Abstract
GNU100 is a novel animal milk oligosaccharide (AMO) biosimilar. In a recent in vitro fermentation study, GNU100 was shown to be fermentable by feline gastrointestinal microbiota and lead to increased short-chain fatty acid production. Our objectives herein were to evaluate the palatability, safety, and gastrointestinal tolerance of GNU100 in healthy adult cats. Exploratory end-points were measured to assess utility. In study 1, 20 adult cats were used to test the palatability of diets containing 0% or 1% GNU100. In study 2, 32 (mean age = 1.9 yr; mean body weight = 4.6 kg) male (n = 12) and female (n = 20) adult cats were used in a completely randomized design. After a 2-wk baseline, cats were assigned to one of the following treatment groups and fed for 26 wk: control (CT, no GNU100), low dose (LD, 0.5% GNU100), medium dose (MD, 1.0% GNU100), and high dose (HD, 1.5% GNU100). On weeks 2, 4, and 26, fresh fecal samples were collected for the measurement of stool quality and immune and inflammatory markers and on weeks 2 and 4 for microbiota and metabolites. On week 4, total feces were collected to measure apparent total tract macronutrient digestibility. On weeks 2, 4, and 26, blood samples were collected for serum chemistry, hematology, and inflammatory marker measurement. The palatability test showed that 1% GNU100 was strongly preferred (P < 0.05), with GNU100 having a 17.6:1 consumption ratio compared with control. In the long-term study, all cats remained healthy, without any signs of gastrointestinal intolerance or illness. All diets were well accepted, resulting in similar (P > 0.05) food intake, fecal characteristics, immunoglobulin A, and calprotectin, and dry matter, organic matter, fat, and crude protein digestibilities. Fecal butyrate was greater (P = 0.02) in cats fed HD than cats fed LD or MD. Fecal indole was lower (P = 0.02) in cats fed HD than cats fed LD. Cats fed CT had a higher (P = 0.003) relative abundance of Actinobacteria than cats fed LD. The relative abundance of Peptococcus was impacted by diet and time. At 4 wk, Campylobacter was lower in fecal samples of cats fed HD. Overall, the data suggest that dietary GNU100 supplementation was highly palatable, well tolerated, did not cause detrimental effects on fecal quality or nutrient digestibility, increased fecal butyrate concentrations, and reduced fecal indole concentrations, supporting the safety of GNU100 for inclusion in feline diets and suggesting potential benefits on gastrointestinal health of cats.
Keywords: feline nutrition, gastrointestinal functionality, microbiome, milk oligosaccharides, nutrient digestibility
Introduction
Mammalian milk contains bioactive molecules that participate in the health and protection of neonates, among which milk oligosaccharides (MO) in their free or conjugated form represent a major component (Liu and Newburg, 2013). MO are capable of providing physiological benefits, including the selective growth promotion of beneficial microbiota and increases in beneficial fermentative products. For instance, in vitro fermentation studies have demonstrated that Bifidobacterium spp. are capable of utilizing MO, resulting in the production of short-chain fatty acids (SCFA) (Ward et al., 2006; LoCascio et al., 2009; Asakuma et al., 2011; Yu et al., 2013b; Garrido et al., 2015). MO have also been shown to inhibit enteric pathogen growth, adhesion to enterocytes, and production of beneficial fermentative products (Kunz et al., 2000; Ruiz-Palacios et al., 2003; Newburg et al., 2005; Asakuma et al., 2011; Zivkovic and Barile, 2011; Bode, 2012; Kavanaugh et al., 2013; Urashima et al., 2013; Vester Boler et al., 2013; Manthey et al., 2014; Musilova et al., 2014; Kong et al., 2019; Oba et al., 2020); aid in the maturation of the gut mucosal barrier and development of the gastrointestinal immune system (Yolken et al., 1992; Kunz et al., 2000; Eiwegger et al., 2004; Urashima et al., 2013; Good et al., 2016; He et al., 2016; Comstock et al., 2017; Plaza-Díaz et al., 2018); possess antiviral function (Bode, 2012; Hester et al., 2013; Comstock et al., 2017; Morozov et al., 2018); modulate intestinal motility (Bienenstock et al., 2013); and enhance brain development and cognitive function of neonates (Wang, 2009; Bode, 2012; Vázquez et al., 2015).
Animal milk oligosaccharides (AMO) are distinct yet structurally similar to human milk oligosaccharides (HMO) (Bubb et al., 1999; Urashima et al., 2001; Macias Rostami et al., 2014). Feline MO have been shown to be structurally diverse with a minimum of 20 structures having been identified by liquid chromatography coupled with mass spectrometry (Hughes et al., 2020; Wrigglesworth et al., 2020). Mimicking the diversity and complexity of MO is critical, as the diverse benefits of MO are almost certainly related to their structural and functional diversity, with multiple components acting together to promote health (Zivkovic and Barile, 2011). However, current substitutes are limited either in structural complexity (e.g., fructooligosaccharides [FOS] and galactooligosaccharides [GOS]) or in diversity (2′-fucosyllactose [2′FL], lacto-N-neotetraose [LNnT]), not to mention the challenge of large-scale supply (Bode et al., 2016).
GNU100 contains a diversity of about 30 complex conjugated oligosaccharides aiming to be a sustainable source of AMO biosimilars. Being isolated from hydrolyzed porcine intestinal mucosa, GNU100 is composed, in part, of O-linked glycans bound to peptidic moieties. The structural features of intestinal mucin glycans are similar to those of MO, in that both classes of compounds have an elongated core structure with galactose and N-acetylglucosamine, and chains that often terminate with fucose or sialic acid groups (Marcobal et al., 2011). Furthermore, mucin glycans and MO enrich for taxonomically similar resident microbes and mucin glycans have been shown to mitigate perturbations to the microbiota and provide benefits to host physiology in a mouse model (Pruss and Sonnenburg, 2020). Finally, mucin-derived glycans have been shown to sustain mucosal immune homeostasis via SCFA production in a rat model (Hino et al., 2020).
A previous study demonstrated that GNU100 was fermented in an in vitro fermentation system inoculated with canine and feline microbiota, resulting in a beneficial shift in bacteria (increased Bifidobacterium and Lactobacillus and reduction in Escherichia/Shigella and Salmonella) and increased production of SCFA (acetate, propionate, and butyrate), branched-chain fatty acids (BCFA; isobutyrate, isovalerate, and valerate), and ammonium (Oba et al., 2020). These organic acids, especially butyrate, serve as an energy source for epithelial cells, modulate colonocyte proliferation, and regulate epithelial barrier integrity, the gastrointestinal immune system, and inflammatory responses (Clausen and Mortensen, 1995; Maeda et al., 2000; Lührs et al., 2002; Wong et al., 2006; Arpaia et al., 2013; Furusawa et al., 2013; van der Beek et al., 2015). Even though AMO have been studied in vitro, to our knowledge, nobody has tested them in cats. Therefore, we initially performed an in vivo study aiming to assess the palatability of GNU100. The study was followed by a second in vivo study aiming to evaluate primarily the safety and gastrointestinal tolerance of GNU100 and exploring its utility in a healthy feline population. We hypothesized that GNU100 would not negatively affect fecal characteristics, serum chemistry, and hematology, or nutrient digestibility, and would modulate the fecal microbiome composition and selected metabolites favorably.
Materials and Methods
Study 1: palatability study
A standard 2-d palatability test was conducted at Kennelwood Inc. (Champaign, IL) to compare the control diet containing no GNU100 and a diet containing 1.0% GNU100. Briefly, 20 adult domestic shorthair cats were housed individually and presented with the test diets coded as diet A or diet B on an individual basis. Diet A and diet B were prepared in an identical manner. A mixed fat source (5% by weight) was sprayed on to a base cat food (Supplementary Table S1) and then was or was not coated with a 1% inclusion rate of GNU100. Two bowls, each containing 110 g of diet, were offered once daily for 2 d. Bowl placement was reversed daily to prevent left–right bias. Food consumption and first choice preference were recorded for each cat. Diet preference assessment was done by comparing the first choice (the first diet consumed) and calculating the intake ratio (intake of diet A/intake of diet A + diet B) of each diet tested in each experiment. To assess first choice diet preference, a chi-square test compared the daily counts for each diet with the counts that represent no preference (i.e., when both diets are chosen equally).
Study 2: safety and tolerability study
Animals and experimental design
All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to animal experimentation (IACUC #18068). The staff conducting the study were blinded to treatments to avoid any potential bias in the evaluation of general health observations, fecal scores, etc. Prior to the study, a physical examination by a licensed veterinarian was conducted, and blood samples for serum chemistry and hematology measurements were collected to confirm health.
Thirty-two (mean age = 1.9 ± 0.05 yr; mean body weight [BW] = 4.6 ± 0.13 kg) male (n = 12) and female (n = 20) adult domestic shorthair cats were used in a completely randomized design (CRD) consisting of 28 wk, including 2 wk of an acclimation phase and 26 wk of an experimental phase. The schedule of sample collection and measurements is described in Supplementary Table S3. Cats were allotted to one of four treatment groups based on BW and sex (n = 8/group; 3 males + 5 females per group), so that mean baseline BW was similar among groups: control diet (CT; no GNU100), low dose (LD; 0.5% GNU100 in diet), medium dose (MD; 1.0% GNU100 in diet), and high dose (HD; 1.5% GNU100 in diet). Cats were housed individually in cages (1.02 × 0.76 × 0.71 m3) during feeding times (8:00 to 10:00 a.m.) and the fecal collection phases, in a temperature- (20 to 22 °C), humidity- (30% to 70%), and light-controlled (14:10 light:dark [L:D] cycle; no access to natural light) room in the Edward R. Madigan Animal Facility at the University of Illinois at Urbana-Champaign, Urbana, IL. At other times, cats were group-housed and able to socialize and exercise outside their cages. Cats were allowed access to various toys and scratching poles for environmental enrichment and playtime with human interaction at least 2 times per week. Freshwater was available ad libitum in stainless steel bowls. Cages and rooms were cleaned daily.
Test diet
Dry, extruded experimental diets formulated to meet all Association of American Feed Control Officials (AAFCO, 2019) nutrient recommendations for adult cats were fed. All diets included poultry byproduct meal (low-ash), brewer’s rice, poultry fat, corn, taurine, vitamin and mineral premixes, a palatant, and the test ingredient (GNU100) and formulated to contain approximately 35% protein, 15% fat, 7% ash, and 5% fiber. Diets were manufactured at Wenger Manufacturing, Inc. (Sabetha, KS). Wenger used their large paddle mixer (capacity = ~550 to 700 kg). For each batch, the appropriate amount of GNU100 was pre-blended into about 50 kg of dry diet mix. The pre-blend was then added to the entire batch. Each batch was mixed for at least 10 to 15 min to ensure complete mixing.
