Abstract
BACKGROUND:
Delayed graft function (DGF), the need for dialysis in the first week following kidney transplant, affects approximately one-quarter of deceased-donor kidney transplant recipients. Donor demographics, donor serum creatinine, and graft cold ischemia time are associated with DGF. However, there is no consensus on the optimal management of hemodynamic instability in organ donors after brain death (DBDs). Our objective was to determine the relationship between vasopressor selection during donor management and the development of DGF.
METHODS:
Prospective observational data, including demographic and critical care parameters, were collected for all DBDs managed by seventeen Organ Procurement Organizations from nine Organ Procurement and Transplantation Network Regions between 2012 and 2018. Recipient outcome data were linked with donor data through donor identification numbers. Donor critical care parameters, including type of vasopressor and doses, were recorded at three standardized time points during donor management. The analysis included only donors who received at least one vasopressor at all three time points. Vasopressor doses were converted to norepinephrine equivalent doses and analyzed as continuous variables. Univariate analyses were conducted to determine the association between donor variables and DGF. Results were adjusted for known predictors of DGF using binary logistic regression.
RESULTS:
Complete data were available for 5,554 kidney transplant recipients and 2,985 DBDs. On univariate analysis, donor serum creatinine, donor age, donor subtype, kidney donor profile index, graft cold ischemia time, phenylephrine dose, and dopamine dose were associated with DGF. After multivariable analysis, increased donor serum creatinine, donor age, kidney donor profile index, graft cold ischemia time, and phenylephrine dose remained independent predictors of DGF.
CONCLUSION:
Higher doses of phenylephrine were an independent predictor of DGF. With the exception of phenylephrine, the selection and dose of vasopressor during donor management did not predict the development of DGF.
LEVEL OF EVIDENCE:
Prognostic study, level III
Keywords: organ donor management, vasopressor, kidney transplant, delayed graft function, brain death
Background
Kidney transplantation improves survival in patients with end-stage chronic kidney disease and is cost-effective compared with long-term dialysis(1,2). According to the Organ Procurement and Transplantation Network (OPTN), 16,301 kidney transplants were performed in the United States in 2018, with the largest proportion of transplanted kidneys from donors after brain death (DBDs). Compared with recipients of living donor kidneys, recipients of DBD kidneys are more likely to develop delayed graft function (DGF), the need for dialysis during the first week following transplantation. DGF is associated with increased health care resource utilization and risk of adverse transplant outcomes, including acute rejection, chronic allograft nephropathy, and graft loss(2–6). Consequently, efforts to improve initial graft function are needed.
Donor demographics and serum creatinine, as well as graft cold ischemia time, have been identified as independent predictors of DGF(7–9). Donor management goals (DMGs) are a set of critical care endpoints that have been established to promote high-quality critical care for DBDs in order to normalize physiological parameters following brain death. Achieving DMGs reduces the risk of DGF in kidney transplant recipients(10,11). The DMGs include a target mean arterial pressure (MAP) of 60 – 100 mmHg, frequently necessitating the use of vasopressors, in addition to fluid resuscitation, during donor management. However, there is no consensus on the preferred vasopressor to achieve the target MAP.
There have been limited studies examining the effect of vasopressor selection and dose during donor management on the development of DGF in kidney transplant recipients. In a randomized clinical trial of 264 DBDs, administration of low-dose dopamine (4 µg/kg/min) during donor management reduced the need for dialysis in the first week post-transplant(12). Additional studies have examined the relationship between administration of vasopressors during donor management of DBDs and high-yield procurement, defined as recovery of four or more organs(13,14). Early initiation of hormone replacement therapy and administration of vasopressin were predictive of increased rate of high-yield procurement whereas administration of norepinephrine was predictive of decreased rate of high-yield procurement. However, neither study included allograft function in the recipients. Observational studies of vasopressor use during donor management, which included analysis of graft outcomes, have been difficult to interpret due to variations in the definition of delayed graft function as well as in organ donation protocols(15,16). Previous studies were also limited by sample size and were generally focused on the impact of a single vasopressor rather than comparison of multiple vasopressors. Using a large prospective registry of linked donor management and recipient outcome data, our objective was to determine the relationship between vasopressor selection and dose during donor management and the development of DGF in kidney transplant recipients.
