Abstract
Hypothermia is risk factor for piglet neonatal mortality, especially for low birth weight piglets. Piglets with intrauterine growth retardation (IUGR) also have a higher mortality risk at birth. This study aimed to validate infrared thermography (IRT) as an alternative to rectal temperature (RT) to measure piglet temperature in the hour postpartum, and to identify piglets with thermoregulation difficulties. At birth (6.3 ± 0.35 min postpartum), 67 piglets were dried, weighed, scored for growth retardation (IUGR; 0–3), and isolated in a plastic box where IRT images were taken, followed by RT. Piglets were then returned to the farrowing pen, and the process repeated at 15, 30, and 60 min postpartum. Piglets were ranked according to their weight (quartiles: 0.57–1.27 kg, 1.27–1.5 kg, 1.5–1.74 kg, 1.74–2.44 kg). Temperatures (ear base and tip; minimum, maximum and average of back) were extracted from IRT images (Thermacam Researcher Pro 2.0). Pearson correlations between temperature measures were calculated, and the effect of time, IUGR score, and weight were included in linear mixed models (SAS 9.4). RT was correlated with all IRT data across time points (P < 0.05); correlations were strongest with the ear base, and weakest with the ear tip and minimum back temperature. Both IUGR score and weight rank affected ear base (P < 0.05) and RTs (P < 0.05). The lightest piglets, and piglets with severe IUGR had the lowest temperature, relative to their counterparts. Indeed, differences between all weights categories were significant for RT. Piglets with the lowest weight (0.27–1.27 kg) had lower ear base temperatures than piglets in the third quartile (1.5–1.74 kg; 35.2 ± 0.36 °C vs. 36.5 ± 0.35 °C, t64.9 = −4.51, P < 0.001) and the heaviest piglets (1.74–2.44 kg; 35.2 ± 0.36 °C vs. 36.4 ± 0.36 °C, t70.4 = −3.97, P < 0.005). Overall, piglets with severe IUGR (score 3) had a lower RT than normal piglets (score 0; 35.8 ± 0.46 °C vs. 37.2 ± 0.42 °C, t43.1 = 3.16, P < 0.05) and piglets with mild IUGR (score 1; 35.8 ± 0.46 °C vs. 37.1 ± 0.40 °C, t45.3 = 2.92, P < 0.05); and they also had lower temperature at the base of the ear than normal piglets (35.1 ± 0.42 °C vs. 36.3 ± 0.36 °C, t63.1 = 3.01, P < 0.05). These results confirmed that IRT is an interesting noninvasive tool for assessing neonatal piglets’ thermoregulatory abilities and could be used in research investigating successful interventions for piglets at risk of hypothermia.
Keywords: birth weight, growth retardation, infrared thermography, pigs, rectal temperature, thermoregulation
INTRODUCTION
Hypothermia is a significant cause of neonatal mortality in piglets (Muns et al., 2016). Indeed a series of studies which investigated piglet survival found that piglets which died before weaning had lower temperatures during the first 24 h (Baxter et al., 2008, 2009, 2012). Low birth weight piglets have difficulties in maintaining their body temperature, and in recovering from the initial drop in body temperature that happens normally during the hour postpartum (Herpin et al., 2002, 2004; Muns et al., 2016). In addition, some piglets do not achieve optimal growth during gestation (intrauterine growth retardation; IUGR) and have two to four times higher risk of dying before weaning than normal piglets, depending on the severity of their condition (Hansen et al., 2019). These piglets often show a disproportional allometry at birth (i.e., abnormally long and thin body; Baxter et al., 2008; Hales et al., 2013), and a “dolphin-shape” head. Interventions such as drying piglets at birth and placing them near a heat source (heated lamp or mat) help them to increase and maintain their body heat and avoid hypothermia. In addition, providing an energy supplement should enhance the thermal status of piglets (Muns et al., 2010). However, providing supplemental energy to piglets is rather costly and labor intensive. Thus, piglets most in need of additional support around the time of birth should be identified, as well as the time at which the benefit is maximized (e.g., if there is a time when their temperature is likely to drop). Monitoring of piglets’ temperature during the first hour postpartum could help to identify this critical time-point.
The use of a digital thermometer for measuring rectal temperature (RT) is considered the gold standard and has been widely used in research on piglet viability. Recently, infrared thermography (IRT) imaging has gained interest as a non-invasive technique to measure thermal status of piglets (e.g., Kammersgaard et al., 2011; Tabuaciri et al., 2012; Soerensen and Pedersen, 2015; Sasaki et al., 2016). Kammersgaard et al. (2013) validated the use of IRT for measuring piglets’ temperature by taking IRT images and RTs of piglets at different times between birth and 48 h post-farrowing. Furthermore, Tabuaciri et al. (2012) and Kammersgaard et al. (2013) both found that the temperature of the base of neonatal piglets’ ears (or maximum ear temperature) was most strongly correlated with their RT. The present study investigated the use of an IRT camera to measure piglets’ temperature at several time points during the hour after birth, relative to the RT (gold standard). This study attempted to maximize control on factors of influence (handling, environment, timing, and behavior) of the temperature at the time of image acquisition. In addition, this study compared known characteristics of piglets’ mortality, that is, weight and IUGR, in order to determine if one is more prevailing in the failure to thermoregulate within 1 h postpartum. We hypothesized that the low birth weight piglets and piglets suffering IUGR would have lower temperatures across time compared with heavier and normal piglets.
MATERIAL AND METHODS
Ethical Approval
Ethical approval for this study was granted by the Teagasc Animal Ethics Committee (approval no. TAEC162/2017). The experiment was carried out in accordance with Irish legislation (SI no. 543/2012) and the EU Directive 2010/63/EU for animal experimentation.