Gnubiotics Sciences SA (Epalinges, Switzerland) provided the test ingredient. GNU100 is a complex of oligosaccharides and peptides isolated from hydrolyzed porcine intestinal mucosa, with the typical physicochemical characteristics depicted in Table 1. The typical oligosaccharide profile of GNU100 measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) is listed in Supplementary Table S2 and shows that GNU100 is made up of a diverse and complex mixture of oligosaccharides with epitopes similar to that present in AMO. All experimental diets had similar ingredient composition except for the GNU100 inclusion (Table 2).
Table 1.
Physicochemical characteristics of GNU100 used for the study
| Parameter | Value |
|---|---|
| Sensorial | White to yellow powder |
| Oligosaccharide and peptide complex | 42.7% DM |
| Free amino acids | 41.7% DM |
| Ash | 15.6% DM |
| Moisture | 4.0% |
| pH (2 w/v%, in deionized water at 20 °C) | 5.6 |
| Molecular weight distribution | |
| >10,000 Da | 0.0% |
| 5,000 to 10,000 Da | 0.2% |
| 2,000 to 5,000 Da | 5.7% |
| 1,000 to 2,000 Da | 38.9% |
| <1,000 Da | 55.3% |
Table 2.
Dietary ingredient and analyzed chemical composition and apparent total tract macronutrient digestibility of GNU100-supplemented diets for adult cats1
| Item | CT | LD | MD | HD | SEM | P-value |
|---|---|---|---|---|---|---|
| Poultry byproduct meal | 43.3 | 43.3 | 43.3 | 43.3 | N/A | N/A |
| Brewer’s rice (US #2) | 39.8 | 39.3 | 38.8 | 38.3 | N/A | N/A |
| Poultry fat | 8.65 | 8.65 | 8.65 | 8.65 | N/A | N/A |
| Corn whole (US #2) | 4.81 | 4.81 | 4.81 | 4.81 | N/A | N/A |
| Palatant | 1.92 | 1.92 | 1.92 | 1.92 | N/A | N/A |
| GNU100 | 0.00 | 0.50 | 1.00 | 1.50 | N/A | N/A |
| Salt (sodium chloride) | 0.48 | 0.48 | 0.48 | 0.48 | N/A | N/A |
| Potassium chloride (50% K) | 0.43 | 0.43 | 0.43 | 0.43 | N/A | N/A |
| Taurine | 0.19 | 0.19 | 0.19 | 0.19 | N/A | N/A |
| Mineral premix2 | 0.18 | 0.18 | 0.18 | 0.18 | N/A | N/A |
| Vitamin premix3 | 0.18 | 0.18 | 0.18 | 0.18 | N/A | N/A |
| Choline chloride | 0.13 | 0.13 | 0.13 | 0.13 | N/A | N/A |
| Chemical composition | ||||||
| DM, % | 93.7 | 92.6 | 93.1 | 93.4 | 0.14 | N/A |
| % DM basis | ||||||
| Organic matter | 93.3 | 93.2 | 93.3 | 93.2 | 0.03 | N/A |
| Ash | 6.69 | 6.77 | 6.73 | 6.78 | 0.04 | N/A |
| Crude protein | 37.2 | 37.0 | 35.9 | 36.2 | 0.08 | N/A |
| Acid-hydrolyzed fat | 17.6 | 17.3 | 15.7 | 16.8 | 0.18 | N/A |
| Gross energy, kcal/g | 5.25 | 5.22 | 5.13 | 5.19 | 0.01 | N/A |
| Digestibility, % | ||||||
| DM | 85.3 | 85.9 | 85.5 | 87.8 | 1.21 | 0.47 |
| Organic matter | 89.1 | 89.9 | 89.1 | 91.1 | 0.92 | 0.39 |
| Acid-hydrolyzed fat | 94.4 | 94.5 | 93.9 | 95.2 | 0.58 | 0.48 |
| Gross energy | 89.6 | 90.3 | 89.3 | 91.5 | 0.89 | 0.35 |
| Crude protein | 84.4 | 85.2 | 84.1 | 87.1 | 1.36 | 0.40 |
1CT, control diet containing (0% GNU); HD, high dose (1.5% GNU); LD, low dose (0.5% GNU); MD, medium dose (1.0% GNU). P-values < 0.05 are listed in bold.
2Provided per kg diet: Mn (as MnSO4), 66.00 mg; Fe (as FeSO4), 120 mg; Cu (as CuSO4), 18.00 mg; Co (as CoSO4), 1.20 mg; Zn (as ZnSO4), 240 mg; I (as KI), 1.80 mg; and Se (as Na2SeO3), 0.24 mg.
3Provided per kg diet: vitamin A, 5.28 mg; vitamin D3, 0.04 mg; vitamin E, 120.00 mg; vitamin K, 0.88 mg; thiamin, 4.40 mg; riboflavin, 5.72 mg; pantothenic acid, 22.00 mg; niacin, 39.60 mg; pyridoxine, 3.52 mg; biotin, 0.13 mg; folic acid, 0.44 mg; and vitamin B12, 0.11 mg.
Daily food intake, weekly BW, and body condition scores
Cats were fed once a day. Following 14 d of acclimation to the CT, cats were randomly assigned to one of four test diets, which served as the sole source of food for the length of the study. All cats were fed to maintain BW throughout the entire study, with adjustments made weekly if needed. The food offered and refusals were measured daily to calculate intake. BW and body condition scores (BCS; 9-point scale) according to Laflamme (1997) were measured at the beginning and end of the study and weekly during the study.
Fecal sample collection and analyses
Fresh fecal samples were collected on weeks 2 and 4 for microbiota, dry matter (DM), and metabolite analysis and on weeks 2, 4, and 26 for immune and inflammatory markers. Additionally, on weeks 0, 2, 4, 8, 12, 16, 20, 24, and 26, fresh fecal samples were collected for measurement of fecal pH and fecal scores. Finally, on week 4, a 5-d total fecal collection was conducted for the measurement of apparent total tract macronutrient digestibility. All collection points are described in Supplementary Table S3. Because testing safety and gastrointestinal tolerance was our primary aim, general stool quality outcomes (fecal pH and scores) and inflammatory markers were measured throughout the entire study. Functional outcomes (e.g., microbiota and metabolites) were measured over a shorter period of time (weeks 2 and 4), which is similar to other gastrointestinal health studies in dogs, cats, and humans. Because fecal metabolites and microbiota populations are relatively stable within 2 wk after a dietary change (Lin et al., 2019), these outcomes were not measured at week 26.
During the total fecal collection period (5 d), total fecal samples were collected, weighed, and scored according to the following scale: 1 = hard, dry pellets, small hard mass; 2 = hard, formed, dry stool, remains firm and soft; 3 = soft, formed, and moist stool, retains shape; 4 = soft, unformed stool, assumes shape of container; and 5 = watery, liquid that can be poured. Samples were then frozen at −20 °C until analysis. Fecal samples used for digestibility analysis were dried at 55 °C in a forced-air oven. All dried dietary treatments and feces were ground in a Wiley mill (model 4, Thomas Scientific, Swedesboro, NJ) through a 2-mm screen.
One fresh fecal sample (within 15 min of defecation) per cat was collected during the fecal collection phase (weeks 2 and 4) for pH, DM, metabolites (SCFA, BCFA, phenols and indoles, and ammonia), microbiota, immunoglobulin A (IgA), and calprotectin analysis (weeks 2, 4, and 26). Fecal pH was measured immediately using an AP10 pH meter (Denver Instrument, Bohemia, NY) equipped with a Beckman Electrode (Beckman Instruments Inc., Fullerton, CA), and then aliquots were collected. Aliquots for analysis of phenols and indoles were frozen at −20 °C immediately after collection. A few aliquots were transferred to sterile cryogenic vials (Nalgene, Rochester, NY), frozen immediately on dry ice, and stored at −80 °C until microbiota, IgA, and calprotectin analyses. Another aliquot for SCFA, BCFA, and ammonia was placed in 2N HCl and stored at −20 °C until analyses. Finally, an aliquot was collected for DM determination.
Fecal SCFA concentrations were determined by gas chromatography according to Erwin et al. (1961) using a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP-1200/1% H3PO4 on 80/100+ mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA). Nitrogen was the carrier with a flow rate of 75 mL/min. Oven, detector, and injector temperatures were 125, 175, and 180 °C, respectively. Fecal phenol and indole concentrations were determined using gas chromatography according to the methods described by Flickinger et al. (2003). Fecal ammonia concentrations were determined according to the methods of Chaney and Marbach (1962).
Fecal proteins were extracted according to Vilson et al. (2016). Fecal samples (250 mg) were vortexed with 750 µL extraction buffer containing 50 mM ethylenediaminetetraacetic acid (EDTA) (ThermoFisher, Waltham, MA) and 100 µg/L soybean trypsin inhibitor (Sigma, St. Louis, MO) in phosphate buffered saline/L percent bovine serum albumin (Tocris Bioscience, Bristol, UK). Phenylmethanesulfonyl fluoride (12.5 µL, 350 mg/L; Sigma, St. Louis, MO) was added into each tube, followed by centrifugation for 10 min. The supernatants were collected for measurement of IgA and calprotectin using commercial enzyme-linked immunosorbent assay (ELISA) kits (IgA: Immunology Consultants Laboratory, Portland, OR; calprotectin: MyBioSource, San Diego, CA).
Blood collection and analyses
On weeks 0, 2, 4, and 26, blood samples were collected for serum chemistry and hematology and, additionally, on weeks 2, 4, and 26, for inflammatory cytokines, immunoglobulin E (IgE), and C-reactive protein (CRP). Just prior to blood collection, cats were sedated by an intramuscular injection of dexmedetomidine (0.04 mg/kg BW) for sedation. After blood was collected, an injection of the reversal agent for dexmedetomidine, atipamezole (0.04 mg/kg BW intramuscularly), was given. Samples were immediately transferred to appropriate vacutainer tubes: approximately 4 mL in #367985 BD Vacutainer glass serum tubes with gel for serum separation (Becton Dickinson, Franklin Lakes, NJ) for blood chemistry profiles, inflammatory cytokines, IgE, and CRP and approximately 2 mL in #367842 BD Vacutainer Plus plastic whole blood tubes with K2EDTA additive (Becton Dickinson, Franklin Lakes, NJ) for hematology.
