Abstract
In calf rearing, the first weeks of life are critical and associated with the highest mortality due to enteric and respiratory diseases. A well-implemented hygiene management can help to protect calves’ health preventively by reducing the load of pathogenic bacteria and interrupting infection chains. The aim of this study was to identify deficiencies in hygiene management of individually housed dairy calves by surveying current practice and examining feeding and housing equipment with different hygiene indicators. On 11 farms, different locations in 2 pens or hutches for individual calf rearing prepared for restocking and 2 feeding buckets per farm, including the inner and outer surfaces of artificial teats, were visually scored for cleanliness and sampled with swabs (housing equipment: n = 167; feeding equipment: n = 120). The sanitation of floors was tested with sock samples (n = 41). A total of 328 samples were analyzed for adenosine triphosphate (ATP) and protein residues, aerobic total viable count (TVC), total coliform count (TCC), Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum β-lactamase-producing bacteria (ESBL), and Salmonella spp. After evaluation of these results, the farmers were informed about the findings and trained on improvement in hygiene management personally. The sampling was repeated after 1 year to detect possible changes in hygiene management. The highest bacterial loads (TVC, TCC, and E. coli) were observed in feeding equipment, especially the inner teat of milk feeding buckets. Environmental samples, primarily the sidewalls and back walls of tested pens and hutches, exhibited the lowest bacterial counts and ATP and protein residues. All samples were negative for MRSA and Salmonella spp. In 10.5% of all samples, ESBL was detected, and in 6.8%, ESBL E. coli was detected, predominately in sock samples, followed by feeding equipment samples. Training in hygiene management showed only limited effects. In conclusion, there is still great potential to improve the implementation of hygiene measures in individual calf housing. In particular, more attention should be paid to the cleaning of feeding buckets and artificial teats, as this is a simple means of interrupting the possible spread of pathogens among calves.
Keywords: cleaning, disease prevention, health risks, hygiene management, suckling calf
Introduction
Enteric diseases are still the most frequent reason for calf morbidity and mortality, with the highest risk in the first 3 wk postpartum (Bendali et al., 1999; Svensson et al., 2006), where calves in Germany are usually kept in single housing. Additionally, diarrhea can depress growth and development of calves and can cause considerable financial losses to commercial farms (de Graaf et al., 1999; Marcé et al., 2010; Torsein et al., 2011). Good housing and hygiene management have the potential to decrease the incidence of diarrhea in young calves (Klein-Jöbstl et al., 2014). Enteric diseases are usually transmitted via the fecal-oral route, so transmission by housing equipment and feeding equipment are likely (Maunsell and Donovan, 2008). Infections caused by transmissions via surfaces hinge on different factors, including the load of pathogens, survival rate on surfaces, resistance to disinfectants, and initial infecting dose (Tuladhar et al., 2012). Irregular or inadequate cleaning is one of the most common problems in calf rearing; therefore, a well-implemented hygiene management in combination with other biosecurity measures can maintain calf´s health by avoiding the transmission of infectious agents (de Graaf et al., 1999; Maunsell and Donovan, 2008; Barry et al., 2019a). The method and frequency of cleaning could significantly reduce the risk for diarrhea outbreaks in calves (Castro-Hermida et al., 2002; Klein-Jöbstl et al., 2014). In pork and poultry production, standardized methods for cleaning and disinfection are more common but still have weaknesses, such as inefficient cleaning of feeders and waterers or drain holes and floor cracks (Mannion et al., 2007; Mueller-Doblies et al., 2010; Luyckx et al., 2015). For calf rearing, practical recommendations and knowledge about critical points in hygiene are rarely implemented in daily routine on farms, even though the importance of hygiene interventions is well known (Weaver et al., 2000; Barrington et al., 2002; Klein-Jöbstl et al., 2014). Additionally, although the EU legal requirements about hygiene in calf rearing state that housing pens for calves must be properly cleaned and disinfected (EU 2008/119/EC), appropriate time intervals, methods for documentation or procedures to evaluate success in sanitation remain unclear. To document sanitation commercial rapid tests for ATP residues and for protein residues were used. Both tests are established in healthcare settings and food industry, but to a lesser extent in animal husbandry (Alfa et al., 2013; Clemensson Lindell et al., 2018; Öz and Arun, 2019). ATP and protein residues are components of soiling, like feces, uneaten, or spilled feed, and other organic debris (Hawronskyi and Holah, 1997). These indicators are easy to use and give a fast reply without further processing in the laboratory, but they are unable to give any information about remaining bacteria (Vilar et al., 2008; Casini et al., 2018; Heinemann et al., 2020). Nevertheless, the test results often correspond to microbiological findings (Alfa et al., 2013; Osimani et al., 2014). The aim of this study was to assess common hygiene management practices on dairy farms and gain information about weak points in the sanitation of newborn calf housing and feeding equipment by comparing different hygiene indicators to interrupt infection chains in the long term. We hypothesize that, despite the difference in management practices, hygiene status can be improved by training as seen in pig fattening (Heinemann et al., 2020). In this study, we focused on a limited number of farms but a high number of sampling locations and different methods per farm to determine the relevant locations and methods for a future study with numerous farms and a shortened sampling protocol.
Materials and Methods
This study was conducted in accordance with federal and institutional animal use guidelines (Az. 84-02.05.40.16.038), the data privacy agreement (University of Bonn, 38/2018), and ethical standards. The first sampling took place from 10/2018 to 12/2018 and the second sampling from 10/2019 to 12/2019.
Selection of the farms
To ensure that the samples can be processed within 24 hr, only farms within a radius of 100 km were eligible to be included in this study. A list of all dairy farms in this area was generated online at the website of the local chamber of agriculture, resulting in a list of 66 farms. Based on telephone availability, 30 farms were contacted and asked about their willingness to participate and their number of cows. Eleven farmers refused participation directly. The reasons given were (1) no time, (2) no interest, (3) simply hung up in conversation, (4) misinformation about the farm (bull fattening instead of dairy farming). Emails with additional information about the overall study goals, including a second visit for hygiene sampling, planned tests, background information about the microbiological species, and time investment by the farmer were sent. Farms with incomparable production systems, such as block calving, free-range rearing, or pair housing were rejected, so 12 farms out of 19 were selected. The size of the selected farms varied from 60 to 700 lactating cows, with a median herd size of 125 animals. On average, dairy farms in North-Rhine Westphalia keep 73 lactating cows, although there may be differences depending on the region (Statistisches Bundesamt, 2019). The animal numbers of the farms participating in the study reflect thus the range of farm sizes in North Rhine-Westphalia. One farm was discarded after the first visit because they changed their calf rearing system from individual housing to free mother-bonded rearing, so neither pens nor hutches were used henceforward. Ultimately, 11 dairy farms, varying between 60 and 700 Holstein–Friesian dairy cows and 60 to 800 calves born per farm per year, were chosen. All farms are individual- or family-owned companies, located in North-Rhine Westphalia, Germany. In Germany, newborn dairy calves are commonly separated from their dams immediately, fed with colostrum, and housed individually for the first 14 d in all-in-all-out systems using either pens or hutches until males are sold and females are kept in groups for restocking. During the first 14 d, newborn calves are fed milk diets (milk replacer, waste milk, or bulk milk) by feeding buckets with artificial teats. The farm visits, including the interview, the visual inspection of the sampling points and further sampling, were always carried out by the same 2 persons, both with experience in sampling for hygiene measurements. Farm characteristics of the sampled farms were assessed by face-to-face interviews with the farm manager or the herd manager using an interview sheet (Supplemental Table 1), which was completed in compliance with the farm or herd manager, and an additional record sheet (Supplemental Table 2), which was completed in the absence of the manager. The in-house-developed interview sheet contained 67 closed-ended and semi-closed-ended questions dealing with hygiene management, feeding management, and health-associated factors of individually housed calves. The interview sheet and record sheet were developed in consultation with 2 experts in dairy calf management and were tested for understanding and plausibility in a preliminary trial with 3 farmers. Based on the results of the preliminary trial, the data sheets were revised. Sampling was performed at 2 different times on each farm. The objectives of the first sampling were to identify critical points in individual calf housing and to assess different hygiene indicators for their suitability in livestock farming. After processing the samples and data analysis, the results were discussed with the farmers for training purposes. Individual results of each farm were compared with the mean results of the first trial. Weak points in hygiene management of each farm were pointed out and inspected together with the farmer or the herd manager directly on site for each farm and improvements for cleaning and disinfection procedure were suggested. Sample collection was repeated after 305 ± 24 d to verify enhancements in hygiene. The ambient temperature during sampling varied between 4 and 24 °C, with a median value of 14.5 °C.