Animals and Management
This study was conducted in July 2018 in the pig research facilities of the Animal and Grassland Research and Innovation Centre, Teagasc Moorepark, Fermoy, Co. Cork, Ireland. The experiment involved 67 piglets from 8 litters (n = 8.4 ± 0.71 piglets/litter). Sow genetic background was pure Large White and piglet genetic background was Large White × Duroc. There was no primiparous sow included in the study: four sows were in their second parity, two sows were in their third parity, and two sows were in their fifth parity. Four of the litters were “large litters” as they had over 14 piglets born (two had 16 and two had 17 piglets born alive), and the other four litters had between 7 and 12 piglets born alive. Only two litters had all the piglets involved in the study. This was due to the timing of farrowing, as some piglets were born before the researcher entered the farrowing room on the experimental day. Animals were managed in conventional farrowing pens (250 × 181 cm) containing a sow crate (225 × 60 cm), a heat pad (155 × 37 cm; 2/3 covered), and a water cup and a feeder for piglets.
Thermal Image Capture
At birth, piglets were dried and isolated in a clear plastic storage box (unknown brand, bought at Toss Bryan, Fermoy, Ireland; 55.4 × 38.0 × 31.7 cm) before acquiring the first thermal image, using a FLIR T420 Infrared camera (thermal resolution: 320 × 240, measurement accuracy: ±2 °C, thermal sensitivity: <0.04 °C; FLIR Systems, Wilsonville, OR, USA). They were then immediately weighed, and scored for IUGR (0–3). This score was attributed based upon on the number of characteristics associated with IUGR which were displayed by the piglets. These characteristics were classified by Hales et al. (2015) as having a dolphin-shape head, bulging eyes and wrinkles around the snout (0 = absence of characteristic, 1 = presence of the characteristic). An overall score of 0 indicated no IUGR while a score of 3 indicated severe IUGR.
The plastic box represented a controlled environment for taking IRT images, because it prevented air flow over the piglets. Before acquiring pictures, reflected room temperature was measured as the mean temperature of a crunched aluminum foil (emissivity = 1). The room temperature was recorded using the same room thermometer (LCD type min/max thermometer, Manotherm; sourced from Ark Animal Care, Newbridge, Ireland) at the time of each image acquisition. These measures allowed to confirm that the room temperature was controlled (each room temperature was individually controlled by Big Farm net program; Big Dutchman AG, Vechta, Germany) and that animals did not suffer heat stress. The skin emissivity of the pig was set at 0.98, as validated by Soerensen et al. (2014). These parameters are important for the correct analysis of the thermal images, as they are used by the software to calculate the subject temperature. Thermal images were acquired at birth (6.3 ± 0.35 min postpartum), 15, 30, and 60 min postpartum, always followed by the taking of a RT. Images of the piglets’ backs were taken at 1 m distance from the piglet with an angle of 75 ° (Supplementary Figure S1A). The consistency of these parameters was ensured by adopting the exact same position and respecting landmarks placed on the floor when capturing images. Thermal images were always taken before RT in order to minimize handling of the piglets, and potential transmission of the experimenter’s heat. In addition, the experimenter wore plastic gloves to further ensure insulation of her hands’ heat and minimize handling bias. The time spent handling the piglet was recorded, especially at birth when the piglets had to be dried. RT measurement took less than 1 min to be obtained, using a digital thermometer (VedoFamily, Pic Solution, Italy).
Animal Behavior
The behavior of each individual piglet was recorded at each time of image acquisition: the behaviors “walking” (i.e., locomotor activity), “suckling” (i.e., active at the udder, with a teat in the mouth), “huddling” (i.e., sleeping or resting in contact with one or more siblings), and “being on the heat pad” (i.e., resting or active in the heat pad area) were scored as present (score 1) or absent (score 0).
Thermal Image Analysis
Thermal images were processed with Thermacam Researcher Pro 2.0. Emissivity, reflected temperature and room temperature were modified for each image so that calculated temperatures were accurate. Point measurements were placed at the bases and the tips of piglets’ ears, and an area was drawn on their back between the shoulders and the rump (Supplementary Figure S1B). From this area, the minimum, maximum and average back temperatures were recorded. Temperature data were then entered in an Excel file and analyzed as normal.
The reliability of the thermal image analysis was assessed with the intraclass correlation coefficients on SPSS Statistics 24 (IBM corp., Armonk, NY, USA). The same experimenter rated several images of the same piglet (inter-image reliability): coefficients were 0.47 for minimum, 0.95 for maximum, 0.96 for average, 0.89 for ear base, and 0.82 for ear tip. Then the same experimenter rated the same images several times (intra-observer reliability): coefficients were 0.44 for minimum, 1.00 for maximum, 1.00 for average, 0.99 for ear base, and 0.86 for ear tip.
Statistical Analysis
Data were analyzed using the software SAS 9.4. The experimental unit was the piglet. Significant terms were determined when the P-value was below 0.05, and tendencies were determined when the P-value was between 0.05 and 0.1.
Pearson correlation tests were performed to investigate the relationships between RT, birth weight, and temperatures obtained from the thermal images of the piglets’ ears (tip and base) and back (minimum, maximum, and average). Correlations were characterized “very strong” if the coefficient r > 0.8, “strong” if 0.8 > r > 0.5, “moderate” if 0.5 > r > 0.3, and “weak” if 0.3 > r.
Whether there was a significant difference in birthweight of piglets with different IUGR scores was tested using a general linear model (GLM, PROC MIXED). The model included fixed effects of IUGR score and sex, and sow as a random effect.
GLMs were also used for the analysis of all temperature data. We initially analyzed the effect of IUGR score on temperature measurements. IUGR score, time postpartum, the interaction, the sex of the piglets, and whether or not the piglet performed suckling behavior, huddling, was located on the heat pad, or was active immediately before being removed for image analysis (i.e., running, playing etc.) and their interaction were included as fixed effects. The random effect of sow and the repeated effect of time were taken into account in all models.
Piglets were then ranked into one of four quartiles, based on their birth weight. A similar analysis to that carried out for the effect of IUGR score was carried out; instead of categorizing the piglets within an IUGR score, they were categorized within a weight category (very light = 0.53–1.23 kg; light = 1.27–1.45 kg; heavy = 1.50–1.72 kg; very heavy = 1.74–2.44 kg).