The blood tubes for serum isolation were centrifuged at 1,300 × g at 4 °C for 10 min (Beckman CS-6R centrifuge; Beckman Coulter Inc., Brea, CA). A fresh sample was transported to the University of Illinois Veterinary Medicine Diagnostics Laboratory for serum chemistry analysis. K2EDTA tubes were cooled (but not frozen), with one aliquot being transported to the University of Illinois Veterinary Medicine Diagnostics Laboratory for hematology analyses.
Serum inflammatory cytokines and IgE were measured by commercial ELISA kits, including those for tumor necrosis factor-alpha (TNF-α; Cat Total Tumor Necrosis Factor ELISA Kit, MyBioSource, San Diego, CA), interleukin-6 (IL-6; Cat Interleukin 6 ELISA Kit, MyBioSource, San Diego, CA), IgE (Cat IgE ELISA Kit, Immunology Consultants Laboratory, Lake Oswego, OR), and CRP (Cat CRP ELISA Kit, Immunology Consultants Laboratory, Lake Oswego, OR).
General health observations
A complete physical examination by a licensed veterinarian was conducted just prior to the start of the study and at study completion. Daily general health observations were noted throughout the study by blinded personnel. Other variables associated with gastrointestinal tolerance such as emesis, poor physical appearance, and abnormal behavior were monitored and recorded.
Hair and skin evaluation
Skin and coat scores were evaluated by the same person (blinded) prior to study initiation, every 4 wk and at study completion according to Rees et al. (2001): hair: 1 = dull, coarse, dry; 2 = poorly reflective, non-soft; 3 = medium reflective, medium soft; 4 = highly reflective, very soft; and 5 = greasy; skin: 1 = dry; 2 = slightly dry; 3 = normal; 4 = slightly greasy; and 5 = greasy.
Diet and fecal chemical analyses
Diet and fecal samples were analyzed for DM, organic matter, and ash according to the methods of Association of Official Analytical Chemists (AOAC, 2006; methods 934.01 and 942.05). Crude protein of diets and feces were determined by Leco Nitrogen/Protein Determinator (FP-2000, Leco Corp., St. Joseph, MI) and total nitrogen values according to AOAC (2006; method 992.15). Total lipid content (acid-hydrolyzed fat) was determined according to the methods of the American Association of Cereal Chemists (AACC, 1983) and Budde (1952). Dietary total dietary fiber was determined according to Prosky et al. (1992). The gross energy of dietary and fecal samples was measured using an oxygen bomb calorimeter (model 1261; Parr Instruments; Moline, IL). Apparent total tract macronutrient digestibility values were calculated using the equation as follows: [nutrient intake (g/d) − fecal output (g/d)/nutrient intake (g/d)] × 100.
Fecal microbiota populations
Fecal bacterial DNA was extracted according to the manufacturer’s instructions using the MO BIO PowerSoil Kit (MO BIO Laboratories, Carlsbad, CA) with bead beating using a vortex adaptor. The concentration of extracted DNA was quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY), and DNA quality was assessed using gel electrophoresis. DNA samples were stored at −80 °C until being sent on dry ice to Gnubiotics Sciences SA for analysis.
16S rRNA Sequencing
Genomic DNA of fecal samples at 2 wk (CT, n = 7; LD, n = 7; MD, n = 8; HD, n = 8) and 4 wk (CT, n = 8; LD, n = 7; MD, n = 7; HD, n = 8) were used for 16S rRNA sequencing. Sequencing was performed by Gnubiotics Sciences SA (Epalinges, Switzerland). In short, a targeted PCR-based sequencing approach was used, where the V1-V2 regions of the 16S rRNA gene were targeted to generate amplicons. The amplicon library was sequenced using the MiSeq Illumina platform with 2 × 150 cycles and paired-end reads. Two negative control reactions without the DNA template and two negative control reactions without the amplification primers were included in the run. Fastq files generated by the sequencer were used to de-multiplex and analyze the raw reads, using a publicly available Divisive Amplicon Denoising Algorithm (DADA2) pipeline. Read counts generated by the pipeline were divided by the total sample read count to obtain relative abundances.
Statistical analyses
All data were analyzed using the Mixed Models procedure of SAS (version 9.4; SAS Institute, Cary, NC). Treatment and period were considered to be fixed effects, and cat was considered to be a random effect. Data normality was checked using the univariate procedure and Shapiro–Wilk statistic, with log transformation being used when normal distribution was lacking. If after logarithmic transformation the data did not reach normality, data were analyzed using the npar1way procedure and Wilcoxon statistic. Microbiota data ordination was done using a principal coordinates analysis plot based on Bray–Curtis dissimilarity, with assessment of differences among microbial profiles of the four groups being done by one-way permutational multivariate analysis of variance (PERMANOVA) (Bray–Curtis dissimilarity distance) using paleontological Statistics (PAST; v3.12) software. Alpha-diversity analyses, including richness (amplicon sequence variants [ASVs]), Shannon diversity index (H), and evenness (e^H/S), were calculated, with significant differences being calculated using Mann–Whitney U test in PAST; v3.12. Differences were considered significant with P < 0.05.
Results
Palatability
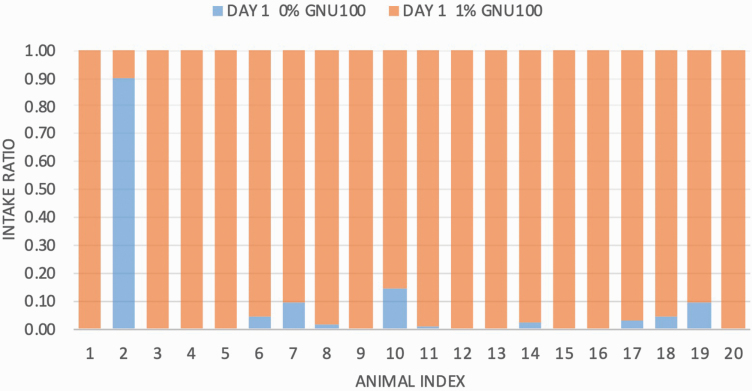
In the palatability test, a 17.58:1 total consumption ratio was observed for the 1% GNU100 diet vs. control diet, demonstrating a strong preference (P < 0.05) for GNU100 (Figure 1). According to a Wilcoxon signed rank test, the consumption difference between the 0% and 1% GNU100 diets was significant (P < 0.0001). Using data from both days, the 1% GNU100 diet was approached first on 28 of the 40 occasions and consumed first on 38 of the 40 occasions, and only one animal consumed more 0% GNU100 than 1% GNU100.
Figure 1.
Mean intake ratios (intake of diet A/intake of diet A + diet B) for each of 20 cats fed diets containing 0% or 1% GNU100.
Diet chemical analyses and apparent total tract macronutrient digestibility
All diets contained similar nutrient concentrations, which was expected. All diets were highly digestible, and apparent total tract macronutrient digestibilities were not affected by treatment (Table 2).
General health observations, food intake, BW, and BCS
All cats remained healthy, without any signs of gastrointestinal intolerance throughout the study. All cats showed a good appetite, with no differences in food intake, BW, or BCS due to treatment (Supplementary Table S4). The condition of skin and hair did not differ among treatments during the experiment, with all having hair that remained in a state described as a medium level of reflectivity and softness (CT, LD, MD, and HD = 3), and the skin was scored as normal (CT = 3.0, LD = 3.1, MD = 3.1, and HD = 3.1).
Fecal scores, pH, DM percentage, and IgA, calprotectin, and metabolite concentrations
Fecal scores, pH, output, and DM percentage did not differ among treatments (Table 3; Supplementary Table S5). For the majority of fecal metabolites (phenol, acetate, propionate, isobutyrate, isovalerate, valerate, and ammonia), there were no differences among treatments (Table 3). However, cats fed HD had higher (P = 0.02) fecal butyrate concentrations than those fed LD or MD (Table 3). Also, cats fed HD had lower (P = 0.02) fecal indole concentrations than those fed LD (Table 3). Fecal IgA and calprotectin were not different among treatments (Table 4).
Table 3.
Mean fecal pH and metabolite concentrations of cats fed GNU100-supplemented diets1
| CT | LD | MD | HD | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 | SEM | Diet | Period | Diet * Period |
| Fecal score | 3.20 | 2.81 | 2.56 | 2.69 | 2.88 | 2.97 | 3.00 | 2.75 | 0.20 | 0.48 | 0.29 | 0.19 |
| Fecal pH | 6.08 | 6.49 | 6.57 | 6.88 | 6.48 | 6.18 | 6.37 | 6.56 | 0.19 | 0.12 | 0.25 | 0.24 |
| Fecal DM (%) | 35.07 | 36.89 | 38.55 | 38.24 | 36.24 | 36.22 | 38.12 | 38.66 | 1.66 | 0.51 | 0.49 | 0.75 |
| umole/g (DM basis) | ||||||||||||
| Phenol | 1.29 | 1.42 | 1.80 | 1.72 | 1.11 | 0.99 | 1.27 | 1.85 | 0.34 | 0.42 | 0.33 | 0.21 |
| Indole | 1.08 | 0.85 | 2.06 | 2.26 | 1.22 | 0.92 | 0.85 | 0.80 | 0.31 | 0.02 | 0.20 | 0.20 |
| Total phenol + indole | 2.37 | 2.27 | 3.86 | 3.98 | 2.33 | 1.90 | 2.12 | 2.65 | 0.55 | 0.07 | 0.89 | 0.44 |
| Acetate | 222 | 197 | 171 | 186 | 212 | 206 | 189 | 161 | 19.92 | 0.36 | 0.23 | 0.32 |
| Propionate | 74.7 | 73.5 | 61.1 | 65.0 | 81.6 | 82.3 | 67.0 | 70.6 | 9.24 | 0.38 | 0.73 | 0.98 |
| Butyrate | 65.7 | 68.3 | 49.3 | 66.1 | 55.6 | 57.1 | 72.2 | 81.0 | 6.63 | 0.02 | 0.12 | 0.63 |
| Total SCFA | 361 | 339 | 282 | 317 | 349 | 347 | 328 | 312 | 28.81 | 0.44 | 0.93 | 0.49 |
| Isobutyrate | 6.54 | 7.41 | 8.30 | 8.98 | 6.55 | 6.65 | 7.31 | 8.69 | 1.19 | 0.53 | 0.15 | 0.85 |
| Isovalerate | 9.74 | 11.5 | 12.9 | 13.9 | 9.67 | 9.57 | 10.8 | 14.1 | 2.00 | 0.47 | 0.08 | 0.55 |
| Valerate | 17.8 | 18.4 | 17.9 | 19.0 | 15.5 | 15.6 | 16.8 | 18.0 | 3.40 | 0.85 | 0.16 | 0.78 |
| Total BCFA | 34.1 | 37.3 | 39.1 | 41.9 | 31.8 | 31.8 | 34.9 | 40.8 | 5.92 | 0.71 | 0.23 | 0.87 |
| Ammonia | 135.3 | 159.2 | 180.6 | 194.9 | 159.8 | 139.9 | 131.5 | 166.4 | 17.81 | 0.25 | 0.08 | 0.08 |
1CT, control diet containing (0% GNU); HD, high dose (1.5% GNU); LD, low dose (0.5% GNU); MD, medium dose (1.0% GNU). P-values < 0.05 are listed in bold.
