Abstract
Gelochelidon nilotica and Sterna hirundo are two sympatric breeding species. The mitogenomes of G. nilotica and S. hirundo are 16,748 bp and 16,707 bp in size. Both two mitogenomes reveal the same gene order and genomic organization to that of typical avian mtDNA. The first conserved blocks with a interrupted poly-C are present in the two species control regions, but not existed in S. albifrons. Seventeen and 11 simple sequence repeats are found in G. nilotica and S. hirundo, respectively. Phylogenetic analysis indicated that Sternidae has the closest relationship with Laridae. We supported that Stercorariidea is a sister group to (Alcidae (Laridae, Sternidae)), G. nilotica is genetically most related to S. hirundo (all belonged to Black cap species), but distant with S. albifrons (White blaze species) in kinship, and suggested that the status of Larus vegae should be further investigated.
Keywords: Gelochelidon nilotica, Sterna hirundo, mitogenome, structure, phylogeny
The terns (Charadriiformes: Sternidae) are a distinctive group of seabirds that occupy aquatic environments the world over and demonstrate an interesting array of variations on a life history centred around aquatic foraging and colonial nesting. Gull-billed Tern (Gelochelidon nilotica) and Common Tern (Sterna hirundo) belonging to Sternidae, are widely distributing coastal seabirds and can be found breeding in most of Europe, Asia and North America, and both of their Northern populations making long-distance southwards migratory movements. The two species both have extremely large range and population size, and are evaluated as Least Concern (IUCN 2016), but they are vulnerable to the deterioration and loss of habitat, human disturbance, pesticide pollution, agricultural intensification, fluctuating water levels (del Hoyo et al. 1996), beach erosion and the development or modification of foraging sites (Molina and Erwin 2006) during the breeding season, and they also may be threatened by future outbreaks of the virus (Melville and Shortridge 2006). The complete mitogenome is an important marker for studies related to taxonomy, biodiversity and conservation (Anmarkrud and Lifjeld 2017). Using complete mitogenomes, one can analyse nucleotide variation, obtain information on haplotypes, and elucidate current population structures of species (Yamamoto et al. 2005). Nevertheless, in terms of phylogenetics, the limited molecular data dampen the diversity and evolution studies in interspecific of Sternidae.
Naturally dead G. nilotica and S. hirundo chicks were collected during the breeding season at Hongjian Nur (39°04′N, 109°53′E), Shaanxi, China. The specimens (proof number: X08, A07) were deposited in the animal specimens museum of Shaanxi Institute of Zoology, Xi’an, China. The mitogenomes were determined after PCR amplification, sequencing and annotation based on previously published sequence (Yang et al. 2016a).
The total length of the G. nilotica (Genbank: MF582631) and S. hirundo (Genbank: MF582632) mitochondrial genomes is 16,748 bp and 16,707 bp, and with the base composition A + T are 56.0% and 55.8%, respectively. Their genes’ arrangement and orientation are identical to that of typical avian mtDNA (Gibb et al. 2007).
The 13 PCGs of G. nilotica and S. hirundo are similar to that observed for other Charadriiformes. The typical ATN (ATG or ATC) start codons are present in G. nilotica and S. hirundo PCGs, with the exception of the COI in two species, which utilize GTG as start codons. Open-reading frames of most PCGs end with AGG, AGA, TAA, or TAG, while COIII and ND4 have the incomplete stop codon T in two species. The ND3 genes of G. nilotica and S. hirundo are all with an extra C nucleotide in 174 sites.
The srRNA and lrRNA in G. nilotica and S. hirundo are 967, 1592 bp and 967, 1596 bp, respectively. All tRNA genes in two species, ranging from 66 to 74 bp in size, can fold into typical cloverleaf secondary structures, with exception of the shortest tRNASer(AGN) (66 bp), in which the DHU arm has been replaced by a simple loop. The control regions are 1198 bp and 1154 bp in length in G. nilotica and S. hirundo. Especially, the interrupted poly-C sequences (5′-CCCCCCCCCTACCCCCCCACCCCCC-3′, bp positions: 15,560–15,584; 5′-CCCCCCCCCCTTCCCCCCCC-3′, bp positions: 15,563–15,582) in domain I of control regions are present in G. nilotica and S. hirundo, but no relevant structure is found in S. albifrons. This sequence is similar to that in the ‘goose hairpin’ in Laridae (Yang et al. 2016b) and to variants found in Himantopus himantopus (Yang et al. 2017). Seventeen simple sequence repeats with 5′-ACAACAA-3′ (bp positions: 16,623–16,741) and 11 repeats with 5′-ACAAACA-3′ (bp positions: 16,624–16,700) are existed in G. nilotica and S. hirundo, respectively.
