Abstract
Hemothorax is a collection of blood in the pleural cavity usually from traumatic injury. Chest X-ray has historically been the imaging modality of choice upon arrival to the hospital. The sensitivity and specificity of point-of-care ultrasound, specifically through the Extended Focal Assessment with Sonography in Trauma (eFAST) protocol has been significant enough to warrant inclusion in most Level 1 trauma centers as an adjunct to radiographs.1,2 If the size or severity of a hemothorax warrants intervention, tube thoracostomy has been and still remains the treatment of choice. Most cases of hemothorax will resolve with tube thoracostomy. If residual blood remains within the pleural cavity after tube thoracostomy, it is then considered to be a retained hemothorax, with significant risks for developing late complications such as empyema and fibrothorax. Once late complications occur, morbidity and mortality increase dramatically and the only definitive treatment is surgery. In order to avoid surgery, research has been focused on removing a retained hemothorax before it progresses pathologically. The most promising therapy consists of fibrinolytics which are infused into the pleural space, disrupting the hemothorax, allowing for further drainage. While significant progress has been made, additional trials are needed to further define the dosing and pharmacokinetics of fibrinolytics in this setting. If medical therapy and early procedures fail to resolve the retained hemothorax, surgery is usually indicated. Surgery historically consisted solely of thoracotomy, but has been largely replaced in non-emergent situations by video-assisted thoracoscopy (VATS), a minimally invasive technique that shows considerable improvement in the patients’ recovery and pain post-operatively. Should all prior attempts to resolve the hemothorax fail, then open thoracotomy may be indicated.
Introduction
Hemothorax has been consistently defined as a collection of blood in the pleural space or a pleural fluid hematocrit of greater than 50%.3,4 It is estimated that 300,000 cases of hemothorax occur annually in the United States5, the majority of which are due to thoracic trauma.4 Although more infrequent, spontaneous and iatrogenic or vascular causes of hemothorax may also occur. Hemorrhage leading to a hemothorax can originate from the chest wall, intercostal vasculature, internal mammary arteries, great vessels, mediastinum, myocardium, lung parenchyma, diaphragm, or abdomen.4,6-9 Management options range from observation to percutaneous drainage [tube thoracostomy, thoracentesis] and/or surgery [Video-Assisted Thoracic Surgery (VATS) or open thoracotomy]. There has been increasing use and reported success with instillation of intrapleural fibrinolytic therapy (IPTF) for retained hemothorax to degrade clotted blood and expedite percutaneous drainage in order to avoid more invasive surgical procedures.
A hematocrit value of pleural fluid below 50% does not necessarily rule out hemothorax. It has been reported that a hemothorax may become diluted with pleural fluid in as little as 3-4 days, mimicking a hemorrhagic effusion with a hematocrit between 25-50%.3,10 Additionally, pleural fluid appears to be clinically indistinguishable from blood at a hematocrit greater than 5%.10 Therefore, it may be prudent to examine a patient more closely for a source of hemorrhage, even when the hematocrit is below 50%.
The severity of a hemothorax is classified according to the amount of blood present within the pleural cavity. Intrapleural blood less than 400 ml is classified as a minimal hemothorax, while 400 ml to 1000 ml is a medium hemothorax. Anything greater than 1000 ml is considered a massive hemothorax.11,12 The clinical consequences may reflect the acuity of the bleeding or exacerbate underlying comorbidities including anemia or coronary artery disease. Most patients who receive appropriate and timely care for hemothorax will have good outcomes with no long term disabilities.
Epidemiology and Etiology
There are three major etiologic categories of hemothorax: spontaneous, iatrogenic, and traumatic. Thoracic trauma is the most common cause4,13-15 contributing to approximately 16,000–30,000 deaths per year16,17 This is followed by iatrogenic, and least commonly, spontaneous.18 In elderly patients the reported incidence is: blunt trauma 73.3%, iatrogenic trauma 25.0%, and spontaneous 1.7%.19 All three origins of hemothorax can potentially involve major arteries such as the aorta, intercostal arteries, and internal mammary arteries leading to tension hemothorax, exsanguination and death.
Spontaneous Hemothorax
Due to the infrequency and variety of causes of spontaneous hemothorax, prior reviews have had to focus on case reports and small series within the literature.3,10,18,20 The most common cause of spontaneous hemothorax is spontaneous pneumothorax.10,21,22 A review of 29 articles by Patrini, et al.3 organized the etiology of spontaneous hemothorax into four main categories: coagulopathic, vascular, neoplastic, and miscellaneous.
Coagulopathic etiologies consist of congenital diseases such as hemophilia or thrombocytopenia, or occur with therapeutic anticoagulation. Most cases of spontaneous hemothorax related to anticoagulation occur following treatment of pulmonary emboli, usually with heparin or warfarin.10,18 Cases in which enoxaparin and tissue plasminogen activator (tPA) were administered have also occurred.23,24
Vascular origins are due to rupture or dissection of the descending thoracic aorta in patients with atherosclerosis or uncontrolled hypertension.25 Genetic diseases such as Ehlers-Danlos Syndrome and Rendu-Osler-Weber Syndrome are associated with spontaneous hemothorax due to physiological alterations of the vasculature.3,10
Various neoplasias are associated with spontaneous hemothorax through various mechanisms including spontaneous rupture of a tumor or direct invasion of vasculature in or near the pleural cavity. Janik, et al.,25 reviewed 145 publications and found 54 cases of spontaneous hemothorax caused by neoplasia, with the largest subset of those related to Von Recklinghausen Disease (neurofibromatosis). Other reported causes include costal exostoses, endometriosis, pulmonary sequestration, infections, pulmonary infarction, pancreatitis, tubal pregnancies, and extramedullary hematopoiesis.3,10,18
Iatrogenic Hemothorax
Iatrogenic hemothorax occurs during surgery or procedures when the lung parenchyma, surrounding vessels, or organs are inadvertently damaged. Thoracentesis, central venous catheterization, tube thoracostomy, and thoracotomy may all result in iatrogenic hemothorax. Some of these procedures are also used in the process to treat hemothorax. Central venous catheterization, specifically subclavian venous catheterization, and placement of chest tubes are among the most common iatrogenic causes of hemothorax,26-30 with a reported incidence of approximately 1%.31 The rate of complications from central venous catheterization is directly related to the experience of the operator,32-36 the anatomic location of insertion,32,37,38 and the use of ultrasound guidance.35,39 Thoracentesis has a reported incidence of hemothorax from 0.1%−0.4%.40,41 The incidence of hemothorax in the elderly is increased secondary to a higher incidence of intercostal artery tortuosity.40
Traumatic Hemothorax
Traumatic hemothorax can be due to either blunt or penetrating injury. Thoracic injuries occur in approximately 60%4,14 of all polytrauma cases and are responsible for 15–30%16,42 of all trauma mortalities.14,43 Hemothorax, hemopneumothorax, and pneumothorax are the most common complications of either penetrating or blunt thoracic trauma, with a frequency ranging from 10–37%.14-16,42,44-47 Hemothorax from blunt thoracic trauma has an overall mortality of 9.4% and is the leading cause of death in the fourth decade of life.48,49 Gunshots and stabbings are the primary means of penetrating thoracic trauma,11,45,50 whereas 70–80% of blunt thoracic trauma is caused by motor vehicle crashes.14,15,42 While blunt thoracic trauma typically occurs far more frequently, mortality is significantly higher in penetrating chest trauma, with up to 90% not surviving transport to the hospital.51 Should penetrating trauma also involve the heart, survival is less than 1%.51 The mortality of penetrating chest trauma can also depend upon the means of injury. Gunshot wounds have a higher mortality than stab wounds.52,53 Additionally, the severity of injuries sustained in trauma correlate with increased complications from hemothorax, as well as mortality.54,55
The distribution of trauma etiology can change rapidly in countries with conflict. At one hospital in Maiduguri, Nigeria 61.5% of all thoracic trauma was penetrating in origin.56 During the Bosnia-Herzegovina conflict 94.9% of thoracic trauma in one hospital was penetrating in origin.57 Most recently, Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have several reports listing penetrating origins in thoracic trauma to be between 88–91%.58-62 The mechanisms of penetrating thoracic injuries have also changed with time, with over 90% of penetrating thoracic injuries being due to gunshots during the civil war, whereas explosions were responsible for 81% of penetrating thoracic trauma in OEF/OIF.61
Detection of Hemothorax
Chest radiography (CXR) has traditionally been the initial tool in the emergency room for rapid evaluation of thoracic injury.14,63-65 Upright CXR is preferred, because in the supine position the blood will be distributed along the entire posterior aspect of the affected pleural space rather than the diaphragmatic surface. This causes the hemothorax to be less apparent, as there is no blunting of the costophrenic angle. In upright radiography, blunting of the costophrenic angle is the most common sign of hemothorax and pleural effusions. The collection of fluid can be altered significantly if there are any intrapleural adhesions, which will cause any blood or fluid to occupy any space available.14
CXR has well known limitations. 300–500 mls of blood is required to blunt the costophrenic angle. This is especially true if the patient is in the supine position, because blood up to 1000 mls can be overlooked.66 Since most severely injured patients arrive in the supine position, and upright CXRs cannot be safely performed, the amount of blood in the chest is often underestimated, and occasionally missed completely. Upright CXR cannot be used to accurately quantify the volume of hemothorax. (See Figures 1 and 2).
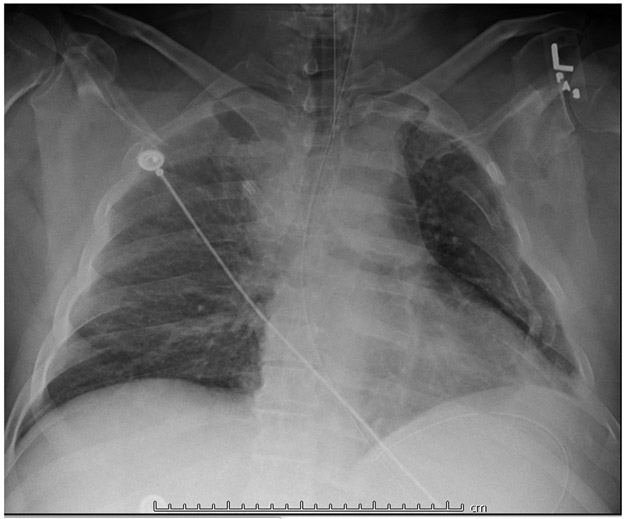
Figure 1.
Patient # 1. Chest X-ray showing multiple L rib fractures. Patient is lying supine, intubated, on mechanical ventilation. The X-ray does not conclusively show a hemothorax.
