Abstract
Transformation of chronic lymphocytic leukemia (CLL) to an aggressive lymphoma, so-called Richter syndrome (RS), usually includes diffuse large B-cell lymphoma (DLBCL) and classic Hodgkin lymphoma (CHL). The transformation can be clonally related to the underlying CLL, and is often Epstein-Barr virus (EBV)-associated. Here we report an 86-year-old female with a newly identified CLL-like monoclonal B-lymphocytosis (MBL) who developed diffuse lymphadenopathy. Biopsy of the left axillary lymph node showed EBV-positive large B-cell lymphoma with morphologic and immunophenotypic features intermediate between DLBCL and CHL, so-called gray zone lymphoma. Comprehensive immunophenotypic, cytogenetics and molecular studies demonstrate a clonal relatedness that suggests a transformation from MBL to EBV+ gray zone lymphoma.
Keywords: Chronic lymphocytic leukemia, monoclonal B-lymphocytosis, Richter syndrome/transformation, EBV+ diffuse large B-cell lymphoma, classic Hodgkin lymphoma, gray zone lymphoma
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (simply CLL) is a low-grade small B-cell lymphoproliferative disorder involving peripheral blood, bone marrow, lymph nodes and/or other lymphoid tissues1. Approximately 10% of CLL patients transform to aggressive B-cell lymphoma, also called Richter syndrome (RS). RS usually includes diffuse large B-cell lymphoma (DLBCL) and rarely classic Hodgkin lymphoma (CHL)1,2. DLBCL variant of RS is often clonally related to the underlying CLL, is more commonly IGHV-unmutated and is associated with a poorer outcome compared to the clonally unrelated transformations, which are usually IGHV-mutated with a similar prognosis to that of de novo DLBCL2-5. CHL-variant transformation can be clonally related or unrelated to the underlying CLL. Clonal relatedness is associated with IGHV mutation but not EBV status or type 1 versus type 2 morphology1,4,6,7. Although only type 2 is considered bona fide CHL transformation1, patients with type 1 morphology can progress to type 2 and both had similar poor outcome7. Here, we present a rare case with concurrent CLL-like monoclonal B-lymphocytosis (MBL) and a clonally related, EBV-positive large B-cell lymphoma with morphologic and immunophenotypic features intermediate between DLBCL and CHL.
Clinical Presentation
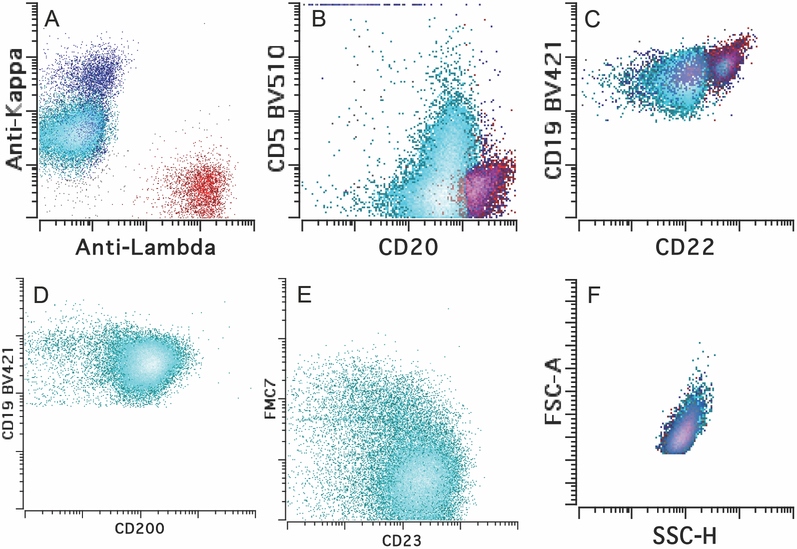
