Abstract
Background:
The optimal level of care for hemodynamically stable patients with isolated blunt hepatic, renal, or splenic injuries (solid organ injuries, or SOIs) is unknown. We sought to characterize inter-hospital variability in intensive care unit (ICU) admission of such patients and to determine whether greater hospital-level ICU use would be associated with improved outcomes.
Methods:
We conducted a retrospective cohort study using the 2015 and 2016 National Trauma Data Bank. We included adult blunt trauma patients with SOIs with an Abbreviated Injury Scale score of 2–4. We excluded patients with other significant injuries, hypotension, or another indication for ICU admission, and hospitals with <10 eligible patients. We categorized hospitals into quartiles based on the proportion of eligible patients admitted to an ICU. The primary outcome was a composite of organ failure (cardiac arrest, acute lung injury/acute respiratory failure, or acute kidney injury), infection (sepsis, pneumonia, or catheter-related blood stream infection), or death during hospitalization.
Results:
Among 14,312 patients at 444 facilities, 7,225 (50%), 5,050 (35%), and 3,499 (24%) had splenic, hepatic, and renal injuries, respectively. The median proportion of ICU use was 44% (IQR 27–59%, range 0–95%). The composite outcome occurred in 180 patients (1.3%), with death in 76 (0.5%), organ failure in 97 (0.7%), and infection in 53 (0.4%). Relative to hospitals with the lowest ICU use (quartile 1), greater hospital-level ICU use was not associated with decreased likelihood of the composite outcome (adjusted odds ratios 1.31 [95% CI 0.88–1.95], 0.81 [95% CI 0.52–1.26], and 0.94 [95% CI 0.62–1.43] for quartiles 2–4, respectively) or its components. Unplanned ICU transfer was no more likely with lower hospital-level ICU use.
Conclusions:
Admission location of stable patients with isolated mild-moderate abdominal SOIs is variable across hospitals, but hospitalization at a facility with greater ICU use is not associated with substantially improved outcomes.
Keywords: solid organ injury, hepatic injury, renal injury, splenic injury, ICU admission
BACKGROUND
Most hepatic, renal, and splenic injuries (solid organ injuries, or SOIs) in hemodynamically stable patients are managed nonoperatively (1–3) with hemodynamic monitoring, serial abdominal examination, and hematocrit measurements. Because of the emphasis on early detection of exam or laboratory abnormalities that may necessitate intervention, physicians sometimes admit these patients to an intensive care unit (ICU). A variety of factors may contribute to the decision of where to admit these patients, including patient physiology, comorbidities, volume of hemoperitoneum, and presence of contrast extravasation. Although ICU admission is common for patients with high-grade SOIs, one third of surgeons would routinely admit even patients with a grade I splenic injury to a continuously monitored setting such as the ICU (4). Among trauma patients, it has been estimated that 22% of ICU admissions may be due to SOIs (5).
For hemodynamically stable patients with blunt splenic or hepatic injury, the 2012 Eastern Association for the Surgery of Trauma (EAST) practice management guidelines recommended in-hospital monitoring but found insufficient evidence to make recommendations about its intensity (ICU versus non-ICU) or duration (1, 2). For hemodynamically stable patients with blunt renal injury, the American Urologic Association recommends non-invasive management, including close hemodynamic monitoring and possible ICU admission (6).
Few studies have evaluated the impact of ICU admission on morbidity and mortality for hemodynamically stable patients with isolated SOIs. We sought to describe national variability in admission disposition (ICU versus non-ICU) of adult trauma patients with isolated blunt SOIs, and determine if hospital-level ICU use is associated with improved inpatient outcomes in this population.
METHODS
Study Design
We performed a retrospective cohort study using the 2015 and 2016 National Trauma Data Bank (NTDB), which includes the majority of U.S. level I and II trauma centers. The study was deemed exempt by the Institutional Review Board at the University of California Davis.
Study Population
We included adult patients (≥18 years old) with a mild to moderate injury of the liver, kidney, or spleen who were admitted to trauma centers participating in the NTDB between January 1, 2015 and December 31, 2016. We defined mild to moderate SOIs on the basis of either Abbreviated Injury Scale (AIS) “predot” codes or diagnosis codes (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]; International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM]; or International Classification of Diseases, 10th Revision, Canada [ICD-10-CA]) corresponding to an AIS score of 2–4 (see Methods, Supplemental Digital Content 1). We used both ICD and AIS codes because neither classification was used for all records in the database. We excluded patients with a penetrating mechanism of injury because their management is inherently different. We focused on isolated SOI, excluding patients with other significant injuries (non-abdominal AIS score >2, or abdominal AIS score ≥2 for injuries unrelated to the SOI [e.g., vascular, gastrointestinal, pelvic, or retroperitoneal structures]; Tables 1 and 2, Supplemental Digital Content 1). We excluded patients identified not to have spent any time in the emergency department (ED), as well as those likely to have had another indication for ICU admission, namely: initial ED systolic blood pressure <90 mmHg; initial ED Glasgow Coma Score (GCS) <9; intubated or assisted respirations in the ED; or no signs of life on arrival in the ED. We restricted the analysis to patients with an ED disposition of floor bed, telemetry/step-down unit, or ICU because others were either not admitted or it was not possible to determine the level of care (e.g., ED disposition to operating room). We did not exclude patients who arrived as transfers from another hospital so long as they met the above criteria for inclusion.