Methods
Study design
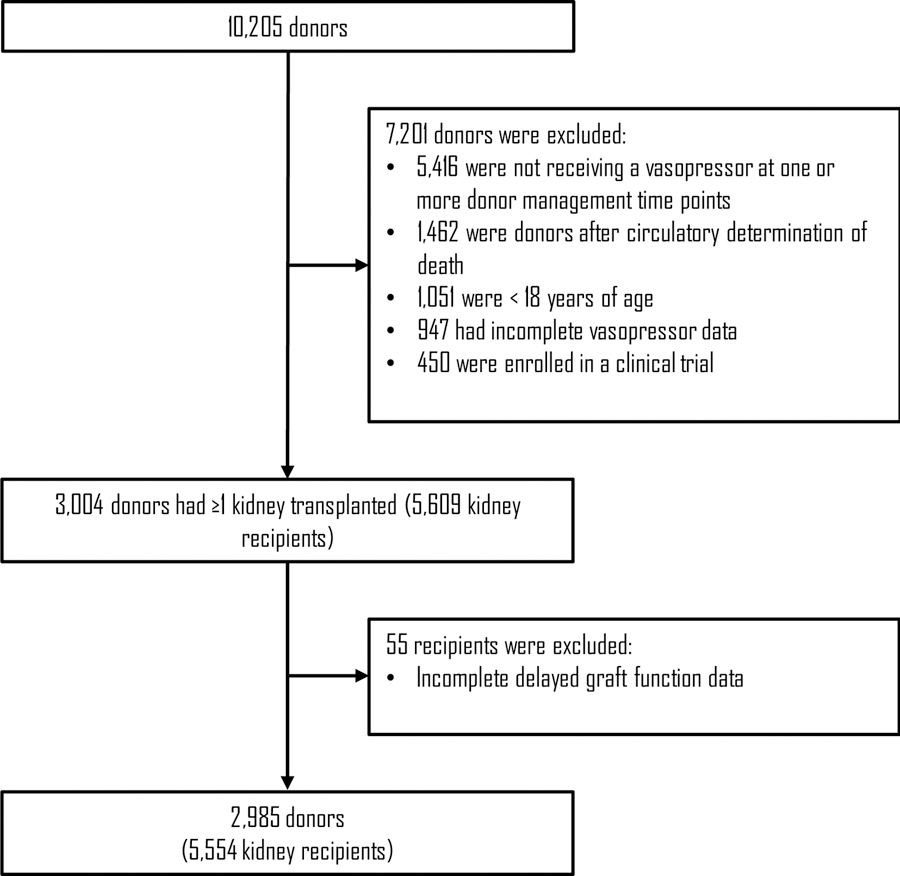
Prospective observational data were collected for all organ donors following neurological determination of death managed by seventeen Organ Procurement Organizations (OPOs) from nine OPTN Regions between 2012 and 2018. The data include donor critical care parameters, including vasopressor doses, at three standardized time points during donor management: (1) Authorization, after authorization for donation when the OPO assumes responsibility for care of the donor, (2) Allocation, at the time of organ allocation, and (3) Prior to Organ Recovery, at the conclusion of donor management immediately prior to organ recovery. Deidentified kidney transplant recipient outcome data were linked with the donor data through the OPTN donor identification numbers. Donors after circulatory determination of death, donors less than 18 years of age, donors enrolled in a clinical trial, donors with incomplete vasopressor data, and donors who were not receiving a vasopressor at one or more donor management time points were excluded from the analysis. Kidney transplant recipients with incomplete DGF data were also excluded.
This study used data from the OPTN. The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, United States Department of Health and Human Services provides oversight to the activities of the OPTN contractor.