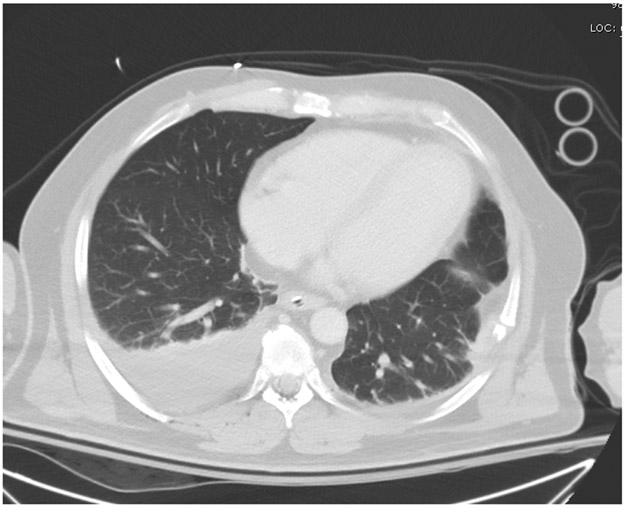
Figure 2.
Thoracic CT scan, Patient # 1. Patient surprisingly had no significant blood in the left hemithorax, but 800 ml of blood from a right hemothorax was drained via tube thoracostomy.
Ultrasound/Fast/eFAST
The Focused Assessment with Sonography in Trauma (FAST) is a bedside test developed in the mid 1990’s for use in acute trauma patients to rapidly assess for intra-abdominal hemorrhage and to rule out clinically significant pericardial tamponade. It is performed by imaging in the sagittal plane the right and left upper quadrants of the abdomen, the suprapubic region in both sagittal and coronal planes, and a subxyphoid or parasternal view of the pericardium. The Extended Focused Assessment with Sonography in Trauma (eFast) adds additional views of the hemithoraces to look for signs of pneumothorax and hemothorax. These include the right and left pleural spaces (anterior axillary line between 6th and 9th intercostal spaces), and left and right anterior pleural spaces (midclavicular line between 2nd and 3rd intercostal spaces).1,2 Multiple investigators report the utility of chest ultrasonography in diagnosing traumatic thoracic injuries.67-74 Ultrasound can quantify volume, detecting 100 ml of pleural fluid with 100% accuracy,67 and as little as 20 ml.75 Specifically related to hemothorax, a recent meta analaysis71 indicates ultrasound has an overall sensitivity of 67% and specificity of 99%, with multiple studies indicating a sensitivity over 90%.68,76 Ultrasound has consistently been shown to be more sensitive than chest radiography for the detection of hemothorax.67,68,74,77,78 Additional benefits to the use of ultrasound include the ability to be used in a pre-hospital setting, portability, reproducibility, non-invasiveness, and employs no contrast or radiation.
Despite the increasing use of ultrasound for diagnosis of traumatic injuries, there are some limitations. The sensitivity of ultrasound is influenced by the experience of the operator and the frequency of the transducer.71,73 While ultrasound typically has a higher sensitivity than chest radiography for detecting hemothoraces, it is not as sensitive as computed tomography2,72,74 and can miss other findings such as mediastinal hematomas.79 (See Figure 3.)
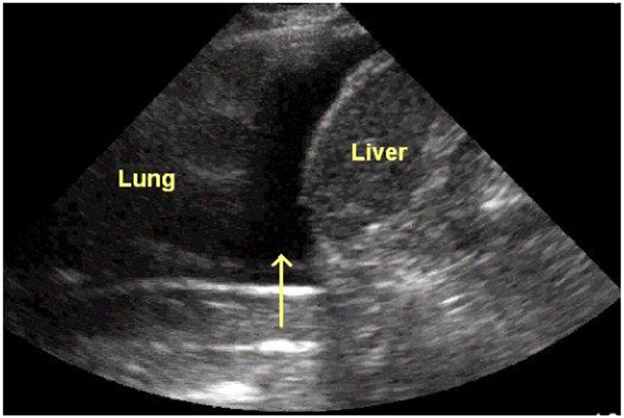
Figure 3.
Sagittal view of the right upper quadrant and lower right hemithorax. The dark area depicted
By the arrow is diagnostic for either fluid or blood in the right hemithorax.
Computed Tomography
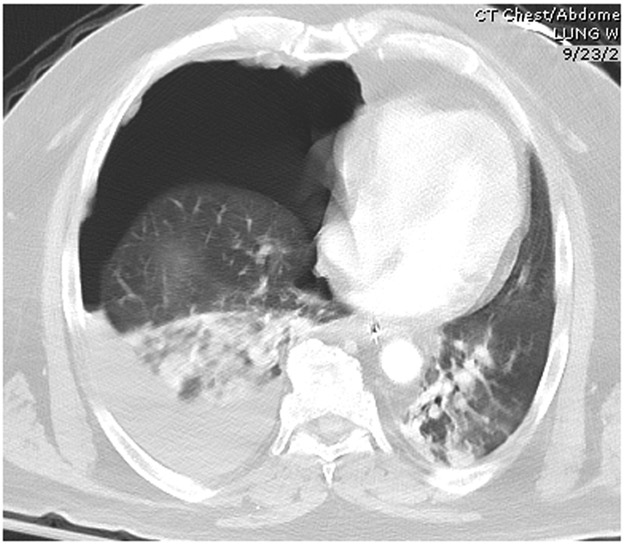
Chest computed tomography (CT) is commonly used to detect additional injuries following chest radiograph and bedside sonography.80-82 The patient may initially be studied using CXR and possibly ultrasound to quickly evaluate the extent of injuries, especially in cases of hemodynamic instability.50 Once stability is achieved, a chest CT, preferably with intravenous contrast, can be performed to evaluate further pathology in detail.65,83-86 CT will identify additional injuries in 20–30% of patients with an initial abnormal CXR. In cases of hemothorax, CT can reveal a collection of blood missed by the chest radiograph.87,88 (See Figure 4 and Figure 5).
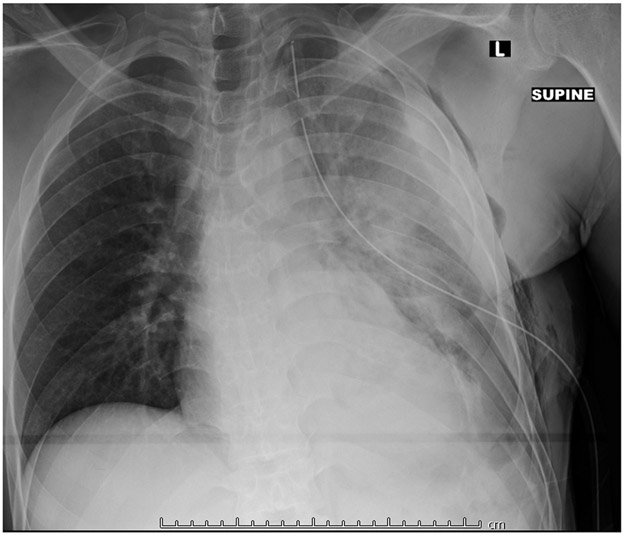
Figure 4.
Chest X-ray showing a massive L hemothorax following blunt trauma from a motor vehicle crash. A tube thoracostomy has already been performed, but the hemothorax is incompletely evacuated.
Figure 5.
CT scan of the chest demonstrating large right hemopneumothorax. Note motion artifact blurring right heart border and thoracic contents
Acute Hemothorax: Early Management
Early identification of patients at risk for hemothorax following trauma significantly decreases morbidity and mortality.89-91 Life threatening injuries require prompt attention either by thoracostomy or in severe cases, thoracotomy. Appropriate management of any patient with suspected hemothorax depends on their hemodynamic status. The Advanced Trauma Life Support Course, sponsored and updated by the American College of Surgeons,92 provides practitioners with guidelines for the appropriate management of chest trauma, including hemothorax. Detailed management of hemothorax is beyond the scope of this review.
Delayed Hemothorax: Recognition and Management
The definition of delayed hemothorax has been described in two different ways. The first definition is any hemothorax that occurs 24h after initial imaging is negative.42 The second definition proposed by Ritter et al.7 states that a delayed hemothorax is any hemothorax that occurs after negative initial imaging regardless of elapsed time.7 Delayed hemothorax has been reported to occur anywhere from 2 hours to 44 days after initial presentation.7,93 Rib fractures are the most common cause of delayed hemothorax and are reported to occur in 30–80%94-96 of patients with thoracic trauma. 94,96-99 Since rib fractures have been found to be associated with both acute and delayed hemothorax, some investigators advocate surgical fixation to improve outcomes in terms of respiratory failure and delayed complications such as recurrent and retained hemothorax, and chronic pain.100-103 Rib fixation remains incompletely studied and controversial. 104
CURRENT TREATMENT OPTIONS:
Expectant Management
Conservative (no intervention) management is determined by the overall severity of the chest injury and/or the acuity of blood loss. If the patient is hemodynamically stable, and the hemothorax is confirmed to be less than 300 ml, conservative management with observation, aggressive respiratory care and pain control can be attempted. 17,105-111 In several studies volumes greater than 260–300 ml were up to 4x more likely to require drainage. 105,106,109,111 Patients with hemothorax volumes less than 260–300cc were 72%−92% likely to resolve without significant complication and with no intervention. Patients that eventually required drainage were more likely to experience an increase in size of the hemothorax, hemodynamic instability or increasing respiratory distress.105,109 In some reports, the only patients with occult hemothorax that developed an empyema were those treated with tube thoracostomy.106,110 If observation is elected, it is suggested that early and frequent imaging (CXR) be repeated during the first 24 hours to ensure stability of the hemothorax and to detect any other potential pathologies. 17,107,108
Needle Thoracentesis
Needle thoracentesis (also called thoracocentesis) has historically been used to evacuate hemothoraces, and is still often used to diagnose new onset pleural effusions suspicious for malignancy or infection.112,113 Thoracentesis is still a common therapeutic method used for the removal of symptomatic pleural effusions.114,115 At present, needle aspiration for the treatment of hemothorax is considered obsolete,17 though small, clinically insignificant collections may still be treated with needle thoracocentesis at the discretion of the physician.116
Tube Thoracostomy
Tube Thoracostomy is the primary intervention for hemothorax. According to international practice management guidelines, all hemothoraces regardless of size should be considered for tube thoracostomy. 117 Small (occult) hemothoraces and pneumothoraces identified only by CT scan are frequently treated expectantly, though a chest tube may be used at the discretion of the physician. Most moderate sized and all massive hemothoraces should be treated with tube thoracostomy. 45,47,118-120 If treated early with adequate drainage, the need for additional surgery for retained or recurrent hemothoraces should be relatively low (10–15%).47,51,118-124 Typically, bloody effusions that remain after tube thoracostomy are reabsorbed in 4–5 weeks without complication. 123
It has been recommended that large bore tubes (28–40 Fr) be used for hemothorax evacuation to better quantify blood loss, and provide a conduit that will remain open and less likely to obstruct from clotting.125,126 However, tube size is based more on surgeon preference and personal experience than rigorous scientific evidence.127,128 Multiple reports have suggested that smaller bore tubes may be just as effective as larger bore tubes. Bore tube sizes as small 14 Fr have been compared to 28 Fr tubes and found to have similar performance.129-131 Interestingly, there is invitro data suggesting that the viscosity of the pleural fluid should be a factor in determining the choice of tube size.132
Once placed, it is very important to continually re-examine the patient and follow the output of the chest tube for signs of ongoing bleeding.133 The initial amount of suction applied to the chest tube remains a matter of debate, but most practitioners apply at least 20 to 40 cm of water negative pressure to the tube using various commercially available devices for collection and fluid measurement. Recent studies suggest that initially placing the chest tube to suction rather than water seal may be associated with improved outcome regarding duration of chest tube therapy, length of hospital stay, persistent air leak,134 and risk of emyema135 Savage et al.136 believe that the benefits of initial suction evacuation are not significant with the exception being significant protection from recurrent pneumothorax. A chest X-ray should be repeated after tube insertion to confirm the proper position.4 Reevaluation of the patient and monitoring of chest tube output should be done every hour for the first 24 hours after installation.