Table 1.
Baseline characteristics of the 14,312 patients with isolated blunt solid organ injuries, by quartile of hospital-level ICU use.
| Patient characteristic | Hospital-level proportion of patients with isolated blunt solid organ injuries admitted to an ICU | |||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| 0–27% | 27–44% | 44–59% | 59–95% | |||
| n = 3788 | n = 3613 | n = 3595 | n = 3316 | P value | ||
| Age (years), mean ± SD | 41 ± 19 | 42 ± 19 | 41 ± 19 | 42 ± 19 | 0.04 | |
| Male sex, n (%) | 2346 (61.9) | 2191 (60.6) | 2175 (60.5) | 2073 (62.5) | 0.23 | |
| Mechanism, n (%) | ||||||
| Fall | 944 (24.9) | 878 (24.3) | 793 (22.1) | 767 (23.1) | 0.09 | |
| Transportation | 2429 (64.1) | 2342 (64.8) | 2419 (67.3) | 2161 (65.2) | ||
| Other or unspecified | 415 (10.9) | 393 (10.9) | 383 (10.6) | 388 (11.7) | ||
| Arrival as a transfer, n (%) | 1456 (38.4) | 1300 (36.0) | 1270 (35.3) | 1145 (34.5) | 0.004 | |
| Comorbidities, n (%) | ||||||
| Current smoker | 1060 (28.0) | 899 (24.9) | 987 (27.4) | 872 (26.3) | 0.014 | |
| Hypertension | 772 (20.4) | 755 (20.9) | 710 (19.8) | 697 (21.0) | 0.54 | |
| Diabetes mellitus | 366 (9.7) | 330 (9.1) | 311 (8.6) | 296 (8.9) | 0.48 | |
| Drug use disorder | 397 (10.5) | 329 (9.1) | 346 (9.6) | 367 (11.1) | 0.031 | |
| Bleeding disorder | 174 (4.6) | 172 (4.8) | 151 (4.2) | 151 (4.6) | 0.71 | |
| Other comorbidity | 1299 (34.3) | 1330 (36.8) | 1196 (33.3) | 1159 (35.0) | 0.014 | |
| ISS, mean ± SD | 9 ± 4 | 9 ± 4 | 9 ± 4 | 10 ± 5 | 0.074 | |
| Kidney injury, n (%) | 861 (22.7) | 922 (25.5) | 896 (24.9) | 820 (24.7) | 0.031 | |
| Liver injury, n (%) | 1306 (34.5) | 1265 (35.0) | 1295 (36.0) | 1184 (35.7) | 0.51 | |
| Spleen injury, n (%) | 1970 (52.0) | 1794 (49.6) | 1766 (49.1) | 1695 (51.1) | 0.053 | |
| Multiple solid organs injured, n (%) | 335 (8.8) | 347 (9.6) | 339 (9.4) | 363 (10.9) | 0.072 | |
| SOI AIS score, n (%) | ||||||
| 2 | 2394 (63.2) | 2252 (62.3) | 2173 (60.4) | 1864 (56.2) | <0.001 | |
| 3 | 931 (24.6) | 969 (26.8) | 958 (26.6) | 913 (27.5) | ||
| 4 | 458 (12.1) | 360 (10.0) | 390 (10.8) | 412 (12.4) | ||
| Not specified | 5 (0.1) | 32 (0.9) | 74 (2.1) | 127 (3.8) | ||
| Other minor injury (AIS ≤2) | ||||||
| Head | 738 (19.5) | 791 (21.9) | 784 (21.8) | 653 (19.7) | 0.010 | |
| Face | 847 (22.4) | 826 (22.9) | 804 (22.4) | 737 (22.2) | 0.92 | |
| Thorax | 1363 (36.0) | 1213 (33.6) | 1228 (34.2) | 1080 (32.6) | 0.020 | |
| Spine | 609 (16.1) | 553 (15.3) | 498 (13.8) | 488 (14.7) | 0.054 | |
| Upper Extremity | 1091 (28.8) | 1016 (28.1) | 1060 (29.5) | 940 (28.4) | 0.59 | |
| Lower Extremity | 1161 (30.6) | 1101 (30.5) | 1099 (30.6) | 1000 (30.2) | 0.97 | |
| Heart rate (beats per minute), n (%) | ||||||
| <50 | 13 (0.3) | 15 (0.4) | 14 (0.4) | 13 (0.4) | 0.16 | |
| 50–99 | 2790 (73.6) | 2627 (72.7) | 2584 (71.9) | 2410 (72.7) | ||
| ≥100 | 941 (24.8) | 931 (25.8) | 962 (26.8) | 836 (25.2) | ||
| Not recorded | 44 (1.2) | 40 (1.1) | 35 (1.0) | 57 (1.7) | ||
| Respiratory rate (breaths per minute), n (%) | ||||||
| <12 | 43 (1.1) | 38 (1.0) | 38 (1.1) | 40 (1.2) | 0.71 | |
| 12–24 | 3428 (90.5) | 3313 (91.7) | 3289 (91.5) | 3029 (91.3) | ||
| ≥25 | 244 (6.4) | 196 (5.4) | 209 (5.8) | 197 (5.9) | ||
| Not recorded | 73 (1.9) | 66 (1.8) | 59 (1.6) | 50 (1.5) | ||
| Supplemental oxygen, n (%) | 581 (15.3) | 430 (11.9) | 495 (13.8) | 573 (17.3) | <0.001 | |
| Early transfusion,a n (%) | 24 (0.6) | 37 (1.0) | 25 (0.7) | 26 (0.8) | 0.25 | |
| Early angioembolization,a n (%) | 50 (1.3) | 38 (1.0) | 24 (0.7) | 31 (0.9) | 0.043 | |
Abbreviations: ICU, intensive care unit; ISS, Injury Severity Score; SOI, solid organ injury; AIS, Abbreviated Injury Scale
Occurring <6 hours after presentation
Table 2.