Outcomes measures
The primary outcome measure of this study was DGF in the kidney transplant recipients, which was defined as the requirement for dialysis in the first week following kidney transplant.
Statistical analysis
Using a conversion scale published by Khanna and colleagues, doses of epinephrine, norepinephrine, dopamine, phenylephrine, and vasopressin were converted to norepinephrine equivalent doses to compare the relative impact of each vasopressor on the occurrence of DGF(17). According to the conversion scale, 0.1 µg/kg/min norepinephrine is equivalent to 0.1 µg/kg/min epinephrine, 15 µg/kg/min dopamine, 1.0 µg/kg/min phenylephrine, or 0.04 U/min vasopressin.
Univariate analyses compared donor demographics, donor factors previously found to be associated with DGF risk, donor subtype, and KDPI in kidney transplants complicated by DGF to those with normal initial graft function. Univariate analyses also compared vasopressor doses in norepinephrine equivalents at all three donor management time points to determine their association with DGF. The analyses were conducted using Pearson’s chi-square test for categorical variables or the independent-samples t-test for equality of means for continuous variables.
Multivariable analysis was performed using binary logistic regression to identify independent predictors of DGF. The models included vasopressor doses in norepinephrine equivalents and serum creatinine at Authorization, Allocation, and Prior to Organ Recovery as well as other donor factors that were statistically significant on univariate analysis. Variables with p < 0.05 on multivariable analysis were considered statistically significant. Separate models were used for inherently related variables. The Hosmer-Lemeshow goodness of fit test and the concordance index (c-statistic) were calculated to evaluate the calibration and discrimination of the multivariable model(18).
Statistical analyses were performed with SPSS Statistics version 25 (IBM Corporation). Values for continuous variables are reported as mean ± standard deviation. Values for categorical variables are reported as percent (%).
Results
During the study period, complete data were available for 2,985 DBDs and 5,554 kidney transplant recipients (Figure 1). The mean donor age was 41 (± 14) years, and 62% of donors were male. The causes of death were head trauma (39%), cerebrovascular accident or stroke (31%), and anoxia (28%). The mean KDPI was 46 (± 28), the mean serum creatinine prior to organ recovery was 1.4 (± 1.1) mg/dL, and the mean duration of cold ischemia time was 17 (± 8) hours. Of the kidney transplant recipients, 27% developed DGF.
Figure 1 -.
Selection diagram for donors after brain death (DBDs) included in the study
Vasopressor use varied over the course of donor management. The percent of DBDs in the study population receiving each vasopressor at the three standardized donor management time points is shown in Table 1. Vasopressin was the most commonly used vasoactive medication, followed by norepinephrine and phenylephrine. Dopamine and epinephrine were used in less than 10 percent of donors.
Table 1.
Vasopressor use in donors receiving one or more vasopressor at all three donor management time points
| Vasopressor | Authorization (%) | Allocation (%) | Prior to Organ Recovery (%) |
|---|---|---|---|
| Dopamine | 7.7 | 8.9 | 9.6 |
| Phenylephrine | 25.5 | 20.2 | 16.4 |
| Norepinephrine | 67.9 | 32.1 | 22.8 |
| Epinephrine | 5.1 | 1.8 | 1.4 |
| Vasopressin | 53.7 | 78.2 | 75.4 |
The results of the univariate analyses of continuous and categorical variables are presented in Tables 2 and 3, respectively. Kidney transplants with DGF had a significantly longer cold ischemia time and were more likely to come from donors who were older and as well as those who had a higher serum creatinine during donor management. Additionally, kidney transplants with DGF were more likely to come from expanded criteria donors and donors with a higher KDPI. DGF occurred more commonly in kidneys from donors who were receiving a higher total dose of all vasopressors in norepinephrine equivalents at the Prior to Organ Recovery time point. Additionally, higher doses of phenylephrine at Allocation and Prior to Organ Recovery as well as higher doses of dopamine at all three donor management time points were associated with DGF. Doses of norepinephrine, epinephrine, and vasopressin were not associated with DGF.
Table 2.