Complication rates from thoracostomy have been reported to be between 6–37%.26,137,138 Proper training is required to perform tube thoracostomy correctly and safely.51,139 Malpositioning is the most common complication following tube thoracostomy, especially during an emergency.27,28,138 Dislodgement, infection, and laceration/perforation of organs may also occur.51 Bleeding can be very serious, and may be due to laceration of an intercostal vein or artery.40,140 Intrapulmonary placement is also a potential adverse event, occurring in up to 38% of thoracostomy complications when caregivers with minimal experience perform the procedure.141
Drainage from the hemithorax should in general be less than 100 ml in 24 hours before removal of the chest tube. Conversely, it has been reported that chest tubes can be removed with output as high as 2 ml/kg without additional intervention required.142 A repeat chest radiograph should be performed prior and after removal to ensure complete lung re-expansion with no residual or recurrent pneumothorax.143 Once discharged it is prudent to re-examine the patient in 1–2 weeks. Some reports suggest that repeat CXR may not be necessary due to low recurrence of hemothorax or pneumothorax.144
Video-Assisted Thoracic Surgery (VATS)
VATS as a means to perform surgery for multiple thoracic complications was developed during the 1990s, and is now accepted and widely applied by trauma surgeons.145-147 This was in no small part due to the improving technology in endoscopes. VATS was initially developed to treat post operative hemothorax, but quickly was adapted to use for major lung resections.148,149 In early trials, VATS conferred lower morbidity, better visualization, and a higher yield of detecting small injuries with less post-operative pain compared to thoracotomy. Improved lung function and the ability to evacuate and decorticate a lung following empyema were added benefits. Most patients also recovered quicker from surgery.13,89,145,149-151 Single lung anesthesia is required for VATS and takes added time to implement. Thus, a major limiting factor of VATS is that the patient must be hemodynamically stable.48,89,123,124,145,150,152-154 The patient can be actively bleeding during VATS as long as they remain hemodynamically stable.10
The timing of VATS is also very important when evacuating a hemothorax. Within 48–72 hours, VATS is usually successful for hemothorax evacuation and allows for early re-expansion of the lung with reduction in short and long term morbidity and mortality.123,152,153 Performing VATS on the sixth post-trauma day or later is associated with a 15.8% possibility of conversion to an open thoracotomy. This delay results in development of pleural thickening and dense adhesions.13,145 Some report that even up to the seventh day there remains significant benefit in performing VATS over thoracotomy,107,155-157 but after seven days complications rise significantly.152,158-160
Uniportal VATS is a relatively new option in thoracic surgery and was originally proposed by two teams desiring to develop a minimally invasive thoracic surgical technique involving only a single incision.161,162 One of the major benefits of Uniportal VATS is less pain secondary to rib retraction for multiple trocars.163-165 Uniportal VATS retains many of the benefits of multiportal VATS for hemothorax evacuation including excellent visibility, decreased hospital length of stay, and avoidance of excess bleeding.166 Placement of the incision is very important in uniportal VATS in order to have the best access for intrathoracic procedures and inspection. Several different approaches have been reported based on the indication.167-171 In multiple studies, there has been no significant difference found between multiport VATS and uniportal VATS with respect to morbidity, mortality, cardiopulmonary complications, and wound infection. These findings support the idea that this procedure is a suitable and safe option for evacuating a hemothorax.163,172-174
Thoracotomy
Thoracotomy is a surgical procedure where the chest wall is incised, and a self-retaining retractor is used to gain access to the thoracic cavity. If the patient is actively bleeding into the thoracic cavity and remains hemodynamically unstable despite initial attempts at resuscitation, thoracotomy is the procedure of choice.175 If the patient can be stabilized, VATS may be considered first.4 Thoracotomy has a reported incidence of 2.6%−6% in all chest trauma cases.47,176 In chest trauma cases involving hemothorax, thoracotomy has been reported to be performed 10% of the time, especially in patients with penetrating chest trauma.11,177
In contrast to the traditional lateral approach in elective surgery, the anterior thoracotomy approach has several indications for emergent hemorrhage control. The first indications for thoracotomy occur during the primary survey. If the patient presents in shock, has a chest wall defect, airway obstruction, tension pneumothorax, pericardial tamponade, or flail chest emergency thoracotomy should be performed. Significant output from a chest tube is also an indication for thoracotomy and is defined as 1000–1500 mls total in 24 hours or 200–500 mls/hour for 2 to 3 consecutive hours.117,124 Specific internal injuries may also be indication for immediate thoracotomy including esophageal injury, diaphragmatic injury, cardiac injury, massive hemothorax and great vessel injury. Thoracotomy is also indicated if the patient develops fibrothorax or high-stage empyema.10,11,46,50,107,154,177,178
The standard approach in emergent thoracotomy is the anterolateral approach which provides an excellent view of the thoracic cavity.47 In some types of injuries, the anterolateral approach may not be sufficient. In this case, either the clamshell or hemiclamshell approach may be used.179 The hemiclamshell approach is also named the cervico-sterno-thoracotomy and involves an arch-shaped incision that begins at the manubrium of the sternum, extends down the midline of the sternum and then curves laterally along the posterior border of the pectoralis major, ending in the midaxiallary region.180 The clamshell approach repeats the process on the opposite side of the chest, exposing the entire thoracic cavity.
There are times when a patient with severe trauma should not receive emergency thoracotomy. It has been found that patients with severe blunt trauma, emergency thoracotomy rarely results in successful resuscitation.181-186 Of those patients in shock upon arrival to the ED, neurologically intact survival ranging from 1.5%−5%, and survival is less than 1% if there are no vital signs. Of those that receive cardiopulmonary resuscitation, 1.5% or less of patients survive neurologically intact with CPR lasting greater than 5–15 minutes.184 Therefore, it has been recommended that emergent thoracotomy not be performed in patients with no signs of life upon arrival to the hospital.187-191
Prophylactic Antibiotics
Prophylactic antibiotics remain a contested issue in virtually every area of medicine, especially with the recent push for antimicrobial stewardship. This situation remains the same when treating hemothorax.192-196 The previous consensus is that a dose of antibiotics is better than none.193 Multiple publications have shown some benefit by using prophylactic antibiotics in patients with hemothoraces. It has been shown that the use of antibiotics at least 24 hours after the start of chest tube drainage for hemothorax can reduce en incidence of pneumonia from 14.8% to 4.1%, and the incidence of empyema from 8.7% to 0.8%.192,193,195,197-199 In contrast, there have been reports that do not show significant reduction in pneumonia or empyema in traumatic hemopenumothorax.192 Other reports have determined there is no significant difference in rates of empyema if the patient were receiving antibiotics for the entirety of chest tube duration, or for only the first 48 hours.179
The duration of antibiotic treatment and recommendations vary from 24 hours after chest tube removal to the point of chest tube removal. Twenty four hours of antibiotic treatment, typically first generation cephalosporins, is advised in traumatic hemothorax within the first 24 hours following chest tube placement.193 Antibiotics should be directed towards Staphyloccus aureus and Streptococcus species which have been shown to grow from empyema cultures with some regularity.179,193 A guideline in 2012 published by Moore. et al200 was not able to make a recommendation on presumptive antibiotics with the evidence to that date. Most recently, a large multi-center study involving 1887 patients determined that there were no significant differences in the primary complications of empyema or pneumonia, as well as no significant difference in secondary measures including length of stay, mortality, Clostridium difficile colitis, or days requiring mechanical ventilation.201
The Life Cycle of Hemothorax: Resolution, Retained Hemothorax, Empyema, and Fibrothorax
There are two different physiologic stages of hemothorax resolution: early and late.17 Some have speculated that an early defibrination of the hemothorax may occur with an increased pleural fluid protein concentration and a corresponding increase in intrapleural hyperosmotic pressure. This promotes the development of a pleural effusion.202,203 As a hemothorax remains within the pleural cavity, it will typically complete spontaneous reabsorption within several weeks, especially if the volume is under 300mls.19,147,204,205 If it does not reabsorb, it will become a retained hemothorax (RH). RH has been defined as blood occupying at least one-third of the pleural space which cannot be drained by thoracostomy after 72 hours or clots of at least 500 mls volume.123,124 RH can begin to form as early as 24 hours after chest tube placement.205 The incidence of retained hemothorax is estimated between 5–30%, based on the results of multiple studies, of which a large percentage requires surgical evacuation.145,202,206 In a level I large trauma service in East Texas that is affiliated with the University of Texas Center of Tyler, the experience has been similar. As there were 1154 trauma centers in the US as of 2002207, the annual incidence of traumatic RH in the US could approach 14,000 cases per year. The incidence of RH in malignant pleural effusions (MPE) is unknown, but with 150,000 cases of MPE annually in the US125, additional cases of RH may occur in a population in which hemostasis is often impaired and for which chest surgery is often contraindicated.
The consequences of RH include fibrothorax, trapped lung or high rates of the development of subsequent empyema; 26.8 percent and pneumonia; 19.5 percent208. Predictors of the development of empyema after RH in trauma patients include an Injury Severity Score of 25 or greater, rib fractures and use of multiple interventions to alleviate the RH209
While the pathogenesis of organizing RH has not been well-delineated in the literature appears to involve derangements of local coagulation and fibrinolysis, as occurs in other forms of pleural organization210,211. The organization of blood in the pleural space involves initial coagulation, which may be due to procoagulants including tissue factor that is expressed by mesothelial and other cells within the pleural compartment212,213. These cells also express plasminogen activators and their inhibitors, including plasminogen activator inhibitor-1, which is strongly implicated in the pathogenesis of pleural organization211,213,214. Intrapleural organization and fibrosis is favored by suppression of local fibrinolysis that occurs concurrently with increased expression of procoagulant activity211. These derangement of pathways of fibrin turnover favor extravascular fibrin deposition within the pleural space and provide a rationale for the use of intrapleural fibrinolytic therapy (IPFT) to lyse RH and thereby avoid its complications.
Evacuating RH early is critical due to the complications that can occur within the late phase of physiological resolution. This has been reported multiple times when VATS was used to evacuate the retained hemothorax, resulting in favorable clinical outcomes.154,178,205,215-217 It has been reported that as many as 25% of patients with a retained hemothorax will require multiple evacuation attempts.208 But if it is managed appropriately, prognosis is usually excellent.