Baseline characteristics of the 444 hospitals by quartile of hospital-level ICU use.
| Hospital characteristic | Hospital-level Proportion of Patients with Isolated Blunt Solid Organ Injuries Admitted to an ICU | P value | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| 0–27% | 27–44% | 44–59% | 59–95% | ||
| n = 111 | n = 112 | n = 110 | n = 111 | ||
| Eligible hospitalizations/year, mean ± SD | 34.1 ± 26.2 | 32.2 ± 21.2 | 32.7 ± 22.2 | 29.9 ± 19.3 | 0.56 |
| Trauma center level, n (%) | |||||
| I | 53 (48) | 43 (38) | 49 (44) | 43 (39) | 0.90 |
| II | 48 (43) | 54 (48) | 51 (46) | 54 (49) | |
| III | 9 (8) | 11 (10) | 7 (6) | 11 (10) | |
| IV | 0 (0) | 1 (1) | 0 (0) | 1 (1) | |
| Not described | 1 (1) | 3 (3) | 3 (3) | 2 (2) | |
| Teaching status, n (%) | |||||
| Community | 48 (43) | 47 (42) | 38 (34) | 61 (55) | 0.008 |
| Non-teaching | 15 (14) | 18 (16) | 31 (28) | 20 (18) | |
| University | 48 (43) | 47 (42) | 41 (37) | 30 (27) | |
| Geographic region, n (%) | |||||
| Northeast | 27 (24) | 26 (23) | 18 (16) | 20 (18) | 0.15 |
| South | 35 (32) | 39 (35) | 40 (36) | 35 (32) | |
| Midwest | 23 (21) | 25 (22) | 26 (24) | 21 (19) | |
| West | 22 (20) | 21 (19) | 26 (24) | 35 (32) | |
| Not described | 4 (4) | 1 (1) | 0 (0) | 0 (0) | |
| Number of adult beds, n (%) | |||||
| ≤250 (or not described) | 18 (16) | 11 (10) | 22 (20) | 22 (20) | 0.081 |
| 251–350 | 27 (24) | 29 (26) | 19 (17) | 34 (31) | |
| 351–500 | 26 (23) | 29 (26) | 28 (25) | 31 (28) | |
| >500 | 40 (36) | 43 (38) | 41 (37) | 24 (22) | |
| Number of trauma ICU beds, n (%) | |||||
| ≤15 | 19 (17) | 24 (21) | 21 (19) | 22 (20) | 0.85 |
| 16–25 | 35 (32) | 34 (30) | 40 (36) | 39 (35) | |
| 26–35 | 25 (22) | 17 (15) | 15 (14) | 19 (17) | |
| >35 | 32 (29) | 37 (33) | 34 (31) | 31 (28) | |
| Telemetry/step-down unit available, n (%) | 91 (82) | 95 (85) | 91 (83) | 93 (84) | 0.95 |
Abbreviations: ICU, intensive care unit.
We defined the exposure as hospital-level “ICU use”—the proportion of eligible patients admitted to the ICU—and ranked hospitals in quartiles based on this proportion. We considered patients admitted to a floor bed or telemetry/step-down unit as “non-ICU” admissions. We excluded hospitals with <10 eligible patients during 2015–2016 to ensure robustness of the exposure status.
Patient and Hospital Characteristics
Patient-level characteristics included age, sex, comorbid conditions, physiologic parameters such as initial ED vital signs (heart rate, respiratory rate, and oxygen saturation) and GCS, mechanism of injury, AIS scores, Injury Severity Score (ISS), number and type of SOIs, and interventions (transfusion, angioembolization, and operation, as defined in the Methods portion of Supplemental Digital Content 1) <6 hours after presentation. We characterized SOIs on the basis of AIS scores rather than Organ Injury Scale (OIS) grades because the NTDB does not contain the latter. Hospital-level characteristics included trauma center level, university affiliation, hospital size, number of ICU beds, number of trauma surgeons, geographical region, telemetry/step-down unit availability (empirically derived from the NTDB), and the proportion of patients with a blunt mechanism of injury (also empirically derived).