Assessment of housing situation and visible inspection
First, information about individual calf housing was recorded, e.g., pens or hutches, dimensions, condition of the floor surface, and slope of the floor. Farm-specific characteristics were captured by photography. Visual cleanliness of the sampled areas was always registered with a 3-score grading system (1 = no remaining soiling visible, 2 = minor soiling visible, 3 = coarse soiling visible).
Sample collection
Samples were collected after the sanitation of empty calf hutches or pens and from feeding buckets before restocking. Sampling was scheduled in consultation with the farmers. In each hutch or pen (2 per farm), samples were taken at the following defined locations: entrance, side wall, back wall, and middle of the floor. Additionally, 2 milk feeding buckets were sampled at the inner bottom of feeding buckets, and inner and outer surface of artificial teats. The 2 hutches or pens and the 2 feeding buckets were chosen randomly; a coin was flipped until only 2 hutches or pens and 2 milk feeding buckets remained. Sample collection was performed by using different kinds of swabs (swabs from rapid tests for the detection of adenosine triphosphate (ATP) and protein residues and swabs for microbiological analysis, see below) by wiping an area of 25 cm2 horizontally and then vertically while rotating the swab. For the different swab tests, the area was shifted except for inner and outer surface of artificial teats where shifting was impossible due to their limited size. Additionally, sock sampling, also known as “boot sock sampling,” which is the recommended method in the EU according to CR (EU) 200/2010 for detection of Salmonella in broiler houses and is routinely used for detection of Salmonella and other bacteria, was performed (Skov et al., 1999; Berghaus et al., 2013). Compared with swabbing, boot sock sampling has the advantage of sampling larger areas of floor. For this purpose, clean and disinfected boots were covered with moistened disposable cotton hairnets as an absorbent coating, and a defined distance of 50 steps was walked through the sampled pen or hutches. Care was taken to ensure that equal numbers of steps were always taken along the sides (20 steps) and diagonally through the pen or hutch (30 steps). The hairnets were then transferred to sterile polyethylene bottles with screw caps in 100 mL of sterile saline solution for transport to the laboratory. All samples were stored in chilled boxes (4 to 8 °C), transported to the microbiological laboratory of the University of Bonn and processed within 24 hr. In total, 328 samples were analyzed.
Rapid tests
Two different kinds of rapid tests were used, which are routinely applied in the control of hygiene procedures of healthcare institutions and in the food industry: one to measure ATP residues (CleanTrace Surface ATP Test Swab UXL100, 3M, Neuss, Germany) and another to measure protein residues (Clean Trace Surface Protein Plus, 3M, Neuss, Germany). Both tests were analyzed directly after sample collection on the farm. For the ATP test, the amount of light is proportional to the amount of ATP residue and is measured by a luminometer (NG III, 3 M, Neuss, Germany). The values obtained are given in relative light units (RLU) per milliliter. The protein test is a semiquantitative system in which a color change occurs, and the change depends on the amount of protein residue. The displayed color was assessed by a defined 5-score scheme (1 = light green, no change, 2 = colorless, 3 = light gray, 4 = light purple, and 5 = dark purple, strong change).
Microbiological tests
Samples for microbiological tests were collected with sterile moistened flocked swabs with 1 mL of Amies medium (eSwab, Copan, Brescia, Italy). An adequate volume of Amies medium was diluted in physiological saline solution (Oxoid, Basingstoke, UK), depending on the expected amount of bacteria. Hairnets from sock samples and the 100 mL of saline solution into which the hairnets were placed for transport were mixed in filter bags with a stomacher to thoroughly dissolve the samples. The saline solution was processed in a manner similar to the swab samples. All samples were investigated for aerobic total viable count (TVC), total coliform count (TCC), and Escherichia coli by pour plating. Nonselective plate count agar (Merck, Darmstadt, Germany) was used for TVC, and Chromocult coliform agar (Merck, Darmstadt, Germany) was used for the enumeration of E. coli and coliform bacteria. TCC and E. coli were used as indicators of fecal contamination. After incubation, the bacteria were counted, and the arithmetic mean was calculated and log-transformed. The results are expressed as log10 colony forming units (cfu) ∙ mL−1. Methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase-producing bacteria (ESBL) were cultivated using the spread-plate technique with selective CHROMAgar plates (Mast Group, Reinfeld, Germany). The Amies medium of the swab samples was directly pipetted onto the agar without prior dilution. To avoid excessive growth of accompanying environmental bacteria, plates were incubated at 41 °C for 24 hr. To distinguish blue colonies (potentially Klebsiella spp. Enterobacter spp., or Citrobacter spp.) from ESBL agar, the colonies were transferred to Columbia sheep blood agar (Mast Group, Reinfeld, Germany), and further species identification was performed based on typical biochemical reactions with an EnteroPluri test (Liofilchem, Roseto Degli Abruzzi, Italy). Pink to purple colonies were classified as ESBL E. coli. White colonies, which are Acinetobacter spp. or Pseudomonas spp., were not further distinguished due to their low pathogenic relevance in bovines. For pre-enrichment of Salmonella spp., the swabs were transferred to peptone water (Merck, Darmstadt, Germany) and incubated (24 hr at 37 °C). Afterward, 1 mL of the pre-enrichment broth was added to 9 mL of Müller Kauffmann tetrathionate broth (BD Diagnostic Systems, Heidelberg, Germany) and incubated for another 24 hr at 37 °C. Additionally, 0.1 mL of the pre-enrichment was added to 10 mL of Rappaport-Vassiliadis broth (BD Diagnostic Systems, Heidelberg, Germany) and incubated for 24 hr at 41.5 °C. Subcultivation of both broths was performed on xylose lysine deoxycholate agar (Merck, Darmstadt, Germany) and mannitol lysine crystal violet brilliant green agar (Merck, Darmstadt, Germany) on 2 consecutive days (incubation: 24 hr at 37 °C).
Data analysis
All data from the record and interview sheet and from laboratory analysis were coded as numbers and summarized in an Excel file (Excel 2016, Microsoft Corp., Redmond, WA). Metric data were checked for normal distribution and log-transformed for cfu from microbiologic tests and RLU from ATP tests. Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC). Based on major differences in cleaning frequency and the number of samples, data regarding hygiene indicators were grouped into “feeding equipment” (results from the inner bottom of feeding buckets and the inner and outer surfaces of artificial teats), “environment” (results from the entrance, sidewalls, back walls, and floors), and “sock samples”. The effects of sampling location within each hygiene indicator were tested with a general linear model with a grouped location as the main effect (Figure 3). Data from individual hygiene indicators were analyzed by the mixed model procedure with time, farm, and time × farm interaction as fixed effects and sample as a random effect (Figures 4 and Supplemental Figure 1). Differences were localized by Tukey’s t test. Correlations between the different hygiene indicators were determined by the Spearman rank correlation procedure (Figures 1 and 2). The results were considered significant at P < 0.05, with P < 0.01, indicating that the results were highly significant and P < 0.10 indicating a tendency. A generalized linear mixed model with dichotomized data (PROC GLIMMIX) was used for modeling the effects of feeding and housing management practices on hygiene indicators. Data were dichotomized using the following cutoff values: according to Böhm (1998), 3.0 log10 cfu∙cm−2 bacteria remain on surfaces in animal houses under practical conditions, even after sufficient cleaning and disinfection. This value, which corresponds to 4.4 log10 cfu∙mL−1 in our study design, was set as a cutoff value for TVC. This approach was not transferable to the sock samples due to the different sampling techniques. For sock samples, a TVC cutoff value of 5.5 log10 cfu∙mL−1 was defined based on previous results (Heinemann et al., 2020). This value for the TVC of sock samples is considered to be achievable under practical conditions with sufficient hygiene management. The TCC and E. coli cutoff values were set at the detection limit (feeding equipment and environmental samples: 2.0 log10 cfu∙mL−1, sock samples: 1.0 log10 cfu∙mL−1). MRSA and ESBL cutoff values were based on the absence of susceptible colonies. The ATP cutoff value of feeding equipment and environment samples was adjusted based on the TVC cutoff value and set at 3.5 log10 RLU∙mL−1. The protein cutoff value was set at a rating of 3 on the color scale.