RH can undergo progressive organization over several days to become an empyema or fibrothorax. The overall incidence of empyema and fibrothorax in cases of retained hemothorax have been estimated at 5% and 1%, respectively.66 Hemothoraces that fail to drain after initial tube thoracostomy may occur in about 20% of cases, 15–30% of those remaining will develop subsequent empyema or fibrothorax.151,209,218 Failure to evacuate the hemothorax in these cases may be due to malposition or poor drainage of the chest tubes, which can influenced by the experience of the clinician.51,139,219 Surgical exploration is an indicated treatment option when either empyema or fibrothorax are diagnosed.46,154,178,215 Development of empyema and fibrothorax can lead to lung entrapment or collapse, potentially leading to respiratory failure.123,135,202
Empyema can occur as a complication of RH due to primary or secondary bacterial contamination of a hemothorax and can originate from broncho-tracheal lesions, esophageal injuries, penetrating missiles, long-standing clotted thoracostomy tube, and post-surgical exposure.17,187 Independent risk factors for empyema are elevated injury score or failure to administer periprocedural antibiotics during tube placement.198,220
Fibrothorax results from organization of the fibrinous neomatrix that coats both the parietal and visceral pleural surfaces.221 Adhesion of these surfaces; pleurodesis, traps the lung in position and prevents full expansion leading to persistent atelectasis and restricted pulmonary function. Fibrothorax often requires surgery, with decortication of the visceral pleura being the therapy of choice to provide lung re-expansion.175,179,221,222
Intrapleural Fibrinolytic Therapy (IPFT) for retained hemothorax
A recent meta-analysis of randomized and nonrandomized controlled trials in the literature suggests that IPFT, now commonly used for non-draining loculated parapneumonic pleural effusions of empyema211, may be effective and well-tolerated for RH, leading to calls for more advanced clinical trial testing223. The use of any fibrinolytic; plasminogen activator agent was found to obviate the need for surgery in 87 percent of patients in this meta-analysis. The use of intrapleural tPA resulted in avoidance of surgery in 83 percent of patients, while the use of other agents was slightly better at 87 percent. As is the case in loculated empyema and complicated parapneumonic pleural effusions with failure to drain224, no agent that has been approved for IPFT for the treatment of RH. The issue is potentially important as the rigors of approval by the Food and Drug Administration and other regulatory agencies includes robust assessment of patient safety. While none of the regimens used to date have undergone rigorous evaluation of patient safety, the weight of clinical evidence to date now suggests that IPFT is generally well-tolerated in patients with RH223. While a pharmacologic approach to treatment of RH is inherently desirable, efficacy and tolerability will need to be tested in future randomized, controlled clinical trials. Dose ranging studies will also be needed to define best practices in terms of selection of the agent to be used in IPFT for RH, as well as the doses and schedules that yield optimal outcomes.
Experimental Techniques for the Management of Hemothorax and its Complications
Several unique techniques have been used to treat hemothorax and its related complications. In one report of 20 patients, intrathoracic lavage was performed at the time of thoracostomy tube placement and resulted in improved secondary intervention rates225,226 In another study involving 110 patients with thoracoabdominal trauma and subsequent billiary-gastroenteric contamination, thoracic irrigation was performed through lesions in the diaphragm resulting in decreased rates of intrathoracic complications.227 Pulsed lavage has been used to assist with the extraction of retained hemothorax and empyema.228,229 Intrapleural epinephrine has been used to control bleeding in cases of massive hemothorax.230
Discussion
The acute management of hemothorax has remained relatively stable over the course of decades. The chest tube has been the crux of management for hemothorax nearly since its discovery, and remains so to this day. Current guidelines support attempted drainage of any sized hemothorax, there is significant evidence that small hemothoraces may be managed expectantly and will likely have good outcomes. Thoracentesis has fallen out of favor for drainage of hemothorax, but is still used for diagnostic purposes and drainage of pleural effusions. For severe or complicated hemothoraces thoracotomy was often the procedure of last resort, and still is for patients who are hemodynamically unstable or with significant comorbidities. In those patients who are hemodynamically stable, VATS has steadily become the preferred procedure when surgery is indicated. VATS offers several benefits over thoracotomy including reduction of post operative pain, improved cosmetic results, and fewer post-operative complications. These benefits seemingly have been improved upon with the advent of Uniportal VATS, which uses a solitary access point to perform surgical procedures which can be as complicated as lobectomies.
The major focus of research and innovation in hemothorax management is the prevention and evacuation of retained hemothoraces. By preventing retained hemothoraces, the incidence of late complications such as empyema and fibrothorax are reduced, improving overall morbidity and mortality. The introduction of intrapleural fibrinolytics has been a major area of focus for both retained hemothorax and empyema, and has been used as early as the 1950s. A major benefit of fibrinolytics in addition to the reduction of late complications is the reduction in invasive surgical procedures to evacuate a retained hemothorax. While there are no major guidelines published recommending the use of intrapleural fibrinolytics, a recent meta-analysis by Hendrickson, et al., shows promise. Additional clinical trials and research into the pathophysiology of fibrinolytics will be needed to gain Food and Drug Administration approval for this indication, but the majority of evidence favors the inclusion of this procedure in the algorithm for hemothorax management.
Acknowledgments
Funding:
Dr. Idell is supported in part by NIH R01 HL130402 Idell S, Florova G and Komissarov A, MPI
Footnotes
Conflict of Interest Statement:
Steven Idell, MD, PhD serves as a Founder and Chief Scientific Officer of LTI and a member of the Board of Directors of LTI, which provided funding for preparation of the drug product and for the trial. He has an equity position (first-tier conflict of interest) in the company, as does the University of Texas Horizon Fund and the UTHSCT. He has a conflict-of-interest plan acknowledging and managing these declared conflicts of interest through the UTHSCT and the UT System. He is an inventor on a patent (USPTO 7332469) held by the UT Board of Regents and licensed to LTI.
The remaining authors declare they have no such conflicts of interest.
References
- 1.Bloom B, Gibbons R, Focused Assessment with Sonography for Trauma (FAST). StatPearls Publishing, 2019. at https://www.ncbi.nlm.gov/books/NBK470479/.) [PubMed] [Google Scholar]
- 2.O׳Keeffe M, Clark S, Khosa F, Mohammed MF, McLaughlin PD, Nicolaou S. Imaging Protocols for Trauma Patients: Trauma Series, Extended Focused Assessment With Sonography for Trauma, and Selective and Whole-body Computed Tomography. Semin Roentgenol 2016;51:130–42. [DOI] [PubMed] [Google Scholar]
- 3.Patrini D, Panagiotopoulos N, Pararajasingham J, Gvinianidze L, Iqbal Y, Lawrence DR. Etiology and management of spontaneous haemothorax. J Thorac Dis 2015;7:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med 2010;104:1583–7. [DOI] [PubMed] [Google Scholar]
- 5.Richardson JD, Miller FB, Carrillo EH, Spain DA. Complex thoracic injuries. Surg Clin North Am 1996;76:725–48. [DOI] [PubMed] [Google Scholar]
- 6.Chang WC, Hsu HH, Chang H, Chen CY. Spontaneous hemothorax caused by a ruptured intercostal artery aneurysm in von Recklinghausen’s neurofibromatosis. J Formos Med Assoc 2005;104:286–9. [PubMed] [Google Scholar]
- 7.Ritter DC, Chang FC. Delayed hemothorax resulting from stab wounds to the internal mammary artery. J Trauma 1995;39:586–9. [DOI] [PubMed] [Google Scholar]
- 8.Chien TM, Yen HW, Chen YF. Ruptured acute type B aortic dissection presenting with bilateral hemathoraces. J Card Surg 2011;26:214–6. [DOI] [PubMed] [Google Scholar]
- 9.Tribble JB, Julian S, Myers RT. Rupture of the liver and right hemidiaphragm presenting as right hemothorax. J Trauma 1989;29:116–8. [DOI] [PubMed] [Google Scholar]
- 10.Ali H, Lippmann M, Mundathaje U, Khaleeq G. Spontaneous Hemothorax - A Comprehensive Review. Chest 2008;134:1056–65. [DOI] [PubMed] [Google Scholar]
- 11.Cakmak M Characteristics of the Patients Undergoing Surgical Treatment for Hemothorax: A Descriptive Study. Bio Res 2017;28:2679–83. [Google Scholar]
- 12.Pohnan R, Blazkova S, Hytych V, et al. Treatment of Hemothorax in the Era of Minimally Invasive Surgery. Mil Med Sci Lett 2019;88:1–8. [Google Scholar]
- 13.Ahmad T, Ahmed S, Hussain N, Sheikh K. Thoracoscopic Evacuation of Retained Post-raumatic Hemothorax. J Col Phys Surg Pak 2013;23:234–6. [PubMed] [Google Scholar]
- 14.Broderick SR. Hemothorax: Etiology, diagnosis, and management. Thorac Surg Clin 2013;23:89–96, vi-vii. [DOI] [PubMed] [Google Scholar]
- 15.Liman ST, Kuzucu A, Tastepe AI, Ulasan GN, Topcu S. Chest injury due to blunt trauma. Eur J Cardiothorac Surg 2003;23:374–8. [DOI] [PubMed] [Google Scholar]
- 16.LoCicero J, Mattox KL. Epidemiology of chest trauma. Surg Clin North Am 1989;69:15–9. [DOI] [PubMed] [Google Scholar]
- 17.Mahoozi H, Volmerig J, Hecker E. Modern Management of Traumatic Hemothorax. J Trauma Treat 2016;5:1–5. [Google Scholar]