An 85-year-old female presented with diffuse lymphadenopathy. She had a remote history (18 years ago) of invasive ductal carcinoma of the breast that was treated with partial mastectomy, radiation and combined chemotherapy of docetaxel and cyclophosphamide, followed by hormonal therapy. There is no known history of immunodeficiency. PET/CT scan confirmed diffuse lymphadenopathy (including left axillary, mediastinal, hilar, bilateral iliac and left superficial inguinal lymph nodes) and increased activities of nodal and extranodal sites (SUV up to 25.3 at left superficial inguinal lymph node; SUV 3.9 at left axilla) including osseous sties (SUV up to 26.1 at L3 vertebra). Metastatic breast carcinoma was clinically suspected. Her complete blood count showed white blood cell count of 5.1 K/μL and absolute lymphocytes of 1.4 K/μL, hemoglobin of 13.1 g/dL and platelets of 202 K/μL. The concurrent peripheral blood flow cytometry detected a small clonal B-cell population with CLL/SLL-like immunophenotype, with abnormal expression of CD19 (dim), CD20 (dim), CD22 (dim), CD200 (bright), co-expression of CD5 (partial) and CD23, and kappa surface light chain restriction and no CD10 and FMC7 expression (Fig. 1). The absolute clonal B-cell count was 0.28 K/μL, far below the cutoff for a diagnosis of CLL. An excisional biopsy of the left axillary lymph node was performed. Histologic examination, together with extensive immunophenotypic, cytogenetics and molecular characterization, established the diagnosis of an EBV+ large B-cell lymphoma with intermediate features between DLBCL and CHL, likely transformed from the underlying MBL. There was no morphologic evidence of CLL/SLL on the nodal biopsy. Consistent with the diagnosis, serum EBV viral titer was markedly increased to 104,504 IU/mL. Serology studies were positive for EBV VCA IgG and EBV nuclear antibody and negative for EBV VCA IgM, indicative of a reactivation of EBV infection. The patient underwent mini-R-CHOP therapy with resolution of lymphadenopathy and no imaging evidence of residual disease currently.
Figure 1.
Immunophenotype of the MBL in Peripheral blood. A-E. The clonal B cells (in cyan) were kappa light chain restricted (dim) and positive for CD19 (dim), CD20 (dim), CD22 (dim), CD5 (partial), CD23 and CD200, while negative for CD10 and FMC-7. Red, normal lambda-expressing B cells; blue, normal kappa-expressing B cells. F. The clonal B cells had similar forward scatter and side scatter to the normal B cells, indicative of small cell size.
Pathologic Findings
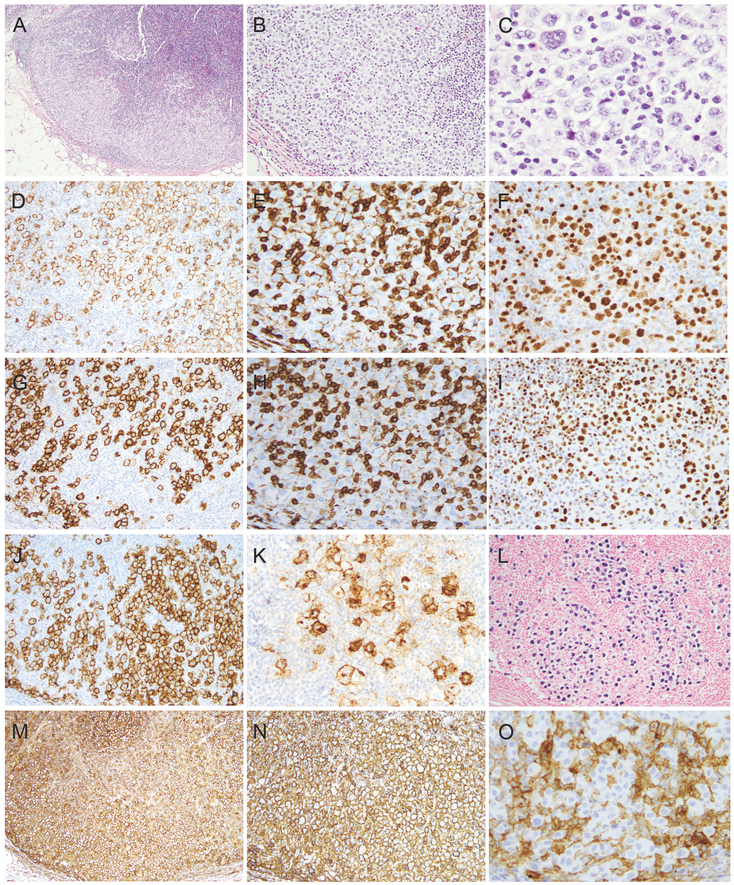
The excisional biopsy revealed largely preserved nodal architecture with partial involvement by sheets of large atypical cells. The larger atypical cells were pleomorphic with abundant pale cytoplasm, bi/multinucleated nuclei with vesicular chromatin and some with prominent eosinophilic nucleoli reminiscent of Hodgkin/Reed-Sternberg (HRS) cells (Fig 2A-C). There were no increased plasma cells, eosinophils, neutrophils and histiocytes seen in the background. There was no morphologic evidence of a low-grade B-cell lymphoma or metastatic carcinoma.