RESULTS
Relationship Between IUGR Score and Weight
There was an effect of IUGR score on piglet birth weight (P < 0.001; Table 1). The range of weights overlapped somewhat, and there was no difference in the weight of piglets with IUGR score of 0 or 1. Nevertheless, there was a significant difference between piglets with an IUGR score of 0 and 2 (t60.4 = 3.38, P < 0.01) or 3 (t60.5 = 4.97, P < 0.001), and between piglets with a score of 1 or 3 (t62.7 = 4.23, P < 0.001). Piglets with a score of 1 tended to be heavier than those with a score of 2 (t62.8 = 2.51, P = 0.07), and of score 2 tended to be heavier than those of score 3 (t60.4 = 2.39, P = 0.09). Therefore, the investigation of the effect of IUGR was quite different from the investigation of the effect of birth weight (category thresholds based on quartiles of the variable weight).
Table 1.
Birth weights of piglets classified into each IUGR category (score 0 = no IUGR to score 3 = severe IUGR)
| N | Minimum | Maximum | LS mean ± SE | |
|---|---|---|---|---|
| IUGR 0 | 15 | 1.03 | 2.44 | 1.68 ± 0.09a |
| IUGR 1 | 22 | 0.98 | 2.08 | 1.57 ± 0.09ab |
| IUGR 2 | 21 | 0.78 | 1.79 | 1.33 ± 0.08bc |
| IUGR 3 | 9 | 0.53 | 1.56 | 1.04 ± 0.12c |
Different superscript letters indicate significant differences between IUGR levels (P < 0.05)
Correlations Between Thermal Data
The correlations between RT, and birth weight and the thermal data acquired from the IRT images, at each different time of data collection (i.e., birth, 15, 30, and 60 min postpartum) are presented in Table 2. RT was positively correlated with all IRT temperatures at all times (P < 0.01). Moderate correlations coefficients (0.3–0.6) were found for the minimum temperature of the back and for the temperature of the ear tip; and strong correlations coefficients (0.5–0.9) were found for the temperature of the ear base and for the maximum and average temperatures of the back.
Table 2.
Pearson correlations coefficients characterizing relationships between RT, and birth weight and thermal data
| Back temperatures | Ear temperatures | |||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Average | Tip | Base | Birth weight | |
| Rectal temperature | ||||||
| 6 min pp.a (birth) | 0.48** | 0.55*** | 0.57*** | 0.59*** | 0.8*** | 0.47** |
| 15 min pp. | 0.37** | 0.45*** | 0.49*** | 0.28* | 0.64*** | 0.57*** |
| 30 min pp. | 0.36** | 0.86*** | 0.78*** | 0.53*** | 0.86*** | 0.56*** |
| 60 min pp. | 0.59*** | 0.9*** | 0.87*** | 0.39** | 0.71*** | 0.36** |
| Birth weight | ||||||
| 6 min pp. (birth) | 0.28* | 0.04 | 0.1 | 0.31* | 0.21 | |
| 15 min pp. | 0.22 | −0.03 | −0.1 | 0.04 | 0.1 | |
| 30 min pp. | −0.05 | 0.28* | 0.15 | 0.03 | 0.32** | |
| 60 min pp. | 0.01 | 0.28* | 0.19 | 0.18 | 0.32** | |
Data from thermal images (temperature of the ear tip, ear base, and minimum, maximum and average back temperature) obtained at birth (6 min) and 15 min, 30 and 60 min postpartum (pp). Numbers in bold show moderate to strong significant correlations.
*P < 0.05.
**P < 0.01.
***P < 0.001.
app. = postpartum.
Birth weight was positively and moderately correlated to RT at all times (Table 2). It was also moderately correlated with ear tip and minimum back temperature at birth, and with ear base and maximum back temperature at 30 and 60 min postpartum. Therefore, the effect of weight on temperature data was studied in more details by using weight ranks (based on weight quartiles) in the analyses.
Effect of Time
The effects of time were the same for both analyses of the effect of IUGR score, and weight rank (see Table 3 for the analysis on weight rank, and Table 4 for the analysis on IUGR score). The RT dropped between birth and 15 min postpartum (P < 0.001) and increased again between 30 and 60 min postpartum (P < 0.001). The same pattern was observed with the temperature at the base of the ear (birth vs. 15 min: P < 0.001; 30 min vs. 60 min: P < 0.001) and the tip of the ear (birth vs. 15 min: P < 0.001; 30 min vs. 60 min: P < 0.01). However, the temperature of the ear tip started to rise again between 15 and 30 min postpartum (P < 0.001).
Table 3.
Mean ± SE temperatures of neonatal piglets, according to the time postpartum and their birth weight category
| Rectal temperature | Ear base temperature | Ear tip temperature | Minimum back temperature | Maximum back temperature | Average back temperature | |
|---|---|---|---|---|---|---|
| Effect of time | ||||||
| Birth (6 min pp.a) | 37.4 ± 0.36A | 36.1 ± 0.33AC | 29.2 ± 0.32AC | 29.3 ± 0.43A | 34.9 ± 0.32A | 33.2 ± 0.35A |
| 15 min postpartum | 36.2 ± 0.34B | 35.4 ± 0.31B | 27.7 ± 0.32B | 28.3 ± 0.43B | 35.4 ± 0.32B | 33.5 ± 0.35A |
| 30 min postpartum | 36.2 ± 0.36)B | 35.7 ± 0.32AB | 28.8 ± 0.35A | 28.7 ± 0.42 | 35.9 ± 0.31C | 34.1 ± 0.35B |
| 60 min postpartum | 37.1 ± 0.37A | 36.6 ± 0.36C | 29.8 ± 0.37C | 29.3 ± 0.44A | 36.7 ± 0.30D | 34.9 ± 0.35C |
| P-value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | <0.001 | <0.001 |
| Effect of weight category | ||||||
| <1.27 kg | 35.7 ± 0.41Aa | 35.2 ± 0.36A | 28.4 ± 0.39 | 28.7 ± 0.44 | 35.4 ± 0.34a | 33.5 ± 0.38A |
| 1.27–1.50 kg | 36.5 ± 0.39b | 35.7 ± 0.35a | 29.2 ± 0.37 | 29.2 ± 0.43 | 35.6 ± 0.34 | 33.9 ± 0.37 |
| 1.50–1.74 kg | 37.3 ± 0.38Bc | 36.5 ± 0.35Bb | 28.9 ± 0.37 | 29.0 ± 0.43 | 36.2 ± 0.33b | 34.4 ± 0.37B |
| >1.74 kg | 37.4 ± 0.39Bc | 36.4 ± 0.36B | 28.9 ± 0.39 | 28.8 ± 0.44 | 35.7 ± 0.34 | 33.8 ± 0.38 |
| P-value | P < 0.001 | P < 0.001 | P = 0.2 | P = 0.2 | P < 0.01 | P < 0.05 |
Superscript letters indicate significant differences within each variable (a,b = P < 0.05, A,B = P < 0.005).
app. = postpartum.