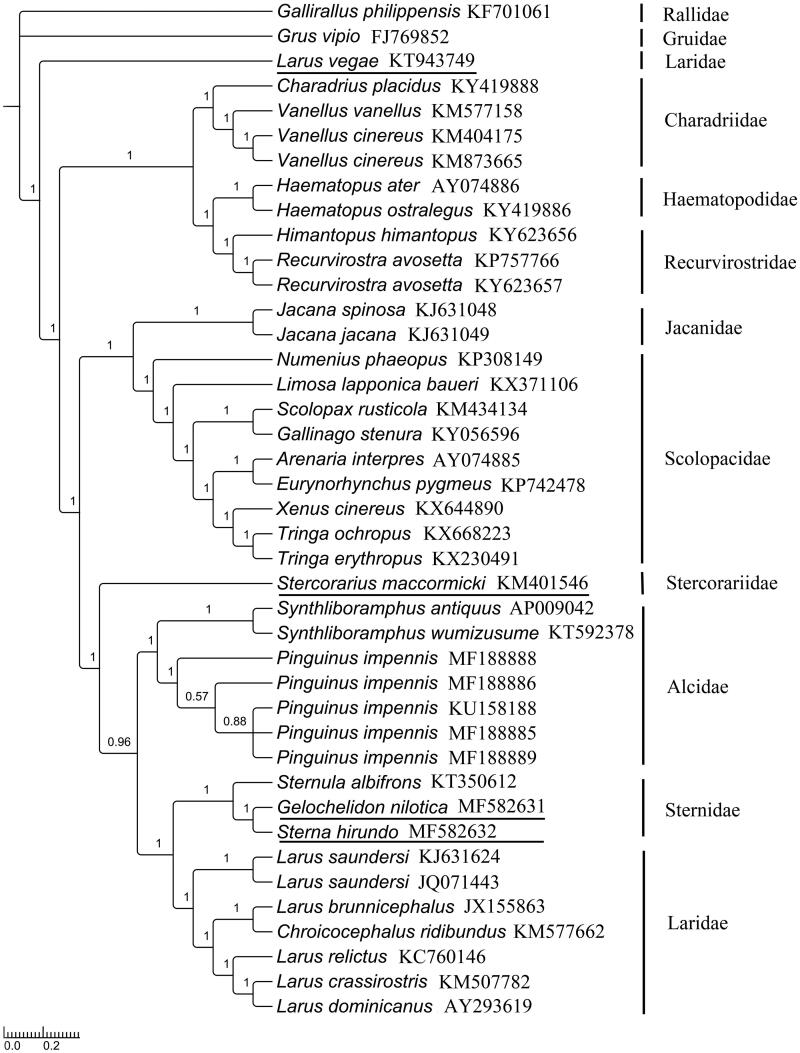
To investigate the phylogenetic positions of G. nilotica and S. hirundo (Sternidae), Bayesian inference (BI) tree and maximum likelihood (ML) tree were reconstructed based on 41 mitogenomes PCGs using MrBayes ver. 3.2.2 (Ronquist et al. 2012) and RAxML (Stamatakis 2006) under the best partitioned scheme and optimal model analysed in Partitionfinder v1.1.1 (Lanfear et al. 2012) (Models GTR + I+G and GTR + G). Gallirallus philippensis and Grus vipio were selected as outgroups. The phylograms obtained from BI and ML (data not shown) were all strongly indicated that Sternidae was a sister group to Laridae (Thomas et al. 2004; Livezey 2010). The first analysis was supported between (Stercorariidea (Alcidae (Laridae, Sternidae))) (bootstrap value 1.00 in BI) and ((Stercorariidea, Alcidae) (Laridae, Sternidae)) (bootstrap value 0.87 in ML) (Maxwell and Harrison 2008). It was also supported that S. hirundo should be belonged to Black cap species with G. nilotica, S. albifrons categorized into the White blaze species (Bridge et al. 2005). Extraordinarily, the Larus vegae was more primitive and located in the root of the tree, but not belonged to the branch of Laridae (Figure 1).