- 18.Martinez FJ, Villanueva AG, Pickering R, Becker FS, Smith DR. Spontaneous hemothorax. Report of 6 cases and review of the literature. Medicine (Baltimore) 1992;71:354–68. [PubMed] [Google Scholar]
- 19.Schweigert M, Beron M, Dubecz A, Stadlhuber R, Stein H. Video-assisted thoracoscopic surgery for posttraumatic hemothorax in the very elderly. Thorac Cardiovasc Surg 2012;60:474–9. [DOI] [PubMed] [Google Scholar]
- 20.Morgan CK, Bashoura L, Balachandran D, Faiz SA. Spontaneous Hemothorax. Ann Am Thorac Soc 2015;12:1578–82. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CC, Wu YL, Lin HJ, Lin MP, Guo HR. Indicators of haemothorax in patients with spontaneous pneumothorax. Emerg Med J 2005;22:415–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu NY, Shih CS, Hsu CP, Chen PR. Spontaneous hemopneumothorax revisited: clinical approach and systemic review of the literature. Ann Thorac Surg 2005;80:1859–63. [DOI] [PubMed] [Google Scholar]
- 23.Mrug M, Mishra PV, Lusane HC, Cunningham JM, Alpert MA. Hemothorax and retroperitoneal hematoma after anticoagulation with enoxaparin. South Med J 2002;95:936–8. [PubMed] [Google Scholar]
- 24.Varnholt V, Ringe H, Nietsch L, Gaedicke G. Hemothorax under thrombolytic therapy with recombinant tissue: plasminogen activator (rt-PA) in a 16-year-old girl. Eur J Pediatr 1999;158 Suppl 3:S140–2. [DOI] [PubMed] [Google Scholar]
- 25.Janik M, Straka L, Krajcovic J, Hejna P, Hamzik J, Novomesky F. Non-traumatic and spontaneous hemothorax in the setting of forensic medical examination: a systematic literature survey. Forensic Sci Int 2014;236:22–9. [DOI] [PubMed] [Google Scholar]
- 26.Menger R, Telford G, Kim P, et al. Complications following thoracic trauma managed with tube thoracostomy. Injury 2012;43:46–50. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez MC, Laan DV, Zimmerman SL, Naik ND, Schiller HJ, Aho JM. Tube thoracostomy: Increased angle of insertion is associated with complications. J Trauma Acute Care Surg 2016;81:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez MC, El Khatib M, Prokop L, Zielinski MD, Aho JM. Complications in tube thoracostomy: Systematic review and meta-analysis. J Trauma Acute Care Surg 2018;85:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson EM, Saltzman DA, Suh G, Dahms RA, Leonard AS. Complications and risks of central venous catheter placement in children. Surgery 1998;124:911–6. [PubMed] [Google Scholar]
- 30.Bagwell CE, Salzberg AM, Sonnino RE, Haynes JH. Potentially lethal complications of central venous catheter placement. J Pediatr Surg 2000;35:709–13. [DOI] [PubMed] [Google Scholar]
- 31.Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access--a systematic review. Crit Care Med 2002;30:454–60. [DOI] [PubMed] [Google Scholar]
- 32.Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med 2007;35:1390–6. [DOI] [PubMed] [Google Scholar]
- 33.Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med 1986;146:259–61. [DOI] [PubMed] [Google Scholar]
- 34.Fares LG, Block PH, Feldman SD. Improved house staff results with subclavian cannulation. Am Surg 1986;52:108–11. [PubMed] [Google Scholar]
- 35.Rezayat T, Stowell JR, Kendall JL, Turner E, Fox JC, Barjaktarevic I. Ultrasound-Guided Cannulation: Time to Bring Subclavian Central Lines Back. West J Emerg Med 2016;17:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bo-Linn GW, Anderson DJ, Anderson KC, McGoon MD. Percutaneous central venous catheterization performed by medical house officers: a prospective study. Cathet Cardiovasc Diagn 1982;8:23–9. [DOI] [PubMed] [Google Scholar]
- 37.Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med 2015;373:1220–9. [DOI] [PubMed] [Google Scholar]
- 38.Parienti JJ, du Cheyron D, Mermel LA, Group SS. Complications of Central Venous Catheterization. N Engl J Med 2016;374:1491–2. [DOI] [PubMed] [Google Scholar]
- 39.Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med 1996;24:2053–8. [DOI] [PubMed] [Google Scholar]
- 40.Carney M, Ravin CE. Intercostal artery laceration during thoracocentesis: increased risk in elderly patients. Chest 1979;75:520–2. [DOI] [PubMed] [Google Scholar]
- 41.Da Rocha RP, Vengjer A, Blanco A, de Carvalho PT, Mongon ML, Fernandes GJ. Size of the collateral intercostal artery in adults: anatomical considerations in relation to thoracocentesis and thoracoscopy. Surg Radiol Anat 2002;24:23–6. [DOI] [PubMed] [Google Scholar]
- 42.Shorr RM, Crittenden M, Indeck M, Hartunian SL, Rodriguez A. Blunt thoracic trauma. Analysis of 515 patients. Ann Surg 1987;206:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horst K, Andruszkow H, Weber CD, et al. Thoracic trauma now and then: A 10 year experience from 16,773 severely injured patients. PLoS One 2017;12:e0186712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inci I, Ozçelik C, Taçyildiz I, Nizam O, Eren N, Ozgen G. Penetrating chest injuries: unusually high incidence of high-velocity gunshot wounds in civilian practice. World J Surg 1998;22:438–42. [DOI] [PubMed] [Google Scholar]
- 45.Afshar MA, Mangeli F, Nakhaei A. Evaluation of injuries caused by penetrating chest traumas in patients referred to the emergency room. Indian journal of surgery 2015;77:191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer DM. Hemothorax related to trauma. Thorac Surg Clin 2007;17:47–55. [DOI] [PubMed] [Google Scholar]
- 47.Kulshrestha P, Munshi I, Wait R. Profile of chest trauma in a level I trauma center. J Trauma 2004;57:576–81. [DOI] [PubMed] [Google Scholar]
- 48.Goodman M, Lewis J, Guitron J, Reed M, Pritts T, Starnes S. Video-assisted thoracoscopic surgery for acute thoracic trauma. J Emerg Trauma Shock 2013;6:106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meredith JW, Hoth JJ. Thoracic trauma: when and how to intervene. Surg Clin North Am 2007;87:95–118, vii. [DOI] [PubMed] [Google Scholar]
- 50.Bertoglio P, Guerrera F, Viti A, et al. Chest drain and thoracotomy for chest trauma. J Thorac Dis 2019;11:S186–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molnar TF. Thoracic Trauma: Which Chest Tube When and Where? Thorac Surg Clin 2017;27:13–23. [DOI] [PubMed] [Google Scholar]
- 52.Onat S, Ulku R, Avci A, Ates G, Ozcelik C. Urgent thoracotomy for penetrating chest trauma: analysis of 158 patients of a single center. Injury 2011;42:900–4. [DOI] [PubMed] [Google Scholar]
- 53.Clarke DL, Quazi MA, Reddy K, Thomson SR. Emergency operation for penetrating thoracic trauma in a metropolitan surgical service in South Africa. J Thorac Cardiovasc Surg 2011;142:563–8. [DOI] [PubMed] [Google Scholar]
- 54.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147:1659–70. [DOI] [PubMed] [Google Scholar]
- 55.Lichtenstein D Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheff) 2017;13:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.N A, BM G. Pattern and Management of Chest Injuries in Maiduguri, Nigeria. Ann Afr Med 2004;3:181–4. [Google Scholar]
- 57.VanRooyen MJ, Sloan EP, Radvany AE, Perić T, Kuliś B, Tabak V. The incidence and outcome of penetrating and blunt trauma in central Bosnia: the Nova Bila Hospital for War Wounded. J Trauma 1995;38:863–6. [DOI] [PubMed] [Google Scholar]
- 58.Stannard A, Morrison JJ, Scott DJ, Ivatury RA, Ross JD, Rasmussen TE. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J Trauma Acute Care Surg 2013;74:830–4. [DOI] [PubMed] [Google Scholar]
- 59.Schofield J, Johnston AM, de Mello WF. Morphine after combat injury and post-traumatic stress disorder. N Engl J Med 2010;362:1341–2; author reply 2. [PubMed] [Google Scholar]
- 60.Hoencamp R, Vermetten E, Tan EC, Putter H, Leenen LP, Hamming JF. Systematic review of the prevalence and characteristics of battle casualties from NATO coalition forces in Iraq and Afghanistan. Injury 2014;45:1028–34. [DOI] [PubMed] [Google Scholar]
- 61.Belmont PJ, Goodman GP, Zacchilli M, Posner M, Evans C, Owens BD. Incidence and epidemiology of combat injuries sustained during “the surge” portion of operation Iraqi Freedom by a U.S. Army brigade combat team. J Trauma 2010;68:204–10. [DOI] [PubMed] [Google Scholar]
- 62.Belmont PJ, McCriskin BJ, Sieg RN, Burks R, Schoenfeld AJ. Combat wounds in Iraq and Afghanistan from 2005 to 2009. J Trauma Acute Care Surg 2012;73:3–12. [DOI] [PubMed] [Google Scholar]
- 63.Ho ML, Gutierrez FR. Chest radiography in thoracic polytrauma. AJR Am J Roentgenol 2009;192:599–612. [DOI] [PubMed] [Google Scholar]
- 64.Wicky S, Wintermark M, Schnyder P, Capasso P, Denys A. Imaging of blunt chest trauma. Eur Radiol 2000;10:1524–38. [DOI] [PubMed] [Google Scholar]
- 65.Manley NR, Maish GO. Blunt Chest Wall Trauma Clinical Algorithms in General Surgery: Springer; 2019:633–5. [Google Scholar]
- 66.Mancini M, Scanlin T, Serebrisky D, Hemothorax. Medscape, 2019. (Accessed Dec 2, 2019, at https://emedicine.medscape.com/article/2047916-overview.) [Google Scholar]
- 67.Brooks A, Davies B, Smethhurst M, Connolly J. Emergency ultrasound in the acute assessment of haemothorax. Emerg Med J 2004;21:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McEwan K, Thompson P. Ultrasound to detect haemothorax after chest injury. Emerg Med J 2007;24:581–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leblanc D, Bouvet C, Degiovanni F, et al. Early lung ultrasonography predicts the occurrence of acute respiratory distress syndrome in blunt trauma patients. Intensive Care Med 2014;40:1468–74. [DOI] [PubMed] [Google Scholar]
- 70.Brun PM, Bessereau J, Levy D, Billeres X, Fournier N, Kerbaul F. Prehospital ultrasound thoracic examination to improve decision making, triage, and care in blunt trauma. Am J Emerg Med 2014;32:817.e1–2. [DOI] [PubMed] [Google Scholar]
- 71.Rahimi-Movaghar V, Yousefifard M, Ghelichkhani P, et al. Application of Ultrasonography and Radiography in Detection of Hemothorax; a Systematic Review and Meta-Analysis. Emerg (Tehran) 2016;4:116–26. [PMC free article] [PubMed] [Google Scholar]