Table 4.
Mean (± SE) temperatures of neonatal piglets, according to the time postpartum and their IUGR score
| Rectal temperature | Ear base temperature | Ear tip temperature | Minimum back temperature | Maximum back temperature | Average back temperature | |
|---|---|---|---|---|---|---|
| Effect of time | ||||||
| Birth (6 min pp.a) | 37.4 ± 0.35A | 36.1 ± 0.33A | 29.3 ± 0.35AC | 29.2 ± 0.47A | 35.0 ± 0.32A | 33.2 ± 0.36A |
| 15 min postpartum | 36.2 ± 0.34B | 35.3 ± 0.30B | 27.8 ± 0.35B | 28.2 ± 0.45B | 35.3 ± 0.32A | 33.4 ± 0.37A |
| 30 min postpartum | 36.1 ± 0.35B | 35.6 ± 0.31ABa | 28.7 ± 0.38A | 28.8 ± 0.45 | 35.8 ± 0.31B | 34.0 ± 0.36B |
| 60 min postpartum | 37.0 ± 0.37A | 36.3 ± 0.37Ab | 29.8 ± 0.39C | 29.4 ± 0.47A | 36.6 ± 0.30C | 34.8 ± 0.37C |
| P-value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | <0.001 | <0.001 |
| Effect of IUGR score | ||||||
| IUGR 0 | 37.2 ± 0.42a | 36.3 ± 0.36a | 29.0 ± 0.40 | 28.8 ± 0.46 | 35.7 ± 0.34 | 33.9 ± 0.39 |
| IUGR 1 | 37.1 ± 0.40a | 36.1 ± 0.34 | 29.0 ± 0.38 | 29.1 ± 0.45 | 35.7 ± 0.33 | 33.9 ± 0.38 |
| IUGR 2 | 36.6 ± 0.38 | 35.9 ± 0.34 | 28.8 ± 0.38 | 28.9 ± 0.45 | 35.9 ± 0.33 | 34.1 ± 0.38 |
| IUGR 3 | 35.8 ± 0.46b | 35.1 ± 0.42b | 28.8 ± 0.49 | 28.9 ± 0.50 | 35.3 ± 0.39 | 33.5 ± 0.44 |
| P-value | P < 0.05 | P < 0.05 | P = 0.89 | P = 0.76 | P = 0.29 | P = 0.25 |
Superscript letters indicate significant differences within each variable (a,b = P < 0.05, A,B = P < 0.005).
app. = postpartum.
The minimum temperature of the back also decreased between birth and 15 min postpartum (P < 0.005), and increased between 15 and 60 min postpartum (P < 0.005). However, the maximum and average temperatures of the back increased constantly across the hour postpartum. Differences were significant between every time points for the maximum temperature of the back (birth vs. 15 min postpartum: P < 0.005; 15 min vs. 30 min postpartum: P < 0.001; 30 min vs. 60 min postpartum: P < 0.001).
Effect of Birth Weight Category
As hypothesized, there was an effect of birth weight rank on RT (F3,44.2 = 14.03; P < 0.001; Table 3). Indeed, there were differences between all rank pairs (P < 0.05), except between the two heaviest ranks (Table 3). A similar effect of birth weight rank was found on the temperature of the base of the ear (F3,65.2 = 8.5, P < 0.001; Table 3). Indeed, piglets in the first quartile (lowest weight; 0.27–1.27 kg) had lower ear base temperatures than piglets in the third quartile (1.5–1.74 kg; 35.2 ± 0.36 °C vs. 36.5 ± 0.35 °C, t64.9 = −4.51, P < 0.001) and piglets in the fourth quartile (1.74–2.44 kg; 35.2 ± 0.36 °C vs. 36.4 ± 0.36 °C, t70.4 = −3.97, P < 0.005), and piglets in the second quartile (1.27–1.5 kg) had lower ear base temperature than piglets in the third quartile (35.27 ± 0.35 °C vs. 36.5 ± 0.35 °C, t62.6 = −2.78, P < 0.05). There was an overall effect of rank for the average (F3,59 = 4.27; P < 0.01) or maximum (F3,57.4 = 3.62, P < 0.05) temperature of the back, although only piglets in the first quartile had significantly lower temperatures than piglets in the third quartile (maximum: 36.2 ± 0.33 °C vs 36.2 ± 0.33 °C, t57.4, P < 0.05; average: 33.5 ± 0.38 °C vs. 34.4 ± 0.37 °C, P < 0.005). There was no effect of birth weight rank on either the temperature at the ear tip (F3,68.3 = 1.59; P = 0.2), or the minimum temperature recorded on the back (F3,90.9 = 1.46; P = 0.2).