Outcomes
We defined the primary outcome as a composite of inpatient death, organ failure (cardiac arrest, acute lung injury/acute respiratory failure, or acute kidney injury), or infection (sepsis, pneumonia, or catheter-related blood stream infection). We included organ failure and infection in the composite because the NTDB does not include information specific to the failure of non-operative management, such as the volume of blood transfusion, or the occurrence of unplanned, urgent operation or angioembolization. In the absence of such information, organ failure and infection may indicate delayed recognition of bleeding (i.e., shock leading to organ failure) or secondary effects of interventions prompted by clinical deterioration (i.e., infection associated with intubation, central venous catheterization, or transfusion). Pre-specified secondary outcomes included: components of the composite outcome (death, organ failure, infection); unplanned ICU transfer; delayed (≥6 hours after presentation) operation for the SOI, angioembolization, and transfusion; ICU length of stay; and ventilator days. Unplanned ICU transfer is defined in the NTDB as transfer to the ICU after initial admission to the floor or return to the ICU after transfer out. We evaluated this outcome to determine whether hospitals with less ICU use on admission compensated for this with an increased rate of subsequent unplanned ICU transfers.
Statistical Analysis
To evaluate the reliability of the exposure status, hospital-level quartile of ICU use, we assessed its agreement between 2015 and 2016 using Cohen’s kappa coefficient, with weights of 1 – [(i – j)/(k – 1)]^2. We performed descriptive analyses of patient and hospital characteristics across quartiles of ICU use. We used multivariable logistic regression to evaluate the association between ICU use and the primary outcome, as well as other binary outcomes. We accounted for correlation of observations within hospitals using robust standard errors. We evaluated for confounding by patient- and hospital-level characteristics using a 10% change-in-estimate approach (7). We used multivariable Poisson regression to evaluate the relationship between ICU use and both ICU length of stay and ventilator days (reported as incidence rate ratios), accounting for correlation of observations within hospitals and using hospital length of stay as the period of exposure.
We performed post hoc sensitivity analyses to examine whether alternative definitions of the study population, exposure status, and outcomes influenced observed associations. These alternative analyses included: (1) restricting the study population to patients with minimal injuries (AIS=1) other than the SOIs; (2) expanding the study population to include patients admitted to observation status (considered as non-ICU); (3) expanding the definition of ICU use to include admission to telemetry/step-down unit status; (4) restricting the study population to patients with SOIs defined by AIS codes (excluding those with SOIs defined only by ICD codes); (5) restricting the study population to patients with SOI AIS scores of only 3 or 4 (and expanding the number of centers to also include those with 5–9 eligible patients during 2015–2016 to allow sufficient patients and centers); (6) expanding the study population to include patients with SOI AIS scores of 5; (7) expanding the study population to also include centers with 5–9 eligible patients during 2015–2016; and (8) expanding the definition of the composite outcome to include discharge to hospice. In each analysis in which the study population or exposure definition changed, we redefined the primary exposure, hospital-level ICU use grouped into quartiles, accordingly.
We used Stata 10 (StataCorp LP, College Station, TX) for all analyses. We conducted two-sided tests and defined statistical significance as P<0.05, without correction for multiple testing.
RESULTS
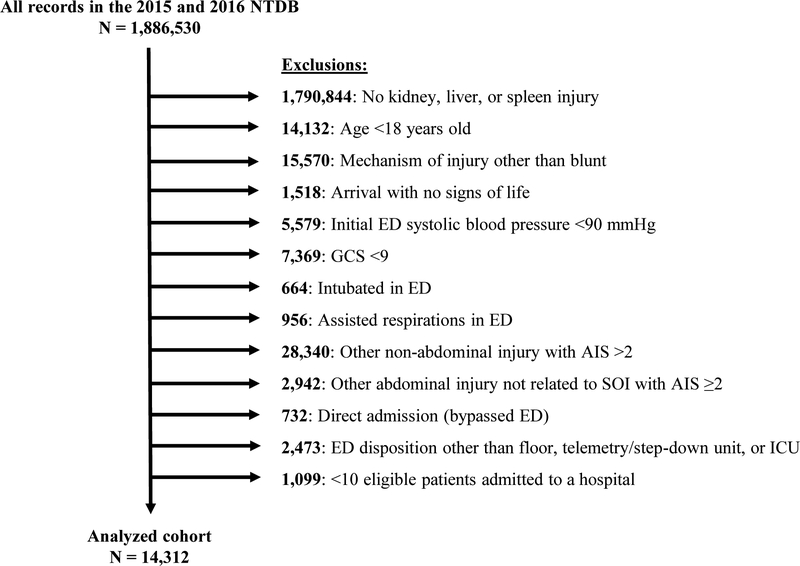
Among 1,886,530 records in the NTDB during 2015–2016, 14,312 hospitalizations at 444 hospitals met inclusion criteria (Figure). The proportion of eligible patients admitted to an ICU at each hospital ranged from 0% to 95% (quartile 1: 0–27%; quartile 2: 27–44%; quartile 3: 44–59%; and quartile 4: 59–95%). ICU admission from the ED occurred for 6,044 patients (42.2% overall; quartile 1: 16.6%; quartile 2: 34.6%; quartile 3: 50.0%; quartile 4: 71.4%; P<0.001). There was substantial agreement in hospital-level quartile of ICU use between 2015 and 2016 (kappa = 0.68).