- 72.Stengel D, Leisterer J, Ferrada P, Ekkernkamp A, Mutze S, Hoenning A. Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. Cochrane Database Syst Rev 2018;12:CD012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abboud PA, Kendall J. Emergency department ultrasound for hemothorax after blunt traumatic injury. J Emerg Med 2003;25:181–4. [DOI] [PubMed] [Google Scholar]
- 74.Hyacinthe AC, Broux C, Francony G, et al. Diagnostic accuracy of ultrasonography in the acute assessment of common thoracic lesions after trauma. Chest 2012;141:1177–83. [DOI] [PubMed] [Google Scholar]
- 75.Röthlin MA, Näf R, Amgwerd M, Candinas D, Frick T, Trentz O. Ultrasound in blunt abdominal and thoracic trauma. The Journal of trauma 1993;34:488–95. [DOI] [PubMed] [Google Scholar]
- 76.Morley EJ, Johnson S, Leibner E, Shahid J. Emergency department evaluation and management of blunt chest and lung trauma (Trauma CME). Emerg Med Pract 2016;18:1–20. [PubMed] [Google Scholar]
- 77.Ebrahimi A, Yousefifard M, Mohammad Kazemi H, et al. Diagnostic Accuracy of Chest Ultrasonography versus Chest Radiography for Identification of Pneumothorax: A Systematic Review and Meta-Analysis. Tanaffos 2014;13:29–40. [PMC free article] [PubMed] [Google Scholar]
- 78.Zieleskiewicz L, Fresco R, Duclos G, et al. Integrating extended focused assessment with sonography for trauma (eFAST) in the initial assessment of severe trauma: Impact on the management of 756 patients. Injury 2018;49:1774–80. [DOI] [PubMed] [Google Scholar]
- 79.Hsu LW, Chong CF, Wang TL, Wu BH. Traumatic mediastinal hematoma: a potentially fatal condition that may be overlooked by traditional Focused Assessment with Sonography for Trauma. Am J Emerg Med 2013;31:262.e1–3. [DOI] [PubMed] [Google Scholar]
- 80.Kea B, Gamarallage R, Vairamuthu H, et al. What is the clinical significance of chest CT when the chest x-ray result is normal in patients with blunt trauma? Am J Emerg Med 2013;31:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez RM, Langdorf MI, Nishijima D, et al. Derivation and validation of two decision instruments for selective chest CT in blunt trauma: a multicenter prospective observational study (NEXUS Chest CT). PLoS Med 2015;12:e1001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez RM, Canseco K, Baumann BM, et al. Pneumothorax and Hemothorax in the Era of Frequent Chest Computed Tomography for the Evaluation of Adult Patients With Blunt Trauma. Ann Emerg Med 2019;73:58–65. [DOI] [PubMed] [Google Scholar]
- 83.Kaewlai R, Avery LL, Asrani AV, Novelline RA. Multidetector CT of blunt thoracic trauma. Radiographics 2008;28:1555–70. [DOI] [PubMed] [Google Scholar]
- 84.Kaewlai R Multidetector-row computed tomography of thoracic trauma. Int J Clin Pract Suppl 2011:3–16. [DOI] [PubMed] [Google Scholar]
- 85.Esposito AA, Zilocchi M, Fasani P, et al. The value of precontrast thoraco-abdominopelvic CT in polytrauma patients. Eur J Radiol 2015;84:1212–8. [DOI] [PubMed] [Google Scholar]
- 86.Strumwasser A, Chong V, Chu E, Victorino GP. Thoracic computed tomography is an effective screening modality in patients with penetrating injuries to the chest. Injury 2016;47:2000–5. [DOI] [PubMed] [Google Scholar]
- 87.Trupka A, Waydhas C, Hallfeldt KK, Nast-Kolb D, Pfeifer KJ, Schweiberer L. Value of thoracic computed tomography in the first assessment of severely injured patients with blunt chest trauma: results of a prospective study. J Trauma 1997;43:405–11; discussion 11–2. [DOI] [PubMed] [Google Scholar]
- 88.Chardoli M, Hasan-Ghaliaee T, Akbari H, Rahimi-Movaghar V. Accuracy of chest radiography versus chest computed tomography in hemodynamically stable patients with blunt chest trauma. Chin J Traumatol 2013;16:351–4. [PubMed] [Google Scholar]
- 89.Ludwig C, Koryllos A. Management of chest trauma. J Thorac Dis 2017;9:S172–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ceran S, Sunam GS, Aribas OK, Gormus N, Solak H. Chest trauma in children. Eur J Cardiothorac Surg 2002;21:57–9. [DOI] [PubMed] [Google Scholar]
- 91.Ekpe EE, Eyo C. Determinants of mortality in chest trauma patients. Niger J Surg 2014;20:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Henry S Advanced Trauma Life Support Student Course Manual. 10 ed. Chicago, IL: American College of Surgeons; 2018. [Google Scholar]
- 93.Yap D, Ng M, Chaudhury M, Mbakada N. Longest delayed hemothorax reported after blunt chest injury. Am J Emerg Med 2018;36:171.e1–.e3. [DOI] [PubMed] [Google Scholar]
- 94.Lin FC, Li RY, Tung YW, Jeng KC, Tsai SC. Morbidity, mortality, associated injuries, and management of traumatic rib fractures. J Chin Med Assoc 2016;79:329–34. [DOI] [PubMed] [Google Scholar]
- 95.Sirmali M, Türüt H, Topçu S, et al. A comprehensive analysis of traumatic rib fractures: morbidity, mortality and management. Eur J Cardiothorac Surg 2003;24:133–8. [DOI] [PubMed] [Google Scholar]
- 96.Sharma OP, Hagler S, Oswanski MF. Prevalence of delayed hemothorax in blunt thoracic trauma. Am Surg 2005;71:481–6. [PubMed] [Google Scholar]
- 97.Chien CY, Chen YH, Han ST, Blaney GN, Huang TS, Chen KF. The number of displaced rib fractures is more predictive for complications in chest trauma patients. Scand J Trauma Resusc Emerg Med 2017;25:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Misthos P, Kakaris S, Sepsas E, Athanassiadi K, Skottis I. A prospective analysis of occult pneumothorax, delayed pneumothorax and delayed hemothorax after minor blunt thoracic trauma. Eur J Cardiothorac Surg 2004;25:859–64. [DOI] [PubMed] [Google Scholar]
- 99.Lin HL, Tarng YW, Wu TH, Huang FD, Huang WY, Chou YP. The advantages of adding rib fixations during VATS for retained hemothorax in serious blunt chest trauma - A prospective cohort study. Int J Surg 2019;65:13–8. [DOI] [PubMed] [Google Scholar]
- 100.Majercik S, Vijayakumar S, Olsen G, et al. Surgical stabilization of severe rib fractures decreases incidence of retained hemothorax and empyema. Am J Surg 2015;210:1112–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 101.Morimoto Y, Sugimoto T, Sakahira H, Matsuoka H, Yoshioka Y, Arase H. Successful management of threatened aortic rupture late after rib fracture caused by blunt chest trauma. Ann Vasc Surg 2014;28:1035.e11–3. [DOI] [PubMed] [Google Scholar]
- 102.Funaki S, Inoue M, Minami M, Okumura M. Video-assisted thoracoscopic resection of fractured ribs to prevent descending aorta injury in patient with chest trauma. Ann Thorac Cardiovasc Surg 2014;20:173–4. [DOI] [PubMed] [Google Scholar]
- 103.Doben AR, Eriksson EA, Denlinger CE, et al. Surgical rib fixation for flail chest deformity improves liberation from mechanical ventilation. J Crit Care 2014;29:139–43. [DOI] [PubMed] [Google Scholar]
- 104.Kasotakis G, Hasenboehler EA, Streib EW, et al. Operative fixation of rib fractures after blunt trauma: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2017;82:618–26. [DOI] [PubMed] [Google Scholar]
- 105.Bilello JF, Davis JW, Lemaster DM. Occult traumatic hemothorax: when can sleeping dogs lie? Am J Surg 2005;190:841–4. [DOI] [PubMed] [Google Scholar]
- 106.Demetri L, Martinez Aguilar MM, Bohnen JD, et al. Is observation for traumatic hemothorax safe? J Trauma Acute Care Surg 2018;84:454–8. [DOI] [PubMed] [Google Scholar]
- 107.Heniford BT, Carrillo EH, Spain DA, Sosa JL, Fulton RL, Richardson JD. The role of thoracoscopy in the management of retained thoracic collections after trauma. Ann Thorac Surg 1997;63:940–3. [DOI] [PubMed] [Google Scholar]
- 108.Zhang M, Teo LT, Goh MH, Leow J, Go KT. Occult pneumothorax in blunt trauma: is there a need for tube thoracostomy? Eur J Trauma Emerg Surg 2016;42:785–90. [DOI] [PubMed] [Google Scholar]
- 109.Mahmood I, Abdelrahman H, Al-Hassani A, Nabir S, Sebastian M, Maull K. Clinical management of occult hemothorax: a prospective study of 81 patients. Am J Surg 2011;201:766–9. [DOI] [PubMed] [Google Scholar]
- 110.Stafford RE, Linn J, Washington L. Incidence and management of occult hemothoraces. Am J Surg 2006;192:722–6. [DOI] [PubMed] [Google Scholar]
- 111.Wells BJ, Roberts DJ, Grondin S, et al. To drain or not to drain? Predictors of tube thoracostomy insertion and outcomes associated with drainage of traumatic hemothoraces. Injury 2015;46:1743–8. [DOI] [PubMed] [Google Scholar]
- 112.Rosenstengel A Pleural infection-current diagnosis and management. J Thorac Dis 2012;4:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010;27:334–47. [DOI] [PubMed] [Google Scholar]
- 114.Argento AC, Murphy TE, Pisani MA, Araujo KL, Puchalski J. Patient-Centered Outcomes Following Thoracentesis. Pleura (Thousand Oaks) 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhuniya S, Arunabha DC, Choudhury S, Saha I, Roy TS, Saha M. Role of therapeutic thoracentesis in tuberculous pleural effusion. Ann Thorac Med 2012;7:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delius RE, Obeid FN, Horst HM, Sorensen VJ, Fath JJ, Bivins BA. Catheter aspiration for simple pneumothorax. Experience with 114 patients. Arch Surg 1989;124:833–6. [DOI] [PubMed] [Google Scholar]
- 117.Mowery NT, Gunter OL, Collier BR, et al. Practice management guidelines for management of hemothorax and occult pneumothorax. J Trauma 2011;70:510–8. [DOI] [PubMed] [Google Scholar]
- 118.Rasmussen OV, Brynitz S, Struve-Christensen E. Thoracic injuries. A review of 93 cases. Scand J Thorac Cardiovasc Surg 1986;20:71–4. [DOI] [PubMed] [Google Scholar]
- 119.Yalçinkaya I, Sayir F, Kurnaz M, Cobanoğlu U. [Chest trauma: analysis of 126 cases]. Ulus Travma Derg 2000;6:288–91. [PubMed] [Google Scholar]
- 120.Villavicencio RT, Aucar JA, Wall MJ. Analysis of thoracoscopy in trauma. Surg Endosc 1999;13:3–9. [DOI] [PubMed] [Google Scholar]
- 121.Kong VY, Oosthuizen GV, Clarke DL. Selective conservatism in the management of thoracic trauma remains appropriate in the 21st century. Ann R Coll Surg Engl 2015;97:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blyth A Thoracic trauma. BMJ 2014;348:g1137. [DOI] [PubMed] [Google Scholar]
- 123.Carrillo EH, Richardson JD. Thoracoscopy in the management of hemothorax and retained blood after trauma. Curr Opin Pulm Med 1998;4:243–6. [DOI] [PubMed] [Google Scholar]