Univariate analysis of continuous data associated with delayed graft function
| Authorization | Allocation | Prior to Organ Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No DGF (n = 4048) |
DGF (n = 1506) |
p valuea | No DGF (n = 4048) |
DGF (n = 1506) |
p valuea | No DGF (n = 4048) |
DGF (n = 1506) |
p valuea | |
| Donor age, years | 38.7 (13.9) | 43.6 (13.2) | < 0.001 | ||||||
| Cold ischemia time, hrs | 16.4 (8.1) | 18.5 (8.6) | < 0.001 | ||||||
| Kidney donor profile index | 41.0 (27.2) | 53.8 (25.9) | < 0.001 | ||||||
| Serum creatinine, mg∙dL−1 | 1.1 (0.7) | 1.6 (1.2) | < 0.001 | 1.1 (0.8) | 1.7 (1.3) | < 0.001 | 1.2 (0.8) | 1.8 (1.4) | < 0.001 |
| Vasopressor doses in norepinephrine equivalents | |||||||||
| Dopamine | 0.035 (0.02) | 0.055 (0.02) | 0.001 | 0.002 (0.01) | 0.004 (0.02) | < 0.001 | 0.002 (0.009) | 0.004 (0.01) | < 0.001 |
| Phenylephrine | 0.038 (0.09) | 0.039 (0.09) | 0.722 | 0.020 (0.06) | 0.027 (0.08) | 0.003 | 0.011 (0.043) | 0.019 (0.07) | < 0.001 |
| Norepinephrine | 0.157 (0.21) | 0.147 (0.18) | 0.076 | 0.055 (0.14) | 0.052 (0.13) | 0.491 | 0.033 (0.118) | 0.034 (0.11) | 0.797 |
| Epinephrine | 0.007 (0.04) | 0.009 (0.04) | 0.180 | 0.002 (0.03) | 0.003 (0.03) | 0.384 | 0.002 (0.022) | 0.003 (0.03) | 0.143 |
| Vasopressin | 0.048 (0.06) | 0.046 (0.06) | 0.492 | 0.058 (0.05) | 0.058 (0.05) | 0.929 | 0.046 (0.047) | 0.047 (0.05) | 0.887 |
| Total norepinephrine equivalents | 0.253 (0.25) | 0.247 (0.23) | 0.370 | 0.137 (0.18) | 0.144 (0.17) | 0.211 | 0.094 (0.14) | 0.106 (0.16) | 0.013 |
DGF, delayed graft function.
Data are presented as mean (standard deviation).
p values were calculated using the independent-samples t-test.
Table 3.
Univariate analysis of categorical data associated with delayed graft function
| Variable | No DGF (n = 4048) |
DGF (n = 1506) |
p valuea |
|---|---|---|---|
| Sex | |||
| Female | 38.0 | 37.0 | 0.480 |
| Male | 62.0 | 63.0 | |
| Race | |||
| Asian | 4.3 | 5.5 | 0.202 |
| Black | 10.8 | 10.5 | |
| Native American | 0.3 | 0.4 | |
| Pacific Islander | 0.2 | 0.5 | |
| White | 83.7 | 82.5 | |
| Multiracial | 0.6 | 0.6 | |
| Ethnicity | |||
| Hispanic/Latino | 30.4 | 31.5 | 0.424 |
| Donor subtype | |||
| Standard criteria donor | 87.1 | 78.9 | < 0.001 |
| Expanded criteria donor | 12.9 | 21.1 |
DGF, delayed graft function. CVA, cerebrovascular accident.
Data are presented as percent (%) of column total.
p values were calculated using Pearson’s chi-square test.