Figure.
Derivation of the study cohort. Abbreviations: NTDB, National Trauma Data Bank; ED, emergency department; GCS, Glasgow Coma Scale; AIS, abbreviated injury scale; ICU, intensive care unit
Most patients were injured in a transportation-related mechanism, including motor vehicle crashes, motorcycle crashes, cyclists, and pedestrians (Table 1). Falls were the second most common mechanism of injury. The most common comorbid conditions were current smoking, hypertension, diabetes, and drug use disorders. For each of the head, facial, thoracic, and extremity AIS categories, over 20% of patients had a minor (AIS ≤2) injury.
There was no significant difference in the mean number of eligible patients admitted per hospital among the quartiles (Table 2). University affiliation was associated with less ICU use. Trauma center level, geographic region, hospital size, and ICU beds were not associated with ICU use. There was no significant difference in the presence of a telemetry/step-down unit across quartiles of ICU use.
Primary Outcome
One hundred eighty patients (1.3%) experienced the composite adverse outcome, including 135 (75%) who were initially admitted to an ICU. Without adjustment for confounders, the odds of the composite outcome were similar across quartiles of ICU use (Table 3). Abdominal AIS score and baseline use of supplemental oxygen met criteria for confounding using the 10% change-in-estimate approach. After adjusting for these confounders, ICU use still was not associated with the composite outcome.
Table 3.
Associations between hospital-level ICU use (modeled as quartiles) and outcomes.a
| Outcome | Quartile 1 n = 3788 |
Quartile 2 n = 3613 |
Quartile 3 n = 3595 |
Quartile 4 n = 3316 |
P-valueb |
|---|---|---|---|---|---|
| Composite, n (%) | 43 (1.1) | 52 (1.4) | 36 (1.0) | 49 (1.5) | NA |
| Unadjusted OR (95% CI) | Reference | 1.27 (0.85–1.90) | 0.88 (0.57–1.35) | 1.31 (0.67–2.54) | 0.34 |
| Adjusted OR (95% CI) | Reference | 1.31 (0.88–1.95) | 0.81 (0.52–1.26) | 0.94 (0.62–1.43) | 0.20 |
| Death, n (%) | 24 (0.6) | 21 (0.6) | 13 (0.4) | 18 (0.5) | NA |
| Unadjusted OR (95% CI) | Reference | 0.92 (0.48–1.74) | 0.57 (0.29–1.11) | 0.86 (0.29–1.59) | 0.40 |
| Adjusted OR (95% CI) | Reference | 0.96 (0.52–1.80) | 0.50 (0.25–1.00)c | 0.71 (0.39–1.30) | 0.21 |
| Organ failure, n (%) | 20 (0.5) | 27 (0.8) | 22 (0.6) | 28 (0.8) | NA |
| Unadjusted OR (95% CI) | Reference | 1.42 (0.78–2.57) | 1.16 (0.61–2.20) | 1.60 (0.62–4.12) | 0.62 |
| Adjusted OR (95% CI) | Reference | 1.46 (0.79–2.71) | 1.06 (0.54–2.07) | 1.07 (0.60–1.93) | 0.60 |
| Infection, n (%) | 13 (0.3) | 18 (0.5) | 8 (0.2) | 14 (0.4) | NA |
| Unadjusted OR (95% CI) | Reference | 1.45 (0.72–2.94) | 0.65 (0.26–1.64) | 1.23 (0.53–2.84) | 0.27 |
| Adjusted OR (95% CI) | Reference | 1.44 (0.70–2.93) | 0.62 (0.24–1.59) | 0.98 (0.43–2.22) | 0.24 |
| Unplanned ICU transfer, n (%) | 30 (0.8) | 39 (1.1) | 52 (1.4) | 43 (1.3) | NA |
| Unadjusted OR (95% CI) | Reference | 1.37 (0.80–2.35) | 1.84 (1.11–3.03) | 1.64 (0.95–2.85) | 0.10 |
| Adjusted OR (95% CI) | Reference | 1.46 (0.84–2.54) | 1.83 (1.14–2.93) | 1.64 (0.93–2.88) | 0.091 |
| Delayed transfusion,d n (%) | 185 (4.9) | 156 (4.3) | 163 (4.5) | 183 (5.5) | NA |
| Unadjusted OR (95% CI) | Reference | 0.88 (0.60–1.29) | 0.92 (0.64–1.35) | 1.14 (0.78–1.66) | 0.41 |
| Adjusted OR (95% CI) | Reference | 0.90 (0.62–1.33) | 0.94 (0.65–1.37) | 1.10 (0.75–1.61) | 0.67 |
| Delayed angioembolization,d n (%) | 171 (4.5) | 117 (3.2) | 125 (3.5) | 151 (4.6) | NA |
| Unadjusted OR (95% CI) | Reference | 0.71 (0.51–0.98) | 0.76 (0.54–1.08) | 1.01 (0.73–1.39) | 0.082 |
| Adjusted OR (95% CI) | Reference | 0.72 (0.52–1.01) | 0.76 (0.54–1.08) | 0.97 (0.70–1.34) | 0.14 |
| Delayed operation,d n (%) | 62 (1.6) | 75 (2.1) | 68 (1.9) | 85 (2.6) | NA |
| Unadjusted OR (95% CI) | Reference | 1.27 (0.85–1.90) | 1.16 (0.79–1.69) | 1.58 (1.11–2.26) | 0.068 |
| Adjusted OR (95% CI) | Reference | 1.35 (0.91–1.99) | 1.17 (0.80–1.72) | 1.48 (1.03–2.12) | 0.16 |
Abbreviations: ICU, intensive care unit; NA, not applicable
The adjusted models include the abdominal Abbreviated Injury Scale score and baseline use of supplemental oxygen as confounders.