Figure 2.
Morphologic and immunophenotypic features of the lymphoma involving left axillary lymph node. A-C. H&E morphology (A, x40; B, x100; C, x400). D-K. Immunohistochemical stains (D, CD20; E, CD3; F, PAX-5; G, CD23; H, CD5; I, LEF1; J, CD30; K, CD15). L. EBER in situ hybridization. M-O. Immune check point antigen expression (M, PDL-1; N, HLA-1; O, B2M).
Immunohistochemical studies showed that the large atypical cells were diffusely positive for CD20 (Figure 2D) and CD79a (not shown). PAX-5 was variably positive (Fig 2F). Interestingly, these cells strongly expressed CD23 and LEF1 but not CD3 or CD5 (Figure 2E, 2G-I). Furthermore, they expressed CD30 and CD15 (Figure 2J and K). EBER (EBV in situ RNA hybridization) (Figure 2L) and EBV-LMP1 (not shown) were diffusely positive in these cells. These cells were also positive for MUM1, C-MYC (subset, dim), TP53 (subset), but were negative for cyclin D1 (not shown). Ki-67 proliferation index was approximately 70% (not shown). There was no up-regulation of PD-L1, PD-L2, or loss of HLA-1 or B2M expression (Figure 2M-O). A component of CLL/SLL was not identified by immunohistochemical studies.
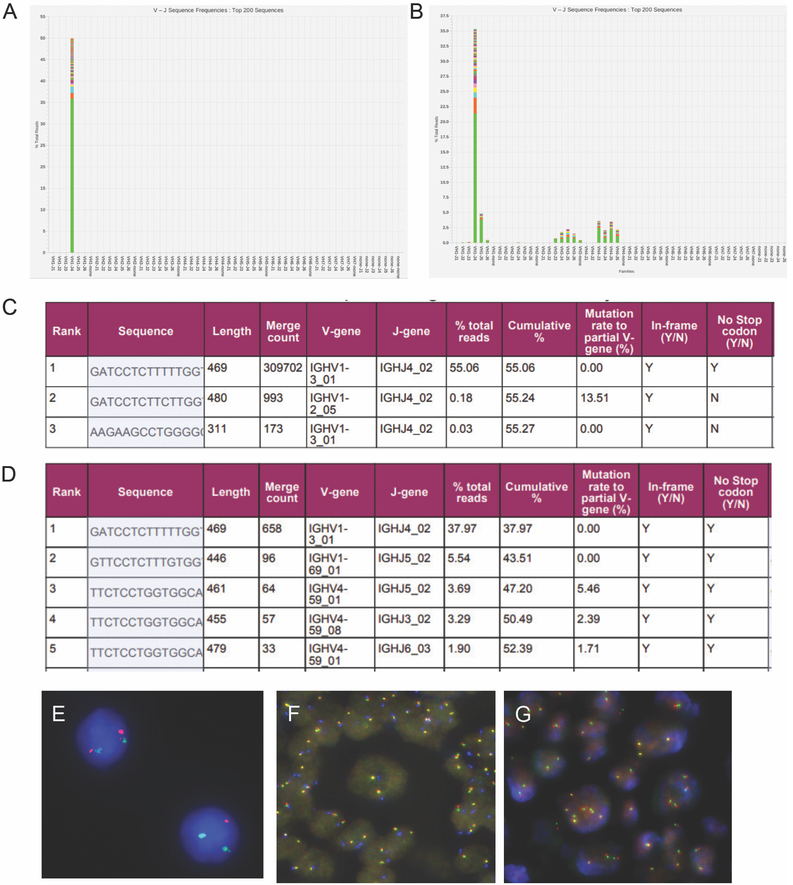
In summary, the neoplastic cells exhibited overlapping morphologic and immunophenotypic features between EBV + DLBCL and CHL. In light of the peripheral blood flow cytometry findings of CLL-like MBL and the expression of CD23 and LEF1 by the large atypical cells, a transformation from the patient’s underlying MBL was suspected. To further characterize the relationship of neoplastic clones of lymph node to the underlying MBL, clonal IGH rearrangement studies using a next generation sequencing (NGS)-based assay (Lymphotrack, Invivoscribe, San Diego, CA) were performed. The results from the peripheral blood showed a single clonal sequence with a 469 bp PCR product, and IGHV-J usage of V1-3 J4, comprising 55% of all IGH sequencing reads. It was unmutated (0.0% mutation rate) and is predicted to be a productive transcript (Figure 3A and C). The identical V1-3 J4 clone was also found in the large B-cell lymphoma using DNA extracted from paraffin sections macrodissected to enrich for areas with highest concentration of large cells (comprising approximately 40% of all IGH sequencing reads, Figure 3B and D), in two replicates, confirming the clonal relatedness between these two processes. FISH studies of the lymph node biopsy detected an interstitial deletion of 13q in 8% of cells analyzed (Figure 3E), a common aberration seen in CLL or CLL-like MBL; there was no evidence of deletion of MYB (6q23), ATM (11q22), or TP53 (17p13), gain of chromosome 12 or IGH translocation. However, gain of one to two intact MYC (8q24) copies were detected in 72% of cells, but no MYC gene rearrangement was detected (not shown). FISH analysis didn’t find any PDL1/PDL2 (9p24) amplification and translocation or CIITA (16p13) translocation, but showed one to two extra copies of PDL1/PDL2 (9p24) and the centromere of chromosome 9 (Figure 3F) and CIITA (Figure 3G), indicative of a triploid or tetraploid clone with gain of these chromosomes in the large HRS like cells. Single nucleotide polymorphism (SNP) array studies showed normal balanced genomic pattern, likely due to the low tumor content below the sensitivity of the array test.