After performing multivariable analysis, independent predictors of DGF were increased donor age, cold ischemia time, and KDPI as well as serum creatinine at Allocation and Prior to Organ Recovery. In the multivariable model that included the norepinephrine equivalent dose for each vasopressor individually at each donor management time point (Table 4), increased phenylephrine dose Prior to Organ Recovery was an independent predictor of DGF (OR 6.93 [1.18 – 40.82]). This multivariable model had acceptable calibration and discrimination (Hosmer and Lemeshow = 0.26, c-statistic = 0.70). In the multivariable model that included the total combined vasopressor dose at each donor management time point (Table 5), the total amount of vasopressor administered was not an independent predictor of DGF. This multivariable model has acceptable calibration (Hosmer and Lemeshow = 0.58). However, the c-statistic was 0.69, below the threshold for acceptable discrimination.
Table 4.
Model 1: Multivariable analysis of donor factors associated with delayed graft function
| Variable | OR (95% CI) | p valuea |
|---|---|---|
| Cold ischemia time, per hour | 1.02 (1.01 – 1.03) | < 0.001 |
| KDPI, per unit | 1.01 (1.01 – 1.02) | < 0.001 |
| Age, per year | 1.01 (1.00 – 1.02) | 0.035 |
| Expanded criteria donor | 0.82 (0.66 – 1.01) | 0.061 |
| Serum creatinine, per mg/dL | ||
| At authorization | 1.11 (0.99 – 1.24) | 0.083 |
| At allocation | 1.29 (1.13 – 1.48) | < 0.001 |
| Prior to organ recovery | 1.21 (1.08 – 1.341) | 0.001 |
| Vasopressor dose at authorization, per µg/kg/min norepinephrine equivalent | ||
| Dopamineb | 3.64 (0.08 – 163.56) | 0.505 |
| Phenylephrineb | 0.69 (0.32 – 1.48) | 0.336 |
| Norepinephrineb | 0.69 (0.48 – 1.01) | 0.053 |
| Epinephrineb | 0.95 (0.19 – 4.82) | 0.948 |
| Vasopressinb | 2.64 (0.80 – 8.67) | 0.110 |
| Vasopressor dose at allocation, per µg/kg/min norepinephrine equivalent | ||
| Dopamineb | 300.51 (0.11 – 792035.16) | 0.156 |
| Phenylephrineb | 0.86 (0.21 – 3.46) | 0.831 |
| Norepinephrineb | 0.77 (0.40 – 1.48) | 0.429 |
| Epinephrineb | 0.60 (0.01 – 30.04) | 0.797 |
| Vasopressinb | 1.65 (0.33 – 8.35) | 0.542 |
| Vasopressor dose prior to organ recovery, per µg/kg/min norepinephrine equivalent | ||
| Dopamineb | 11.53 (0.003 – 38908.72) | 0.555 |
| Phenylephrineb | 6.93 (1.18 – 40.82) | 0.032 |
| Norepinephrineb | 0.86 (0.40 – 1.82) | 0.687 |
| Epinephrineb | 1.03 (0.02 – 69.20) | 0.991 |
| Vasopressinb | 2.04 (0.36 – 11.61) | 0.422 |
DGF, delayed graft function. KDPI, kidney donor profile index. OR, odds ratio. CI, confidence interval.
p values were calculated using binary logistic regression.
Vasopressor doses were converted to norepinephrine equivalent doses prior to analysis.
Table 5.
Model 2: Multivariable analysis of donor factors associated with delayed graft function
| Variable | OR (95% CI) | p valuea |
|---|---|---|
| Cold ischemia time, per hour | 1.02 (1.01 – 1.02) | < 0.001 |
| KDPI, per unit | 1.01 (1.01 – 1.02) | < 0.001 |
| Age, per year | 1.01 (1.003 – 1.02) | 0.013 |
| Expanded criteria donor | 0.81 (0.66 – 1.003) | 0.053 |
| Serum creatinine, per mg/dL | ||
| At authorization | 1.10 (0.99 – 1.24) | 0.089 |
| At allocation | 1.28 (1.12 – 1.46) | < 0.001 |
| Prior to organ recovery | 1.22 (1.10 – 1.36) | < 0.001 |
| Total norepinephrine equivalent dose, per µg/kg/min | ||
| At authorization | 0.77 (0.57 – 1.04) | 0.093 |
| At allocation | 0.91 (0.54 – 1.53) | 0.717 |
| Prior to organ recovery | 0.21 (0.82 – 2.46) | 0.208 |
DGF, delayed graft function. KDPI, kidney donor profile index. OR, odds ratio. CI, confidence interval.
p values were calculated using binary logistic regression.