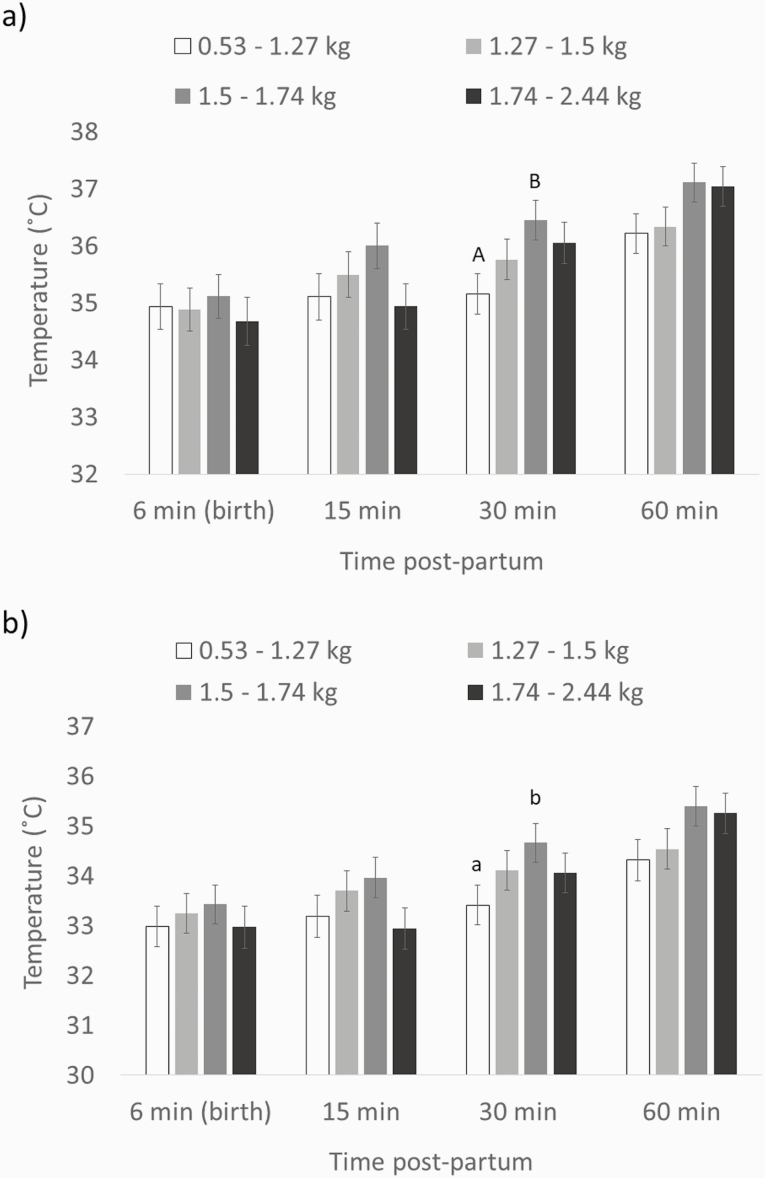
There was an interaction between time and weight rank on the maximum (F9,123 = 2.59, P < 0.01; Figure 1a) and average (F9,131 = 2.51, P < 0.05; Figure 1b) temperatures of the back. For both temperatures, the only significant difference was between lightest piglets (first quartile) and piglets in the third weight quartile at 30 min postpartum (maximum: 35.2 ± 0.36 °C vs. 36.4 ± 0.35 °C, t54.3 = −4.34, P < 0.005; average: 33.4 ± 0.4 °C vs. 34.7 ± 0.39 °C, t55.9 = −4.12, P < 0.01).
Figure 1.
Maximum (a) and average (b) back temperatures (LS mean ± SE) of piglets in different weight categories. Different letters indicate differences between weight categories (lowercase: P < 0.05; uppercase P < 0.005). P-value for the effect of the interaction between time and weight rank was P < 0.01 for the maximum back temperature, and P < 0.05 for the average back temperature.
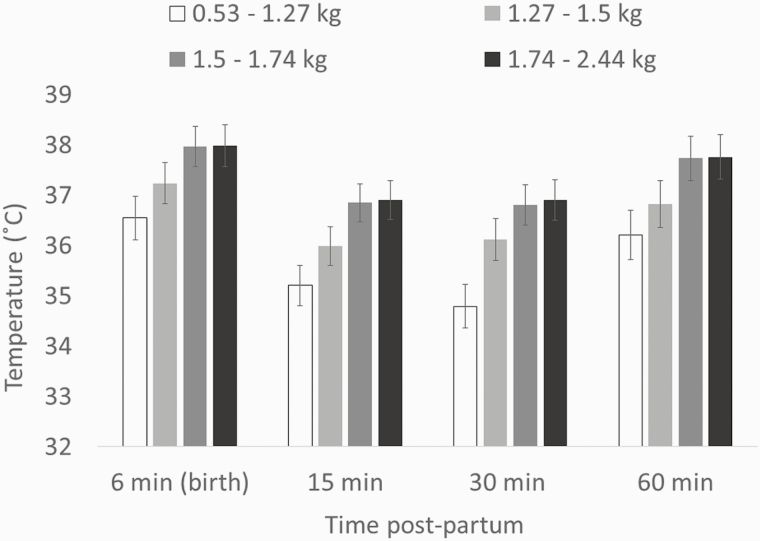
The interaction between weight rank and time was not significant for RT (F9,101 = 1.1, P = 0.37), minimum temperature of the back (F9,132 = 0.71; P = 0.70), or temperature at the base (F9,125 = 1.01, P = 0.44) or the tip (F9,134 = 0.97; P = 0.47) of the ear. However, within each time point there were differences in RT between the weight ranks (birth: F3,42.3 = 7.31, P < 0.001; 15 min postpartum: F3,45.9 = 15.23, P < 0.001; 30 min postpartum: F3,48.5 = 14.6, P < 0.001; 60 min postpartum: F3,45.7 = 5.42, P < 0.005; Figure 2). These differences were mainly due to the lightest piglets (first quartile) having lower RT than heaviest piglets (third and fourth quartiles) at birth (36.6 ± 0.43 °C vs. 38.0 ± 0.40 °C, t41.6 = −4.07, P < 0.01; 36.6 ± 0.43 °C vs. 38.0 ± 0.42 °C, t44.8 = −3.91, P < 0.05; respectively), at 15 min postpartum (35.2 ± 0.40 °C vs. 36.8 ± 0.37 °C, t47.8 = −5.66, P < 0.001; 35.2 ± 0.40 °C vs. 36.9 ± 0.38 °C, t48.5 = −5.84, P < 0.001; respectively) and at 30 min postpartum (34.8 ± 0.43 °C vs. 36.8 ± 0.40 °C, t48.7 = −5.69, P < 0.001; 34.8 ± 0.43 °C vs. 36.8 ± 0.40 °C, t48.2 = −6.04, P < 0.001; respectively; Figure 2). At 30 min postpartum, piglets in the first quartile also had a lower RT than piglets in the second quartile (34.8 ± 0.43 °C vs. 36.1 ± 0.41 °C, t49.4 = −3.57, P < 0.05). Similarly, there were differences between the weight categories in ear base temperatures at 15 min (F3,67.6 = 3.53; P < 0.05), 30 min (F3,55.8 = 5.38; P < 0.005) and 60 min postpartum (F3,57.2 = 6.13; P < 0.005). However, the differences were only significant between piglets in the first quartile and piglets in the third quartile at 30 min postpartum (t55.4 = −3.61; P < 0.05), and between piglets in the first quartile and piglets in the fourth quartile at 60 min postpartum (t58.5 = −3.87; P < 0.05).
