Abstract
Takotsubo syndrome(TTS) is attributed to catecholamine surge, which is also observed in COVID-19 disease due to the cytokine storm. We performed a systematic literature search using PubMed, Embase, and the Cochrane Central Register of Controlled Trials retrospectively to identify COVID-19-associated TTS case reports and evaluated patient-level demographics, laboratory markers clinical attributes, treatment given, and outcomes. There are 27 cases reported of TTS associated with COVID-19 infection of which 44.5% were male. Reported median age was 57 years (IQR: 39–65) and 62.95 years (IQR: 50.5–73.5) in case series and individual patients’ cases in database, respectively. The time interval from the symptom onset to TTS diagnosis was median 6.5 days (IQR: 1.0–8.0) in case series and 6.7 days (IQR: 4–10) in individual patients’ database. The median LVEF was 36% (IQR: 35–37) and 38.15%(IQR: 30–42.5%—[male: 40.33% (IQR: 33–44.2)] and female [37.15% (IQR: 30–40)] in case series and individual-patients’ database, respectively. Troponin was elevated in all patients except one patient. 77.2% patients of TTS with COVID-19 had an elevated C-reactive protein and/or D-dimer. Twelve out of 22 (54.5%) patients developed cardiac complication such as cardiogenic-shock, atrial fibrillation, acute heart failure, supraventricular tachycardia, and biventricular heart failure. Nineteen out of 26 (73.07%) patients were discharged, and three were hospitalized due to acute respiratory distress syndrome and needed extracorporeal membrane oxygenation or ongoing maternal age. There were 4 (14.8%) mortality. There was no major gender difference observed in development of TTS in COVID-19 unlike COVID-19 per se. Older median age group for TTS in COVID-19 patients irrespective of cardiovascular comorbidities and gender probably reflects age as an independent risk factor. Patients who developed TTS had higher mortality rate especially if they developed cardiogenic shock.
Keywords: Takotsubo cardiomyopathy, COVID-19, SARS-COV-2, Broken heart syndrome, Stress cardiomyopathy, Takotsubo syndrome, Apical ballooning syndrome
Introduction
As of 25 November 2020, COVID-19 has affected 59,204,902 people and 13,97,139 death with CFR of 2.35% globally as per WHO Situation Report. Emerging evidence suggests that COVID-19 not only affects the respiratory system but can affect multiple organ systems [1]. Takotsubo syndrome (TTS) is a reversible cardiac condition, characterized by acute left ventricular dysfunction usually in the setting of physical or emotional stress [2]. In a study, Shi et al. reported a high burden of myocardial injury (19.7–27.8%), contributing to significantly high mortality reported in these patients [3]. COVID-19 patients with cardiovascular injury have been reported to have a high burden of underlying cardiovascular comorbidities [3, 4]. One of the recent cohorts has reported an evidence of substantial (78%) cardiac involvement in magnetic resonance imaging (MRI) among 100 German patients, who were diagnosed, treated, and recovered from COVID-19 illness. Compared to risk factor matched controls, COVID-19 patients had lower left ventricular ejection fraction (LVEF), higher left ventricle (LV) volume, high sensitive troponin T (hsTnT), and higher MRI markers of inflammation (T1 and T2 measures, 60%) and enhancement at a median time of 71 days after diagnosis [5]. Current evidence suggests that fulminant cases of COVID-19 might have a cytokine storm or cytokine releasing syndrome, demonstrated by elevation of inflammatory cytokines [6]. Hyperinflammatory states can lead to acute stress and injury, characterized by elevated markers of inflammation and/or myocardial injury such as C-reactive protein, pro-calcitonin, creatine kinase, myoglobin, troponins, and N-terminal pro b-type natriuretic peptide (NT-proBNP) in these patients [6]. It has been observed that cytokine release syndrome is often accompanied by catecholamine surge [7], which can predispose to TTS in COVID-19 patients. Due to paucity of data on TTS in COVID-19, we aimed to perform a systemic review of individual case reports and case series of TTS in COVID-19 patients.
Method
Search Strategy
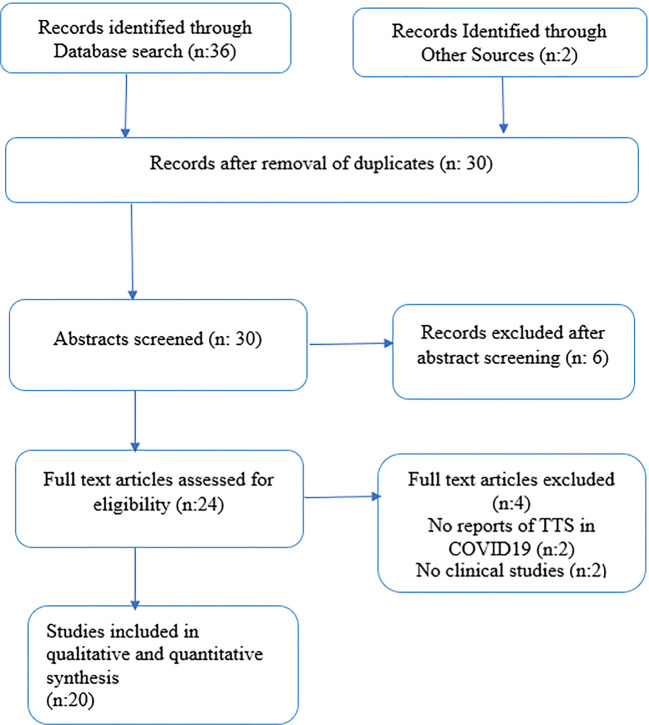
Online databases including PubMed, Google scholar, Web of Science, and the Cochrane Central Register of Controlled Trials were retrospectively searched until inception. Search strategy followed Preferred Reporting Items for Systematic Reviews and Meta-Analyze (PRISMA) guidelines by using MeSH and keywords like “Stress cardiomyopathy”, “Takotsubo syndrome”, Takotsubo cardiomyopathy”, “Broken heart syndrome”, “COVID-19″, and “SARS-COV-2″. The search items were combined using Boolean operators (“OR”; “AND”). No filters including language, country of publication, and type of articles, including abstracts and posters, were applied. The references of individual case reports were sifted to find any relevant cases. The available results were downloaded into an EndNote library. The full search strategy is shown in the PRISMA diagram (Fig. 1).