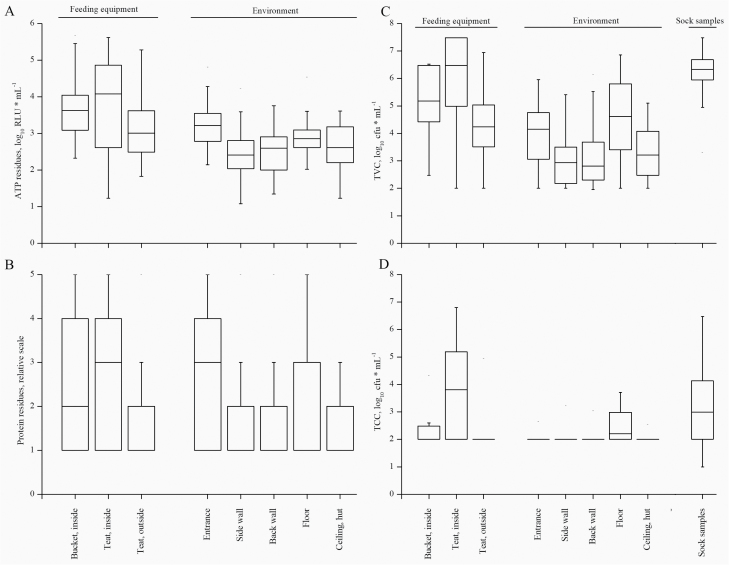
Figure 3.
Results for ATP residues (A), protein residues (B), aerobic TVC (C), and TCC (D) depending on the sampling areas: feeding equipment, environment or floor as done by sock samples shown as boxplots (lower whisker: 25% quartile, median, upper whisker: 75% quartile). Different letters indicate significant differences (P < 0.05) between the sampling areas.
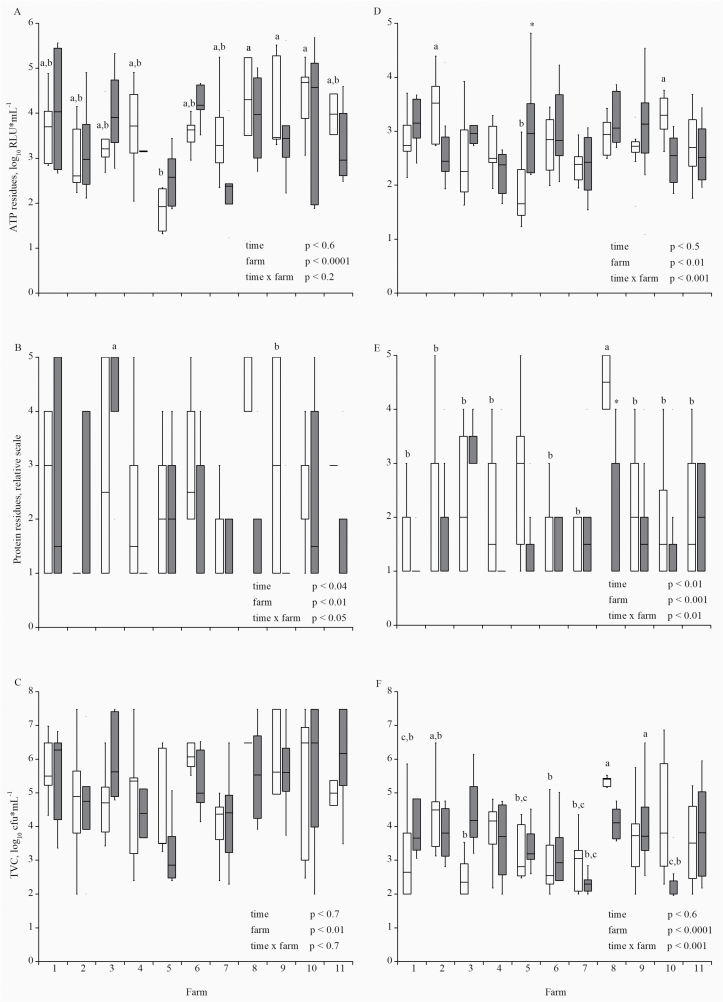
Figure 4.
Results for ATP residues, protein residues, and aerobic TVC from feeding equipment samples (A, B, and C) and environment samples (D, E, and F) from the first sampling (white bars) and the second sampling (gray bars) depending on the farm. Results are shown as boxplots (lower whisker: 25% quartile, median, upper whisker: 75% quartile). Boxplots with different superscripts differ (P < 0.05).
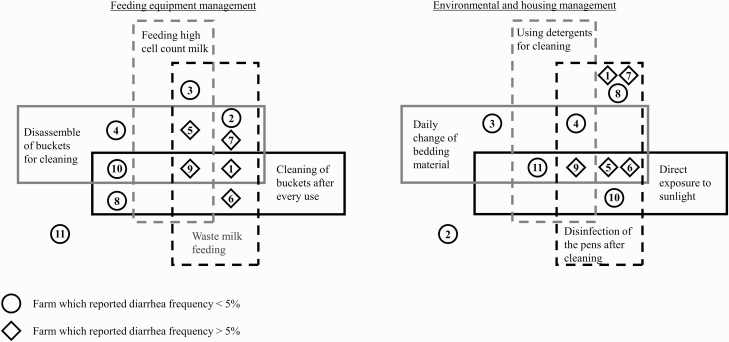
Figure 1.
Comparison of different feeding and housing management practices depending on the reported diarrhea frequency of the farms. Different numbers represent different farms. Symbols in boxes indicate used management practices on each farm, whereby symbols out of the boxes show that these management practices were not implemented at the respective farm.
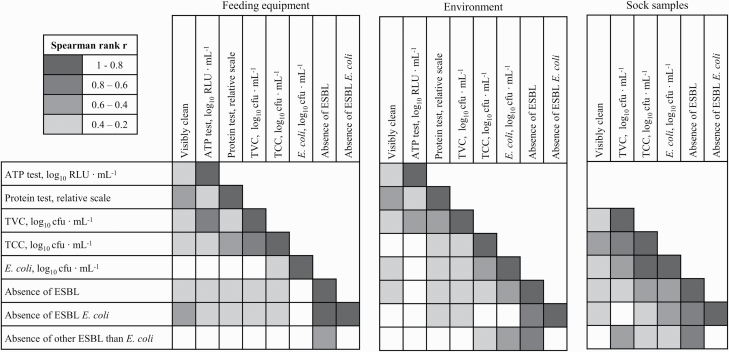
Figure 2.
Spearman rank correlations (P ≤ 0.05) between the hygiene indicators ATP, protein, aerobic TVC, TCC, E. coli, extended-spectrum β-lactamase-producing bacteria (ESBL), ESBL E. coli, and other ESBL independent of the effects of sampling points.
