JP, Krailo MD, Dang H, et al. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning from the past to move forward J Clin Oncol 343031–30382016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmerini E, Jones RL, Marchesi E, et al. Gemcitabine and docetaxel in relapsed and unresectable high-grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer. 2016;16:280. doi: 10.1186/s12885-016-2312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan X-L, Cai G-P, Zhu L-L, et al. Efficacy and safety of ifosfamide-based chemotherapy for osteosarcoma: A meta-analysis Drug Des Devel Ther 95925–59322015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. doi: 10.1093/annonc/mdr151. Grignani G, Palmerini E, Dileo P, et al: A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann Oncol 23:508-516, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors Clin Cancer Res 182658–26672012 [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.1080/0284186X.2018.1503714. Longhi A, Paioli A, Palmerini E, et al: Pazopanib in relapsed osteosarcoma patients: report on 15 cases. Acta Oncol 58:124-128, 2019. [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1634/theoncologist.2018-0542. Xie L, Xu J, Sun X, et al: Apatinib for advanced osteosarcoma after failure of standard multimodal therapy: An open label phase 2 clinical trial. J Clin Oncol [epub ahead of print December 17, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaspar N, Casanova M, Sirvent F, et al: Single-agent expansion cohort of lenvatinib (LEN) and combination dose-finding cohort of LEN + etoposide (ETP) + ifosfamide (IFM) in patients (pts) aged 2 to ≤ 25 years with relapsed/refractory osteosarcoma (OS). J Clin Oncol 36, 2018 (suppl; abstr 11527) [Google Scholar]
- 12. Italiano A, Penel N, Toulmonde M, et al: Cabozantinib in patients with advanced osteosarcomas and Ewing sarcomas: A French Sarcoma Group (FSG)/US National Cancer Institute phase II collaborative study. Ann Oncol 29, 2018 (suppl; abstr LBA67)
- 13. doi: 10.1002/cam4.5044. Attia S, Bolejack V, Ganjoo KN, et al: A phase II trial of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS) of soft tissue and bone: SARC024 trial results. J Clin Oncol 35, 2017 (suppl; abstr 11005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1634/theoncologist.2020-0679. Riedel RF, Ballman KV, Lu Y, et al: A randomized, double-blind, placebo-controlled, phase II study of regorafenib vs placebo in advanced/metastatic, treatment-refractory liposarcoma: Results from the SARC024 study. J Clin Oncol 36, 2018 (suppl; abstr 11505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer 45228–2472009 [DOI] [PubMed] [Google Scholar]
- 16. doi: 10.1016/S1470-2045(18)30742-3. Duffaud F, Mir O, Boudou-Rouquette P, et al: Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 20:120-133, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Laverdiere C, Kolb EA, Supko JG, et al. Phase II study of ecteinascidin 743 in heavily pretreated patients with recurrent osteosarcoma Cancer 98832–8402003 [DOI] [PubMed] [Google Scholar]
- 18.Grimer RJ, Cannon SR, Taminiau AM, et al. Osteosarcoma over the age of forty Eur J Cancer 39157–1632003 [DOI] [PubMed] [Google Scholar]
- 19.McHugh JB, Thomas DG, Herman JM, et al. Primary versus radiation-associated craniofacial osteosarcoma: Biologic and clinicopathologic comparisons Cancer 107554–5622006 [DOI] [PubMed] [Google Scholar]
- 20.Grignani G, Palmerini E, Ferraresi V, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial Lancet Oncol 1698–1072015 [DOI] [PubMed] [Google Scholar]
- 21.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial Lancet Oncol 181493–15012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials Lancet Oncol 19416–4262018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makker V, Rasco DW, Vogelzang NJ, et al: Lenvatinib + pembrolizumab in patients with advanced endometrial cancer: Updated results. J Clin Oncol 36, 2018 (suppl; abstr 5596) [Google Scholar]
- 24.Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: An overview of rationale, preclinical evidence, and initial clinical data. Front Oncol. 2015;5:202. doi: 10.3389/fonc.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial Lancet Oncol 19405–4152018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor MH, Rasco DW, Brose MS, et al: A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J Clin Oncol 36, 2018 (suppl; abstr 6016) [Google Scholar]
- 27.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial Lancet 381303–3122013 [DOI] [PubMed] [Google Scholar]
- 28.Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial Lancet Oncol 171732–17422016 [DOI] [PubMed] [Google Scholar]
- 29. doi: 10.1186/s12885-016-2440-9. Adenis A, de la Fouchardiere C, Paule B, et al: Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 16:412-412, 2016 [Erratum: BMC Cancer 16:518, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study Eur J Cancer 493412–34192013 [DOI] [PubMed] [Google Scholar]
- 31. Bekaii-Saab TS, Ou F-S, Anderson DM, et al: Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)—An ACCRU Network study. J Clin Oncol 36, 2018 (suppl; abstr 611)