Fig. 1.
PRISMA flowchart of study selection
Study Selection
We selected all these case reports and case series. Two authors (H.D and K.S) independently reviewed the abstracts, titles, and types of studies that meet eligibility criteria during phase 1. The disagreements were resolved by consensus with a third author (D.J). The second phase of the search included full text review of articles to enable identification of items for data extraction based on the inclusion criteria. Data from the article were curated and summarized in the form of country of origin, age, gender, clinical features on admission, PMH/ comorbidities, complication during hospitalization, triggering events, medical intervention during hospitalization, and their outcome. Analysis and data collection were performed using Microsoft Excel software.
Results
The search yielded 27 patients (case reports n = 17; case series n = 3). The “baseline demographic, clinical and laboratory characteristics” and “clinical outcomes and medical management “of individual patient data are shown in Table 1 and Table 2, respectively, and case series in Table 3. Of all reported 27 cases, 22 patients’ individual data was incompletely available on search. 55.5% of TTS (n = 15) patients were women and were more than 60 years of age (n = 8; 53.3%). Most of the reported cases were from the USA (43.7%) [8–18], Italy (31.25%) [19–23], and Spain (12.5%) [24, 25], while Belgium [26] and Switzerland [27] contributed 1 case each. Among the cohort, 19 out of 26 (73.07%) patients were discharged, and three were hospitalized due to acute respiratory distress syndrome and on extracorporeal membrane oxygenation or ongoing maternal age. There were 4 (14.81%) deaths in TTS with COVID-19, which is higher than mortality reported in COVID-19 with pre-existing cardiovascular diseases otherwise (14.81% vs 5.8%) [28].
Table 1.
Baseline demographic and clinical characteristics, laboratory markers in COVID-19 patients with TTS
| Author | Country | Age/sex | Comorbidities/PMH | Clinical features during admission | ECG finding | Echocardiograhy/ventriculography/TTE | Troponin | CRP (mg/L) | D-DIMER (ng/ml) | Ejection Fraction (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Roca | Italy | 87/F | Breast cancer | Fever, chills, fatigue, dry cough, SOB | Negative T waves and repolarization phase alteration | Alterations in the left ventricle: apical akinetic expansion (apical ballooning) and hypokinesia of the mid-ventricular segments | 5.318 ng/mL | 205.6 | – | 48 |
| Moderato | Italy | 59/F | HTN, DM, obesity, and anxiety disorders | Fever, acute dyspnea, chest pain | Lateral elevation of the ST tract with lateral giant symmetric negative T waves in front and elongated QTc (511 ms) | Apical akinesia with “apical ballooning” as per TTE | 1.137 ng/mL | 160 | – | 40–45 |
| Debbagh | USA | 67/F | Non-ischemic cardiomyopathy with LVEF 15% managed with improvement of 40% pericardial effusion | Left shoulder pain, cough, SOB, worsening dyspnea and orthopnea | Deep T wave inversion in precordial leads (V2 to V6) | Hypokinesis of the apical and periapical walls | 2.410 ng/mL | 159 | 6520 | 40 |
| Fadi | USA | 52/M | Fever, shortness of breath | ST segment elevations in the inferior leads—II, III, aVF; | Apical ballooning on ventriculography | <0.015 ng/mL | 276 | 3450 | 45 | |
| Minhas | USA | 58/F | DM type 2, HTN, and dyslipidemia | Fever, fatigue, productive cough, diarrhea, SOB | Sinus tachycardia and 1-mm upsloping ST segment elevations in leads 1 and aVL, mild diffuse PR interval | Akinetic middle to distal anterior, anteroseptal, anterolateral, and apical segments, moderately hypokinetic middle and distal inferolateral segments, and hyperdynamic basal segments. Apical ballooning and distal third or apical right ventricular (RV) free wall was akinetic, with hyperdynamic RV basal wall motion | 11.02 ng/mL | – | – | 20 |
| Nguyen | Belgium | 71/F | Hypercholesterolemia, normotensive hydrocephalus with VP shunt, HTN | Dyspnea, afebrile, fainting | ECG showed sinus rhythm with prolonged QT interval (QTc 521 ms) | Ventriculogaram: regional wall motion abnormality unrelated to the coronary lesions, compatible with a median Takotsubo | 0.412 ng/mL | – | – | – |