Figure 2.
RT (LS mean ± SE) of piglets in different weight rank across the first hour postpartum (effect time × weight rank: P > 0.1).
Effect of IUGR score
IUGR score had a significant effect on piglets’ RT (F3,43.6 = 4.07, P < 0.05) and temperature at the base of the ear (F3,62.1 = 3.14, P < 0.05; Table 4). Indeed, piglets with severe IUGR (score 3) had an overall lower RT than normal piglets (score 0; 35.8 ± 0.46 °C vs. 37.2 ± 0.42 °C, respectively, t43.1 = 3.16, P < 0.05) and piglets with mild IUGR (score 1; 35.8 ± 0.46 °C vs. 37.1 ± 0.40 °C, respectively, t45.3 = 2.92, P < 0.05). Piglets with severe IUGR also had lower temperature at the base of the ear than normal piglets (35.1 ± 0.42 °C vs. 36.3 ± 0.36 °C, respectively, t63.1 = 3.01, P < 0.05). The effect of IUGR score was not significant on any of the ear tip temperature (F3,68.5 = 0.21, P = 0.89) and the back temperatures (minimum: F3,87.7 = 0.4, P = 0.76; average: F3,56.9 = 1.42, P = 0.25; maximum: F3,54.9 = 1.28, P = 0.29; Table 4).
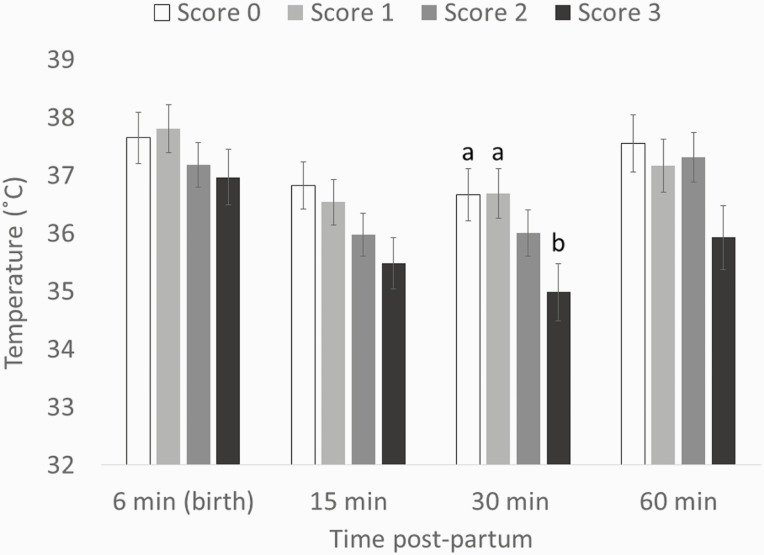
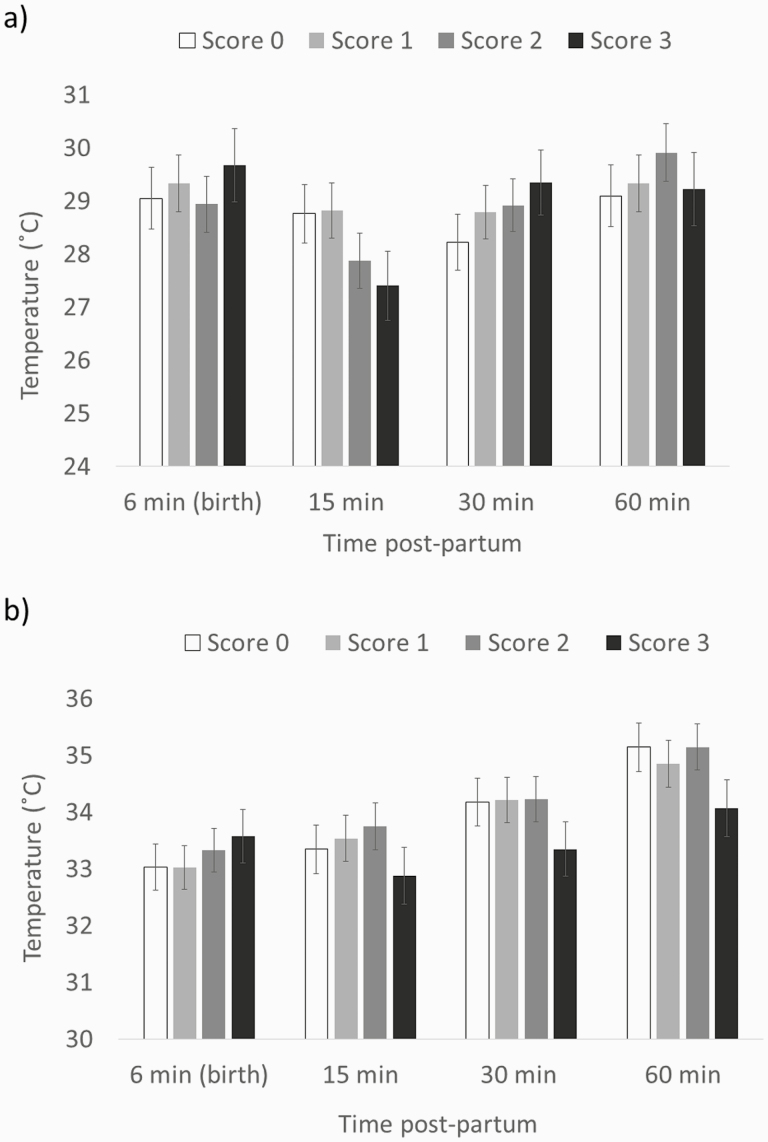
There was an interaction between time and IUGR score for RT (F9,105 = 2.42, P < 0.05; Figure 3). Indeed, at 30 min postpartum, piglets with severe IUGR (score 3) had lower RT than normal (IUGR score 0) piglets (35 ± 0.49 °C vs. 36.7 ± 0.45 °C, respectively; t46.6 = 3.52, P < 0.05) and piglets with an IUGR score 1 (35 ± 0.49 °C vs. 36.7 ± 0.42 °C, respectively; t50 = 3.64, P < 0.05). There also was an interaction between time and IUGR score on the minimum (F9,131 = 2, P < 0.05; Figure 4a) and average (F9,136 = 2.09, P < 0.05; Figure 4b) temperatures of the back, but pair-wise comparisons were not different. There was no interaction between time and IUGR score for the ear temperatures (ear base: F9,128 = 1.45, P = 0.18; ear tip: F9,134 = 0.52, P = 0.86) or the maximum back temperature (F9,128 = 1.85, P = 0.06).