Table 4.
Mean serum TNF, CRP, IL-6, and IgE concentrations and mean fecal calprotectin and IgA concentrations of cats fed GNU100-supplemented diets1
| CT | LD | MD | HD | P-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Week 2 | Week 4 | Week 26 | Week 2 | Week 4 | Week 26 | Week 2 | Week 4 | Week 26 | Week 2 | Week 4 | Week 26 | SEM | Diet | Period | Diet* Period |
| Serum | ||||||||||||||||
| TNF, pg/mL | 182 | 175 | 162 | 177 | 178 | 177 | 174 | 181 | 169 | 167 | 170 | 216 | 18.09 | 0.88 | 0.89 | 0.53 |
| CRP, mg/L | 110 | 108 | 120 | 111 | 103 | 117 | 99 | 97 | 106 | 117 | 112 | 124 | 8.66 | 0.53 | 0.0003 | 0.98 |
| IL-6, pg/mL | 51 | 57 | 91 | 68 | 78 | 66 | 46 | 55 | 62 | 57 | 69 | 74 | 11.03 | 0.35 | 0.12 | 0.41 |
| IgE, ug/mL | 7.9 | 7.0 | 3.8 | 6.7 | 6.8 | 6.3 | 9.9 | 6.0 | 6.9 | 5.5 | 5.3 | 7.0 | 1.58 | 0.64 | 0.45 | 0.44 |
| Fecal | ||||||||||||||||
| Calprotectin, ug/g feces | 0.3 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 | 0.2 | 0.08 | 0.04 | 0.95 | <0.0001 | 0.50 |
| IgA, mg/g feces | 12.6 | 8 | 10.7 | 9 | 8.3 | 11.1 | 9.7 | 7.2 | 8.7 | 8.1 | 8.5 | 7.8 | 1.60 | 0.40 | 0.21 | 0.66 |
1CT, control diet containing (0% GNU); HD, high dose (1.5% GNU); LD, low dose (0.5% GNU); MD, medium dose (1.0% GNU). P-values < 0.05 are listed in bold.
Serum chemistry, taurine, inflammatory cytokines, and hematology
Although a few measures were outside the reference ranges, serum chemistry, taurine, and hematology did not differ among treatments (Supplementary Tables S6 and S7). For all animals, regardless of treatment, the albumin:globulin ratio was highest on week 26. Data for week 2 had an intermediate value, which was similar to weeks 0 and 26, and data for week 4 had the lowest value. Circulating phosphorus was lower on week 2 compared with week 0. Circulating sodium was lower on week 4 than all other weeks. Circulating potassium was higher on weeks 0 and 26, with week 4 being intermediate and similar to weeks 26 and 2 data, and week 2 data being the lowest. Sodium:potassium ratio was lower on week 2 compared with all the other weeks. Bicarbonate was higher on weeks 2 and 26, week 26 had an intermediate value similar to all weeks, and week 4 was the lowest. Creatinine was higher on week 26 compared with week 4. Blood urea nitrogen was lower on week 26 compared with all the other weeks. Albumin was higher on week 4, followed by weeks 0 and 2 (similar), and lowest on week 26. Globulin was higher on week 26, week 2 had an intermediate value similar to weeks 26 and 0, week 0 had a lower value than week 26, and week 4 was the lowest. Calcium was higher on week 26, followed by week 4, and similar lower at weeks 2 and 0. Glucose was higher on week 26, week 2 had a similar value to weeks 26 and 0, week 0 had a similar value to weeks 2 and 4, and week 4 had the lowest value. Alkaline phosphatase was higher on weeks 0 and 2, followed by week 4 and the lowest on week 26. Cholesterol was higher on week 26, followed by week 4, and similar lower on weeks 0 and 2. Taurine was lower on week 2 compared with all the other weeks. Serum inflammatory cytokines, IgE, and CRP were not affected by dietary treatment (Table 4).
Fecal microbiota
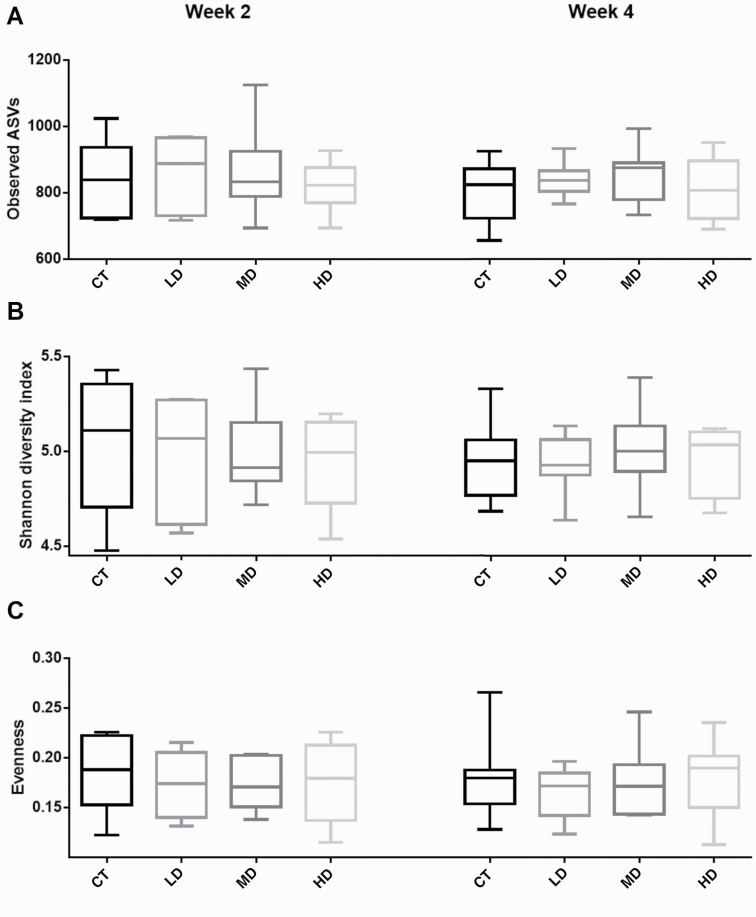
A total of 20,056,145 high-quality reads were obtained. The overall number of ASVs detected was 3,285. The number of reads per sample ranged from 116,834 to 621,005. After the calculation of relative abundance per sample, further analyses were performed. Principal component analysis of the microbial communities did not show significant clustering by group after 2 wk (P = 0.8767, F = 0.7679, PERMANOVA) or after 4 wk (P = 0.9959, F = 0.5405, PERMANOVA; Supplementary Figure S1). Alpha-diversity measures suggested that GNU100 did not affect species richness, Shannon diversity index, or evenness (Figure 2).
Figure 2.
Alpha-diversity indices of fecal microbiota from cats fed GNU100-supplemented diets. (A) richness (observed ASVs), (B) Shannon diversity index (H), and (C) evenness (e^H/S). CT, control diet containing (0% GNU); HD, high dose (1.5% GNU); LD, low dose (0.5% GNU); MD, medium dose (1.0% GNU).
The predominant bacterial phyla in feces were Firmicutes (>70% of the total number of reads) in all treatment and points of collection (Table 5). Cats fed the CT diet had a higher (P = 0.003) relative abundance of Actinobacteria than cats fed the LD diet (Table 5). At the genus level, 124 taxa were observed in the samples. The most abundant bacterial genus was Prevotella (>8% of the total number of reads) in all treatment and points of collection (Table 6). A significant diet * period interaction (P = 0.01) was observed for the relative abundance of Peptococcus (Table 6). Peptococcus relative abundance was higher (P < 0.01) in cats fed MD than those fed CT or HD at week 2, but was not different among treatments at week 4. Time effects were observed for the relative abundances of Bacteroides, Gemmiger, Lactobacillus, and Megasphaera. While the relative abundances of fecal Bacteroides (P = 0.02) and Gemmiger (P = 0.002) were higher at week 2 than at week 4, the relative abundances of Lactobacillus (P < 0.0001) and Megasphaera (P < 0.0001) were higher at week 4 than week 2 (Table 6). When treatment effects were evaluated with week, the data show that GNU100 did not have any effect on bacterial genera on week 2 (Supplementary Table S8), but Campylobacter was higher in cats fed CT than cats fed HD (Supplementary Table S9) at week 4.
Table 5.
Mean relative abundance (% of sequences) of bacterial phyla in fecal samples of cats fed GNU100-supplemented diets1
| CT | LD | MD | HD | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 | SEM | Diet | Period | Diet * Period |
| Actinobacteria | 8.97 | 5.55 | 2.22 | 2.95 | 4.68 | 4.9 | 5.88 | 7.27 | 1.43 | 0.003 | 0.832 | 0.545 |
| Bacteroidetes | 13.9 | 14.9 | 15.9 | 11.5 | 12.8 | 13.8 | 14.4 | 12.6 | 2.54 | 0.997 | 0.974 | 0.631 |
| Firmicutes | 72.7 | 75.9 | 75.3 | 82.3 | 78.5 | 77.3 | 75.1 | 76.7 | 2.81 | 0.213 | 0.340 | 0.376 |
| Fusobacteria | 0.51 | 0.38 | 0.95 | 0.54 | 0.45 | 0.68 | 0.3 | 0.25 | 0.15 | 0.158 | 0.259 | 0.324 |
| Proteobacteria | 3.26 | 2.78 | 4.96 | 2.34 | 3.07 | 2.77 | 3.82 | 2.81 | 0.72 | 0.980 | 0.086 | 0.684 |
1CT, control diet containing (0% GNU); HD, high dose (1.5% GNU); LD, low dose (0.5% GNU); MD, medium dose (1.0% GNU). P-values < 0.05 are listed in bold.
Table 6.