Results
Characteristic farm management factors
The number of dairy calves born per year ranged from 60 to 800 animals. The median reported calf loss during the first 14 d including losses at birth was 0.8% (0% to 5.9%) (Table 1). Generally, the farms showed substantial differences in management practices pertaining to housing and feeding (Table 1 and Figure 1). The time at which the separation from the dam occurred varied between 1 and 24 hr postpartum (median: 12 hr). Newborn calves were kept in individual housing for the first 14 d on average (minimum at 1 farm: 10 d, maximum at 2 farms: 21 d). During the individual housing period, calves were housed in pens on 7 farms (64%) (1.78 ± 0.29 m2) and in hutches on 4 farms (36%) (2.27 ± 0.13 m2). The hutches and pens were mostly built on hard artificial surfaces such as concrete or asphalt (45.5%), wood (45.5%), or plastic (9%). None of the pens or hutches stood on gravel or earth. All farms used straw as bedding material, and 6 (55%) renewed the bedding on a daily basis by adding clean straw on top (one farm (9%): every other day; 3 farms (27%): only on demand; 1 farm (9%): only at rehousing). Five farms (46%) housed newborn calves in direct sunlight instead of closed barns. All farms fed whole milk by feeding buckets, and 7 (64%) additionally fed waste milk (milk from cows suffering from mastitis or being administered antibiotics) or milk with a high somatic cell count. All farms fed milk without prior pasteurization. Most commonly, milk was fed warm (10 of 11 farms; 91%) and nonacidified (7 of 11 farms; 64%). It was recorded whether medicinal agents are regularly used in individual calf husbandry and which are these. None of the farms used regular deworming agents. On 7 farms (64%), calves were treated with medicinal agents in the last 6 months (2× amoxicillin (β-lactam antibiotic), 2× halofuginone (against cryptosporidiosis), 1× meloxicam (nonsteroidal anti-inflammatory drug), 1× treatment against acidosis, and 1× bromhexine (against respiratory disorders). In the interview, farmers were asked how often diarrhea occurs during single housing, with the possible replies <5% and >5%. Five of 11 farms (46%) reported diarrhea in calves at an incidence rate >5%. Four out of these 5 farms (80%) with diarrheic problems commissioned a fecal analysis, which resulted in the detection of Cryptosporidium spp. and rotavirus or only Cryptosporidium spp. Three farms (27%) reported occasional respiratory disorders, and 1 farm (9%) reported a few umbilical infections. With regard to sanitation, all farms regularly cleaned the pens or boxes used for individual housing, but none of the farms used a fixed cleaning protocol. Cleaning was reported to be performed with pressure washers with water only (8 of 11 farms; 73%) and more rarely with the additional use of detergents (3 of 11 farms; 27%). Usually, farms routinely disinfect the pens after cleaning (7 of 11 farms; 64%) with disinfectants containing p-chlorocresol or combinations of glutaraldehyde, quaternary ammonium compounds, and organic acids. All farms reported cleaning the buckets, but with substantial differences in the interval between cleanings. Four farms (36%) cleaned the buckets after every use, which meant twice a day, 1 farm (9%) cleaned the buckets weekly and the remaining farms (55%) cleaned them after each calf, which was equivalent to a 14-d interval. Cleaning methods differed between the farms and included cleaning with cold water only (7 farms; 64%), cleaning with cold water with detergents (2 farms; 18%), cleaning with hot water (1 farm; 9%), and cleaning with hot water with detergents (1 farm; 9%). The feeding bucket was disassembled for cleaning on 6 farms (55%). Disinfecting the buckets was uncommon and was only performed on 2 farms (18%). The reported diarrhea frequency correlated positively with disinfection of the pens after cleaning (rSpearman = 0.56; P < 0.001) and waste milk feeding (rSpearman = 0.66; P < 0.001, Figure 1).
Table 1.
Reported calf production data and rearing practices of the visited dairy farms sorted by number of calves per year
| Farm | No. calves per year | Losses during first 14 d | Losses during first 14 d, % | No. of rearing places | Contact between calves impossible | Calf hutches | Calf pens |
|---|---|---|---|---|---|---|---|
| 1 | 800 | 4 | 0.5 | 96 | X | X | |
| 2 | 350 | 2 | 0.6 | 24 | X | ||
| 3 | 250 | 2 | 0.8 | 20 | X | ||
| 4 | 200 | 5 | 2.5 | 23 | X | ||
| 5 | 130 | 1 | 0.8 | 16 | X | X | |
| 6 | 115 | 4 | 3.5 | 10 | X | ||
| 7 | 110 | 1 | 0.1 | 15 | X | X | |
| 8 | 110 | 0 | 0.0 | 14 | X | ||
| 9 | 85 | 5 | 5.6 | 5 | X | ||
| 10 | 65 | 0 | 0.0 | 9 | X | ||
| 11 | 60 | 0 | 0.0 | 8 | X | X |
Comparability of hygiene indicators
Spearman rank correlations between the applied hygiene indicators are presented in Figure 2 and were all positive. For the sock samples, the ATP test or protein test was not feasible due to the sampling technique. Visible soiling showed correlations with almost all other hygiene indicators used (0.2 < r < 0.6, P < 0.01). The E. coli load in the feeding equipment showed a correlation with only TCC (r = 0.3, P < 0.001). The ATP load from environmental samples was correlated only with the results from the rapid protein tests (r = 0.3, P < 0.001) and TVC (r = 0.4, P < 0.001). In contrast, the protein test and TVC results were correlated with TCC, E. coli, and the absence of ESBL and ESBL E. coli.
Critical points in hygiene management
All samples were negative for Salmonella spp. and MRSA. In 34 of 324 samples, ESBL was detected (10.5%), with 22 detections of ESBL E. coli (6.8%), 14 detections of ESBL Acinetobacter spp. or Pseudomonas spp. (4.3%) and 3 detections of ESBL Klebsiella spp. (0.9%). In four samples, more than one ESBL species was found after sanitation. ESBL species were predominantly found in sock samples (41.2%), followed by feeding equipment (35.3%) and environment samples (23.5%). At the farm level, 8 out of the 11 farms were positive for ESBL, which also included all farms with reported diarrhea problems. The highest bacterial loads, expressed as the TVC, were found on feeding equipment and in sock samples (Figure 3). Because of the different sampling techniques and on the basis of the scale findings, the sock samples could not be directly compared with other samples, so interpretation of the results must be considered with care. Environmental samples, primarily from the sidewalls and back walls, exhibited the lowest results for TVC, TCC, protein, and ATP (Figure 3). TCC and ATP were highest on the feeding equipment, especially the inner surface of the artificial teat (Figure 3). The TCC was often below the detection limit of 2.0 log10 cfu ∙ mL−1 in swab samples and 1.0 log10 cfu ∙ mL−1 in sock samples (Supplemental Table 3). Positive detections of TCC were obtained in 81.0% of the sock samples, 40.2% of the samples from feeding equipment, and 11.5% of the environmental samples. The detection rate varied between the farms from 12.5% to 48.2% for TCC. In 80.5% of the sock samples, 5.1% of the samples from feeding equipment and 4.8% of the environmental samples, E. coli was detectable, with detection rates ranging from 3.1% to 24%.
Risk factors for hygiene impairments
Based on the results of a previous literature research, risk factors that could have a negative impact on hygiene if they did not fit the expected demands for feeding equipment and housing equipment were defined. These risk factors were recorded by the questionnaire as well as by own observations on the farms. For feeding equipment, these tested factors were the feeding of waste milk, the feeding of high cell count milk, the lack of cleaning of feeding buckets after every use, the lack of disassembly of feeding buckets for cleaning, using only water for the cleaning of feeding buckets instead of cleaning with detergents and failing to use disinfectants when cleaning feeding buckets. For risk factors associated with housing, the evaluated variables were direct sunlight, the absence of a slope to the back wall, the absence of cracks in the ground, smooth surfaces, the absence of contact between the calves, the use of individual pens or hutches, rearing in hutches, shifting of hutches after use, daily changing of the bedding material, the use of detergents while cleaning, the use of disinfectants and regular disinfection of the pens after every calf. To determine the risk factors associated with poor hygiene, odds ratios (OR) were calculated for the results of the different hygiene indicators used to measure hygiene in feeding equipment samples (Table 2), environmental samples (Table 3), and sock samples (Table 4). Only significant risk factors (P ≤ 0.05) were considered. The use of detergents while cleaning feeding buckets resulted in higher visible cleanliness. Feeding of waste milk, feeding of high cell count milk, and cleaning the buckets after every use were associated with a lower pass rate on the ATP tests (Table 2). Additionally, cleaning after every use decreased the odds for TVC, meaning a lower chance of being below the cutoff value. Disassembly of the feeding buckets, meaning unscrewing the artificial teat from the bucket prior to cleaning, resulted in higher odds of being below the cutoff values for ATP and TVC (Table 2). The management factor of the absence of a slope to the back wall indicates that fluids, such as cleaning water soiled with urine, feces, or milk, could run off instead of contaminating the area where the calf lies, which is normally at the back wall. In environmental samples, the factor “absence of slope to the back wall” increased the odds of visible cleanliness and the odds of being below the cutoff values for the protein test, E. coli, ESBL in all and ESBL E. coli (Table 3). The use of non-adjacent pens or hutches for single housing, implying a greater distance between the calves, resulted in greater visible cleanliness and a greater likelihood of being below the cutoff value for the TVC. If hutches were used on the farms, the factor “shifting of hutches” was recorded, with the hypothesis that shifting the hutches to another place lowers the risk of soiling remaining from the last calf and the risk of vertical transfer of pathogens between calves. Shifting hutches increased the odds of visible cleanliness and being below the cutoff value for the TVC. The absence of cracks in the ground leads to a lower amount of protein residues. The use of disinfectants, independent of the frequency of usage, resulted in higher visible cleanliness and higher rates of being below the cutoff values for protein residues, the TVC and the TCC. A higher odd ratio for passing the protein test was associated with regular disinfection of pens after every calf. Smooth surfaces lowered the risk of detection of TCC and E. coli in environmental samples (Table 3). “Rearing in hutches” was associated with lower odds for visible cleanliness instead of “rearing in pens”, as well as for the absence of ESBL Pseudomonas spp. and Acinetobacter spp. in sock samples. Daily changes in bedding material lead to lower odds for TCC (Table 4). The absence of cracks in the ground increased the odds for being below the cutoff values for TCC and E. coli and for the absence of total ESBL, and the number of cracks in the ground affected the total ESBL detection in sock samples (Table 4).