| Sattar | USA | 67/F | HTN, type 2 DM | Fever, chill, cough, malaise, and myalgias, anxious on examination | T inversion in V1,V3; RBBB; atrial fibrillation at rapid ventricular response | Diffuse anterior wall, apical akinesia, and apical ballooning | 0.423 ng/mL | 222 | 1681 | 30 |
| Tsao | USA | 59/F | Obesity | Fever, chills, fatigue, myalgia, cough | ST segment elevation with non-specific T wave inversion | Severe hypokinesia of mid left ventricular cavity, normal contractility of basal, and apical segment | 1 ng/mL | >300 | 2184 | 36 |
| Sala | Italy | 43/F | None | Chest pain, dyspnea | Mild ST elevation in V1-V2 and aVR, Reciprocal ST depression in V4-V6, QT prolongation | Hypokinesia LV mid and basal segment normal apical contraction S/Oreverse TTS | hs 135 ng/mL | 18 | – | 43 |
| Mayer p | Switzerland | 83/F | HTN | Acute chest pain, dry cough, SOB | ST elevation in precordial leads with T wave inversion | Apical ballooning with hyperkinetic basal segment | 1.142 ng/mL | NR | ||
| Pasqualetto | Italy | 84/M | HTN, DM | Fever, dyspnea, cough, chest pain | NR | Apical ballooning with basal wall hypercontractility and systolic dysfunction | hs 70 ng/mL | 168.8, | 1381 | 53 |
| 85/F | HTN | Fever, dyspnea, cough, chest pain | 647 ng/mL | 170.9 | 1227 | 30 | ||||
| 81/M | HTN, DM | Fever, dyspnea, cough, chest pain | 596 ng/mL | 190.4 | 3340 | 42 | ||||
| Kariyanna | USA | 72/F | Obesity, DM, HTN, hyperlipidemia, penicillin allergy | Dry cough, loss of appetite, STROKE | Q waves in V1-V2 leads suggestive of septal infarct and Q waves with ST segment elevation V3,V4,V5, and deep T wave inversion in V6 | Apical ballooning with basal wall hypercontractility and systolic dysfunction | 4250 ng/mL | 270 | 6518 | 30 |
| S Lopez | Spain | 50/M | Benign mediastina tumor | Fever, dyspnea, cough, chest pain | 2-mm ST segment elevation in the inferior and lateral leads |
LV angiography: presented basal segment akinesia and hypercontractility of the mid-apical segments with elevated diastolic pressure s/o Inverted TTS TTE: akinesia of all basal segments |
64 ng/mL | – | – | 42 |
| Recalde | Spain | 50/M | Copper metabolism disorder, benign right mediastinal tumor | Fever, dyspnea, cough | Sinus tachycardia with lateral ST segment elevation, 2 mm |
No LV dilation, akinesis of all basal segments and hypercontractility of mid-apical segments Normal right chambers No pericardial effusion Ventriculography shows an inverted takotsubo pattern |
64.1 ng/mL | 379.5 | 2442 | NR |
| Bernardi | Italy | 74/M | Hypertension, dyslipidemia, and impaired fasting blood sugar | Fever, dyspnea, cough | ST segment elevation in anterolateral leads |
TTE: Dilated left ventricle with akinesis of the mid and apical ventricle segments with hyperkinesis of the basal segments and severe systolic dysfunction. + first grade diastolic dysfunction, partial LVOT obstruction, SAM of mitral valve, a/w severe MR + 2 large apical thrombotic formations: the posterior one was elongated (maximum: 31 mm) and mobile, and the anterior one was wide and oval |
0.775 ng/mL | 14.2 mg/l | 2931 | 30 |
| Bopat A | USA | 61/F | HTN | Exertional dyspnea,tachycardic | T wave inversions (V3-V6) with progressive deepening of TWI and progressive Prolongstion in QT interval, ST elevations and biphasic T | apical hypokinesis | 10 ng/L | 138.4 mg/L | – | 61 |
| Chitturi K | USA | 65/F | HTN, DM, hyperlipidemia, obesity, ischemic heart disease | Fever, dyspnea, dry cough | T wave inversion in leads V1-V2 and QTc 457 ms | severe global right and left ventricular hypokinesis with paradoxical septal motion | 1.9 ng/mL | 368.2 | 20,000 | 25 |
| Faqihi F | USA | 40/M | – | Chest pain, fatigue, myalgia, dry cough, dyspnea | Non-specific ST segment and T wave abnormalities in the precordial leads | basal and midventricular LV akinesia with apical sparing and “ace of spades” configuration typical of RTTC | 4.7 ng/mL | 82.5 | Normal | 30 |
| Juusela A | USA | 45/F | Obesity and materal age | Fever, tachycardia | Non-specific T wave abnormalities | Global hypokinesis | 0.9 ng/mL | – | – | 40 |
| 26/F | PCOD, maternal age | SOB, dyspnea | – | – | 76.8 | 40 |
NR not reported, M male, F female, TTE transthoracic echocardiography, HTN hypertension, DM diabetes mellitus, TTS Takotsubo syndrome, LV left ventricle, EF ejection fraction, MR mitral regurgitation, SAM systemic anterior motion, S/O signs of, SOB shortness of breath
Table 2.