Mean relative abundance (% of sequences) of bacterial genera in fecal samples of cats fed GNU100-supplemented diets1
| CT | LD | MD | HD | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus2 | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 | SEM | Diet | Period | Diet * Period |
| Acetanaerobacterium | 1.31 | 1.24 | 1.37 | 1.36 | 1.24 | 1.03 | 1.10 | 1.20 | 0.20 | 0.79 | 0.53 | 0.47 |
| Allisonella | 1.95 | 2.14 | 1.73 | 2.07 | 2.50 | 2.55 | 1.94 | 1.90 | 0.82 | 0.94 | 0.44 | 0.87 |
| Alloprevotella | 0.81 | 1.50 | 1.32 | 1.24 | 1.25 | 1.08 | 0.97 | 0.97 | 0.42 | 0.95 | 0.54 | 0.30 |
| Bacteroides | 1.39 | 1.18 | 2.52 | 1.10 | 1.26 | 1.16 | 1.16 | 0.95 | 0.28 | 0.33 | 0.02 | 0.26 |
| Bifidobacterium | 2.68 | 2.71 | 0.40 | 0.98 | 1.57 | 1.17 | 0.98 | 2.68 | 0.78 | 0.19 | 0.29 | 0.36 |
| Blautia | 5.61 | 4.74 | 6.56 | 5.45 | 6.77 | 6.08 | 5.48 | 4.66 | 0.94 | 0.51 | 0.13 | 1.00 |
| Catenibacterium | 1.30 | 0.69 | 0.68 | 1.24 | 0.56 | 0.72 | 1.14 | 0.57 | 0.34 | 0.79 | 0.95 | 0.09 |
| Clostridium_XI | 7.35 | 5.74 | 6.48 | 6.75 | 6.74 | 5.66 | 5.07 | 5.90 | 0.83 | 0.65 | 0.36 | 0.17 |
| Clostridium_XlVa | 0.93 | 0.65 | 0.74 | 0.72 | 0.73 | 0.80 | 0.71 | 0.65 | 0.09 | 0.59 | 0.24 | 0.23 |
| Collinsella | 5.49 | 2.16 | 1.74 | 1.56 | 2.54 | 2.45 | 4.32 | 3.28 | 0.97 | 0.14 | 0.07 | 0.24 |
| Dialister | 0.76 | 1.06 | 0.73 | 0.93 | 1.19 | 1.31 | 0.85 | 0.92 | 0.56 | 0.95 | 0.18 | 0.93 |
| Gemmiger | 1.70 | 0.80 | 1.60 | 0.91 | 1.44 | 0.91 | 1.40 | 0.62 | 0.39 | 0.71 | 0.002 | 0.86 |
| Holdemanella | 0.83 | 0.69 | 1.02 | 0.88 | 0.85 | 1.11 | 0.79 | 0.50 | 0.21 | 0.44 | 0.49 | 0.36 |
| Lactobacillus | 1.51 | 4.95 | 2.76 | 6.79 | 2.58 | 4.41 | 1.21 | 4.33 | 0.92 | 0.18 | <0.0001 | 0.68 |
| Megamonas | 4.52 | 4.31 | 8.23 | 6.87 | 10.59 | 5.08 | 7.41 | 5.33 | 2.17 | 0.44 | 0.12 | 0.59 |
| Megasphaera | 5.65 | 7.85 | 3.90 | 5.21 | 4.25 | 6.29 | 4.78 | 7.15 | 1.49 | 0.70 | <0.0001 | 0.84 |
| Peptococcus | 0.41b | 0.59ab | 0.52ab | 0.66ab | 0.72a | 0.58ab | 0.45b | 0.54ab | 0.06 | 0.08 | 0.04 | 0.01 |
| Phascolarctobacterium | 0.86 | 0.91 | 1.71 | 1.05 | 1.02 | 1.08 | 1.32 | 1.10 | 0.35 | 0.72 | 0.19 | 0.28 |
| Prevotella | 10.96 | 11.44 | 10.94 | 8.45 | 9.54 | 10.75 | 11.40 | 9.67 | 2.46 | 0.96 | 0.67 | 0.78 |
| Romboutsia | 0.95 | 0.58 | 0.80 | 0.76 | 0.79 | 0.65 | 0.77 | 0.71 | 0.13 | 0.97 | 0.08 | 0.50 |
| Roseburia | 2.01 | 2.42 | 1.49 | 2.41 | 1.62 | 2.64 | 3.21 | 3.85 | 0.93 | 0.51 | 0.08 | 0.95 |
| Sutterella | 1.01 | 1.08 | 0.99 | 0.74 | 0.89 | 0.95 | 0.89 | 0.62 | 0.17 | 0.54 | 0.34 | 0.42 |
1CT, control diet containing (0% GNU); HD, high dose (1.5% GNU); LD, low dose (0.5% GNU); MD, medium dose (1.0% GNU). P-values < 0.05 are listed in bold.
2All genera with relative abundance >0.5% of total sequences are presented.
a,bMean values within a row with unlike superscript letters differ (P < 0.05).
Discussion
The primary aim of this study was to evaluate the palatability, safety, and gastrointestinal tolerance of GNU100 in healthy adult cats over a 26-wk period. A secondary aim was to test its functionality (selective growth of beneficial microbiota, increased beneficial fermentative products, and positive modulation of gut immunity) in cats. As is typical in gastrointestinal health studies, these functional outcomes were only measured over a shorter period of time (weeks 2 and 4) because fecal metabolites and microbiota populations have been shown to be stable after only 2 wk following a dietary change (Lin et al., 2019). As long as the same diets continued to be consumed throughout the study, changes to these outcomes would not be expected beyond the first few weeks.
When testing a novel ingredient for use in pet food, it is important to confirm its acceptance by the target animal. If the animal does not voluntarily consume the ingredient, its potential benefits will not be realized. Cats are strictly carnivorous and show a preference for amino acids described as “sweet” (such as proline, cysteine, ornithine, lysine, histidine, and alanine) and reject the “bitter” amino acids (such as arginine, isoleucine, phenylalanine, and tryptophan) (Bradshaw et al., 1996). Cats have a dysfunctional sweet taste receptor and have been reported to dislike sweet taste from sugars (Boudreau et al., 1985). Cats also reject the monophosphate nucleotides that accumulate in mammalian tissues after death (Bradshaw, 1991) and substances such as quinine, alkaloids, and tannic, malic, and phytic acids that are perceived by the cat as bitter (Levesque, 1999). GNU100 has a high content of amino acids, peptides, and glycopeptides that could explain the higher palatability or preference of this ingredient for the cats. Having this in mind, the high palatability of the 1% GNU100 diet vs. 0% control is of great importance.
It is always important to assess the gastrointestinal tolerance of a novel ingredient to confirm that it does not cause unintended side effects. Intolerance in terms of poor stool quality is common with fermentable substances, including soluble fibers and oligosaccharides, in cats. For example, Barry et al. (2010) reported that cats fed a pectin-supplemented diet presented with softer feces, an indicator of increased fermentation and/or change in microbial ecology, possibly causing the cat to experience intestinal discomfort. Increased fecal output has also been reported in cats fed a FOS + GOS-supplemented diet (Kanakupt et al., 2011). Cats supplemented with dietary oligofructose have been reported to have greater fecal moisture and output compared with controls (Hesta et al., 2005). In the current study, all doses of GNU100 were well tolerated, with no signs of gastrointestinal distress. Based on our previous in vitro study that showed moderate gas production upon GNU100 fermentation (Oba et al., 2020), this was anticipated but is important to confirm in vivo.
All cats remained healthy throughout the study, with all having a good appetite and no differences in food intake, BW, or BCS due to treatment. Although a few blood measures were outside the reference ranges, serum chemistry, taurine, and hematology did not differ among treatments. Intestinal immunity (fecal IgA), inflammation (serum CRP, IL-6, and TNF-α; fecal calprotectin), and allergy (serum IgE) markers were not affected by dietary treatment, demonstrating that this ingredient does not cause allergic reactions, intestinal inflammation, or an immune reaction in animals consuming this ingredient. Additionally, the condition of skin and hair did not differ among treatments during the experiment, with all having hair that remained in a state described as a medium level of reflectivity and softness, and the skin was considered normal. Fecal scores, pH, and DM percentage did not differ among treatments, demonstrating that this ingredient does not cause constipation or diarrhea in cats consuming it.
HMO have been shown to decrease pH in in vitro studies using colonic microbiota from neonatal piglets and infant humans (Li et al., 2012; Yu et al., 2013a). In an in vitro fermentation study of GNU100 by microbiota of a feline donor, pH decreased over time, but differences were not observed among GNU100 (0.5% and 1%) and control (0% GNU100) incubations (Oba et al., 2020). Similar results were observed in the current study and in cats receiving probiotic or FOS supplements, where their fecal pH did not differ from the control group (Rodrigues et al., 2020).
Another consideration of a novel ingredient is whether it negatively affects the formulated diet, reducing its digestibility and ultimately compromising the nutrient availability to the animal. In previous studies, cats that have consumed a diet supplemented with FOS, GOS, pectin, oligofructose, or inulin have had decreased crude protein digestibility, which is generally attributed to enhanced microbial concentrations in the colon (Hesta et al., 2001; Barry et al., 2010; Kanakupt et al., 2011). The diets of the current study were formulated to be iso-nutrient, isonitrogenous, and isocaloric. All diets contained similar nutrient concentrations and were highly digestible, with apparent total tract macronutrient digestibilities not being affected by treatment. These data suggest that the use of GNU100 will not affect nutrient digestibility when used up to 1.5% of the diet.
In regard to functionality, GNU100 did not alter the concentration of most of the fecal metabolites. Cats fed HD, however, had higher fecal butyrate concentrations than those fed LD or MD. These results are similar to data derived from a recent in vitro study using feline fecal inocula, whereby GNU100 increased the production of propionate and butyrate compared with 0% controls (Oba et al., 2020). In an in vitro study using fecal inocula of human infants, HMO, LNnT, and 2′FL increased the production of acetate and total SCFA (Vester Boler et al., 2013). In that study, LNnT was shown to result in the highest production of gas, total SCFA, acetate, and butyrate for most of the donor groups, with acetate being the major SCFA produced in neonatal piglet fecal inocula incubation, compared with 2:1 mixture of polydextrose and GOS and short-chain FOS (Li et al., 2012). Diets supplemented with oligofructose increased fecal butyrate concentrations in adult cats (Barry et al., 2010; Shinohara et al., 2020). Butyrate serves as an energy source for epithelial cells, modulates colonocyte proliferation, and regulates epithelial barrier integrity, the gastrointestinal immune system, and inflammatory responses (Clausen and Mortensen, 1995; Maeda et al., 2000; Lührs et al., 2002; Blottière et al., 2003; Wong et al., 2006; Arpaia et al., 2013; Furusawa et al., 2013; van der Beek et al., 2015). Therefore, increased butyrate concentrations are a marker of gastrointestinal health.