Figure 3. NGS and FISH studies.
A-D. NGS (Lymphotrack assay) studies demonstrate clonal relatedness (A and C, peripheral blood; B and D, lymph node). E-G. FISH studies on lymph node (E, 13q probe; F, PDL1/2 probes; G, CIITA probe). FISH probes: 13q14 (labeled with orange), 13q34 (labeled with green, both from Abbott Molecular, Des Plaines, IL), PDL1 and PDL2 (labeled with orange and green, respectively, from Empire Genomics, Buffalo, NY), CEP9 (labeled with aqua, from Abbott Molecular), CIITA (5’ and 3’ probes, labeled with orange and green, respectively, from Empire Genomics).
DISCUSSION
This is a rare case of EBV+ large B-cell lymphoma with intermediate features between DLBCL and CHL that is clonally related to the underlying CLL-like MBL. Aggressive B-cell lymphoma of RS can be clonally related or unrelated to the underlying CLL. The cumulative evidence of clonal relatedness between these two processes in our case includes: similar immunophenotype (CD23 and LEF1), and an identical clonal IGH rearrangement sequence. Although PCR studies with IGH primers and capillary or gel electrophoresis separation of product sizes have been historically utilized to compare the clonal relatedness, NGS-based studies show unparalleled advantage by identifying the exact clonal IGH sequences, increasing confidence in the clonal relationship between two samples, and providing information on the IGHV mutation status. The data from our study are unable to distinguish linear versus branching clonal evolution between MBL and gray zone lymphoma.
The classification of this aggressive B-cell lymphoma is challenging. The neoplastic cells have cytologic and immunophenotypic feature of HRS cells, suggestive of a CHL transformation. Both type 1 and type 2 morphology have been described in the setting of CLL to CHL transformation4,7-10. Type 1 pattern shows scattered HRS cells in a background of CLL while type 2 pattern shows HRS cells in a mixed inflammatory background1. Currently, only the type 2 pattern is considered as bona fide CHL transformation1. Type 1 can progress to type 2 and both type 1 and type 2 had similarly poor outcomes7. Therefore, type 1 is likely an early phase of type 2. Both type 1 and type 2 can be clonally related to the underlying CLL and both can be EBV positive6,7,11-16. Nearly all the clonally related HRS cells occurred in IGHV mutated CLL4,7. However, there is no obvious inflammatory background in our case. In addition, there is no morphologically identifiable CLL in the tissue. Therefore, it does not fit into either the type 1 or type 2 pattern. The unmutated IGHV sequence and the presence of neoplastic cells in the cohesive sheet with a relatively intact B-cell program also argue against CHL. Therefore, a diagnosis of EBV positive gray zone lymphoma is favored.