Table 2.
Results for risk factors with calculated OR, 95% confidence intervals (CI), and P-values for calf feeding equipment failing to meet the expectations
| Expectations1 | Percent failing to meet expectations, % | Total no. | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Visibly clean (score 1) | |||||
| Use of detergents | 17.5 | 120 | 4.75 | 1.03 to 21.97 | 0.05 |
| ATP test (3.5 log10 RLU ∙ mL−1) | |||||
| Feeding of waste milk | 17.1 | 119 | 0.41 | 0.18 to 0.92 | 0.03 |
| Feeding of high cell count milk | 47.1 | 119 | 0.29 | 0.12 to 0.70 | 0.01 |
| Cleaning feeding buckets after every use | 51.3 | 119 | 0.22 | 0.10 to 0.48 | < 0.001 |
| Disassembling feeding buckets for cleaning | 47.1 | 119 | 2.20 | 1.01 to 4.83 | 0.05 |
| TVC (4.4 log10 cfu ∙ mL−1) | |||||
| Cleaning feeding buckets after every use | 67.8 | 118 | 0.34 | 0.15 to 0.79 | 0.01 |
| Disassembling feeding buckets for cleaning | 68.3 | 120 | 3.35 | 1.30 to 8.60 | 0.01 |
1For the different tests used (in italic), a cutoff value (given in brackets) was defined. Samples below the cutoff value meet the recommended expectation. OR >1 represent a higher chance to achieve a better hygiene status in regard to the respective test.
Table 3.
Results for risk factors with calculated OR, (CI), and P-values for calf environmental samples failing to meet expectations
| Expectations1 | Percent failing to meet expectations, % | Total no. | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Visibly clean (score 1) | |||||
| Absence of slope to the back wall | 19.2 | 167 | 7.28 | 2.12 to 25.00 | 0.01 |
| No use of adjacent pens or hutches | 19.2 | 167 | 3.93 | 1.12 to 13.77 | 0.05 |
| Shifting of hutches after use | 13.4 | 112 | 12.12 | 1.50 to 98.01 | 0.02 |
| Use of disinfectants | 19.2 | 167 | 12.38 | 4.67 to 32.81 | < 0.001 |
| Protein test (score 3) | |||||
| Absence of slope to the back wall | 29.3 | 167 | 5.56 | 1.58 to 19.63 | 0.01 |
| Absence of cracks in the ground | 29.3 | 167 | 2.11 | 1.02 to 4.35 | 0.04 |
| Use of disinfectants | 29.3 | 167 | 3.03 | 1.51 to 6.07 | 0.01 |
| Regular disinfection of pens after every calf | 29.3 | 167 | 2.48 | 1.12 to 5.17 | 0.02 |
| TVC (4.4 log10 cfu ∙ mL−1) | |||||
| Use of individual pens or hutches | 25.9 | 166 | 4.36 | 1.44 to 13.17 | 0.01 |
| Shifting of hutches after use | 25.9 | 166 | 2.34 | 1.05 to 5.20 | 0.04 |
| Use of disinfectants | 25.9 | 166 | 3.49 | 1.69 to 7.22 | < 0.001 |
| TCC below detection limit | |||||
| Smooth surfaces | 12.1 | 166 | 3.78 | 1.41 to 10.11 | 0.01 |
| E. coli below detection limit | |||||
| Absence of slope to the back wall | 4.2 | 166 | 5.96 | 1.01 to 35.11 | 0.05 |
| Smooth surfaces | 3.6 | 165 | 10.75 | 1.96 to 58.83 | 0.01 |
| Use of disinfectants | 4.4 | 158 | 11.67 | 1.35 to 100.98 | 0.03 |
| Absence of ESBL | |||||
| Absence of slope to the back wall | 4.8 | 166 | 9.93 | 2.02 to 48.87 | 0.01 |
| Absence of ESBL E. coli | |||||
| Absence of slope to the back wall | 3 | 166 | 25.33 | 3.69 to 173.76 | 0.001 |
1For the different tests used (in italic), a cutoff value (given in brackets) was defined. Samples below the cutoff value meet the recommended expectation. OR >1 represent a higher chance to achieve a better hygiene status in regard to the respective test.
Table 4.
Results for risk factors with calculated OR, CI, and P-values for sock samples from calf individual housing pens failing to meet expectations
| Expectations1 | Percent failing to meet expectations, % | Total no. | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Visibly clean (score 1) | |||||
| Rearing in hutches | 46.3 | 41 | 0.09 | 0.02 to 0.45 | 0.01 |
| TCC below detection limit | |||||
| Absence of cracks in the ground | 82.9 | 41 | 14.40 | 1.42 to 145.60 | 0.03 |
| Daily change in bedding material | 82.9 | 41 | 0.09 | 0.01 to 0.91 | 0.04 |
| E. coli below detection limit | |||||
| Absence of cracks in the ground | 80.5 | 41 | 6.90 | 1.12 to 42.61 | 0.04 |
| Absence of ESBL | |||||
| Absence of cracks in the ground | 34.2 | 41 | 6.46 | 1.15 to 36.45 | 0.04 |
| Absence of ESBL Acinetobacter spp. and ESBL Pseudomonas spp. | |||||
| Rearing in hutches | 17.1 | 41 | 0.06 | 0.01 to 0.61 | 0.02 |
1For the different tests used (in italic), a cutoff value (given in brackets) was defined. Samples below the cutoff value meet the recommended expectation. OR >1 represent a higher chance to achieve a better hygiene status in regard to the respective test.
Training effects
The results for the ATP, protein, and TVC measures from the first and second visits were compared, depending on the farm, to observe a possible training effect (Figure 4). In general, the training on individual farms showed limited time effects: only the levels of protein residues in feeding equipment and environment samples were significantly lower after training. Sock samples showed great variations in TVC and TCC between the first and second sampling without any consistent training effect but with time × farm interactions (Supplemental Figure 1). Almost all hygiene indicators differed among individual farms (Figures 4 and supplemental Figure 1). The proportions of samples above the detection limit for the TCC and E. coli in the feeding equipment, environment, and sock samples were lower after training (Supplemental Table 3).
Discussion
Healthy calves are the prerequisite for low antibiotic usage and economic success (Bendali et al., 1999; Walker et al., 2012). For that purpose, hygiene plays a key role in maintaining calves’ health (Lorenz et al., 2011; Relić et al., 2020). This study emphasizes a risk-oriented approach and the sampling of individual housing and feeding equipment after sanitation and preparation for restocking, which is a critical step in hygiene management. Maunsell and Donovan (2008) defined risk factors as those factors that reduced the ability of calves to resist diseases at a given level of pathogens and those that increased the level of pathogen exposure. In addition to appropriate colostrum management (Godden et al., 2009), hygiene management is an important risk factor, as it prevents the carryover of diarrhea-causing pathogens. Reported calf losses within the first 14 d on the participating farms ranged from 0% to 5.9%, and only 5 farms reported a diarrhea rate >5%, which seems low. Due to nonuniformly data recording in Germany, differentiation between reasons of calf mortality such as stillbirth or diseases is not possible. The mortality rates vary between 10% and 15% in Germany and between 6% and 14% during rearing in other countries (Sanftleben, 2010; Johnson et al., 2011; Tautenhahn, 2017). An underestimation may have occurred because the farms reported data on calf mortality and reported numbers were not proven by documents. In addition, these farms participated voluntarily. Svensson et al. (2003) mentioned that farms participating voluntarily in scientific studies might be primarily well-managed farms. Even the reported incidence rate of calf diarrhea probably displays not the actual situation but reflects the self-awareness of the farmers of management problems. A weak relationship was observed between visible cleanliness, which is generally used by farmers to assess the level of cleanliness after sanitation, and the results for ATP, protein, TVC, the absence of ESBL and additional E. coli load in the environmental and sock samples. This does not seem to be true for assessing the sanitation of feeding equipment based on visible cleanliness because it was not possible to draw conclusions about the presence of E. coli, ATP, or protein or the TVC. This shows the limits of visible perception and might be the reason for the very high bacterial loads on feeding equipment. The relationship between visual cleanliness and microbial contamination of surfaces and especially the value of visual inspection is controversially discussed in the scientific literature (Huneau-Salaün et al., 2010; Luyckx et al., 2015; Renaud et al., 2017). Nevertheless, even if bacterial or viral soiling is not necessarily visibly perceptible (Sherlock et al., 2009), visual inspection should always be carried out after cleaning and prior to disinfection, as perceptible soiling can interfere with disinfectants and hinder the success of disinfection. Independently of being switched to the side or resampled, it should be kept in mind, that sampling area only represented a random sample. This has a high variation in regard to results of hygienic testing intrinsically. This mirrors non-uniform soiling in animal husbandry. Therefore, conclusions about the hygienic status of a farm should be made based on sampling of various locations.