Clinical outcomes and medical management in COVID-19 patients with TTS
| Author | Triggering events | Complications | Time from symptoms onset to TTS (days) | Total hospital length of stay (days) | Outcome | Medical management |
|---|---|---|---|---|---|---|
| Roca | Pneumonia and SARS-COV-2 | Hypoxemia | 14 | NR | Recovered | Azithromycin, ceftriaxone, methylprednisolone, bisoprolol, and fondaparinux |
| Moderato | Agitated and anxiety disorder | ARDS | 7 | 10 | Recovered | IV beta-blocker, diuretic, nitrate, HCQ, darunavir cobicistat, azithromycin, LMWH |
| Dabbagh | Pericardiocentesis | Cardiac tamponade | 12 | NR | Recovered | Pericardiocentesis, elective intubation, HCQ, colchicine, low-dose glucocorticoids |
| Fadi | Altered mental status, schizophrenia | ARDS, acute respiratory failure | NR | NR | Recovered | Colchicine, methylprednisolone, intravenous continuous heparin infusion |
| Minhas | – | ARDS, cardiogenic shock | 5 | 6 | Recovered | Antiplatelet therapy and anticoagulation with continuous intravenous heparin and discontinued HCQ, dobutamine |
| Nguyen | – | Hypoxemia | NR | NR | NR | Mechanical ventilation, two drugs eluting stents |
| Sattar | Anxious | Atrial fibrillation | 14 | NR | Recovered | HCQ, azithromycin, aspirin, clopidogrel, high dose statin, amiodarone, rivaroxaban |
| Tsao | – | Hypoxemia, monomorphic VT | 1 | NR | Recovered | Supportive care, IV norepinephrine, vasopressin, lidocaine |
| Sala | – | 3 | 13 | Recovered | HCQ, lopinavir/ritonavir | |
| Mayer p | Emotional stress | Heart failure | 4 | 10 | Recovered | Conventional heart failure medication |
| Pasqualetto | – | Hypertensive crisis | 10 | NR | Recovered | Antiviral, HCQ, fondaparinux, aspirin, nitroglycerine, metoprolol |
| – | Septic shock, respiratory failure | 10 | NR | Death | Antiviral, HCQ, fondaparinux, aspirin, inotropic support | |
| – | – | 10 | NR | Recovered | Antiviral, HCQ, fondaparinux, aspirin, metoprolol | |
| Kariyanna | Stroke and SARS-COV-2 | Cardiogenic shock | 4 | NR | Death | Low-dose aspirin, and high-intensity statin therapy azithromycin, plaquenil, aztreonam, gentamicin, vasopressin, dopamine, norepinephrine, epinephrine, dobutamine |
| S Lopez | – | Cardiogenic shock | 8 | NR | Recovered | – |
| Recalde | – | Mixed shock–cardiogenic and septic | 8 | 11 | Recovered | HCQ, lopinavir-ritonavir, tocilizumab, azithromycin, methylprednisolone |
| Bernardi | – | LV thrombi, cardiogenic shock-hypotension | – | 21 days | Recovered |
Initially: azithromycin hydroxychloroquine, and dexamethasone + enoxaparin, intravenous dobutaminee |
| Bopat A | SARS-COV-2 infection | Hypoxic respiratory failure | 4 | 18 | Recovered | Hydroxychloroquine, remdesivir, or biologic monoclonal antibodies, norepinephrine, metoprolol |
| Chitturi K | SARS-COV-2 infection | Multisystem organ failure, biventricular heart failure, AKI, hepatitis, ARDS, | 8 | – | Recovered | Remdesivir, norepi, vasopresin, dobutamine, inhaled epoprostenol, hydrocortison, tocilizumab |
| Faqihi F | Cardiogenic Shock | – | 17 | Recovered | Norepi, proning, lopinavir-ritonavir, enoxaparine, milrinone, esmolol, hydrocortisone | |
| Juusela A | Preeclampsia, acute heart failure | – | – | Hospitalized | Magnesium sulfate, methylprednisolone, norepinephrine, hydroxychloroquine, tocilizumab, CPR | |
| Supraventricular tachycardia | Hospitalized | Metoprolol, antibiotics |
TTS Takotsubo syndrome, VT ventricular tachycardia, LV left ventricle, ARDS acute respiratory distress syndrome, LMWH low molecular weight heparin, HCQ hydroxychloroquine, NR/(−) data not reported
Table 3.