P-value for the null hypothesis that the coefficients of all of the indicator variables representing quartiles of ICU use are 0 (“chunk” test)
The 95% confidence interval contains 1, but the boundary is depicted as 1.00 due to rounding.
Occurring ≥6 hours after presentation (see Methods portion of Supplemental Digital Content 1 for definitions of transfusion, angioembolization, and operation)
Secondary Outcomes
There were no significant differences in the odds of death, organ failure, or infection by quartile of ICU use (Table 3). An ICU stay occurred at some time during hospitalization for 6,289 patients (43.9% overall; quartile 1: 19.9%; quartile 2: 36.4%; quartile 3: 51.1%; quartile 4: 71.8%; P<0.001). The median (IQR) ICU length of stay over the entire hospitalization was 0 (0, 2) days (quartile 1: 0 [0, 0] days; quartile 2: 0 [0, 2] days; quartile 3: 1 [0, 2] days; quartile 4: 2 [0, 3] days; P<0.001). Relative to quartile 1, greater hospital-level ICU use was associated with longer patient-level ICU length of stay by a factor of 1.63 (95% CI 1.38–1.92) for quartile 2, 2.22 (95% CI 1.90–2.60) for quartile 3, and 2.73 (95% CI 2.34–3.20) for quartile 4. Compared to quartile 1, patients in quartile 3 had an increased adjusted odds of unplanned ICU transfer (OR 1.83, 95% CI 1.14–2.93). There was no significant association between ICU use and ventilator days. Delayed operation occurred in 290 patients (2.0%) and delayed transfusion in 687 (4.8%). Relative to quartile 1, delayed operation was more common among patients in quartile 4 (OR 1.48, 95% CI 1.03–2.12). Delayed angioembolization occurred in 564 patients (3.9%) and did not differ across quartiles of ICU use.
Sensitivity Analyses
All sensitivity analyses we performed, including restricting the study population to patients with SOI AIS scores of only 3 or 4 (and expanding the number of centers to also include those with 5–9 eligible patients), and expanding the cohort to also include patients with an SOI AIS score of 5, suggested hospital-level ICU use was not associated with the composite outcome (Table 4).
Table 4.
Sensitivity analyses evaluating the association between hospital-level ICU use (modeled as quartiles) and the primary composite outcome,a using alternative definitions of the study cohort, exposure status, and outcome.
| Sensitivity analysis | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-valueb | |
|---|---|---|---|---|---|---|
| (1) Restricting cohort to patients with minimal injuries (AIS≤1) other than the SOIs | Events/total | 17/1650 | 18/1535 | 16/1602 | 35/1605 | NA |
| OR (95% CI) | Reference | 1.06 (0.60–1.89) | 0.89 (0.41–1.91) | 1.13 (0.59–2.17) | 0.94 | |
| (2) Including patients admitted to observation status (considered as non-ICU) | Events/total | 5/029 | 53/3824 | 34/3554 | 47/3380 | NA |
| OR (95% CI) | Reference | 1.12 (0.76–1.64) | 0.69 (0.43–1.09) | 0.80 (0.54–1.19) | 0.16 | |
| (3) Expanding the definition of ICU to include admission to telemetry/step-down units | Events/total | 46/3483 | 44/4017 | 34/3132 | 56/3680 | NA |
| OR (95% CI) | Reference | 0.80 (0.51–1.23) | 0.79 (0.50–1.26) | 0.85 (0.56–1.28) | 0.69 | |
| (4) Restricting cohort to those with SOI AIS scores defined by AIS codes | Events/total | 43/3783 | 48/3644 | 35/3448 | 34/3182 | NA |
| OR (95% CI) | Reference | 1.25 (0.83–1.89) | 0.90 (0.59–1.37) | 0.86 (0.55–1.35) | 0.39 | |
| (5) Restricting cohort to SOIs with AIS 3–4 (and expanding the number of hospitals to also include those with 5–9 eligible patients) | Events/total | 16/1280 | 26/1392 | 26/1438 | 9/1120 | NA |
| OR (95% CI) | Reference | 1.53 (0.84–2.79) | 1.44 (0.75–2.77) | 0.58 (0.26–1.26) | 0.037 | |
| (6) Expanding cohort to include SOIs with AIS=5 | Events/total | 47/3815 | 55/3756 | 41/3673 | 51/3381 | NA |
| OR (95% CI) | Reference | 1.26 (0.85–1.86) | 0.81 (0.53–1.24) | 0.94 (0.63–1.41) | 0.28 | |
| (7) Expanding cohort to also include hospitals with 5–9 eligible patients | Events/total | 44/3805 | 57/3940 | 40/3974 | 47/3367 | NA |
| OR (95% CI) | Reference | 1.32 (0.90–1.95) | 0.82 (0.54–1.26) | 0.90 (0.59–1.37) | 0.14 | |
| (8) Expanding definition of the composite outcome to include discharge to hospice | Events/total | 49/3788 | 54/3613 | 42/3595 | 56/3316 | NA |
| OR (95% CI) | Reference | 1.19 (0.82–1.74) | 0.84 (0.56–1.28) | 0.99 (0.65–1.50) | 0.49 | |
Abbreviations: AIS, abbreviated injury scale; ICU, intensive care unit; NA, not applicable; SOI, solid organ injury
Adjusted for abdominal AIS score and baseline use of supplemental oxygen as confounders
P-value for the null hypothesis that the coefficients of all of the indicator variables representing quartiles of ICU use are 0 (“chunk” test)
DISCUSSION
