Abstract
In this study, we determined the complete plastome sequence of Averrhoa carambola L. (Oxalidaceae) (NCBI acc. no. KX364202). To the best of our knowledge, this is the first reported complete plastome sequence from the order Oxalidales. The gene order and structure of the A. carambola plastome are collinear with the typical plastome of land plants. The complete plastome size is 155,965 bp in length and consists of a large single copy region of 87,217 bp and a small single copy region of 17,496 bp, which are separated by a pair of 25,626-bp-long inverted repeat regions. The overall A-T content of the plastome sequence is 61.2%. The plastome contains 111 genes, of which 77 are protein-coding genes, 30 are tRNA genes, and 4 are rRNA genes. Sixteen genes contain one intron and two genes have two introns. A total of 91 simple sequence loci were identified from the genome. Phylogenetic analysis revealed that A. carambola is a sister group of Euonymus japonicus with 100% bootstrap support.
Keywords: Averrhoa carambola, Oxalidaceae, plastome, tropical fruit
Averrhoa carambola L. is commonly referred to as carambola or starfruit. It is a widely cultivated tropical fruit that originated in tropical Asia (Kim 2011). The fruit of this species is used for both food and medicine. Averrhoa carambola belongs to the family Oxalidaceae, which is one of the seven families in the order Oxalidales (Byng et al. 2016). To date, there have been no published plastome sequences for plants in Oxalidales, even though there have been several phylogenetic studies using chloroplast gene markers (Soltis et al. 2000, 2003). Oxalidaceae consists of six genera and approximately 770 species, several of which, such as starfruit (A. carambola) and oca (Oxalis tuberosa), are plants of economic importance. The complete plastome sequence of A. carambola will aid us in developing molecular markers for the identification and improvement of cultivars of this species. In addition, being the first plastome data for the order Oxalidales, the complete plastome sequence of A. carambola is expected to become a standard reference for elucidating plastome evolution and phylogenetic relationships in this order.
The leaves of A. carambola used in this study were collected from the Korea University greenhouse, where we grew the plants from seeds that were originally collected in Thailand. The plants flowered and fruited in the greenhouse. A voucher specimen was deposited in the Korea University Herbarium (KUS acc. no. 2014-0241). Fresh leaves were ground into powder in liquid nitrogen and the total DNA was extracted using the CTAB method (Doyle & Doyle 1987). The DNAs were further purified by ultracentrifugation and dialysis (Palmer 1986). The genomic DNAs are deposited in the Plant DNA Bank in Korea (PDBK acc. no. 2014-0241). The complete plastome sequence was generated using an Illumina HiSeq 2000 system (Illumina, Inc., San Diego, CA). Annotations were performed using the National Center for Biotechnology Information (NCBI) BLAST, DOGMA (Wyman et al. 2004), and tRNAscan-SE programs (Lowe & Eddy 1997). For the phylogenetic analysis, we selected and downloaded 37 complete plastome sequences based on the APG IV system (Byng et al. 2016) from the NCBI database. All the 37 plastome sequences were from super-rosid plants.
The gene order and structure of the A. carambola plastome are similar to those of a typical angiosperm (Shinozaki et al. 1986; Kim & Lee 2004; Yi & Kim 2012). The complete plastome is 155,965 bp in length, and consists of a large single copy (LSC) region of 87,217 bp and a small single copy (SSC) region of 17,496 bp separated by two inverted repeats (IR) of 25,626 bp. The plastome comprises 111 unique genes (77 protein-coding genes, 30 tRNA genes and four rRNA genes). Seven protein-coding, seven tRNA and four rRNA genes are duplicated in the IR regions. The infA and rpl32 genes are pseudogenes. The major portion of the A. carambola plastome consists of protein-coding genes (54.8%), tRNA genes (1.8%), and rRNA genes (5.8%). The average A-T content of the plastome is 61.2%, whereas that in the LSC, SSC, and IR regions is 65.7%, 69.8% and 57.5%, respectively. Sixteen genes have one intron and two genes, ycf3 and clpP have two introns. A total of 91 simple sequence repeat (SSR) loci, which can be defined as having more than 10 duplications of simple nucleotide(s), are scattered among the noncoding regions of the genome. Among these, 56, 12 and 23 are mono-SSR, di-SSR, and tri-SSR loci, respectively. Some of these loci will be useful in identifying cultivars of A. carambola.