Clinical and laboratory characteristics and outcome in COVID-19 with TTS of case series
| Variables | Values in median/n (% or IQR) |
|---|---|
| Age, years | 57 (39–65) |
| Male n (%) | 5 (100%) |
| Clinical features | |
| Dyspnea n (%) | 4 (80%) |
| Chest Pain n (%) | 1 (20%) |
| Cardiac and inflammatory markers | |
| Troponin I (ng/mL) | 11.4 (0.55–12.55) |
| D-dimer (microgram/mL) | 1.8 (1.3–11.5) |
| CRP (mg/dL) | 207 (162–277) |
| CK-MB (ng/mL) | 26.9 (23.9–101.6) |
| ECG and echocardiographic finding | |
| Wall motion abnormalities n (%) | 5(100%) |
| Right ventricular dysfunction n (%) | 1 (20%) |
| Pericardial effusion | 1 (20%) |
| ST Segment elevation n (%) | 2 (40%) |
| T wave inversion n (%) | 1 (20%) |
| Sinus tachycardia n (%) | 1 (20%) |
| In-hospital complication | |
| ARDS n (%) | 4 (80%) |
| AKI n (%) | 4 (80%) |
| Outcome | |
| Recovered and Discharged n (%) | 2 (40%) |
| Hospitalized n (%) | 1 (20%) |
| Death n (%) | 2 (40%) |
IQR interquartile range, ECG electrocardiography, ARDS acute respiratory destress syndrome, AKI acute kidney injury, CRP C-reactive protein, CK-MB creatine-kinase MB
Case Series
Case series of Giustino G and colleagues reported 5 male patients of TTS with COVID-19 positive. Reported median age was 57 years (IQR: 39–65). The study noted that median time from symptoms onset to TTS diagnosis was 6.5 days (IQR: 1.0–8.0), with median value of troponin,1.4 ng/mL (IQR: 0.55–12.55); CK-MB, 26.9 ng/mL; BNP, 153 pg/mL; CRP, 207 mg/dL; and IL, 6–56 pg/mL. Ejection fraction was 36% (IQR: 35–37). Four out 5 (80%) patients suffer from acute kidney injury and acute respiratory distress syndrome during hospitalization (Table 3).
Individual Reported Cases of TTS in COVID-19
For other 22 individual patient’s data, calculated median age was 62.95 years (IQR: 50.5–73.5). The time interval from the symptom onset to TTS diagnosis was 6.7 days (IQR: 4–10). Out of these 22 cases of TTS, comorbidities reported included hypertension (54.5%), diabetes (36.6%), psychiatric illness (9.1%), malignancy (13.6%), dyslipidemia or hyperlipidemia (18.1%), and obesity (22.7%), and two each had ischemic cardiomyopathy and PCOD (4.54%). Twelve out of 22 (54.5%) patients had cardiac complications such as atrial fibrillation, pericardial effusion, cardiogenic shock, heart failure, supraventricular tachycardia, and biventricular heart failure. Twenty out of 22 (90.9%) patients reported an elevated troponin T/I, reduced left ventricular ejection fraction with a median of 38.15% (IQR: 30%–42.5%) [male: 40.33% (IQR: 33–44.2)] and female [37.15% (IQR: 30–40)]. 17/22 (77.27%) reported an elevated CRP. Eleven patients also reported an elevated D-dimer with a median of 2686.5 ng/mL (IQR: 1606–4217). 5/22 (22.72%) reported an elevated IL-6 of mean 350.46 pg/mL (range: 67–724.38). One patient had both right and left ventricular wall motion abnormalities. All patient had specific abnormalities on ECG and cardiac imaging suggestive of TTS. Eleven out of 22 (50%) patients were treated with anticoagulation, nine out of 22 (40.9%) needed vasopressors during hospitalization, and seven (31.8%) were treated with beta-blocker or diuretics. 5/22 (22.72%) were administered tocilizumab. Nineteen out of 22 (86.3%) had improvement either clinically or on repeat echocardiography from LV dysfunction.
Discussion
This is the first ever systemic review of COVID-19 patients with Takotsubo syndrome. As COVID-19 pandemic progresses globally, clear picture of cardiovascular manifestation and associated complications is emerging. Takotsubo syndrome is one of the cardiovascular complications reported in literature among COVID-19 patients. Furthermore, studies also reported increasing incidence of development of stress cardiomyopathy due to COVID-19 pandemic in COVID-19-negative patients [29]. In our own previous study, we had reported male gender is equally affected by TTS with COVID-19 [30]. The current study confirms that TTS equally involves both genders in COVID-19 patients unlike TTS without COVID-19 (male 44.5% and female 55.5% vs male 10.2% and female 89.8%) that might be due to COVID-19 affects predominantly male gender as per current evidence [2]. TTS is found to be more prevalent in postmenopausal women among females. This may be due to possible decreased levels of estrogens, as had been evident in animal models which reported that estrogen may have protective effects on cardiac myocyte by downregulation of adrenoreceptors, hypothalamo-sympathoadrenal axis, and rise in cardioprotective substance such as atrial natriuretic peptide [31, 32]. It is hypothesized that development of TTS in COVID-19 could be due to acute stress leading to surge in the catecholamines. In this study, we have observed that various physical and emotional triggers were captured in development of TTS in COVID-19, such as infection due to SARS-CoV-2, psychiatric illness (schizophrenia and anxiety disorder), stroke, medical procedure (pericardiocentesis), and COVID-19 pneumonia [8, 9, 13, 19, 20]. Majority of patients (88.8%) developed various complications during hospitalization such as ARDS, cardiogenic or septic shock, pericardial effusion, acute kidney injury, hypoxemia, atrial fibrillation, cardiac asystole, hypertensive crisis, and heart failure, supraventricular tachycardia, preeclampsia, and biventricular ventricular failure. Emotional factors such as fear of severity of COVID-19 itself, contact with hospitalized family member, worry about socioeconomic costs, nightmares, and intrusive thoughts of COVID-19 related to morbidity all may lead to central sympathetic hyperactivation and may contribute in development of stress syndrome/Takotsubo syndrome.