This study demonstrates the wide variability in ICU use for hemodynamically stable patients with isolated mild to moderate SOIs. Despite similar patient characteristics across quartiles, ICU use ranged from 0% to 95%. These findings agree with a survey of members of the American Association for the Surgery of Trauma showing considerable disagreement in the appropriate level of care for patients with blunt splenic injury: ICU use ranged from 9–73% for OIS grade 1–2 injuries and from 18–82% for grade 3–5 injuries (8). Variability may be due to minimal literature on the effect of ICU care in this population, and therefore no evidence-based guidelines for ICU admission. In a survey of EAST members, only 30% reported having institutional guidelines for management of blunt splenic injury (4). Other explanations include variation in resource availability and physician preference.
The only hospital factor we identified that was associated with ICU use was teaching status. Patients treated at university-affiliated hospitals were less likely to be admitted to the ICU. In contrast, a retrospective review of blunt hepatic injuries in Michigan showed ICU admission was more common at level I trauma centers compared to level II trauma centers (9). In our study, we observed no significant difference in ICU use based on trauma center level, but there was a tendency for level I centers to use ICUs less. Differences may be explained by a larger sample size and inclusion of splenic and renal injuries in our study.
In our study, there was a low incidence of the composite adverse outcome; only 180 patients (1.3%) died or had organ failure or infection, which is lower than in other studies (3, 10). This may be a result of limiting our analysis to patients with isolated injuries who were not initially hypotensive or have other characteristics likely to result in ICU admission. ICU use was not associated with the composite adverse outcome, and this finding was robust to several alternative approaches to defining the study population, exposure, and outcome. Because the NTDB cannot distinguish between OIS grade 1 and 2 injuries (both of which are categorized as AIS 2), our analysis included some patients with SOIs of minimal severity. Notably, results were consistent when we limited the analysis to patients with more severe injuries (SOI AIS 3–4).
While we could not assess from the NTDB why individual patients were admitted to the ICU, it seems likely that clinicians desired closer monitoring of hemodynamic, laboratory, and other clinical parameters. However, the time spent in the ICU may not have been necessary, or any benefits may have been offset by countervailing risks. Disturbances in the sleep-wake cycle, for example, are associated with increased rates of delirium and worse outcomes (11), and surgical ICUs have the most nocturnal nursing interventions (12). Additionally, ICU admission may result in decreased mobility, increased sedating medications, and increased exposure to drug-resistant organisms. Among hemodynamically stable patients, the risks of ICU admission may counterbalance the potential benefits.
A retrospective review of Pennsylvania level I and II trauma centers examined the observed to expected ratios for ICU use among patients with blunt splenic injuries and found no association with mortality (10). Furthermore, the authors reported that 73% of patients admitted to the ICU had no ICU procedure performed, and therefore may not have required ICU admission for observation. A retrospective review of blunt hepatic injury sought to characterize which patients would require an ICU intervention, which they defined as transfusion within 24 hours, angiography, or laparotomy (13). Among hemodynamically stable patients with low-grade (grade 1–3) injury, 74% had no intervention.
Interestingly, we found some evidence that ICU use was associated with an increase in unplanned ICU transfer. Unplanned ICU transfer presumably signifies some deterioration of or increased risk to the patient, suggesting failure of treatment in the non-ICU setting. If ICU care were an integral part of treating SOIs, then one would expect hospitals with greater ICU use upon admission to have decreased unplanned ICU transfer. Alternatively, hospitals that have a low threshold to admit patients to the ICU from the ED may also liberally transfer patients to the ICU later in the hospital stay. Whatever the explanation, the lack of an association of greater ICU use on admission with less subsequent unplanned ICU transfer—combined with the lack of an observed improvement in clinical outcomes with increased ICU use—raises the possibility that ICU care is not always necessary for these patients.
The main limitation of this study is the potential for unidentified confounding factors. We performed a thorough evaluation of the variables provided within the NTDB, but other important characteristics were not available, including additional information on patient physiology, comorbidities, and the nature of the SOI (e.g., volume of hemoperitoneum and presence of contrast extravasation), all of which may have influenced whether to admit a patient to the ICU. Because this was a hospital-level analysis, the most plausible unidentified confounders may be hospital characteristics, such as multidisciplinary teams, nurse-patient ratios, availability of mid-level practitioners, presence of algorithms for SOI management, quality of rescue processes, or other bundles of care.