- 124.Casós SR, Richardson JD. Role of thoracoscopy in acute management of chest injury. Curr Opin Crit Care 2006;12:584–9. [DOI] [PubMed] [Google Scholar]
- 125.American Thoracic S. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987–2001. [DOI] [PubMed] [Google Scholar]
- 126.Laws D, Neville E, Duffy J, Pleural Diseases Group SoCC, B.itish Thoracic Society. BTS guidelines for the insertion of a chest drain. Thorax 2003;58 Suppl 2:ii53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shalli S, Saeed D, Fukamachi K, et al. Chest tube selection in cardiac and thoracic surgery: a survey of chest tube-related complications and their management. J Card Surg 2009;24:503–9. [DOI] [PubMed] [Google Scholar]
- 128.Light RW. Pleural controversy: optimal chest tube size for drainage. Respirology 2011;16:244–8. [DOI] [PubMed] [Google Scholar]
- 129.Niinami H, Tabata M, Takeuchi Y, Umezu M. Experimental assessment of the drainage capacity of small silastic chest drains. Asian Cardiovasc Thorac Ann 2006;14:223–6. [DOI] [PubMed] [Google Scholar]
- 130.Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg 2018;42:107–13. [DOI] [PubMed] [Google Scholar]
- 131.Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg 2012;73:1423–7. [DOI] [PubMed] [Google Scholar]
- 132.Park JK, Kraus FC, Haaga JR. Fluid flow during percutaneous drainage procedures: an in vitro study of the effects of fluid viscosity, catheter size, and adjunctive urokinase. AJR Am J Roentgenol 1993;160:165–9. [DOI] [PubMed] [Google Scholar]
- 133.Waydhas C, Sauerland S. Pre-hospital pleural decompression and chest tube placement after blunt trauma: A systematic review. Resuscitation 2007;72:11–25. [DOI] [PubMed] [Google Scholar]
- 134.Feenstra TM, Dickhoff C, Deunk J. Systematic review and meta-analysis of tube thoracostomy following traumatic chest injury; suction versus water seal. Eur J Trauma Emerg Surg 2018;44:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramanathan R, Wolfe LG, Duane TM. Initial suction evacuation of traumatic hemothoraces: a novel approach to decreasing chest tube duration and complications. Am Surg 2012;78:883–7. [PubMed] [Google Scholar]
- 136.Savage SA, Cibulas GA, Ward TA, Davis CA, Croce MA, Zarzaur BL. Suction evacuation of hemothorax: A prospective study. J Trauma Acute Care Surg 2016;81:58–62. [DOI] [PubMed] [Google Scholar]
- 137.Sritharen Y, Hernandez MC, Haddad NN, et al. External Validation of a Tube Thoracostomy Complication Classification System. World J Surg 2018;42:736–41. [DOI] [PubMed] [Google Scholar]
- 138.Filosso PL, Guerrera F, Sandri A, et al. Errors and Complications in Chest Tube Placement. Thorac Surg Clin 2017;27:57–67. [DOI] [PubMed] [Google Scholar]
- 139.Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii61–76. [DOI] [PubMed] [Google Scholar]
- 140.Kesieme EB, Dongo A, Ezemba N, Irekpita E, Jebbin N, Kesieme C. Tube thoracostomy: complications and its management. Pulm Med 2012;2012:256878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harris EA, Arheart KL, Penning DH. Endotracheal tube malposition within the pediatric population: a common event despite clinical evidence of correct placement. Can J Anaesth 2008;55:685–90. [DOI] [PubMed] [Google Scholar]
- 142.Younes RN, Gross JL, Aguiar S, Haddad FJ, Deheinzelin D. When to remove a chest tube? A randomized study with subsequent prospective consecutive validation. J Am Coll Surg 2002;195:658–62. [DOI] [PubMed] [Google Scholar]
- 143.Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Tube thoracostomy; chest tube implantation and follow up. Journal of thoracic disease 2014;6:S470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Golden P Follow-up chest radiographs after traumatic pneumothorax or hemothorax in the outpatient setting: a retrospective review. Int J Trauma Nurs 1999;5:88–94. [DOI] [PubMed] [Google Scholar]
- 145.Morales Uribe CH, Villegas Lanau MI, Petro Sánchez RD. Best timing for thoracoscopic evacuation of retained post-traumatic hemothorax. Surg Endosc 2008;22:91–5. [DOI] [PubMed] [Google Scholar]
- 146.Fabbrucci P, Nocentini L, Secci S, et al. Video-assisted thoracoscopy in the early diagnosis and management of post-traumatic pneumothorax and hemothorax. Surg Endosc 2008;22:1227–31. [DOI] [PubMed] [Google Scholar]
- 147.Oosthuizen GV, Clarke DL, Laing GL, et al. Introducing video-assisted thoracoscopy for trauma into a South African township hospital. World J Surg 2013;37:1652–5. [DOI] [PubMed] [Google Scholar]
- 148.Landreneau RJ, Hazelrigg SR, Ferson PF, et al. Thoracoscopic resection of 85 pulmonary lesions. Ann Thorac Surg 1992;54:415–9; discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 149.Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–44. [DOI] [PubMed] [Google Scholar]
- 150.Samra SS, Samra NS, Jain A, Mehta V. Video-assisted thoracic surgery for hemothorax following coronary artery bypass. Asian Cardiovasc Thorac Ann 2006;14:e19–20. [DOI] [PubMed] [Google Scholar]
- 151.Cetindag IB, Neideen T, Hazelrigg SR. Video-assisted thoracic surgical applications in thoracic trauma. Thorac Surg Clin 2007;17:73–9. [DOI] [PubMed] [Google Scholar]
- 152.Carrillo EH, Heniford BT, Etoch SW, et al. Video-assisted thoracic surgery in trauma patients. J Am Coll Surg 1997;184:316–24. [PubMed] [Google Scholar]
- 153.Lin HL, Huang WY, Yang C, et al. How early should VATS be performed for retained haemothorax in blunt chest trauma? Injury 2014;45:1359–64. [DOI] [PubMed] [Google Scholar]
- 154.Liu DW, Liu HP, Lin PJ, Chang CH. Video-assisted thoracic surgery in treatment of chest trauma. J Trauma 1997;42:670–4. [DOI] [PubMed] [Google Scholar]
- 155.Lang-Lazdunski L, Mouroux J, Pons F, et al. Role of videothoracoscopy in chest trauma. Ann Thorac Surg 1997;63:327–33. [DOI] [PubMed] [Google Scholar]
- 156.Lang-Lazdunski L, Chapuis O, Pons F, Jancovici R. [Videothoracospy in thoracic trauma and penetrating injuries]. Ann Chir 2003;128:75–80. [DOI] [PubMed] [Google Scholar]
- 157.Lang-Lazdunski L, Pilling JE. Video assisted thoracoscopic surgery is still the standard. BMJ 2007;334:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Milfeld DJ, Mattox KL, Beall AC. Early evacuation of clotted hemothorax. Am J Surg 1978;136:686–92. [DOI] [PubMed] [Google Scholar]
- 159.Vassiliu P, Velmahos GC, Toutouzas KG. Timing, safety, and efficacy of thoracoscopic evacuation of undrained post-traumatic hemothorax. Am Surg 2001;67:1165–9. [PubMed] [Google Scholar]
- 160.Villegas MI, Hennessey RA, Morales CH, Londoño E. Risk factors associated with the development of post-traumatic retained hemothorax. Eur J Trauma Emerg Surg 2011;37:583–9. [DOI] [PubMed] [Google Scholar]
- 161.Migliore M, Deodato G. A single-trocar technique for minimally invasive surgery of the chest. Surgical endoscopy 2001;15:899–901. [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto H, Okada M, Takada M, Mastuoka H, Sakata K, Kawamura M. Video-assisted thoracic surgery through a single skin incision. Arch Surg 1998;133:145–7. [DOI] [PubMed] [Google Scholar]
- 163.Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434–8. [DOI] [PubMed] [Google Scholar]
- 164.Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43–6. [DOI] [PubMed] [Google Scholar]
- 165.Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg 2013;8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sanna S, Bertolaccini L, Brandolini J, et al. Uniportal video-assisted thoracoscopic surgery in hemothorax. J Vis Surg 2017;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bertolaccini L, Rocco G, Viti A, Terzi A. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5 Suppl 3:S214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bertolaccini L, Viti A, Terzi A, Rocco G. Geometric and ergonomic characteristics of the uniportal video-assisted thoracoscopic surgery (VATS) approach. Ann Cardiothorac Surg 2016;5:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bertolaccini L, Rocco G, Pardolesi A, Solli P. The Geometric and Ergonomic Appeal of Uniportal Video-Assisted Thoracic Surgery. Thorac Surg Clin 2017;27:331–8. [DOI] [PubMed] [Google Scholar]
- 170.Suda T, Ashikari S, Tochii S, Sugimura H, Hattori Y. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718–9. [DOI] [PubMed] [Google Scholar]
- 171.Liu Z, Yang R, Shao F, Pan Y. Modified procedure of uniportal video-assisted thoracoscopic lobectomy with muscle sparing incision. Ann Transl Med 2016;4:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gonzalez-Rivas D Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pastina M, Menna C, Andreetti C, Ibrahim M. The era of uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2017;9:462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reinersman JM, Passera E, Rocco G. Overview of uniportal video-assisted thoracic surgery (VATS): past and present. Ann Cardiothorac Surg 2016;5:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yeam I, Sassoon C. Hemothorax and chylothorax. Curr Opin Pulm Med 1997;3:310–4. [DOI] [PubMed] [Google Scholar]
- 176.Demirhan R, Onan B, Oz K, Halezeroglu S. Comprehensive analysis of 4205 patients with chest trauma: a 10-year experience. Interact Cardiovasc Thorac Surg 2009;9:450–3. [DOI] [PubMed] [Google Scholar]
- 177.Lowdermilk GA, Naunheim KS. Thoracoscopic evaluation and treatment of thoracic trauma. Surg Clin North Am 2000;80:1535–42. [DOI] [PubMed] [Google Scholar]
- 178.Hecker E, Hamouri S. [Video-assisted thoracoscopic surgery (VATS) for the management of parapneumonic pleural empyema]. Zentralbl Chir 2008;133:212–7. [DOI] [PubMed] [Google Scholar]
- 179.Karmy-Jones R, Jurkovich GJ. Blunt chest trauma. Curr Probl Surg 2004;41:211–380. [DOI] [PubMed] [Google Scholar]
- 180.Lebreton G, Baste JM, Thumerel M, Delcambre F, Velly JF, Jougon J. The hemiclamshell approach in thoracic surgery: indications and associated morbidity in 50 patients. Interact Cardiovasc Thorac Surg 2009;9:965–9. [DOI] [PubMed] [Google Scholar]
- 181.Grove CA, Lemmon G, Anderson G, McCarthy M. Emergency thoracotomy: appropriate use in the resuscitation of trauma patients. Am Surg 2002;68:313–6; discussion 6–7. [PubMed] [Google Scholar]