Figure 3.
RT (LS mean ± SE) of piglets with different IUGR score across the first hour postpartum (effect time × IUGR score: P < 0.05). Different letters indicate differences between weight categories (P < 0.05).
Figure 4.
Minimum (a) and average (b) back temperatures (LS mean ± SE) of piglets with different IUGR score. Different letters indicate differences between weight categories (lowercase: P < 0.05; uppercase P < 0.005). P-value for the effect of the interaction between time and IUGR score was P < 0.05 for both the minimum and average back temperatures.
DISCUSSION
This study aimed at validating the use of IRT to assess the thermal status of piglets across the first hour postpartum, to provide an estimate of their thermoregulatory abilities. Correlations with all temperatures obtained from IRT images with RT confirmed that IRT can be a valid tool to assess thermal status of piglets around birth. More specifically, images of the ears showed the same thermal patterns as RT over time. The second aim of the study was to identify whether the level of IUGR and birth weight could influence thermoregulatory ability; the aim was to determine whether the pattern of temperature change over time was affected by weight and IUGR score. The results showed clearly that both factors did indeed influence thermoregulatory abilities of piglets.
Correlations between RT and ear (base and tip) and back (minimum, maximum, and average temperatures of the back area, from shoulders to rumps) temperatures confirmed earlier work showing that IRT is a valid tool for assessing piglets temperature (Tabuaciri et al., 2012; Kammersgaard et al., 2013; Soerensen and Pedersen, 2015). Furthermore, strong correlations with RTs at each time point suggested that maximum back and ear base temperatures are best locations for approximating piglet body temperature. The levels of correlation found in the present study are similar to the ones found in other studies (Tabuaciri et al., 2012; Kammersgaard et al., 2013). Higher correlations could be expected in the present study as the time of acquisition of images (relative to the piglet’s birth) and environmental factors (isolation in a plastic box) were controlled, which was not the case in previous work (Tabuaciri et al., 2012; Kammersgaard et al., 2013). Indeed, piglets were handled during image acquisition in the study of Kammersgaard et al. (2013), which can cause stress and elevation of body temperature, and the time postpartum was not accounted for in the study of Tabuaciri et al. (2012). Moreover, there are potential confounding factors not accounted for in these studies, such as environmental temperature, piglets’ behavior (e.g., huddling; Llamas Moya et al., 2006), and presence of birth fluids.
During the first hour postpartum, piglets’ RT, the gold standard in core temperature measurement, decreased between birth and 15 min postpartum, then increased again between 30 and 60 min postpartum. The ear base, ear tip, and the minimum back temperatures followed the same pattern. This shows thermoregulation process, that is, the change of temperature overtime to reach or maintain thermal homeostasis, and such pattern (decrease shortly after birth and increase afterwards) was previously reported by Herpin et al. (2002). However, the maximum and average back temperatures increased steadily overtime, which confirms the findings of Kammersgaard et al. (2013) that the back temperature may not provide an accurate estimate of the core body temperature.
The effect of level of IUGR and weights on temperature data were investigated separately, as they describe different populations of piglets. Indeed, even if lower average weights correspond to a greater severity of IUGR, it is important to make a distinction between piglets born with low birth-weight (also called “small for gestational age”) and piglets which experience IUGR, because their survival chance and growth potential might be different (Rutherford et al., 2013). In the present study, both the level of IUGR and weight ranks (based on weight quartiles) affected piglets’ rectal and ear base temperatures. However, significant differences between the IUGR levels were only found at 30 min postpartum between severe IUGR piglets (score 3) and normal piglets (score 0), whereas weight ranks differed more across time. The second difference was found in the pattern of RT, as IUGR level did not affect piglets’ RT at birth but weight rank did. Indeed, piglets with the lowest birth weight (0.57–1.27 kg) had a lower RT compared to heavier piglets from birth until 30 min postpartum. Moreover, at 60 min postpartum, piglets with a body weight above 1.5 kg had a RT greater than 37 °C whereas the RT of piglets under 1.27 kg was just above 36 °C, showing that the later might have some difficulty in ensuring thermoregulation (i.e., piglets should reach the thermal homeostasis of 39 °C within 48 h of age; Herpin et al., 2002). The same difference of temperature at 60 min postpartum was also observed between piglets with severe IUGR, compared with all other levels. Altogether, our results suggest that IUGR and low birth weight are two separated conditions, often affecting the same piglets, but which may have separate influences on piglet thermoregulatory abilities. It is possible that a low birth weight, independent of the level of IUGR, is an unfavorable condition for thermoregulation, due to the greater surface to body mass ratio and consequent greater heat loss (Herpin et al., 2002), and that IUGR level may prevent these small piglets from ensuring their thermoregulation within 1 h postpartum. Therefore, affected piglets may require a greater amount of time to reach thermal comfort, or a greater supply of energy from colostrum. The present results may suggest that piglets with low birth weights rather than IUGR should be targeted by energy supplementation interventions.