EBV+ gray zone lymphoma has been thought to be rare. Nicolae et al included a few cases of gray zone lymphoma pattern in EBV+ large B-cell lymphoma of the young17. Retrospectively, these cases are probably best classified as EBV+ gray zone lymphoma. A recent study from LYSA group showed up to 20% of gray zone lymphomas are EBV positive18. The discrepancies might be due to different patient populations. As shown by Elsayed et al19, synonymous to the gray zone lymphoma, which represents the continuous spectrum between DLBCL and CHL, EBV+ gray zone lymphoma may very well represent the continuous spectrum between EBV+ DLBCL and prototypic EBV+ CHL. Taken together, EBV+ large B-cell lymphomas with gray zone pattern do exist. Whether we should classify them as EBV positive large B-cell lymphoma versus gray zone lymphoma needs further studies.
Gains/amplifications in 9q24.1 (JAK2/PDL2) (55% cases) has been reported in gray zone lymphoma, similar to CHL and primary mediastinal B-cell lymphoma (PMBL)20. Correspondingly, PD-L1 and PD-L2 expression are highly expressed and PD-L1/2 inhibitors are highly effective in these lymphomas21,22. Although PD-L1 expression is also significantly upregulated in EBV+ related malignancies including DLBCL23, there are usually no gains/amplifications of PD-L1/224. In this regard, gains of PD-L1/2 favor a diagnosis of gray zone lymphoma in our case.
Rearrangements involving CIITA (16p13.13), which might facilitate escape from immune surveillance of the tumor cells, are detected in 27% gray zone lymphoma, a frequency between that observed for CHL (15%) and PMBL (38%)20. In contrast, this recurrent rearrangement is only present in 4% DLBCL25. Furthermore, EBV+ DLBCL has no characteristic abnormalities, and they show even fewer chromosomal alterations than their EBV-negative counterparts, suggesting predominantly an EBV driven pathogenesis26. EBV infection causes CIITA gene repression27. CIITA rearrangement status in EBV+ DLBCL and EBV+ gray zone lymphoma remains to be studied even although our case had no evidence of CIITA rearrangement.
Low-count MBL as seen in our case generally has an indolent clinical course1, and a transformation of MBL to gray zone lymphoma has never been reported before. However, since the patient only had one lymph node biopsied, we cannot completely exclude the possibility that other lymph nodes may show overt CLL/SLL involvement. The EBV+ gray zone lymphoma in our patient responded to the mini-CHOP regimen and she achieved complete remission, suggesting that EBV+ gray zone lymphoma might not be as aggressive as EBV- gray zone lymphoma.
CONCLUSION
We report an exceedingly rare EBV associated gray zone lymphoma that is clonally related to the underlying CLL-like MBL. A comprehensive approach including morphologic, immunophenotypic, cytogenetics and molecular studies is required for rendering an accurate diagnosis. Deregulation of immune check points may play an important role in the transformation. Based on the available clinical and molecular data, it appears that gray zone type EBV+ large B-cell transformation may be less aggressive than EBV- gray zone lymphoma.
Acknowledgement
This study was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. WX is supported by a startup fund from Department of Pathology at MSKCC.
Footnotes
Conflict-of-interest disclosure: The authors have no disclosure.