Cats are obligate carnivores and, therefore, require greater concentrations of dietary protein to meet their amino acid (AA) requirements (NRC, 2006). Clostridial species are one of the primary microbial taxa that ferment undigested AA, producing putrefactive compounds, such as ammonia, BCFA, indole, and phenol (Barker, 1981). These putrefactants (phenols, indoles, and ammonia) are considered potentially carcinogenic, as they are capable of altering cellular DNA (Macfarlane and Cummings, 1991; Corpet et al., 1995; Matsui et al., 1995; Pedersen et al., 2002) and are responsible for the malodor of the feces (Hussein et al., 1999). Thus, the reduction of fecal indole is deemed to be beneficial to the animal. In addition to having greater fecal butyrate concentrations, cats fed HD had lower fecal indole concentrations than those fed LD. In dogs, fecal BCFA, indole, and phenol concentrations have been shown to be decreased with the feeding of FOS (Swanson et al., 2002a, 2002b) or a fiber-prebiotic (Nogueira et al., 2019). In cats, fecal ammonia and indole concentrations have been shown to be decreased after lactosucrose supplementation (Terada et al., 1993). However, cats that received supplementation of FOS or pectins increased fecal concentrations of several proteolytic metabolites, including ammonia, BCFA, 4-methylphenol, indole, cadaverine, putrescine, and tryptamine (Barry et al., 2010). In a previous study in cats supplemented with short-chain FOS, GOS, or both, fecal indole concentrations ranged from 1.7 to 1.9 ± 0.2 umol/g DM and were not different among treatments (Kanakupt et al., 2011). In another study assessing cats supplemented with different fiber types (4% cellulose, FOS, or pectin), fecal indole concentrations ranged from 1.4 to 2.4 ± 0.3 umol/g DM, with fecal indole being reduced (P = 0.049) by FOS treatment compared with the cellulose treatment, whereas those for pectin was intermediate (Barry et al., 2010). In the present study, fecal indole concentrations ranged from 0.8 to 2.3 ± 0.3 umol/g DM and were similar to that of previous studies.
This study tested whether GNU100 was a microbiota modulator. Our data showed that the consumption of GNU100 (LD) decreased the relative abundance of Actinobacteria compared with cats fed CT. A higher proportion of Actinobacteria was previously observed in obese cats compared with the lean cats (Tal et al., 2020), and the phenomenon was associated with BCS (Kieler et al., 2019). In studies in dogs, Actinobacteria were shown to be increased in dogs with chronic enteropathies compared with healthy control dogs (Allenspach et al., 2010) and idiopathic inflammatory bowel disease (Suchodolski et al., 2012). These data suggest that a decrease in fecal Actinobacteria is potentially beneficial for the animal, but more research is needed to confirm.
The diet * period change in fecal Peptococcus was also observed. Members of Peptococcaceae, including Peptococcus, are present in the feces of healthy cats (Bermingham et al., 2013; Bell et al., 2014; Suchodolski et al., 2015) and have been shown to be decreased in cats with diabetes (Bell et al., 2014) and diarrhea (Suchodolski et al., 2015). The relevance of the Peptococcus diet * period interaction is unknown at this time. A lower relative abundance of Campylobacter was noticed in fecal samples of cats fed HD after 4 wk (Supplementary Table S8). Although the majority of cats are subclinically infected, some will develop mild to moderate enteritis (Acke, 2018). Moreover, Campylobacter is among the most common causes of bacterial gastroenteritis in humans, and cats appear to be potential important sources of human infection (Thépault et al., 2020). In a previous study, reduced colonization of this genus was shown in avians supplemented with prebiotics (Kim et al., 2019). Interestingly, there is a body of evidence showing that MO and glycoconjugates contribute to protection against pathogens. Notably, MO and glycoconjugates have been shown to reduce the adhesion of Campylobacter to intestinal cells and gastrointestinal colonization, a protective effect that may be accomplished by serving as a soluble decoy, acting as a prebiotic, or producing metabolites that create favorable conditions for commensals (Ruiz-Palacios et al., 2003; Yu et al., 2016; Lis-Kuberka and Orczyk-Pawiłowicz, 2019). Megasphaera and Roseburia spp. are described as important butyrate producers in humans and cats (Geirnaert et al., 2017; Shinohara et al., 2020). Even though fecal butyrate was greatest in cats fed the highest dose of GNU100, these genera were only numerically increased with increasing dosage of GNU100 at weeks 2 and 4.
This study had many strengths, including the long length of study. Because the primary aim was to test the safety of GNU100, AAFCO study guidelines were used in regard to experimental design (CRD), length of study (6 mo), and animal numbers (n = 8/treatment). Another strength was the testing of several doses of GNU100, including four groups of cats. The study also has a few limitations. First, because safety studies require such a long intervention period, a CRD was used. While this design is ideal for safety testing, it likely limited our ability to observe differences in fecal microbiota and metabolites, which are highly variable among animals. A crossover or Latin square design, which would allow each animal to serve as their own control, likely would have reduced variation and increased statistical power. Also, because the AAFCO protocol requires the study of a healthy animal population, the ability to observe differences is more challenging than a clinical population.
Overall, the data of this study suggest that GNU100 was highly palatable and its dietary supplementation over a long period of time (6 mo) was well tolerated and did not cause detrimental effects on fecal quality. GNU100 also did not negatively affect macronutrient digestibility or any safety biomarkers, therefore supporting the safety of GNU100 for inclusion in feline diets. Finally, the highest dosage of GNU100 favorably impacted fecal metabolite concentrations of butyrate (higher) and indole (lower). Although the relative abundances of fecal microbiota were not dramatically modified, the overall results suggest potential benefits on gastrointestinal health of cats.
Supplementary Material
Acknowledgment
The funding for this study was provided by Gnubiotics Sciences SA, Epalinges, Switzerland.
Glossary
Abbreviations
- 2′FL
2′-α-l-fucopyranosyl-d-lactose
- AMO
animal milk oligosaccharides
- ASV
amplicon sequence variant
- ATTD
apparent total tract digestibility
- BCFA
branched-chain fatty acids
- BCS
body condition scores
- BW
body weight
- CRD
completely randomized design
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- DM
dry matter
- FOS
fructooligosaccharides
- GOS
galactooligosaccharides
- HMO
human milk oligosaccharides
- IgA
immunoglobulin A
- IgE
immunoglobulin E
- IL-6
interleukin-6
- LNnT
lacto-N-neotetraose
- PERMANOVA
permutational multivariate analysis of variance
- MO
milk oligosaccharides
- SCFA
short-chain fatty acids
- TNF-α
tumor necrosis factor-alpha
Conflict of interest statement
S.V., R.W., Y.M., and Y.A. are employed by Gnubiotics Sciences SA. K.S.S. has served as a paid consultant for Gnubiotics Sciences SA. All other authors have no conflicts of interest.
Literature Cited
- AACC. 1983. Approved methods. 8th ed. In: AACC, editor. St Paul (MN): American Association of Cereal Chemists. [Google Scholar]
- Acke, E 2018. Campylobacteriosis in dogs and cats: a review. N. Z. Vet. J. 66:221–228. doi: 10.1080/00480169.2018.1475268 [DOI] [PubMed] [Google Scholar]
- Allenspach, K., A. House, K. Smith, F. M. McNeill, A. Hendricks, J. Elson-Riggins, A. Riddle, J. M. Steiner, D. Werling, O. A. Garden, . et al. 2010. Evaluation of mucosal bacteria and histopathology, clinical disease activity and expression of Toll-like receptors in German shepherd dogs with chronic enteropathies. Vet. Microbiol. 146:326–335. doi: 10.1016/j.vetmic.2010.05.025 [DOI] [PubMed] [Google Scholar]
- AOAC 2006. Official methods of analysis of AOAC International. 17th ed. Arlington (VA): Association of Official Analysis Chemists International. [Google Scholar]
- Arpaia, N., C. Campbell, X. Fan, S. Dikiy, J. van der Veeken, P. deRoos, H. Liu, J. R. Cross, K. Pfeffer, P. J. Coffer, . et al. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakuma, S., E. Hatakeyama, T. Urashima, E. Yoshida, T. Katayama, K. Yamamoto, H. Kumagai, H. Ashida, J. Hirose, and M. Kitaoka. . 2011. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 286:34583–34592. doi: 10.1074/jbc.M111.248138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of American Feed Control Officials (AAFCO) 2019. Official publication 2019. Oxford (IN): AAFCO. [Google Scholar]
- Barker, H. A 1981. Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 50:23–40. doi: 10.1146/annurev.bi.50.070181.000323 [DOI] [PubMed] [Google Scholar]
- Barry, K. A., B. J. Wojcicki, I. S. Middelbos, B. M. Vester, K. S. Swanson, and G. C. Fahey Jr. 2010. Dietary cellulose, fructooligosaccharides, and pectin modify fecal protein catabolites and microbial populations in adult cats. J. Anim. Sci. 88:2978–2987. doi: 10.2527/jas.2009-2464 [DOI] [PubMed] [Google Scholar]
- van der Beek, C. M., J. G. Bloemen, M. A. van den Broek, K. Lenaerts, K. Venema, W. A. Buurman, and C. H. Dejong. . 2015. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J. Nutr. 145:2019–2024. doi: 10.3945/jn.115.211193 [DOI] [PubMed] [Google Scholar]
- Bell, E. T., J. S. Suchodolski, A. Isaiah, L. M. Fleeman, A. K. Cook, J. M. Steiner, and C. S. Mansfield. . 2014. Faecal microbiota of cats with insulin-treated diabetes mellitus. PLoS One. 9:e108729. doi: 10.1371/journal.pone.0108729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham, E. N., S. Kittelmann, W. Young, K. R. Kerr, K. S. Swanson, N. C. Roy, and D. G. Thomas. . 2013. Post-weaning diet affects faecal microbial composition but not selected adipose gene expression in the cat (Felis catus). PLoS One. 8:e80992. doi: 10.1371/journal.pone.0080992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock, J., R. H. Buck, H. Linke, P. Forsythe, A. M. Stanisz, and W. A. Kunze. . 2013. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One. 8:e76236. doi: 10.1371/journal.pone.0076236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blottière, H. M., B. Buecher, J. P. Galmiche, and C. Cherbut. . 2003. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc. 62:101–106. doi: 10.1079/PNS2002215 [DOI] [PubMed] [Google Scholar]