Feeding equipment
The cleaning process for feeding buckets and artificial teats varied among the farms in regard to the cleaning frequency, temperature of the water, and use of detergents. In a study from Austria, 97% of the investigated farms reported cleaning the feeding buckets after every use. Of these farms, only 25% used water with detergents while cleaning, whereas 25% cleaned with water alone (Klein-Jöbstl et al., 2014). This finding also corresponds to our results. The cleaning and disinfection of feeding buckets and teats are recommended after every use (Maunsell and Donovan, 2008), but we found that 4 farms (36%) cleaned the feeding buckets after every use. In other studies, cleaning was reported more often, with 83.3% (Lundborg et al. 2005) or 77% (colostrum buckets; Renaud et al. 2018). Furthermore, a stronger distinction between the terms cleaning and rinsing might be advisable. The feeding buckets showed the highest loads for all considered parameters (ATP, protein, TVC, TCC, and E. coli). Diarrhea-causing pathogens may multiply in feeding equipment and will be ingested or transmitted when feeding buckets are exchanged between the calves. Confusingly, the risk of ATP and TVC residues increases with the superficial cleaning of feeding buckets. This is probably because only rinsing with water without disassembling the teat only leads to an improved appearance, without improving the inner cleanliness. The ATP and TVC results were considerably better when the artificial teats were removed prior to cleaning. Unscrewing the teats from the feeding buckets during every cleaning is time-consuming, which is often cited as a limiting factor (Gosling, 2018). Commercial available feeding systems with quick locks for artificial teats may help to convince farmers to invest in better hygiene practices, but currently are rarely used in practice. The use of detergents to clean feeding equipment increased visible cleanliness and is already mentioned as a protective measure to reduce the prevalence of C. parvum (Trotz-Williams et al., 2008). Barry et al. (2019b) found no associations between feeding equipment hygiene and mortality rate. In their study, hygiene was assessed physically and by protein swabs. Both methods predominately reflect adhering dirt and feed residues and do not necessarily represent the bacterial burden. Aust et al. (2013) showed a considerable recontamination of pasteurized milk caused by irregular cleaning of feeding equipment. Bruning-Fann and Kaneene (1992) suspected a connection between calf mortality rates and the sanitation of feeding buckets. To avoid diarrhea or septicemia in newborn calves, proper hygiene of feeding equipment is crucial (Godden, 2008), and more attention should be paid to this topic.
Housing equipment
Individual housing is associated with lower risks of disease transmission and calf mortality (Svensson et al., 2003; Hotchkiss et al., 2015) and a lower burden of pathogenic factors (Barrington et al., 2002). The OR for visible cleanliness and TVC below the cutoff value increased when hutches were shifted between uses. The movement of calf pens is recommended by Hotchkiss et al. (2015) to reduce the enrichment of bacteria in the environment. The type of flooring seems to have an impact on C. parvum prevalence (Castro-Hermida et al., 2002, Trotz-Williams et al., 2008). We assume that this effect is transferable to other pathogens since concrete and other smooth surfaces are easier to clean and reduce the survival of pathogenic residues. This is in line with our results, showing lower ATP and protein residues and bacterial loads and an increased odds ratio for the absence of E. coli on smooth surfaces. The absence of cracks in the ground leads to superior removal of TCC and E. coli and the absence of ESBL in sock samples. To avoid the accumulation of soiling and possible deposits of pathogens, farmers should take care of smooth surfaces and fix undesirable cracks. Direct exposure to sunlight is mentioned as a factor that decreases pathogens (Barrington et al., 2002), but we did not observe an association between direct sunlight and microbiological parameters. Daily cleaning of pens resulted in an 87% lower risk of infection with C. parvum in calves compared with a monthly cleaning interval (Castro-Hermida et al., 2002). Some tested farms in this study cleaned the pens after every calf, which is equivalent to an average interval of 14 d. Soaking with detergents resulted in significantly reduced counts of TVC and Enterobacteriaceae on metal and concrete surfaces and is recommended in livestock housing (Hancox et al., 2013). In our study, only a minority of farms used detergents to clean pens, and this was not associated with reductions in the levels of hygiene indicators. Bartels et al. (2010) found that consistent cleaning of calf housing areas was a protective factor against infections with coronavirus, which emphasizes the importance of proper sanitation. For the within-farm prevalence of C. parvum and cleaning of calf housing areas, no significant association was found (Trotz-Williams et al., 2008). Disinfection of the pens was part of the routine on the farms in this study. Odd ratios for visible cleanliness and meeting expectations on protein tests, for the TVC and for the TCC increased with the use of disinfectants after every calf. Disinfection led to significant reductions in the TVC and Enterobacteriaceae load on concrete surfaces in livestock housing (Hancox et al. 2013). In other studies, no associations between hygiene in calf pens and the occurrence of diarrhea were observed (Lundborg et al., 2005; Klein-Jöbstl et al., 2015).
Training effect and farm-specific practices
Farmers were informed about hygiene weaknesses after the first visit and trained for better performance. In a personal conversation, it was found that most farmers were well aware of their weaknesses in cleaning and disinfection before the study. However, this awareness did not guarantee conceptual implementation and understanding of the consequences, as has been observed before (Lüdtke, 2004; Boersema, 2008). Additionally, the farmers reported a rather low incidence of mortality compared with Germany average, which might be another reason for their low motivation for improvement. This might explain why information given in the training was only acted on at a few farms (based on the interaction) and translated into improvements in sanitation, contrary to what has been seen in pig fattening (Heinemann et al., 2020). Veterinary consultants should probably regularly draw farmers´ attention to the importance of hygiene in calf rearing and frequently point out weak points to achieve long-term changes. Differences among the farms also indicate that practical measures that are easy to implement are still missing. Despite an extensive literature review, we could not find studies that are directly comparable to our study, as they have primarily focused on risk assessments in occupied pens, with hygiene as an additional factor. Since all-in all-out practice is well established in newborn dairy calf rearing, precise recommendations for proper sanitation or self-monitoring systems with practical hygiene plans, as is common for pig and poultry, are still rare. Lundborg et al. (2005) mentioned that scientific data dealing with calf health and the effects of management and feeding procedures are surprisingly sparse, and Barry et al. (2019b) stated that hygiene practices in newborn calf rearing show substantial potential for improvement. It should be noted that calf mortality can be caused by multiple factors, with calf diarrhea being the highest risk factor (Torsein et al., 2011; Johnson et al., 2017). Therefore, it is hardly possible to identify a specific factor since the various factors under field conditions affect each other (Bruning-Fann and Kaneene, 1992). In future investigations, the number of samples per farm can be reduced with the focus on frequently occurring weak points, a greater number of farms should be visited, and fecal samples should be obtained from rehoused calves and analyzed for diarrhea-causing pathogens. A great opportunity to reduce the number of samples per farm would be to rinse the buckets with a saline solution beforehand, which would then serve as an analysis matrix, similar to Renaud et al. (2017). In the long term, we can imagine that verified risk factors associated with hygiene could be part of a Hazard Analysis and Critical Control Points concept for calf rearing, as suggested by Boersema et al. (2008) and supported by supervising veterinarians in the prevention of calf diseases. In conclusion, the feeding equipment showed the highest potential for improvements in hygiene management. The protein test has no added value compared with the ATP test and microbiological tests and can therefore be omitted. Training in hygiene management has no further effect if the calves’ health level is already high.