Additionally, the composite outcome we used does not capture all clinically relevant endpoints, such as patient-reported outcomes and failure of non-operative management, because the NTDB does not collect them. We attempted to derive measures of failed non-operative management, such as delayed operation, angioembolization, and transfusions, using ICD procedure codes, but there may have been variability in reporting among institutions, the 6-hour cutoff we used to define “delayed” probably oversimplifies ascertainment, and ICD codes do not reliably indicate the volume of transfusions. Because the composite outcome was rare, we may not have identified a true association between hospital-level ICU use and the outcome (type II error). We do not have any information about outcomes subsequent to the initial hospitalization. Lastly, while we did not observe improved patient outcomes with greater ICU use, there may be a subset of patients who do benefit from ICU admission.
Non-operative management of blunt SOIs often includes admission to an ICU for close monitoring and detection of clinical abnormalities that would prompt intervention, but use of ICUs for this purpose varies markedly across major trauma centers. While ICU admission intuitively seems like a safer level of care during this observation period, our study showed there was no large association between ICU use and a composite outcome of death, organ failure, or infection. Further studies are necessary to elucidate which factors influence surgeons in their use of ICU admission for SOIs, identify which patients benefit from ICU care, determine whether ICU care may have a negative impact on certain patients, and inform evidence-based guidelines for ICU admission in this patient population.
Supplementary Material
SOURCES OF FUNDING
Dr. Bowman was supported by training grants from the Agency for Healthcare Research and Quality (T32HS022236) and the National Center for Advancing Translation Sciences, National Institutes of Health (UL1 TR001860).
Findings from this study were presented at the 90th Annual Meeting of the Pacific Coast Surgical Association, Tucson, Arizona, February 15-18, 2019.
Footnotes
CONFLICTS OF INTEREST
The authors do not report any conflicts of interest.
SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1. Methods describing the definitions of solid organ injury, transfusion, operation, and angioembolization and tables listing the Abdominal Abbreviated Injury Scale “predot” codes that were used to define solid organ injuries (inclusion criteria) and other significant injuries (exclusion criteria). docx
REFERENCES
- 1.Stassen NA, Bhullar I, Cheng JD, Crandall M, Friese R, Guillamondegui O, Jawa R, Maung A, Rohs TJ Jr., Sangosanya A et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S288–93. [DOI] [PubMed] [Google Scholar]
- 2.Stassen NA, Bhullar I, Cheng JD, Crandall ML, Friese RS, Guillamondegui OD, Jawa RS, Maung AA, Rohs TJ Jr., Sangosanya A, et al. Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S294–300. [DOI] [PubMed] [Google Scholar]
- 3.Tinkoff G, Esposito TJ, Reed J, Kilgo P, Fildes J, Pasquale M, Meredith JW. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. 2008;207(5):646–55. [DOI] [PubMed] [Google Scholar]
- 4.Fata P, Robinson L, Fakhry SM. A survey of EAST member practices in blunt splenic injury: a description of current trends and opportunities for improvement. J Trauma. 2005;59(4):836–41; discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney C, Kaur M, Gupta B, Singh PM, Gupta A, Kumar S, Misra MC. Critical care issues in solid organ injury: Review and experience in a tertiary trauma center. Saudi J Anaesth. 2014;8(Suppl 1):S29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morey AF, Brandes S, Dugi DD 3rd, Armstrong JH, Breyer BN, Broghammer JA, Erickson BA, Holzbeierlein J, Hudak SJ, Pruitt JH, et al. Urotrauma: AUA guideline. J Urol. 2014;192(2):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37. [DOI] [PubMed] [Google Scholar]
- 8.Zarzaur BL, Kozar RA, Fabian TC, Coimbra R. A survey of American Association for the Surgery of Trauma member practices in the management of blunt splenic injury. J Trauma. 2011;70(5):1026–31. [DOI] [PubMed] [Google Scholar]
- 9.Tignanelli CJ, Joseph B, Jakubus JL, Iskander GA, Napolitano LM, Hemmila MR. Variability in management of blunt liver trauma and contribution of level of American College of Surgeons Committee on Trauma verification status on mortality. J Trauma Acute Care Surg. 2018;84(2):273–9. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman EJ, Wiebe DJ, Martin ND, Pascual JL, Reilly PM, Holena DN. Variation in intensive care unit utilization and mortality after blunt splenic injury. J Surg Res. 2016;203(2):338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson PL, Ceriana P, Fanfulla F. Delirium: is sleep important? Best Pract Res Clin Anaesthesiol. 2012;26(3):355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le A, Friese RS, Hsu CH, Wynne JL, Rhee P, O’Keeffe T. Sleep disruptions and nocturnal nursing interactions in the intensive care unit. J Surg Res. 2012;177(2):310–4. [DOI] [PubMed] [Google Scholar]
- 13.Perumean JC, Martinez M, Neal R, Lee J, Olajire-Aro T, Imran JB, Williams BH, Phelan HA. Low-grade blunt hepatic injury and benefits of intensive care unit monitoring. Am J Surg. 2017;214(6):1188–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.