Vasopressor doses were converted to norepinephrine equivalent doses prior to analysis.
Discussion
Despite the pathophysiological effects of brain death, vasopressors are not universally required to normalize hemodynamic parameters and achieve DMGs in DBDs. The decision to select one vasopressor over another is most relevant in donors who receive one or more vasopressors throughout donor management. Given the importance of optimizing donor management to improve kidney transplantation outcomes as well as the uncertainty regarding the optimal use of vasopressors in DBDs, this report investigated the relationship between vasopressor selection and dose and the development of DGF in kidney transplant recipients. In so doing, we determined that increased phenylephrine dose during donor management is independently associated with DGF in kidney transplant recipients. None of the other vasopressors nor total combined vasopressor dose were found to be independent predictors of DGF.
The relationship between phenylephrine dose during donor management and recipient DGF has not been previously investigated. This analysis determined kidney transplant recipients who received organs from donors receiving an increased phenylephrine dose Prior to Organ Recovery were nearly seven times more likely to develop DGF. One possible explanation for these findings could be the fact that phenylephrine leads to selective α1 receptor-mediated vasoconstriction, which could lead to relative ischemia of visceral organs(22).
With regards to other vasopressors, dopamine has both inotropic and vasopressor effects and was historically chosen as the first-line vasopressor during donor management(20). In a randomized clinical trial, Schneulle et al investigated the administration of low dose dopamine (4 µg/kg/min) in 264 DBDs who were stable while receiving a norepinephrine dose less than or equal to 0.4 µg/kg/min. Recipients of kidneys from dopamine-treated donors were less likely to require multiple dialysis treatments during the first week after transplant (24.7% vs 35.4%, p = 0.01)(12). We failed to observe a similar effect; however, less than 10 percent of donors in our study population received dopamine and dopamine was not administered in a standardized fashion across all participating OPOs.
Vasopressin has a number of effects which are beneficial in the management of brain death pathophysiology. These effects were observed in a small randomized clinical trial of 24 DBDs conducted by Pennefather et al. In this trial, vasopressin decreased plasma hyperosmolality and increased mean arterial pressure(21). Additionally, in DBDs who received vasopressin, the dopamine dose was reduced without adverse hemodynamic consequences(21). Plurad et al found that use of vasopressin during donor management was an independent predictor of high-yield organ procurement, defined as recovery of four or more organs, in an observational study of 10,431 DBDs(14). While this study suggests a beneficial role for vasopressin in donor management, allograft outcomes were not investigated. Rather than focusing on hemodynamic parameters in the donor or organ procurement rates, our study focuses on recipient graft function and did not find a significant relationship between vasopressin dose in the donor and recipient DGF.
There have been limited investigations of the effect of epinephrine during donor management on kidney transplant recipient outcomes. In a single-center observational study, use of epinephrine during donor management was found to be an independent predictor of prolonged DGF, which the investigators defined as six or more days to reach a Cockroft calculated creatinine clearance of 10 mL/min(15). In our study, using a large database of prospective observational donor data linked with recipient DGF outcome data, we did not observe a significant relationship between epinephrine dose in the donor and recipient DGF.
Lastly, although norepinephrine is commonly used during donor management, its effect on kidney transplant recipient outcomes has not been investigated. Concerns have been expressed about the potential harms of norepinephrine α-receptor agonist activity(20). However, our study did not find a significant relationship between norepinephrine dose in the donor and recipient DGF, perhaps suggesting that the balanced α-receptor and β-receptor agonism provided by norepinephrine ameliorates the negative effects of the isolated α-receptor agonism provided by phenylephrine.