One cohort study has found a significant increase in the incidence of TTS during the COVID-19 pandemic and reported that these patients had significantly longer median hospital stay than pre-pandemic era (8 days vs 4–5 days) [33]. Conditions of acute stress leading to central sympathetic activation have been suggested as potential pathophysiological mechanisms [34]. Hypercytokinemia is a hallmark of SARS-CoV-2 as it has been reported that COVID-19 is often a state of both immunodeficiency and hyperinflammation, with the latter getting manifested by a cytokine storm [6]. The elevated cytokine levels may also be responsible for the lethal complications of COVID-19 which are significantly associated with mortality. Studies have reported that rise in pro-inflammatory cytokines such as IL-6, IL-10, TNF-a, and IFN-y are observed in TTS and are associated with increase in-hospital adverse events (< 0.001) and mortality (< 0.05) [35, 36]. These itself can present with an increase in catecholamine levels [37], and this response itself can induce myocardial inflammation [38]. We have observed that patients with underlying cardiovascular comorbidities such as non-ischemic cardiomyopathy, hypertension, and diabetes had an elevation of pro-inflammatory cytokines such as IL-6.
This study also reports that majority of TTS patients had an elevated CRP level, and this finding was consistent with the study done by Morel et al. [39], which suggests that inflammatory markers might be related to impairment of left ventricular function. Furthermore, significant elevation of troponin and ECG changes suggestive of myocardial injury was also noted in almost all patients except one case which had normal Troponin level.
Current evidence suggests that D-dimer level significantly increases with increasing severity of COVID-19, which correlates with thrombogenic mellieu. Battrawy et al. [40] stated that patients with elevated C-reactive protein, D-dimers, and impaired left ventricular function have increased risk of development of thromboembolism in TTS patients. One case report of TTS with COVID-19 has reported a thrombus formation in left ventricle, which was successfully managed with enoxaparin [23]. Study done by Zhou et al. [41] has reported that patients who were treated with heparin had lower 28-day mortality compared to those who were not. In this review, we observed that (77.2%) patients of TTS with COVID-19 had an elevated C-reactive protein and/or D-dimer. Majority of patients were treated with anticoagulation (such as low molecular weight heparin, fondaparinux, rivaroxaban, clopidogrel). Oral anticoagulation therapy should be considered in high-risk COVID-19 patients with TTS such as older age group and those with reduced LVEF. Furthermore, ten out of 17 (58.8%) were treated with HCQ, five out of 17 (29.4%) with antivirals, five out of 17 (29.4%) with vasopressors, and 4 patients (23.5%) were treated with beta-blocker. Five patients were treated with tocilizumab (IL-6 inhibitor). We observed that all patients who were treated with tocilizumab had improvement either clinically or in laboratory findings. A larger study may aid in assessing benefits of tocilizumab in high-risk cardiac COVID-19 patients and who have a significantly elevated inflammatory markers such as CRP, IL-6.
In current study, cardiac imaging demonstrated apical ballooning in most of the cases [9, 19, 20, 22, 27], apical hypo/akinesia with or without basal hyperkinesia [8, 10, 20], basal or mid and basal segment hypo/akinesia [10, 19, 24], and median TTS [26] characteristic of TTS variants. Four (14.81%) patients have reported a reverse or inverted Takotsubo syndrome on echocardiography or ventriculography [16, 21, 24, 25]. One patient reported both right and left ventricular wall motion abnormalities [10]. Furthermore, Majority of the patients reported non-specific ST or T wave changes {such as ST segment elevation in Inferior leads (II, III, aVF) or septal leads (V1, V2) or lateral leads (1 and aVL) with or without T wave depression in precordial leads} followed by diffuse PR interval and QTc prolongation. A wide range of cardiovascular complication reported on electrocardiographic changes in COVID-19 patients such as acute coronary syndromes, rhythm disorders, non-specific ST-T wave changes or ischemic changes, acute pericarditis, pulmonary embolism, and left ventricular hypertrophy [42]. Therefore, focused cardiac ultrasound study and critical care echocardiography performed at bedside are effective options to screen for cardiovascular complications of COVID-19 infection.
Even though most of cases recovered and were discharged (74.1%) successfully, ten out of 27 (37.03%) patients developed at least one of the cardiac complication such as cardiogenic shock, atrial fibrillation, heart failure, supraventricular tachycardia, or biventricular heart failure. We observed that patients with cardiogenic shock had 33.3% (2/6) mortality. Prognosis of TTS in COVID-19 patients depends on underlying triggering events as it has been reported that physical triggers in older age group could lead to worse outcomes due to larger extent of myocardial damage, and the cohort had higher mortality rate, while those with emotional trigger had better outcomes in the cohort [43, 44]. The prognosis of TTS in COVID-19 patients was worse with 14.8% mortality as compared to 5.8% mortality in COVID-19 patients having pre-existing cardiovascular disease without TTS. This can partially be explained by physical trigger such as COVID-19/SARS-Cov-2 and underlying comorbidities [13, 28].