Reference:
- 1.Swerdlow SCE, Harris NE, et al. WHO classification of tumours of haematopoietic and lymphoid tissue. Lyon, IARC press;. 2017. [Google Scholar]
- 2.Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123(11):1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timar B, Fulop Z, Csernus B, et al. Relationship between the mutational status of VH genes and pathogenesis of diffuse large B-cell lymphoma in Richter’s syndrome. Leukemia. 2004;18(2):326–330. [DOI] [PubMed] [Google Scholar]
- 4.Mao Z, Quintanilla-Martinez L, Raffeld M, et al. IgVH mutational status and clonality analysis of Richter’s transformation: diffuse large B-cell lymphoma and Hodgkin lymphoma in association with B-cell chronic lymphocytic leukemia (B-CLL) represent 2 different pathways of disease evolution. Am J Surg Pathol. 2007;31(10):1605–1614. [DOI] [PubMed] [Google Scholar]
- 5.Rossi D, Spina V, Deambrogi C, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117(12):3391–3401. [DOI] [PubMed] [Google Scholar]
- 6.Kazmierczak M, Kroll-Balcerzak R, Balcerzak A, et al. Hodgkin lymphoma transformation of chronic lymphocytic leukemia: cases report and discussion. Med Oncol. 2014;31(1):800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao W, Chen WW, Sorbara L, et al. Hodgkin lymphoma variant of Richter transformation: morphology, Epstein-Barr virus status, clonality, and survival analysis-with comparison to Hodgkin-like lesion. Hum Pathol. 2016;55:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morscio J, Bittoun E, Volders N, et al. Secondary B-cell lymphoma associated with the Epstein-Barr virus in chronic lymphocytic leukemia patients. J Hematop. 2016;9:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Leval L, Vivario M, De Prijck B, et al. Distinct clonal origin in two cases of Hodgkin’s lymphoma variant of Richter’s syndrome associated With EBV infection. Am J Surg Pathol. 2004;28(5):679–686. [DOI] [PubMed] [Google Scholar]
- 10.Ohno T, Smir BN, Weisenburger DD, Gascoyne RD, Hinrichs SD, Chan WC. Origin of the Hodgkin/Reed-Sternberg cells in chronic lymphocytic leukemia with “Hodgkin’s transformation”. Blood. 1998;91(5):1757–1761. [PubMed] [Google Scholar]
- 11.Momose H, Jaffe ES, Shin SS, Chen YY, Weiss LM. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin’s disease. Mediation by Epstein-Barr virus. Am J Surg Pathol. 1992;16(9):859–867. [DOI] [PubMed] [Google Scholar]
- 12.Fong D, Kaiser A, Spizzo G, Gastl G, Tzankov A. Hodgkin’s disease variant of Richter’s syndrome in chronic lymphocytic leukaemia patients previously treated with fludarabine. Br J Haematol. 2005;129(2):199–205. [DOI] [PubMed] [Google Scholar]
- 13.Kanzler H, Kuppers R, Helmes S, et al. Hodgkin and Reed-Sternberg-like cells in B-cell chronic lymphocytic leukemia represent the outgrowth of single germinal-center B-cell-derived clones: potential precursors of Hodgkin and Reed-Sternberg cells in Hodgkin’s disease. Blood. 2000;95(3):1023–1031. [PubMed] [Google Scholar]
- 14.Pescarmona E, Pignoloni P, Mauro FR, et al. Hodgkin/Reed-Sternberg cells and Hodgkin’s disease in patients with B-cell chronic lymphocytic leukaemia: an immunohistological, molecular and clinical study of four cases suggesting a heterogeneous pathogenetic background. Virchows Arch. 2000;437(2):129–132. [DOI] [PubMed] [Google Scholar]
- 15.Kuppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin’s disease and either follicular lymphoma or B-CLL. Mol Med. 2001;7(5):285–292. [PMC free article] [PubMed] [Google Scholar]
- 16.Ansell SM, Li CY, Lloyd RV, Phyliky RL. Epstein-Barr virus infection in Richter’s transformation. Am J Hematol. 1999;60(2):99–104. [DOI] [PubMed] [Google Scholar]
- 17.Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126(7):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkozy C, Copie-Bergmand C, Damotte D, et al. Gray-zone Lymphoma Between cHL and Large B-Cell Lymphoma: A Histopathologic Series From the LYSA. Am J Surg Pathol. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Elsayed AA, Satou A, Eladl AE, Kato S, Nakamura S, Asano N. Grey zone lymphoma with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a clinicopathological study of 14 Epstein-Barr virus-positive cases. Histopathology. 2017;70(4):579–594. [DOI] [PubMed] [Google Scholar]
- 20.Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. 2011;24(12):1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1(26):2643–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2018 update on diagnosis, risk-stratification and management. Am J Hematol. 2018;93(7):953–962. [DOI] [PubMed] [Google Scholar]
- 24.Brown AF, Salama ME, Patel JL, et al. D-L1 protein expression in most EBV-driven lymphoproliferative disorders is not associated with 9p24.1 amplification. Hematopathology. 2017;2(1):11. [Google Scholar]
- 25.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin JH, Lin JY, Chou YC, et al. Epstein-Barr virus LMP2A suppresses MHC class II expression by regulating the B-cell transcription factors E47 and PU.1. Blood. 2015;125(14):2228–2238. [DOI] [PubMed] [Google Scholar]