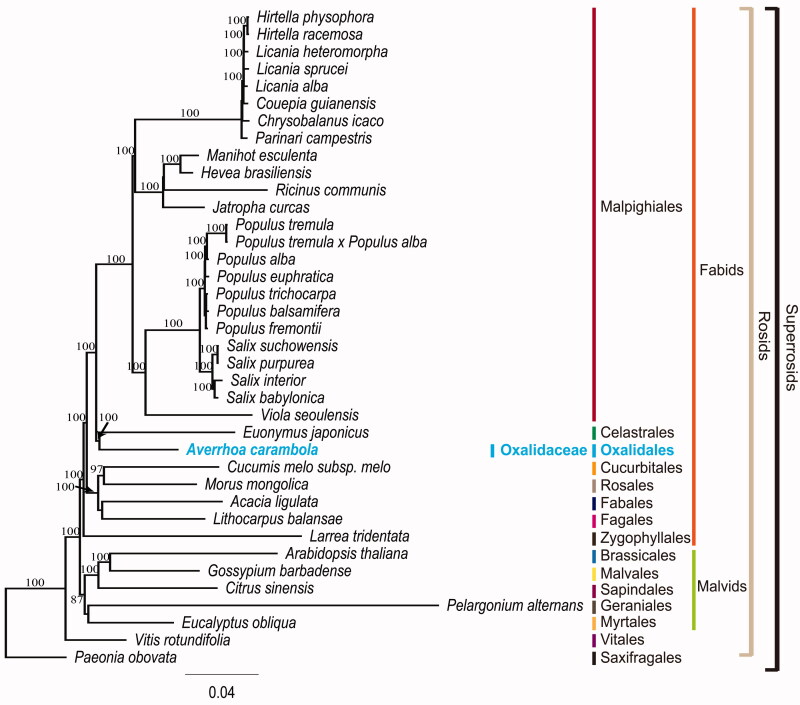
To validate the phylogenetic relationships of A. carambola in rosids, we constructed a maximum likelihood (ML) tree by using 38 super-rosid taxa. Phylogenetic analysis was performed on a data set that included 76 protein-coding genes (excluding infA, rpl32, and rps16) and four rRNA genes from the 38 taxa using RAxML v. 7.7.1 (Figure 1; Stamatakis et al. 2008). The 80 gene sequences (82,922 bp in length) were aligned with the MUSCLE program using Geneious v. 6.1.8 (Biomatters Ltd., Auckland, New Zealand). The results showed that A. carambola is included in a clade containing plants in the orders Celastrales and Malpighiales (COM clade) with 100% bootstrap support. Similar results were obtained using the APG IV system (Byng et al. 2016). However, the relationships within the COM clade identified in the present study differ from those identified using the APG IV system. A sister-group relationship between Oxalidales and Geraniales was proposed based on the results obtained using the APG IV system. In contrast, a sister-group relationship between Oxalidales and Celastrales is suggested in the present study. Currently, there is only one complete plastome sequence available for each of the orders Oxalidales and Celastrales (Choi & Park 2016). In order to resolve the relationships within the COM clade, further complete plastome sequences are needed from Oxalidales and Celastrales.
Figure 1.
Maximum likelihood (ML) tree based on 76 protein-coding and four rRNA genes from 38 plastid genomes as determined by RAxML. The numbers at each node indicate the ML bootstrap values. Genbank accession numbers of taxa are shown below, Acacia ligulata (NC_026134), Arabidopsis thaliana (NC_000932), Averrhoa carambola (KX364202), Chrysobalanus icaco (NC_024061), Citrus sinensis (NC_008334), Couepia guianensis (NC_024063), Cucumis melo subsp. melo (NC_015983), Eucalyptus obliqua (NC_022378), Euonymus japonicus (NC_028067), Gossypium barbadense (NC_008641), Hevea brasiliensis (NC_015308), Hirtella physophora (NC_024066), Hirtella racemosa (NC_024060), Jatropha curcas (NC_012224), Larrea tridentata (NC_028023), Licania alba (NC_024064), Licania heteromorpha (NC_024062), Licania sprucei (NC_024065), Lithocarpus balansae (NC_026577), Manihot esculenta (NC_010433), Morus mongolica (NC_025772), Paeonia obovata (NC_026076), Parinari campestris (NC_024067), Pelargonium alternans (NC_023261), Populus alba (NC_008235), Populus balsamifera (NC_024735), Populus euphratica (NC_024747), Populus fremontii (NC_024734), Populus tremula (NC_027425), Populus tremula × Populus alba (NC_028504), Populus trichocarpa (NC_009143), Salix babylonica (NC_028350), Salix interior (NC_024681), Salix purpurea (KP019639), Salix suchowensis (NC_026462), Viola seoulensis (NC_026986) and Vitis rotundifolia (NC_023790).
Acknowledgments
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This work was supported by the National Research Foundation of Korea (NRF) under Grants [NRF-2015M3A9B8030588 and NRF-2015M3A9B8047398] and the Eco-Innovation project [416-111-007] of the Ministry of Environment from KEITI to KJK.
References
- Byng JW, Chase MW, Christenhusz MJ, Fay MF, Judd WS, Mabberley DJ, Sennikov AN, Soltis DE, Soltis PS, Stevens PF.. 2016. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc. 181:1–20. [Google Scholar]
- Choi KS, Park S.. 2016. The complete chloroplast genome sequence of Euonymus japonicus (Celastraceae). Mitochondrial DNA. 27:3577–3578. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL.. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15. [Google Scholar]
- Kim K-J. 2011. Tropical fruit resources. Seoul: Geobook. [Google Scholar]
- Kim K-J, Lee HL.. 2004. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 11:247–261. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR.. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JD. 1986. Isolation and structural analysis of chloroplast DNA. Method Enzymol. 118:167–186. [Google Scholar]
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K.. 1986. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 5:2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis DE, Senters AE, Zanis MJ, Kim S, Thompson JD, Soltis PS, De Craene LPR, Endress PK, Farris JS.. 2003. Gunnerales are sister to other core eudicots: implications for the evolution of pentamery. Am J Bot. 90:461–470. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis MJ, Savolainen V, Hahn WH, Hoot SB, Fay MF.. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot J Linn Soc. 133:381–461. [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J.. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 57:758–771. [DOI] [PubMed] [Google Scholar]
- Wyman SK, Jansen RK, Boore JL.. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255. [DOI] [PubMed] [Google Scholar]
- Yi D-K, Kim K-J.. 2012. Complete chloroplast genome sequences of important oilseed crop Sesamum indicum L. PLoS One. 7:e35872. [DOI] [PMC free article] [PubMed] [Google Scholar]