Our study was limited by small sample size, publication bias, missing data, and lack of generalizability in demographics of the series analyzed.
Conclusion
Though the current evidence suggests that male gender is one the risk factor in COVID-19, however, there was no major gender differences observed in development of TTS in COVID-19. This may reflect females especially postmenopausal age group having an increased risk of development of TTS, thus yielding almost similar prevalence rates of TTS in COVID-19. Older median age among TTS in COVID-19 patients irrespective of cardiovascular comorbidities and gender probably reflects age as an independent risk factor. Patients with TTS in COVID-19 have higher mortality rate, more so among those who developed cardiogenic shock. Early diagnosis and management of TTS in COVID-19 may decrease in-hospital mortality and cardiovascular complication. All high-risk patients with COVID-19 should be screened for TTS, and optimal treatment may be considered with antiplatelet medication, statin, and beta-blockers if indicated.
Authors’ Contribution
Dr. Hardik D. Desai and Dr. Kamal Sharma have equally contributed.
Data Availability
Not Applicable.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not Applicable.
Consent for Publication
Not applicable.
Code Availability
Not Applicable.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kamal Sharma, Email: drkamalsharma@unmicrc.org.
Hardik D. Desai, Email: Hardikdesai198@yahoo.com
Jaimini V. Patoliya, Email: Jaiminipatoliya20@gmail.com
Dhigishaba M. Jadeja, Email: Dhigishabajadeja@gmail.com
Dhruv Gadhiya, Email: Dhruavgadhiya1992@gmail.com.
References
- 1.Spuntarelli V, Luciani M, Bentivegna E, Marini V, Falangone F, Conforti G, Rachele ES, Martelletti P. COVID-19: is it just a lung disease? a case-based review. SN Compr Clin Med. 2020;28:1–6. doi: 10.1007/s42399-020-00418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) [published online ahead of print, 2020 Jul 27]. JAMA Cardiol 2020;e203557. doi:10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed]
- 6.Mehta P, McAuley D, Brown M, Sanchez E, Tattersall R, Manson J, et al. COVID-19: consider cytokine storm syndromes and immufnosuppression HLH across speciality collaboration, UK. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staedtke V, Bai R-Y, Kim K, Darvas M, Davila ML, Riggins GJ, Rothman PB, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564:273–277. doi: 10.1038/s41586-018-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabbagh M, Aurora L, D’Souza P, Weinmann A, Bhargava P, Basir M, et al. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2:1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taza F, Zulty M, Kanwal A, Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13:e236561. doi: 10.1136/bcr-2020-236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minhas A, Scheel P, Garibaldi B, Liu G, Horton M, et al. Takotsubo syndrome in the setting of COVID-19. JACC Case Rep. 2020;2:1321–1325. doi: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sattar Y, Connerney M, Ullah W, Philippou A, Slack D, McCarthy B, Kroll S, Luddington S, Maya T et al COVID-19 presenting as takotsubo cardiomyopathy complicated with atrial fibrillation IJC H&V August 2020,100580 doi:10.1016/j.ijcha.2020.100580 [DOI] [PMC free article] [PubMed]
- 12.Tsao CW, Strom JB, Chang JD, Manning WJ. COVID-19-associated stress (Takotsubo) cardiomyopathy. Circ Cardiovasc Imaging. 2020;13(7):e011222. doi: 10.1161/CIRCIMAGING.120.011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariyanna PT, Chandrakumar HP, Jayarangaiah A, et al. Apical Takotsubo cardiomyopathy in a COVID-19 patient presenting with stroke: a case report and pathophysiologic insights. Am J Med Case Rep. 2020;8(10):350–357. doi: 10.12691/ajmcr-8-10-8. [DOI] [Google Scholar]
- 14.Bapat A, Maan A, Heist EK. Stress-induced cardiomyopathy secondary to COVID-19. Case Rep Cardiol. 2020;2020:8842150. doi: 10.1155/2020/8842150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chitturi K, Tacker S, Saadi M, et al. Successful treatment of heart failure in COVID-19 induced cytokine storm with tocilizumab: a case report. Eur HF J Case Rep. 10.1093/ehjcr/ytaa188. [DOI] [PMC free article] [PubMed]
- 16.Faqihi F, Alharthy A, Alshaya R, Papanikolaou J, Kutsogiannis DJ, Brindley PG, Karakitsos D. Reverse Takotsubo cardiomyopathy in fulminant COVID-19 associated with cytokine release syndrome and resolution following therapeutic plasma exchange: a case-report. BMC Cardiovasc Disord. 2020;20(1):389. doi: 10.1186/s12872-020-01665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juusela A, Nazir M, Gimovsky M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020;2(2):100113. doi: 10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giustino G, Croft LB, Oates CP, Rahman K, Lerakis S, Reddy VY, Goldman M. Takotsubo cardiomyopathy in males with Covid-19. J Am Coll Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roca E, Lombardi C, Campana M, et al. Takotsubo syndrome associated with COVID-19. Eur J Case Rep Intern Med. 2020;7(5):001665. doi: 10.12890/2020_001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moderato L, Monello A, Lazzeroni D, et al. Sindrome Takotsubo in corso di polmonite da SARS-CoV-2: una possibile complicanza cardiovascolare. G Ital Cardiol (Rome) 2020;21(6):417–420. doi: 10.1714/3359.33323. [DOI] [PubMed] [Google Scholar]