There are several important limitations to this study. First, this is an observational study which only allows us to determine associations between vasopressor selection and dose during donor management and DGF in kidney transplant recipients; however, we cannot determine causality. Second, the vasopressor doses were recorded only at the three standardized donor management time points rather than throughout donor management. This limitation prevented us from calculating the total exposure of the donors to each vasopressor. Additionally, the equation used to convert the vasopressor doses into norepinephrine equivalents was derived from the effects of these medications on hemodynamic parameters in patients with septic shock. The relative effects of these medications may differ between patients with septic shock and DBDs. It is possible that donor fluid balance may influence the development of DGF in kidney recipients; however, we were not able to include this variable in our analyses because the data were not included in the database used for this study. Additionally, our analysis did not examine whether the relationship between vasopressor selection during donor management and the development of DGF in kidney recipients is modified by donor hemodynamics. In kidney transplant recipients with DGF, the primary outcome measure, the decision to initiate dialysis posttransplant is not standardized and there may be variability in the use of posttransplant dialysis across transplant centers. However, there is no reason to believe that the decision by a transplant center to initiate dialysis would be influenced by the use of vasopressors in the donor. Finally, the database used for this study did not include recipient characteristics that could be related to the development of DGF.
Decreasing the incidence of DGF has the potential to improve kidney transplant recipient outcomes and reduce health care resource utilization. This study investigated the relationship between vasopressor selection and dose during donor management and the development of DGF in kidney transplant recipients. Analysis of 2,985 DBDs and 5,554 kidney transplant recipients from nine OPTN regions determined increased phenylephrine dose during donor management is independently associated with the risk of DGF in kidney transplant recipients. None of the other vasopressors nor total combined vasopressor dose were found to be independent predictors of recipient DGF.
Acknowledgments
The authors would like to acknowledge and thank the donors, recipients, staff, and leadership of the following Organ Procurement Organizations for their support of this study, as well as their assistance in collection and management of data: Donor Network of Arizona, California Transplant Donor Network, Golden State Donor Services, OneLegacy, New Mexico Donor Services, Nevada Donor Network, DonorConnect, Lifesharing – A Donate Life Organization, LifeGift, Pacific Northwest Transplant Bank, Gift of Life Michigan, LifeLink of Georgia, Gift of Hope, New England Organ Bank, Southwest Transplant Alliance, Tennessee Donor Services, and Donor Alliance.
Conflicts of Interest and Source of Funding: EAS is currently receiving a grant (F30 DK114980) from the National Institutes of Health. For the remaining authors, no conflicts were declared. Support for work related to this manuscript was obtained from a grant from Arnold Ventures.
Footnotes
Meetings: Presented at the 78th Annual Meeting of the American Association for the Surgery of Trauma, September 18 – 21, 2019 in Dallas, Texas.
Publisher's Disclaimer: Disclaimer: The data reported here have been supplied by UNOS as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Conflicts of Interest
The authors have no commercial interests to disclose.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999. December 2;341(23):1725–30. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, Kasiske BL, Alhamad T, Lentine KL. An economic assessment of contemporary kidney transplant practice. Am J Transplant 2018. May;18(5):1168–76. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca I, Teixeira L, Malheiro J, Martins LS, Dias L, Castro Henriques A, Mendonça D. The effect of delayed graft function on graft and patient survival in kidney transplantation: an approach using competing events analysis. Transpl Int 2015. June;28(6):738–50. [DOI] [PubMed] [Google Scholar]