- 182.Fialka C, Sebök C, Kemetzhofer P, Kwasny O, Sterz F, Vécsei V. Open-chest cardiopulmonary resuscitation after cardiac arrest in cases of blunt chest or abdominal trauma: a consecutive series of 38 cases. J Trauma 2004;57:809–14. [DOI] [PubMed] [Google Scholar]
- 183.Stockinger ZT, McSwain NE. Additional evidence in support of withholding or terminating cardiopulmonary resuscitation for trauma patients in the field. J Am Coll Surg 2004;198:227–31. [DOI] [PubMed] [Google Scholar]
- 184.Powell DW, Moore EE, Cothren CC, et al. Is emergency department resuscitative thoracotomy futile care for the critically injured patient requiring prehospital cardiopulmonary resuscitation? J Am Coll Surg 2004;199:211–5. [DOI] [PubMed] [Google Scholar]
- 185.Cothren CC, Moore EE. Emergency department thoracotomy for the critically injured patient: Objectives, indications, and outcomes. World J Emerg Surg 2006;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000;190:288–98. [DOI] [PubMed] [Google Scholar]
- 187.Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: A comprehensive review of complications and related topics. Int J Crit Illn Inj Sci 2014;4:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Seamon MJ, Pathak AS, Bradley KM, et al. Emergency department thoracotomy: still useful after abdominal exsanguination? J Trauma 2008;64:1–7; discussion −8. [DOI] [PubMed] [Google Scholar]
- 189.Seamon MJ, Fisher CA, Gaughan JP, Kulp H, Dempsey DT, Goldberg AJ. Emergency department thoracotomy: survival of the least expected. World J Surg 2008;32:604–12. [DOI] [PubMed] [Google Scholar]
- 190.Seamon MJ, Chovanes J, Fox N, et al. The use of emergency department thoracotomy for traumatic cardiopulmonary arrest. Injury 2012;43:1355–61. [DOI] [PubMed] [Google Scholar]
- 191.Seamon MJ, Haut ER, Van Arendonk K, et al. An evidence-based approach to patient selection for emergency department thoracotomy: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015;79:159–73. [DOI] [PubMed] [Google Scholar]
- 192.Maxwell RA, Campbell DJ, Fabian TC, et al. Use of presumptive antibiotics following tube thoracostomy for traumatic hemopneumothorax in the prevention of empyema and pneumonia--a multi-center trial. J Trauma 2004;57:742–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 193.Luchette FA, Barrie PS, Oswanski MF, et al. Practice Management Guidelines for Prophylactic Antibiotic Use in Tube Thoracostomy for Traumatic Hemopneumothorax: the EAST Practice Management Guidelines Work Group. Eastern Association for Trauma. J Trauma 2000;48:753–7. [DOI] [PubMed] [Google Scholar]
- 194.LoCurto JJ, Tischler CD, Swan KG, et al. Tube thoracostomy and trauma--antibiotics or not? J Trauma 1986;26:1067–72. [DOI] [PubMed] [Google Scholar]
- 195.Gonzalez RP, Holevar MR. Role of prophylactic antibiotics for tube thoracostomy in chest trauma. Am Surg 1998;64:617–20; discussion 20–1. [PubMed] [Google Scholar]
- 196.Aguilar MM, Battistella FD, Owings JT, Su T. Posttraumatic empyema. Risk factor analysis. Arch Surg 1997;132:647–50; discussion 50–1. [DOI] [PubMed] [Google Scholar]
- 197.Wilson RF, Nichols RL. The EAST Practice Management Guidelines for Prophylactic Antibiotic Use in Tube Thoracostomy for Traumatic Hemopneumothorax: a commentary. Eastern Association for Trauma. J Trauma 2000;48:758–9. [DOI] [PubMed] [Google Scholar]
- 198.Bradley M, Okoye O, DuBose J, et al. Risk factors for post-traumatic pneumonia in patients with retained haemothorax: results of a prospective, observational AAST study. Injury 2013;44:1159–64. [DOI] [PubMed] [Google Scholar]
- 199.O’Connor JV, Chi A, Joshi M, DuBose J, Scalea TM. Post-traumatic empyema: aetiology, surgery and outcome in 125 consecutive patients. Injury 2013;44:1153–8. [DOI] [PubMed] [Google Scholar]
- 200.Moore FO, Duane TM, Hu CK, et al. Presumptive antibiotic use in tube thoracostomy for traumatic hemopneumothorax: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73:S341–4. [DOI] [PubMed] [Google Scholar]
- 201.Cook A, Hu C, Ward J, et al. Presumptive antibiotics in tube thoracostomy for traumatic hemopneumothorax: a prospective, Multicenter American Association for the Surgery of Trauma Study. Trauma Surg Acute Care Open 2019;4:e000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cohen NS, Braig Z, Collins JN. Prevalence and Management of Posttraumatic Retained Hemothorax in a Level 1 Trauma Center. Am Surg 2018;84:e369–e71. [PubMed] [Google Scholar]
- 203.Inci I, Ozçelik C, Nizam O, Eren N. Thoracic trauma in the elderly. Eur J Emerg Med 1998;5:445–50. [PubMed] [Google Scholar]
- 204.Chang YT, Dai ZK, Kao EL, et al. Early video-assisted thoracic surgery for primary spontaneous hemopneumothorax. World J Surg 2007;31:19–25. [DOI] [PubMed] [Google Scholar]
- 205.Chou YP, Lin HL, Wu TC. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Curr Opin Pulm Med 2015;21:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Scott MF, Khodaverdian RA, Shaheen JL, Ney AL, Nygaard RM. Predictors of retained hemothorax after trauma and impact on patient outcomes. Eur J Trauma Emerg Surg 2017;43:179–84. [DOI] [PubMed] [Google Scholar]
- 207.MacKenzie EJ, Hoyt DB, Sacra JC, et al. National inventory of hospital trauma centers. JAMA 2003;289:1515–22. [DOI] [PubMed] [Google Scholar]
- 208.DuBose J, Inaba K, Demetriades D, et al. Management of post-traumatic retained hemothorax: a prospective, observational, multicenter AAST study. J Trauma Acute Care Surg 2012;72:11–22; discussion −4; quiz 316. [DOI] [PubMed] [Google Scholar]
- 209.DuBose J, Inaba K, Okoye O, et al. Development of posttraumatic empyema in patients with retained hemothorax: results of a prospective, observational AAST study. J Trauma Acute Care Surg 2012;73:752–7. [DOI] [PubMed] [Google Scholar]
- 210.Tucker T, Idell S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin Thromb Hemost 2013;39:373–81. [DOI] [PubMed] [Google Scholar]
- 211.Komissarov AA, Rahman N, Lee YCG, et al. Fibrin turnover and pleural organization: bench to bedside. Am J Physiol Lung Cell Mol Physiol 2018;314:L757–L68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Idell S, Zwieb C, Kumar A, Koenig KB, Johnson AR. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. American Journal of Respiratory Cell & Molecular Biology 1992;7:414–26. [DOI] [PubMed] [Google Scholar]
- 213.Idell S The pathogenesis of pleural space loculation and fibrosis. Curr Opin Pulm Med 2008;14:310–5. [DOI] [PubMed] [Google Scholar]
- 214.Karandashova S, Florova G, Azghani AO, et al. Intrapleural adenoviral delivery of human plasminogen activator inhibitor-1 exacerbates tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 2013;48:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cobanoğlu U, Sayir F, Mergan D. [Should videothorascopic surgery be the first choice in isolated traumatic hemothorax? A prospective randomized controlled study]. Ulus Travma Acil Cerrahi Derg 2011;17:117–22. [DOI] [PubMed] [Google Scholar]
- 216.Huang FD, Yeh WB, Chen SS, et al. Early Management of Retained Hemothorax in Blunt Head and Chest Trauma. World J Surg 2018;42:2061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chou YP, Kuo LC, Soo KM, et al. The role of repairing lung lacerations during video-assisted thoracoscopic surgery evacuations for retained haemothorax caused by blunt chest trauma. Eur J Cardiothorac Surg 2014;46:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.MacLeod JB, Ustin JS, Kim JT, Lewis F, Rozycki GS, Feliciano DV. The Epidemiology of Traumatic Hemothorax in a Level I Trauma Center: Case for Early Video-assisted Thoracoscopic Surgery. Eur J Trauma Emerg Surg 2010;36:240–6. [DOI] [PubMed] [Google Scholar]
- 219.John M, Razi S, Sainathan S, Stavropoulos C. Is the trocar technique for tube thoracostomy safe in the current era? Interact Cardiovasc Thorac Surg 2014;19:125–8. [DOI] [PubMed] [Google Scholar]
- 220.O’Connor JV, DuBose JJ, Scalea TM. Damage-control thoracic surgery: Management and outcomes. J Trauma Acute Care Surg 2014;77:660–5. [DOI] [PubMed] [Google Scholar]
- 221.Jantz MA, Antony VB. Pleural fibrosis. Clin Chest Med 2006;27:181–91. [DOI] [PubMed] [Google Scholar]
- 222.Sinha P, Sarkar P. Late clotted haemothorax after blunt chest trauma. J Accid Emerg Med 1998;15:189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hendriksen BS, Kuroki MT, Armen SB, Reed MF, Taylor MD, Hollenbeak CS. Lytic Therapy for Retained Traumatic Hemothorax: A Systematic Review and Meta-analysis. Chest 2019;155:805–15. [DOI] [PubMed] [Google Scholar]
- 224.Idell S, Rahman NM. Intrapleural Fibrinolytic Therapy for Empyema and Pleural Loculation: Knowns and Unknowns. Ann Am Thorac Soc 2018;15:515–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kugler NW, Carver TW, Paul JS. Thoracic irrigation prevents retained hemothorax: a pilot study. J Surg Res 2016;202:443–8. [DOI] [PubMed] [Google Scholar]
- 226.Kugler NW, Carver TW, Milia D, Paul JS. Thoracic irrigation prevents retained hemothorax: A prospective propensity scored analysis. J Trauma Acute Care Surg 2017;83:1136–41. [DOI] [PubMed] [Google Scholar]
- 227.Zellweger R, Navsaria PH, Hess F, Omoshoro-Jones J, Kahn D, Nicol A. Transdiaphragmatic pleural lavage in penetrating thoracoabdominal trauma. Br J Surg 2004;91:1619–23. [DOI] [PubMed] [Google Scholar]
- 228.Tomaselli F, Maier A, Renner H, Smolle-Jüttner FM. Thoracoscopical water jet lavage in coagulated hemothorax. Eur J Cardiothorac Surg 2003;23:424–5. [DOI] [PubMed] [Google Scholar]
- 229.Nakamura H, Taniguchi Y, Miwa K, Adachi Y, Fujioka S, Haruki T. Surgical outcome of video-assisted thoracic surgery for acute thoracic empyema using pulsed lavage irrigation. Gen Thorac Cardiovasc Surg 2010;58:126–30. [DOI] [PubMed] [Google Scholar]
- 230.Huang PM, Chou NK, Lin TH, Chen CN. Intrapleural Epinephrine Irrigation for Massive Malignant Hemothorax. Thorac Cardiovasc Surg 2016;64:263–5. [DOI] [PubMed] [Google Scholar]