- 21.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, de Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasqualetto MC, Secco E, Nizzetto M, et al. Stress cardiomyopathy in COVID-19 disease. Eur J Case Rep Intern Med. 2020;7(6):001718. doi: 10.12890/2020_001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardi N, Calvi E, Cimino G, Pascariello G, Nardi M, Cani D, Faggiano P et al COVID-19 pneumonia, takotsubo syndrome, and left ventricle thrombi heart failure and imaging JACC 2020 15th July. 1359–1364 doi: 10.1016/j.jaccas.2020.06.008 [DOI] [PMC free article] [PubMed]
- 24.Solano-López J, Sánchez-Recalde A, Zamorano JL, SARS-CoV-2, a novel virus with an unusual cardiac feature: inverted takotsubo syndrome, Eur Heart J, ehaa390, doi:10.1093/eurheartj/ehaa390 [DOI] [PMC free article] [PubMed]
- 25.Sánchez-Recalde Á, Solano-López J, Miguelena-Hycka J, Martín-Pinacho JJ, Sanmartín M, Zamorano JL. COVID-19 and cardiogenic shock. Different cardiovascular presentations with high mortality. Rev Esp Cardiol (Engl Ed). 2020;73(8):669–672. doi:10.1016/j.rec.2020.04.012 [DOI] [PMC free article] [PubMed]
- 26.Nguyen D, Nguyen T, De Bels D, Castro Rodriguez J. A case of Takotsubo cardiomyopathy with COVID 19 [published online ahead of print, 2020 May 12]. Eur Heart J Cardiovasc Imaging. 2020;jeaa152. doi:10.1093/ehjci/jeaa152 [DOI] [PMC free article] [PubMed]
- 27.Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41(19):1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra M, Desai S, Kuy S, Henry T, Patel A, et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Desai HD, Sharma K, Jadeja DM, Desai HM, Moliya P. COVID-19 pandemic induced stress cardiomyopathy: a literature review. Int J Cardiol Heart Vasc. 2020;31:100628. doi: 10.1016/j.ijcha.2020.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai HD, Jadeja DM, Sharma K. Takotsubo syndrome a rare entity in patients with COVID-19: an updated review of case-reports and case-series. IJC Heart Vasc. 2020;29:100604. doi: 10.1016/j.ijcha.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003; 42 Suppl 1:S117–9. [DOI] [PubMed]
- 32.Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci. 2008;1148:479–485. doi: 10.1196/annals.1410.079. [DOI] [PubMed] [Google Scholar]
- 33.Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa’N A, Hedrick DP, Sleik KM, Mehta N, Chung MK, Khot UN, Kapadia SR, Puri R, Reed GW. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(7):e2014780. doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 35.Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, Spath N, Yucel-Finn A, Yuecel R, Oldroyd K, Dospinescu C, Horgan G, Broadhurst P, Henning A, Newby DE, Semple S, Wilson HM, Dawson DK. Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation. 2019;139(13):1581–1592. doi: 10.1161/CIRCULATIONAHA.118.037975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoro F, Tarantino N, Ferraretti A, Ieva R, Musaico F, Guastafierro F, di Martino L, di Biase M, Brunetti ND. Serum interleukin 6 and 10 levels in Takotsubo cardiomyopathy: increased admission levels may predict adverse events at follow-up. Atherosclerosis. 2016;254:28–34. doi: 10.1016/j.atherosclerosis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 38.Roghi A, Pedrotti P, Milazzo A, Bonacina E, Bucciarelli-Ducci C. Adrenergic myocarditis in pheochromocytoma. J Cardiovasc Magn Reson. 2011;13:4. doi: 10.1186/1532-429X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morel O, Sauer F, Imperiale A, Cimarelli S, Blondet C, Jesel L, Trinh A, de Poli F, Ohlmann P, Constantinesco A, Bareiss P. Importance of inflammation and neurohumoral activation in Takotsubo cardiomyopathy. J Card Fail. 2009;15(3):206–213. doi: 10.1016/j.cardfail.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 40.El-Battrawy I, Borggrefe M, Akin I. Takotsubo syndrome and embolic events. Heart Fail Clin. 2016;12(4):543–550. doi: 10.1016/j.hfc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angeli F, Spanevello A, De Ponti R, Visca D, Marazzato J, Palmiotto G, Feci D, Reboldi G, Fabbri LM, Verdecchia P. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. 2020;78:101–106. doi: 10.1016/j.ejim.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Lüscher TF, Templin C, for the International Takotsubo (InterTAK) Registry Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: data from the international Takotsubo registry. JAMA Cardiol. 2016;1:335–340. doi: 10.1001/jamacardio.2016.0225. [DOI] [PubMed] [Google Scholar]
- 44.Pelliccia F, Pasceri V, Patti G, Tanzilli G, Speciale G, Gaudio C, Camici PG. Long-term prognosis and outcome predictors in Takotsubo syndrome: a systematic review and meta-regression study. JACC Heart Fail. 2019;7(2):143–154. doi: 10.1016/j.jchf.2018.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.