Supplementary Material
Acknowledgments
We gratefully acknowledge the voluntary participation of the dairy farmers and their support of the study and thank Simone M. Schmid, Miriam Guse, Nuala Connolly for their assistance help in preparing the farm visits and the microbiological examination in the laboratory. This project was supported by funds from the Government’s Special Purpose Fund held at Landwirtschaftliche Rentenbank (project no. 20870245).
Glossary
Abbreviations
- ATP
adenosine triphosphate
- cfu
colony-forming unit
- CI
confidence intervall
- ESBL
extended-spectrum β-lactamase-producing bacteria
- MRSA
methicillin-resistant Staphylococcus aureus
- OR
odds ratio
- RLU
relative light unit
- TCC
total coliform count
- TVC
aerobic total viable count
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Alfa, M. J., I. Fatima, and N. Olson. . 2013. Validation of adenosine triphosphate to audit manual cleaning of flexible endoscope channels. Am. J. Infect. Control 41:245–248. doi: 10.1016/j.ajic.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Aust, V., K. Knappstein, H. J. Kunz, H. Kaspar, J. Wallmann, and M. Kaske. . 2013. Feeding untreated and pasteurized waste milk and bulk milk to calves: effects on calf performance, health status and antibiotic resistance of faecal bacteria. J. Anim. Physiol. Anim. Nutr. (Berl.). 97:1091–1103. doi: 10.1111/jpn.12019. [DOI] [PubMed] [Google Scholar]
- Barrington, G. M., J. M. Gay, and J. F. Evermann. . 2002. Biosecurity for neonatal gastrointestinal diseases. Vet. Clin. North Am. Food Anim. Pract. 18:7–34. doi: 10.1016/s0749-0720(02)00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, J., E. A. M. Bokkers, D. P. Berry, I. J. M. de Boer, J. McClure, and E. Kennedy. . 2019b. Associations between colostrum management, passive immunity, calf-related hygiene practices, and rates of mortality in preweaning dairy calves. J. Dairy Sci. 102:10266–10276. doi: 10.3168/jds.2019-16815. [DOI] [PubMed] [Google Scholar]
- Barry, J., E. Kennedy, R. Sayers, I. J. M. de Boer, and E. A. M. Bokkers. . 2019a. Development of a welfare assessment protocol for dairy calves from birth through weaning. Anim. Welf. 28:331–344. 10.7120/09627286.28.3.331 [DOI] [Google Scholar]
- Bartels, C. J., M. Holzhauer, R. Jorritsma, W. A. Swart, and T. J. Lam. . 2010. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 93:162–169. doi: 10.1016/j.prevetmed.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendali, F., H. Bichet, F. Schelcher, and M. Sanaa. . 1999. Pattern of diarrhoea in newborn beef calves in south-west France. Vet. Res. 30:61–74. https://pubmed.ncbi.nlm.nih.gov/10081113/ [PubMed] [Google Scholar]
- Berghaus, R. D., S. G. Thayer, B. F. Law, R. M. Mild, C. L. Hofacre, and R. S. Singer. . 2013. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Appl. Environ. Microbiol. 79:4106–4114. doi: 10.1128/AEM.00836-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersema, J., J. Noordhuizen, A. Vieira, J. Lievaart, and W. Baumgartner. . 2008. Imbedding HACCP principles in dairy herd health and production management: case report on calf rearing. Ir. Vet. J. 61:594–602. doi: 10.1186/2046-0481-61-9-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm, R 1998. Disinfection and hygiene in the veterinary field and disinfection of animal houses and transport vehicles. Int. Biodeter. Biodegr. 41:217–224. 10.1016/S0964-8305(98)00030-4 [DOI] [Google Scholar]
- Bruning-Fann, C., and J. B. Kaneene. . 1992. Environmental and management risk factors associated with morbidity and mortality in perinatal and pre-weaning calves: a review from an epidemiological perspective. Vet. Bull. 62:399–413. https://www.researchgate.net/publication/259382731_Environmental_and_management_risk_factors_associated_with_morbidity_and_mortality_in_perinatal_and_pre-weaning_calves_A_review_from_an_epidemiological_perspective [Google Scholar]
- Casini, B., B. Tuvo, M. Totaro, F. Aquino, A. Baggiani, and G. Privitera. . 2018. Evaluation of the cleaning procedure efficacy in prevention of nosocomial infections in healthcare facilities using cultural method associated with high sensitivity luminometer for ATP detection. Pathogens 7:1–9. 10.3390/pathogens7030071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hermida, J. A., Y. A. González-Losada, and E. Ares-Mazás. . 2002. Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galicia (NW Spain). Vet. Parasitol. 106:1–10. doi: 10.1016/s0304-4017(02)00036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemensson Lindell, I., A. Lundh, K. Svennersten Sjaunja, and M. Cederholm. . 2018. Adenosine triphosphate bioluminescence for hygiene testing of rubber liners and tubes on dairy farms. J. Dairy Sci. 101:2438–2447. 10.3168/jds.2017-13466 [DOI] [PubMed] [Google Scholar]
- Commission Regulation 200/2010 of 10 March 2010 implementing Regulation (EC) No. 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of Salmonella serotpyes in adult breeding flocks of Gallus gallus. Official Journal of the European Union, L 61/1; https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R0200 [Google Scholar]
- Council Directive 2008/119/EC of 18 December 2008 laying down minimum standards for the protection of calves. Official Journal of the European Union, L 10/7 https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008L0119
- Godden, S 2008. Colostrum management for dairy calves. Vet. Clin. North Am. Food Anim. Pract. 24:19–39. doi: 10.1016/j.cvfa.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden, S. M., D. M. Haines, K. Konkol, and J. Peterson. . 2009. Improving passive transfer of immunoglobulins in calves. II: Interaction between feeding method and volume of colostrum fed. J. Dairy Sci. 92:1758–1764. 10.3168/jds.2008-1847 [DOI] [PubMed] [Google Scholar]
- Gosling, R. J 2018. A review of cleaning and disinfection studies in farming environments. Livestock. 23:232–237. 10.12968/live.2018.23.5.232 [DOI] [Google Scholar]
- de Graaf, D. C., E. Vanopdenbosch, L. M. Ortega-Mora, H. Abbassi, and J. E. Peeters. . 1999. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 29:1269–1287. doi: 10.1016/s0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancox, L. R., M. Le Bon, C. E. R. Dodd, and K. H. Mellits. . 2013. Inclusion of detergents in a cleaning regime and effect on microbial load in livestock housing. Vet. Rec. 2013:1–4. 10.1136/vr.101392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawronskyi, J.-M., and J. Holah. . 1997. ATP: a universal hygiene monitor. Trends Food Sci. Technol. 8:79–84. doi: 10.1016/S0924-2244(97)01009-1 [DOI] [Google Scholar]
- Heinemann, C., I. Meyer, F. T. Bögel, S. M. Schmid, J. J. Hayer, and J. Steinhoff-Wagner. . 2020. Individual training for farmers based on results from protein and ATP rapid tests and microbiological conventional cultural methods improves hygiene in pig fattening farms. J. Anim. Sci. 98:1–10. 10.1093/jas/skz389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss, E., S. Thomson, B. Wells, E. Innes, and F. Katzer. . 2015. Update on the role of cryptosporidiosis in calf diarrhea. Livestock 20:2–6. 10.12968/live.2015.20.6.316 [DOI] [Google Scholar]
- Huneau-Salaün, A., V. Michel, L. Balaine, I. Petetin, F. Eono, F. Ecobichon, and S. L. Bouquin. . 2010. Evaluation of common cleaning and disinfection programmes in battery cage and on-floor layer houses in France. Br. Poult. Sci. 51:204–212. doi: 10.1080/00071661003745794. [DOI] [PubMed] [Google Scholar]
- Johnson, K., C. C. Burn, and D. C. Wathes. . 2011. Rates and risk factors for contagious disease and mortality in young dairy heifers. CAB Rev. 6:1–10. 10.1079/PAVSNNR20116059 [DOI] [Google Scholar]