- Bode, L 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22:1147–1162. doi: 10.1093/glycob/cws074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, L., N. Contractor, D. Barile, N. Pohl, A. R. Prudden, G. J. Boons, Y. S. Jin, and S. Jennewein. . 2016. Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application. Nutr. Rev. 74:635–644. doi: 10.1093/nutrit/nuw025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau, J. C., L. Sivakumar, L. T. Do, T. D. White, J. Oravec, and N. K. Hoang. . 1985. Neurophysiology of geniculate ganglion (facial nerve) taste systems: species comparisons. Chem. Senses 10:89–127. doi: 10.1093/chemse/10.1.89 [DOI] [Google Scholar]
- Bradshaw, J. W. S 1991. Sensory and experiential factors in the design of foods for domestic dogs and cats. Proc. Nutr. Soc. 50:99–106. doi: 10.1079/PNS19910015 [DOI] [PubMed] [Google Scholar]
- Bradshaw, J. W., D. Goodwin, V. Legrand-Defrétin, and H. M. Nott. . 1996. Food selection by the domestic cat, an obligate carnivore. Comp. Biochem. Physiol. A. Physiol. 114:205–209. doi: 10.1016/0300-9629(95)02133-7 [DOI] [PubMed] [Google Scholar]
- Bubb, W. A., T. Urashima, K. Kohso, T. Nakamura, I. Arai, and T. Saito. . 1999. Occurrence of an unusual lactose sulfate in dog milk. Carbohydr. Res. 318:123–128. doi: 10.1016/s0008-6215(99)00102-0 [DOI] [PubMed] [Google Scholar]
- Budde, E. F 1952. The determination of fat in baked biscuit type of dog foods. J. Assoc. Off. Agric. Chem. 35:799–805. doi: 10.1093/jaoac/35.3.799 [DOI] [Google Scholar]
- Chaney, A. L., and E. P. Marbach. . 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. doi: 10.1093/clinchem/8.2.130 [DOI] [PubMed] [Google Scholar]
- Clausen, M. R., and P. B. Mortensen. . 1995. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut 37:684–689. doi: 10.1136/gut.37.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock, S. S., M. Li, M. Wang, M. H. Monaco, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, and S. M. Donovan. . 2017. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in noninfected and rotavirus-infected neonatal piglets. J. Nutr. 147:1041–1047. doi: 10.3945/jn.116.243774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet, D. E., Y. Yin, X. M. Zhang, C. Rémésy, D. Stamp, A. Medline, L. Thompson, W. R. Bruce, and M. C. Archer. . 1995. Colonic protein fermentation and promotion of colon carcinogenesis by thermolyzed casein. Nutr. Cancer 23:271–281. doi: 10.1080/01635589509514381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiwegger, T., B. Stahl, J. Schmitt, G. Boehm, M. Gerstmayr, J. Pichler, E. Dehlink, C. Loibichler, R. Urbanek, and Z. Szépfalusi. . 2004. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr. Res. 56:536–540. doi: 10.1203/01.PDR.0000139411.35619.B4 [DOI] [PubMed] [Google Scholar]
- Erwin, E. S. S., G. J. J. Marco, and E. M. M. Emery. . 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Flickinger, E. A., E. M. Schreijen, A. R. Patil, H. S. Hussein, C. M. Grieshop, N. R. Merchen, and G. C. Fahey Jr. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Furusawa, Y., Y. Obata, S. Fukuda, T. A. Endo, G. Nakato, D. Takahashi, Y. Nakanishi, C. Uetake, K. Kato, T. Kato, . et al. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- Garrido, D., S. Ruiz-Moyano, D. G. Lemay, D. A. Sela, J. B. German, and D. A. Mills. . 2015. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Sci. Rep. 5:13517. doi: 10.1038/srep13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirnaert, A., M. Calatayud, C. Grootaert, D. Laukens, S. Devriese, G. Smagghe, M. De Vos, N. Boon, and T. Van de Wiele. . 2017. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 7:11450. doi: 10.1038/s41598-017-11734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, M., C. P. Sodhi, Y. Yamaguchi, H. Jia, P. Lu, W. B. Fulton, L. Y. Martin, T. Prindle, D. F. Nino, Q. Zhou, . et al. 2016. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 116:1175–1187. doi: 10.1017/S0007114516002944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., S. Liu, D. E. Kling, S. Leone, N. T. Lawlor, Y. Huang, S. B. Feinberg, D. R. Hill, and D. S. Newburg. . 2016. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut. 65:33–46. doi: 10.1136/gutjnl-2014-307544 [DOI] [PubMed] [Google Scholar]
- Hesta, M., E. Hoornaert, A. Verlinden, and G. P. Janssens. . 2005. The effect of oligofructose on urea metabolism and faecal odour components in cats. J. Anim. Physiol. Anim. Nutr. (Berl). 89:208–214. doi: 10.1111/j.1439-0396.2005.00551.x [DOI] [PubMed] [Google Scholar]
- Hesta, M., G. P. Janssens, J. Debraekeleer, and R. De Wilde. . 2001. The effect of oligofructose and inulin on faecal characteristics and nutrient digestibility in healthy cats. J. Anim. Physiol. Anim. Nutr. (Berl). 85:135–141. doi: 10.1046/j.1439-0396.2001.00308.x [DOI] [PubMed] [Google Scholar]
- Hester, S. N., X. Chen, M. Li, M. H. Monaco, S. S. Comstock, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, and S. M. Donovan. . 2013. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 110:1233–1242. doi: 10.1017/S0007114513000391 [DOI] [PubMed] [Google Scholar]
- Hino, S., T. Mizushima, K. Kaneko, E. Kawai, T. Kondo, T. Genda, T. Yamada, K. Hase, N. Nishimura, and T. Morita. . 2020. Mucin-derived O-glycans act as endogenous fiber and sustain mucosal immune homeostasis via short-chain fatty acid production in rat cecum. J. Nutr. 150:2656–2665. doi: 10.1093/jn/nxaa097 [DOI] [PubMed] [Google Scholar]
- Hughes, K., P. G. Jones, C. Lebrilla, and D. J. Wrigglesworth. . 2020. Pet food products comprising oligosaccharides and methods of use. WO2020033708A1. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020033708 [Google Scholar]
- Hussein, H. S., E. A. Flickinger, and G. C. Fahey Jr. 1999. Petfood applications of inulin and oligofructose. J. Nutr. 129(7 Suppl):1454S–1456S. doi: 10.1093/jn/129.7.1454S [DOI] [PubMed] [Google Scholar]
- Kanakupt, K., B. M. Vester Boler, B. R. Dunsford, and G. C. Fahey Jr. 2011. Effects of short-chain fructooligosaccharides and galactooligosaccharides, individually and in combination, on nutrient digestibility, fecal fermentative metabolite concentrations, and large bowel microbial ecology of healthy adults cats. J. Anim. Sci. 89:1376–1384. doi: 10.2527/jas.2010-3201 [DOI] [PubMed] [Google Scholar]
- Kavanaugh, D. W., J. O′Callaghan, L. F. Buttó, H. Slattery, J. Lane, M. Clyne, M. Kane, L. Joshi, and R. M. Hickey. . 2013. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS One. 8:e67224. doi: 10.1371/journal.pone.0067224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieler, I. N., M. Osto, L. Hugentobler, L. Puetz, M. T. P. Gilbert, T. Hansen, O. Pedersen, C. E. Reusch, E. Zini, T. A. Lutz, . et al. 2019. Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria. Sci. Rep. 9:4822. doi: 10.1038/s41598-019-41195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. A., M. J. Jang, S. Y. Kim, Y. Yang, H. O. Pavlidis, and S. C. Ricke. . 2019. Potential for prebiotics as feed additives to limit foodborne Campylobacter establishment in the poultry gastrointestinal tract. Front. Microbiol. 10:1–12. doi: 10.3389/fmicb.2019.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C., M. Elderman, L. Cheng, B. J. de Haan, A. Nauta, and P. de Vos. . 2019. Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non-digestible carbohydrates. Mol. Nutr. Food Res. 63:e1900303. doi: 10.1002/mnfr.201900303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, C., S. Rudloff, W. Baier, N. Klein, and S. Strobel. . 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20:699–722. doi: 10.1146/annurev.nutr.20.1.699 [DOI] [PubMed] [Google Scholar]
- Laflamme, D 1997. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract. 25:13–18. [Google Scholar]
- Levesque, A 1999. No Il gusto nel cane e nel gatto. Summa. 16:15–25. [Google Scholar]
- Li, M., L. L. Bauer, X. Chen, M. Wang, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, G. C. Fahey Jr, and S. M. Donovan. . 2012. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J. Nutr. 142:681–689. doi: 10.3945/jn.111.154427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.-Y., M. Carroll, X. Yang, S. Do, W. Shi, T. Phungviwatnikul, F. He, and K. S. Swanson. . 2019. Effects of dietary macronutrient content on fecal microbiota populations and metabolite concentrations of healthy adult dogs. J. Anim. Sci. 97 (Suppl. 3):61–62. (Abstr.) doi: 10.1093/jas/skz258.5128 [DOI] [Google Scholar]
- Lis-Kuberka, J., and M. Orczyk-Pawiłowicz. . 2019. Sialylated oligosaccharides and glycoconjugates of human milk. The impact on infant and newborn protection, development and well-being. Nutrients 11:306. doi: 10.3390/nu11020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B., and D. S. Newburg. . 2013. Human milk glycoproteins protect infants against human pathogens. Breastfeed. Med. 8:354–362. doi: 10.1089/bfm.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio, R. G., M. R. Niñonuevo, S. R. Kronewitter, S. L. Freeman, J. B. German, C. B. Lebrilla, and D. A. Mills. . 2009. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb. Biotechnol. 2:333–342. doi: 10.1111/j.1751-7915.2008.00072.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lührs, H., T. Gerke, J. G. Müller, R. Melcher, J. Schauber, F. Boxberger, W. Scheppach, and T. Menzel. . 2002. Butyrate inhibits NF-κB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 37:458–466. doi: 10.1080/003655202317316105 [DOI] [PubMed] [Google Scholar]
- Macfarlane, G. T., and J. H. Cummings. . 1991. The colonic flora, fermentation and large bowel digestive function. In: Phillips, S. F., J. H. Pemberton, and R. G. Shorter, editors. The large intestine: physiology, pathophysiology and disease. New York (NY): Raven Press; p. 51–92. [Google Scholar]