- 4.Moreira P, Sá H, Figueiredo A, Mota A. Delayed renal graft function: risk factors and impact on the outcome of transplantation. Transplant Proc 2011. February;43(1):100–5. [DOI] [PubMed] [Google Scholar]
- 5.Redfield RR, Scalea JR, Zens TJ, Muth B, Kaufman DB, Djamali A, Astor BC, Mohamed M. Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl Int 2016. January;29(1):81–7. [DOI] [PubMed] [Google Scholar]
- 6.Wu WK, Famure O, Li Y, Kim SJ. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int 2015. October;88(4):851–8. [DOI] [PubMed] [Google Scholar]
- 7.Jeldres C, Cardinal H, Duclos A, Shariat SF, Suardi N, Capitanio U, Hébert MJ, Karakiewicz PI. Prediction of delayed graft function after renal transplantation. Can Urol Assoc J 2009. October;3(5):377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapal M, Le Borgne F, Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F, Rostaing L, Kamar N, et al. A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int 2014. December;86(6):1130–9. [DOI] [PubMed] [Google Scholar]
- 9.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant 2010. October;10(10):2279–86. [DOI] [PubMed] [Google Scholar]
- 10.Malinoski DJ, Patel MS, Ahmed O, Daly MC, Mooney S, Graybill CO, Foster CE, Salim A. The Impact of Meeting Donor Management Goals on the Development of Delayed Graft Function in Kidney Transplant Recipients: Donor Management Goals and Renal DGF. Am J Transplant 2013. April;13(4):993–1000. [DOI] [PubMed] [Google Scholar]
- 11.Cardinal H, Lamarche F, Grondin S, Marsolais P, Lagacé AM, Duca A, Albert M, Houde I, Boucher A, Masse M, et al. Organ donor management and delayed graft function in kidney transplant recipients: A multicenter retrospective cohort study. Am J Transplant 2019. January;19(1):277–84. [DOI] [PubMed] [Google Scholar]
- 12.Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, Fischereder M, Jauch KW, Heemann U, Zeier M, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA 2009. September 9;302(10):1067–75. [DOI] [PubMed] [Google Scholar]
- 13.Plurad DS, Bricker S, Falor A, Neville A, Bongard F, Putnam B. Donor hormone and vasopressor therapy: Closing the gap in a transplant organ shortage. J Trauma Acute Care Surg 2012. September;73(3):689–94. [DOI] [PubMed] [Google Scholar]
- 14.Plurad DS, Bricker S, Neville A, Bongard F, Putnam B. Arginine vasopressin significantly increases the rate of successful organ procurement in potential donors. Am J Surg 2012. December;204(6):856–60; discussion 860–861. [DOI] [PubMed] [Google Scholar]
- 15.Giral M, Bertola JP, Foucher Y, Villers D, Bironneau E, Blanloeil Y, Karam G, Daguin P, Lerat L, Soulillou JP. Effect of Brain-Dead Donor Resuscitation on Delayed Graft Function: Results of a Monocentric Analysis. Transplantation. 2007. May;83(9):1174–81. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Huang Z, Zhou H, Lin M, Hua X, Hong L, Na N, Cai R, Wang G, Sun Q. New Factors Predicting Delayed Graft Function: a Multi-Center Cohort Study of Kidney Donation After Brain Death Followed by Circulatory Death. Kidney Blood Press Res 2018;43(3):893–903. [DOI] [PubMed] [Google Scholar]
- 17.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, Busse LW, Altaweel L, Albertson TE, Mackey C, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med 2017. August 3;377(5):419–30. [DOI] [PubMed] [Google Scholar]
- 18.LaValley MP. Logistic Regression. Circulation. 2008. May 6;117(18):2395–9. [DOI] [PubMed] [Google Scholar]
- 19.Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining Delayed Graft Function after Renal Transplantation: Simplest Is Best. Transplantation. 2013. November;96(10):885–9. [DOI] [PubMed] [Google Scholar]
- 20.Kotloff RM, Blosser S, Fulda GJ, Malinoski D, Ahya VN, Angel L, Byrnes MC, DeVita MA, Grissom TE, Halpern SD, et al. Management of the Potential Organ Donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med 2015. June;43(6):1291–325. [DOI] [PubMed] [Google Scholar]
- 21.Pennefather SH, Bullock RE, Mantle D, Dark JH. Use of low dose arginine vasopressin to support brain-dead organ donors. Transplantation. 1995. January 15;59(1):58–62. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Mao Z, Zeng X, Kang H, Liu H, Pan L, Hou PC. Vasopressors in septic shock: a systematic review and network meta-analysis. Ther Clin Risk Manag 2015;11:1047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]