In conclusion, this study confirmed that IRT, and especially images of the base of the ear, is a valid noninvasive tool to assess thermal status of neonatal piglets. Images taken during the first hour postpartum could be used to determine piglets with difficulties in maintaining body temperature under the experimental conditions. Further research work could use IRT to test the effects of energy supplementation on the thermoregulatory abilities of neonatal piglets, in order to identify the most successful timing of supplementation.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the Irish Department of Agriculture, Food and the Marine through the IRM/RSF/CoFoRD 2013 Research Call (project no. 13S428). We would like to thank the farm staff of the Moorepark pig research unit for their help during data collection.
Conflict of interest statement. None declared.
LITERATURE CITED
- Baxter E. M., Jarvis S., D’Eath R. B., Ross D. W., Robson S. K., Farish M., Nevison I. M., Lawrence A. B., and Edwards S. A.. . 2008. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69:773–783. doi: 10.1016/j.theriogenology.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Baxter E. M., Jarvis S., Palarea-Albaladejo J., and Edwards S. A.. . 2012. The weaker sex? The propensity for male-biased piglet mortality. Plos One 7:e30318. doi: 10.1371/journal.pone.0030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter E. M., Jarvis S., Sherwood L., Robson S. K., Ormandy E., Farish M., Smurthwaite K. M., Roehe R., Lawrence A. B., and Edwards S. A.. . 2009. Indicators of piglet survival in an outdoor farrowing system. Livest. Sci. 124:266–276. 10.1016/J.LIVSCI.2009.02.008. Available from https://www.sciencedirect.com/science/article/pii/S1871141309000614 [DOI] [Google Scholar]
- Hales J., Moustsen V. A., Devreese A. M., Nielsen M. B. F., and Hansen C. F.. . 2015. Comparable farrowing progress in confined and loose housed hyper-prolific sows. Livest. Sci. 171:64–72. 10.1016/j.livsci.2014.11.009 [DOI] [Google Scholar]
- Hales J., Moustsen V. A., Nielsen M. B., and Hansen C. F.. . 2013. Individual physical characteristics of neonatal piglets affect preweaning survival of piglets born in a noncrated system. J. Anim. Sci. 91:4991–5003. doi: 10.2527/jas.2012-5740 [DOI] [PubMed] [Google Scholar]
- Hansen C. F., Hales J., Amdi C., and Moustsen V. A.. . 2019. Intrauterine growth-restricted piglets defined by their head shape have impaired survival and growth during the suckling period. Anim. Prod. Sci. 59:1056–1062. 10.1071/AN17581 [DOI] [Google Scholar]
- Herpin P., Vincent A., and Damon M.. 2004. Effect of breed and body weight on thermoregulatory abilities of European (Pietrain×(Landrace× Large White)) and Chinese (Meishan) piglets at birth. Livest. Prod. Sci. 88:17–26. [Google Scholar]
- Herpin P., Damon M., and Le Dividich J.. . 2002. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 78:25–45. 10.1016/S0301-6226(02)00183-5 [DOI] [Google Scholar]
- Kammersgaard T. S., Malmkvist J., and Pedersen L. J.. . 2013. Infrared thermography—a non-invasive tool to evaluate thermal status of neonatal pigs based on surface temperature. Animal 7:2026–2034. doi: 10.1017/S1751731113001778 [DOI] [PubMed] [Google Scholar]
- Kammersgaard T. S., Pedersen L. J., and Jørgensen E.. . 2011. Hypothermia in neonatal piglets: interactions and causes of individual differences. J. Anim. Sci. 89:2073–2085. 10.2527/jas.2010-3022 [DOI] [PubMed] [Google Scholar]
- Llamas Moya S., Boyle L. A., Lynch P. B., and Arkins S.. . 2006. Influence of teeth resection on the skin temperature and acute phase response in newborn piglets. Anim. Welf. 15:291–297. [Google Scholar]
- Muns R., Nuntapaitoon M., and Tummaruk P.. . 2016. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 184:46–57. 10.1016/j.livsci.2015.11.025. Available from 10.1016/j.livsci.2015.11.025 [DOI] [Google Scholar]
- Muns R., de la Torre R., Agostini P. S., Manteca X., and Gasa J.. . 2010. The effect of colostrum supplementation on piglets’ body temperature recovery and lactation performance. J. Anim. Sci. 88:306.19820061 [Google Scholar]
- Rutherford K. M. D., Baxter E. M., D’Eath R. B., Turner S. P., Arnott G., Roehe R., Ask B., Sandøe P., Moustsen V. A., Thorup F., . et al. 2013. The welfare implications of large litter size in the domestic pig I: biological factors. Anim. Welf. 22:199–218. 10.7120/09627286.22.2.199 [DOI] [Google Scholar]
- Sasaki Y., Furusho K., Ushijima R., Tokunaga T., Uemura R., and Sueyoshi M.. . 2016. Body surface temperature of suckling piglets measured by infrared thermography and its association with body weight change. Japan Agric. Res. Q. 50:361–368. [Google Scholar]
- Soerensen D. D., Clausen S., Mercer J. B., and Pedersen L. J.. . 2014. Determining the emissivity of pig skin for accurate infrared thermography. Comput. Electron. Agric. 109:52–58. 10.1016/j.compag.2014.09.003. Available from http://www.sciencedirect.com/science/article/pii/S0168169914002178 [DOI] [Google Scholar]
- Soerensen D. D., and Pedersen L. J.. . 2015. Infrared skin temperature measurements for monitoring health in pigs: a review. Acta Vet. Scand. 57:5. doi: 10.1186/s13028-015-0094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuaciri P., Bunter K. L., and Graser H.-U.. . 2012. Thermal imaging as a potential tool for identifying piglets at risk. AGBU Pig Genetics Workshop; Armidale; p. 23–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.