- Macias Rostami, S., T. Bénet, J. Spears, A. Reynolds, E. Satyaraj, N. Sprenger, and S. Austin. . 2014. Milk oligosaccharides over time of lactation from different dog breeds. PLoS One. 9:e99824. doi: 10.1371/journal.pone.0099824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, T., M. Towatari, H. Kosugi, and H. Saito. . 2000. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 96:3847–3856. doi: 10.1182/blood.V96.12.3847.h8003847_3847_3856 [DOI] [PubMed] [Google Scholar]
- Manthey, C. F., C. A. Autran, L. Eckmann, and L. Bode. . 2014. Human milk oligosaccharides protect against enteropathogenic Escherichia coli attachment in vitro and EPEC colonization in suckling mice. J. Pediatr. Gastroenterol. Nutr. 58:165–168. doi: 10.1097/MPG.0000000000000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal, A., M. Barboza, E. D. Sonnenburg, N. Pudlo, E. C. Martens, P. Desai, C. B. Lebrilla, B. C. Weimer, D. A. Mills, J. B. German, . et al. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10:507–514. doi: 10.1016/j.chom.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T., Y. Matsukawa, T. Sakai, K. Nakamura, A. Aoike, and K. Kawai. . 1995. Effect of ammonia on cell-cycle progression of human gastric cancer cells. Eur. J. Gastroenterol. Hepatol. 7(Suppl 1):S79–S81. [PubMed] [Google Scholar]
- Morozov, V., G. Hansman, F. G. Hanisch, H. Schroten, and C. Kunz. . 2018. Human milk oligosaccharides as promising antivirals. Mol. Nutr. Food Res. 62:e1700679. doi: 10.1002/mnfr.201700679 [DOI] [PubMed] [Google Scholar]
- Musilova, S., V. Rada, E. Vlkova, and V. Bunesova. . 2014. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef. Microbes 5:273–283. doi: 10.3920/BM2013.0080 [DOI] [PubMed] [Google Scholar]
- Newburg, D. S., G. M. Ruiz-Palacios, and A. L. Morrow. . 2005. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553 [DOI] [PubMed] [Google Scholar]
- Nogueira, J. P. S., F. He, H. F. Mangian, P. M. Oba, and M. R. C. De Godoy. . 2019. Dietary supplementation of a fiber-prebiotic and saccharin-eugenol blend in extruded diets fed to dogs. J. Anim. Sci. 97:4519–4531. doi: 10.1093/jas/skz293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2006. Nutrient requirements of dogs and cats. Washington (DC): National Academies Press. [Google Scholar]
- Oba, P. M., S. Vidal, R. Wyss, Y. Miao, Y. Adesokan, and K. S. Swanson. . 2020. Effect of a novel animal milk oligosaccharide biosimilar on the gut microbial communities and metabolites of in vitro incubations using feline and canine fecal inocula. J. Anim. Sci. 98:1–11. doi: 10.1093/jas/skaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, G., J. Brynskov, and T. Saermark. . 2002. Phenol toxicity and conjugation in human colonic epithelial cells. Scand. J. Gastroenterol. 37:74–79. doi: 10.1080/003655202753387392 [DOI] [PubMed] [Google Scholar]
- Plaza-Díaz, J., L. Fontana, and A. Gil. . 2018. Human milk oligosaccharides and immune system development. Nutrients. 10:1038. doi: 10.3390/nu10081038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosky, L., N. G. Asp, T. F. Schweizer, J. W. Devries, and I. Furda. . 1992. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J. AOAC Int. 75:360–367. [PubMed] [Google Scholar]
- Pruss, K. M., and J. Sonnenburg. . 2020. Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. ISME J. doi: 10.1038/s41396-020-00798-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, C. A., J. E. Bauer, W. J. Burkholder, R. A. Kennis, B. L. Dunbar, and K. E. Bigley. . 2001. Effects of dietary flax seed and sunflower seed supplementation on normal canine serum polyunsaturated fatty acids and skin and hair coat condition scores. Vet. Dermatol. 12:111–117. doi: 10.1046/j.1365-3164.2001.00234.x [DOI] [PubMed] [Google Scholar]
- Rodrigues, B. M., P. M. Olivo, M. P. Osmari, R. S. Vasconcellos, L. B. Ribeiro, F. I. Bankuti, and M. S. S. Pozza. . 2020. Microencapsulation of probiotic strains by lyophilization is efficient in maintaining the viability of microorganisms and modulation of fecal microbiota in cats. Int. J. Microbiol. 2020:1293481. doi: 10.1155/2020/1293481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios, G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguia, and D. S. Newburg. . 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278:14112–14120. doi: 10.1074/jbc.M207744200 [DOI] [PubMed] [Google Scholar]
- Shinohara, M., M. Kiyosue, T. Tochio, S. Kimura, and Y. Koga. . 2020. Activation of butyrate-producing bacteria as well as bifidobacteria in the cat intestinal microbiota by the administration of 1-kestose, the smallest component of fructo-oligosaccharide. J. Vet. Med. Sci. 82:866–874. doi: 10.1292/jvms.19-0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski, J. S., S. E. Dowd, V. Wilke, J. M. Steiner, and A. E. Jergens. . 2012. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One. 7:e39333. doi: 10.1371/journal.pone.0039333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski, J. S., M. L. Foster, M. U. Sohail, C. Leutenegger, E. V. Queen, J. M. Steiner, and S. L. Marks. . 2015. The fecal microbiome in cats with diarrhea. PLoS One. 10:e0127378. doi: 10.1371/journal.pone.0127378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, K. S., C. M. Grieshop, E. A. Flickinger, L. L. Bauer, J. Chow, B. W. Wolf, K. A. Garleb, and G. C. Fahey Jr. 2002a. Fructooligosaccharides and Lactobacillus acidophilus modify gut microbial populations, total tract nutrient digestibilities and fecal protein catabolite concentrations in healthy adult dogs. J. Nutr. 132:3721–3731. doi: 10.1093/jn/132.12.3721 [DOI] [PubMed] [Google Scholar]
- Swanson, K. S., C. M. Grieshop, E. A. Flickinger, L. L. Bauer, H. P. Healy, K. A. Dawson, N. R. Merchen, and G. C. Fahey Jr. 2002b. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 132:980–989. doi: 10.1093/jn/132.5.980 [DOI] [PubMed] [Google Scholar]
- Tal, M., J. S. Weese, D. E. Gomez, M. Hesta, J. M. Steiner, and A. Verbrugghe. . 2020. Bacterial fecal microbiota is only minimally affected by a standardized weight loss plan in obese cats. BMC Vet. Res. 16:112. doi: 10.1186/s12917-020-02318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada, A., H. Hara, S. Kato, T. Kimura, I. Fujimori, K. Hara, T. Maruyama, and T. Mitsuoka. . 1993. Effect of lactosucrose (4G-BETA-d-Galactosylsucrose) on fecal flora and fecal putrefactive products of cats. J. Vet. Med. Sci. 55:291–295. doi: 10.1292/jvms.55.291 [DOI] [PubMed] [Google Scholar]
- Thépault, A., V. Rose, M. Queguiner, M. Chemaly, and K. Rivoal. . 2020. Dogs and cats: reservoirs for highly diverse Campylobacter jejuni and a potential source of human exposure. Animals 10:838. doi: 10.3390/ani10050838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima, T., T. Saito, T. Nakamura, and M. Messer. . 2001. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 18:357–371. doi: 10.1023/a:1014881913541 [DOI] [PubMed] [Google Scholar]
- Urashima, T., E. Taufik, K. Fukuda, and S. Asakuma. . 2013. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 77:455–466. doi: 10.1271/bbb.120810 [DOI] [PubMed] [Google Scholar]
- Vázquez, E., A. Barranco, M. Ramírez, A. Gruart, J. M. Delgado-García, E. Martínez-Lara, S. Blanco, M. J. Martín, E. Castanys, R. Buck, . et al. 2015. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J. Nutr. Biochem. 26:455–465. doi: 10.1016/j.jnutbio.2014.11.016 [DOI] [PubMed] [Google Scholar]
- Vester Boler, B. M., M. C. Rossoni Serao, T. A. Faber, L. L. Bauer, J. Chow, M. R. Murphy, and G. C. Fahey Jr. 2013. In vitro fermentation characteristics of select nondigestible oligosaccharides by infant fecal inocula. J. Agric. Food Chem. 61:2109–2119. doi: 10.1021/jf305056f [DOI] [PubMed] [Google Scholar]
- Vilson, Å., Å. Hedhammar, A. Reynolds, J. Spears, E. Satyaraj, R. Pelker, C. Rottman, B. Björkstén, and H. Hansson-Hamlin. . 2016. Immunoglobulins in dogs: correspondence and maturation in 15 litters of German shepherd dogs and their dams. Vet. Rec. Open 3:e000173. doi: 10.1136/vetreco-2016-000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B 2009. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 29:177–222. doi: 10.1146/annurev.nutr.28.061807.155515 [DOI] [PubMed] [Google Scholar]
- Ward, R. E., M. Niñonuevo, D. A. Mills, C. B. Lebrilla, and J. B. German. . 2006. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl. Environ. Microbiol. 72:4497–4499. doi: 10.1128/AEM.02515-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, J. M., R. de Souza, C. W. Kendall, A. Emam, and D. J. Jenkins. . 2006. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40:235–243. doi: 10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]
- Wrigglesworth, D. J., E. Goonatilleke, R. Haydock, K. R. Hughes, C. B. Lebrilla, K. S. Swanson, P. Jones, and P. Watson. 2020. High-throughput glycomic analyses reveal unique oligosaccharide profiles of canine and feline milk samples. PLoS ONE. 15:e0243323. doi: 10.1371/journal.pone.0243323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken, R. H., J. A. Peterson, S. L. Vonderfecht, E. T. Fouts, K. Midthun, and D. S. Newburg. . 1992. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Invest. 90:1984–1991. doi: 10.1172/JCI116078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z.-T., C. Chen, D. E. Kling, B. Liu, J. M. McCoy, M. Merighi, M. Heidtman, and D. S. Newburg. . 2013a. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 23:169–177. doi: 10.1093/glycob/cws138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z. T., C. Chen, and D. S. Newburg. . 2013b. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 23:1281–1292. doi: 10.1093/glycob/cwt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z.-T., N. N. Nanthakumar, and D. S. Newburg. . 2016. The human milk oligosaccharide 2′-fucosyllactose quenches Campylobacter jejuni-induced inflammation in human epithelial cells HEp-2 and HT-29 and in mouse intestinal mucosa. J. Nutr. 146:1980–1990. doi: 10.3945/jn.116.230706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic, A. M., and D. Barile. . 2011. Bovine milk as a source of functional oligosaccharides for improving human health. Adv. Nutr. 2:284–289. doi: 10.3945/an.111.000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.