Figure 1.
Topology of Bayesian tree for 41 species based on mitogenome PCG sequences. GenBank accession numbers are indicated following species name (numbers on nodes are bootstrap values).
Disclosure statement
The authors declare no competing interests.
References
- Anmarkrud JA, Lifjeld JT.. 2017. Complete mitochondrial genomes of eleven extinct or possibly extinct bird species. Mol Ecol Resour. 17:334–341. [DOI] [PubMed] [Google Scholar]
- Bridge ES, Jones AW, Baker AJ.. 2005. A phylogenetic framework for the terns (Sternini) inferred from mtDNA sequences: implications for taxonomy and plumage evolution. Mol Phylogenet Evol. 35:459–469. [DOI] [PubMed] [Google Scholar]
- del Hoyo J, Elliott A, Sargatal J.. 1996. Handbook of the birds of the world, Vol. 3 Barcelona: Lynx Edicions. [Google Scholar]
- Gibb GC, Kardailsky O, Kimball RT, Brawn EL, Penny D.. 2007. Mitochondrial genomes and avian phylogeny: complex characters and resolvability without explosive radiations. Mol Biol Evol. 24:269–280. [DOI] [PubMed] [Google Scholar]
- IUCN 2016. The IUCN Red List of Threatened Species. Version 2017-1. [accessed 2017 Aug 01]. www.iucnredlist.org. [Google Scholar]
- Lanfear R, Calcott B, Ho SY, Guindon S.. 2012. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 29:1695–1701. [DOI] [PubMed] [Google Scholar]
- Livezey BC. 2010. Phylogenetics of modern shorebirds (Charadriiformes) based on phenotypic evidence: analysis and discussion. Zool J Linn Soc. 160:567–618. [Google Scholar]
- Maxwell EE, Harrison LB.. 2008. Ossification sequence of the common tern (Sterna hirundo) and its implications for the interrelationships of the Lari (Aves, Charadriiformes). J Morphol. 269:1056–1072. [DOI] [PubMed] [Google Scholar]
- Melville DS, Shortridge KF.. 2006. Spread of H5N1 avian influenza virus: an ecological conundrum. Lett Appl Microbiol. 42:435–437. [DOI] [PubMed] [Google Scholar]
- Molina KC, Erwin RM.. 2006. The distribution and conservation status of the Gull-billed Tern (Gelochelidon nilotica) in North America. Waterbirds. 29:271–295. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP.. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690. [DOI] [PubMed] [Google Scholar]
- Thomas GH, Wills MA, Székely T.. 2004. A supertree approach to shorebird phylogeny. BMC Evol Biol. 4:28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kakizawa R, Yamagishi S.. 2005. Mitochondrial genome project of endangered birds in Japan: 1. Ancient Murrelet, Synthliboramphus antiquus. J Yamashina Inst Ornithol. 37:20–29. [Google Scholar]
- Yang C, Lian T, Wang QX, Huang Y, Xiao H.. 2016b. Structural characteristics of the Relict Gull (Larus relictus) mitochondrial DNA control region and its comparison to other Laridae. Mitochondrial DNA A. 27:2487–2491. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang QX, Huang Y, Xiao H.. 2016a. Complete mitochondrial genome of Relict Gull, Larus relictus (Charadriiformes: Laridae). Mitochondrial DNA A. 27:411–412. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang QX, Li XJ, Xiao H, Huang Y.. 2017. Characterization of the mitogenomes for two sympatric breeding species in Recurvirostridae (Charadriiformes) and their phylogenetic implications. Mitochondrial DNA B. 2:182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]