- Johnson, K. F., N. Chancellor, C. C. Burn, and D. C. Wathes. . 2017. Prospective cohort study to assess rates of contagious disease in pre-weaned UK dairy heifers: management practices, passive transfer of immunity and associated calf health. Vet. Rec. Open 4:e000226. doi: 10.1136/vetreco-2017-000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Jöbstl, D., T. Arnholdt, F. Sturmlechner, M. Iwersen, and M. Drillich. . 2015. Results of an online questionnaire to survey calf management practices on dairy cattle breeding farms in Austria and to estimate differences in disease incidences depending on farm structure and management practices. Acta Vet. Scand. 57:44. doi: 10.1186/s13028-015-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Jöbstl, D., M. Iwersen, and M. Drillich. . 2014. Farm characteristics and calf management practices on dairy farms with and without diarrhea: a case-control study to investigate risk factors for calf diarrhea. J. Dairy Sci. 97:5110–5119. doi: 10.3168/jds.2013-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, I., J. Fagan, and S. J. More. . 2011. Calf health from birth to weaning. II. Management of diarrhoea in pre-weaned calves. Ir. Vet. J. 64:9 10.1186/2046-0481-64-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdtke, K 2004. Erhebungen zum Umgang der Landwirte mit ihren Rindern [PhD thesis]. Germany: Klinik für Wiederkäuer, tierärztliche Fakultät, Ludwig-Maximilians-Universität München. [Google Scholar]
- Lundborg, G. K., E. C. Svensson, and P. A. Oltenacu. . 2005. Herd-level risk factors for infectious diseases in Swedish dairy calves aged 0–90 days. Prev. Vet. Med. 68:123–143. 10.1016/j.prevetmed.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Luyckx, K., J. Dewulf, S. Van Weyenberg, L. Herman, J. Zoons, E. Vervaet, M. Heyndrickx, and K. De Reu. . 2015. Comparison of sampling procedures and microbiological and non-microbiological parameters to evaluate cleaning and disinfection in broiler houses. Poult. Sci. 94:740–749. doi: 10.3382/ps/pev019. [DOI] [PubMed] [Google Scholar]
- Mannion, C., F. C. Leonard, P. B. Lynch, and J. Egan. . 2007. Efficacy of cleaning and disinfection on pig farms in Ireland. Vet. Rec. 161:371–375. doi: 10.1136/vr.161.11.371. [DOI] [PubMed] [Google Scholar]
- Marcé, C., R. Guatteo, N. Bareille, and C. Fourichon. . 2010. Dairy calf housing systems across Europe and risk for calf infectious diseases. Animal 4:1588–1596. doi: 10.1017/S1751731110000650. [DOI] [PubMed] [Google Scholar]
- Maunsell, F., and G. A. Donovan. . 2008. Biosecurity and Risk Management for Dairy Replacements. Vet. Clin. North Am. Food Anim. Pract. 24:155–190. 10.1016/j.cva.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Doblies, D., J. J. Carrique-Mas, A. R. Sayers, and R. H. Davies. . 2010. A comparison of the efficacy of different disinfection methods in eliminating Salmonella contamination from turkey houses. J. Appl. Microbiol. 109:471–479. doi: 10.1111/j.1365-2672.2010.04667.x. [DOI] [PubMed] [Google Scholar]
- Osimani, A., C. Garofalo, F. Clementi, S. Tavoletti, and L. Aquilanti. . 2014. Bioluminescence ATP monitoring for the routine assessment of food contact surface cleanliness in a university canteen. Int. J. Environ. Res. Public Health 11:10824–10837. doi: 10.3390/ijerph111010824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz, P., and Ö. Ö. Arun. . 2019. Evaluating the performance of ATP bioluminescence method by comparison with classical cultural method. Food Health 5:77–82. 10.3153/FH19008 [DOI] [Google Scholar]
- Relić, R., J. Starič, and J. Ježek. . 2020. Management practices that influence the welfare of calves on small family farms. J. Dairy Res. 87: 93–98. 10.1017/S0022029920000539 [DOI] [PubMed] [Google Scholar]
- Renaud, D. L., D. F. Kelton, S. J. LeBlanc, D. B. Haley, A. B. Jalbert, and T. F. Duffield. . 2017. Validation of commercial luminometry swabs for total bacteria and coliform counts in colostrum-feeding equipment. J. Dairy Sci. 100:9459–9465. doi: 10.3168/jds.2017-13228. [DOI] [PubMed] [Google Scholar]
- Renaud, D. L., D. F. Kelton, S. J. LeBlanc, D. B. Haley, and T. F. Duffield. . 2018. Calf management risk factors on dairy farms associated with male calf mortality on veal calf farms. J. Dairy Sci. 101:1785–1794. 10.3168/jds.2017-13578 [DOI] [PubMed] [Google Scholar]
- Sanftleben, P 2010. Kälberhaltung – prima Klima. 28. Wissenschaftliche Fachtagung für Landwirte und Tierärzte. 27. Oktober 2010. – [accessed March 11, 2020] https://www.mrv-eg.de/downloads/vortraege/ft28_2.pdf.
- Sherlock, O., N. O’Connell, E. Creamer, and H. Humphreys. . 2009. Is it really clean? An evaluation of the efficacy of four methods for determining hospital cleanliness. J. Hosp. Infect. 72:140–146. doi: 10.1016/j.jhin.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Skov, M. N., B. Carstensen, N. Tornøe, and M. Madsen. . 1999. Evaluation of sampling methods for the detection of Salmonella in broiler flocks. J. Appl. Microbiol. 86:695–700. doi: 10.1046/j.1365-2672.1999.00715.x. [DOI] [PubMed] [Google Scholar]
- Statistisches Bundesamt (Destatis) 2019. German statistical annual book 2019 (Statistisches Jahrbuch 2019). https://www.destatis.de/DE/Themen/Querschnitt/Jahrbuch/statistisches-jahrbuch-2019-dl.pdf?__blob=publicationFile
- Svensson, C., A. Linder, and S. O. Olsson. . 2006. Mortality in Swedish dairy calves and replacement heifers. J. Dairy Sci. 89:4769–4777. doi: 10.3168/jds.S0022-0302(06)72526-7. [DOI] [PubMed] [Google Scholar]
- Svensson, C., K. Lundborg, U. Emanuelson, and S. O. Olsson. . 2003. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev. Vet. Med. 58:179–197. doi: 10.1016/s0167-5877(03)00046-1. [DOI] [PubMed] [Google Scholar]
- Tautenhahn, A 2017. Risk factors associated with calf mortality and growth of calves in dairy farms located in northeastern Germany [PhD thesis]. Germany: Klinik für Klauentiere, Fachbereich Veterinärmedizin, Freie Universität Berlin; 10.17169/refubium-7653 [DOI] [Google Scholar]
- Torsein, M., A. Lindberg, C. H. Sandgren, K. P. Waller, M. Törnquist, and C. Svensson. . 2011. Risk factors for calf mortality in large Swedish dairy herds. Prev. Vet. Med. 99:136–147. doi: 10.1016/j.prevetmed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams, L. A., S. W. Martin, K. E. Leslie, T. Duffield, D. V. Nydam, and A. S. Peregrine. . 2008. Association between management practices and within-herd prevalence of Cryptosporidium parvum shedding on dairy farms in southern Ontario. Prev. Vet. Med. 83:11–23. doi: 10.1016/j.prevetmed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar, E., W. C. Hazeleger, M. Koopmans, M. H. Zwietering, R. R. Beumer, and E. Duizer. . 2012. Residual viral and bacterial contamination of surfaces after cleaning and disinfection. Appl. Environ. Microbiol. 78:7769–7775. doi: 10.1128/AEM.02144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar, M. J., J. L. Rodríguez-Otero, F. J. Diéguez, M. L. Sanjuán, and E. Yus. . 2008. Application of ATP bioluminescence for evaluation of surface cleanliness of milking equipment. Int. J. Food Microbiol. 125:357–361. doi: 10.1016/j.ijfoodmicro.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Walker, W. L., W. B. Epperson, T. E. Wittum, L. K. Lord, P. J. Rajala-Schultz, and J. Lakritz. . 2012. Characteristics of dairy calf ranches: morbidity, mortality, antibiotic use practices, and biosecurity and biocontainment practices. J. Dairy Sci. 95:2204–2214. doi: 10.3168/jds.2011-4727. [DOI] [PubMed] [Google Scholar]
- Weaver, D. M., J. W. Tyler, D. C. VanMetre, D. E. Hostetler, and G. M. Barrington. . 2000. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 14:569–577. doi:. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.