Abstract
Background:
Although the use of sedation is commonly practiced to keep infants still while receiving magnetic resonance imaging, non-pharmacological strategies are a potential alternative.
Objectives:
The purpose of this study was to determine the success rate of obtaining usable magnetic resonance images in infants with the sole use of non-pharmacological strategies.
Design:
Systematic literature review and meta-analysis
Setting:
A search was conducted in PubMed, CINAHL and Cochrane Library.
Participants:
Human infants from birth to 24 months of age who did not receive any sedation or anesthesia during magnetic resonance imaging
Method:
Articles that reported the success rate of obtaining usable images were included.
Results:
Of the 521 non-duplicate articles found, 58 articles were included in the systematic review with sample sizes ranging from 2–457, an average success rate of 87.8%, and an average scan time of 30 minutes. The most common non-pharmacological technique included feeding and swaddling infants before imaging to encourage infants to sleep during the scan. Meta-analysis performed on 53 articles comprising 3,410 infants found a success rate of 87%, but significant heterogeneity was found (I2 = 98.30%). It was more difficult to obtain usable images solely with non-pharmacological techniques if infants were critically ill or a structural magnetic resonance imaging of the brain was required.
Conclusion:
Non-pharmacological techniques are effective for obtaining usable magnetic resonance imaging scans in most but not all infants.
Keywords: Infants, Sedation, MRI, Safety
Tweetable abstract:
Non-pharmacological techniques are effective for obtaining usable magnetic resonance imaging scans in most infants.
1. Introduction
Magnetic resonance imaging (MRI) is valuable for identifying and confirming the diagnosis of numerous birth abnormalities, including perinatal stroke (Govaert, Ramenghi, Taal, de Vries, & Deveber, 2009; Ramaswamy, Miller, Barkovich, Partridge, & Ferriero, 2004), hydrocephalus (McGirt et al., 2005; Vanneste, 2000), and heart structure abnormalities (Boxer, Singh, LaCorte, Goldman, & Stein, 1986; Krishnamurthy, 2008), among others (Alamo, Gudinchet, & Meuli, 2015; Prada et al., 2015). In a research setting, the application of MRI to infant populations have provided a wealth of information regarding the process and timing of brain development (Giedd & Rapoport, 2010; Lebel & Deoni, 2018), brain alterations in neurodevelopmental and psychiatric illnesses (Giedd & Rapoport, 2010; Hendren, De Backer, & Pandina, 2000), as well as examining the underlying relationships between brain anatomy and observed cognition and behavior (Casey, Tottenham, Liston, & Durston, 2005; Durston & Casey, 2006). Furthermore, MRI is promising for the potential development of non-invasive markers of pediatric diseases and disorders, such as pediatric epilepsy (Spader et al., 2013; Hermann et al., 2002), multiple sclerosis (Banwell, Ghezzi, Bar-Or, Mikaeloff, & Tardieu, 2007; Callen et al., 2009), and autism spectrum disorders (Ameis & Catani, 2015; Travers et al., 2012). Thus, it is essential to acquire high-quality MRI scans in infants. Yet this population remains one of the most challenging groups to acquire such scans.
Arguably the most critical limitation of MRI, particularly for use in infants, is the modality’s sensitivity to motion. Significant intra-scan motions can quickly degrade the quality of the acquired images, rendering them unusable (Darge, Anupindi, & Jaramillo, 2011; Le Bihan, Poupon, Amadon, & Lethimonnier, 2006). Thus, obtaining images of usable quality typically requires the infant to remain still throughout the entire exam. This can be challenging for infants as scan times can often last up to 20 minutes or longer (Edwards & Arthurs, 2011). For these reasons, pharmacological strategies such as sedatives or general anesthesia are routinely used to obtain diagnostically usable images (Edwards & Arthurs, 2011; Woodthorpe, Trigg, Alison, & Sury, 2007). While pharmacological strategies have advantages for increasing patient cooperation and minimizing image artefacts, these agents are expensive and can produce troublesome side effects such as respiratory depression (Edwards & Arthurs, 2011; Flick et al., 2011). Consideration of the potential adverse pharmacological side effects and cost burdens has motivated the use of alternative, non-pharmacological strategies for obtaining usable images in infants. The purpose of this study was to determine the success rate of obtaining usable MRI scans in infants with the sole use of non-pharmacological strategies.
2. Methods
2.1. Search Strategy
Articles published in English in peer-reviewed journals in PubMed, CINAHL and Cochrane Library were searched through May 30, 2019, with no restriction on how long ago an article was published or the study design. Inclusion criteria was comprised of human infants from birth to two years of age who received no sedation or anesthesia during an MRI and the number of usable images was reported. Gray literature was excluded, such as conference proceedings, procedural papers, policy documents, newsletters, and opinion papers. Table 1 describes the search terms in each of the databases. Two authors independently assessed each article for eligibility. After the elimination of duplicates, articles were first selected by title and abstract. Then full-text publications were examined. Articles that could not answer our research question were excluded, as well as literature reviews. Disagreements resulted in the inclusion of the article. Finally, the reference lists of the included full text articles were examined. Prior to initiating this review, a draft protocol was written but not registered or published. We followed the steps of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati et al., 2009).
Table 1.
Search terms
| Databases | Search terms |
|---|---|
| PubMed | Search (((((“Anesthesia, Inhalation”[Mesh] OR “Anesthesia, General”[Mesh] |
| CINAHL | OR “Anesthesia, Intravenous”[Mesh] OR “Anesthesia and Analgesia”[Mesh]) |
| OR “Anesthesia”[Mesh]) OR (“Deep Sedation”[Mesh] OR “Conscious | |
| Cochrane Library | Sedation”[Mesh] OR “Anti-Anxiety Agents”[Mesh]) OR sedat* OR anesthes*)) |
| AND | |
| ((“Infant, Extremely Premature”[Mesh] OR “Infant, Extremely Low Birth Weight”[Mesh] OR “Infant, Very Low Birth Weight”[Mesh] OR “Infant Behavior”[Mesh] OR “Infant, Small for Gestational Age”[Mesh] OR “Infant, Premature, Diseases”[Mesh] OR “Infant, Premature”[Mesh] OR “Infant, Postmature”[Mesh] OR “Infant, Newborn”[Mesh] OR “Infant, Low Birth Weight”[Mesh] OR “Term Birth”[Mesh] OR “Intensive Care, Neonatal”[Mesh] OR “Infant”[Mesh]) OR “Premature Birth”[Mesh] OR infant OR premature OR preemie OR neonat* OR NICU OR baby OR child OR toddler)) | |
| AND | |
| (“Magnetic Resonance Imaging”[Mesh] OR “Magnetic Resonance Imaging, Interventional”[Mesh] OR “Diffusion Magnetic Resonance Imaging”[Mesh] OR “Magnetic Resonance Angiography”[Mesh] OR “Magnetic Resonance Imaging, Cine”[Mesh] OR MRI OR “magnetic resonance”) | |
| AND | |
| (image OR quality) | |
| ------------------------------------ | |
| Filters: Humans; English; Child: birth-18 years; Infant: birth-23 months |
2.2. Data collection process
The following information from each article was gathered and classified to facilitate comparison; design, country where participants received imaging, description of sample including size, inclusion/exclusion criteria, intervention (i.e. how infants were kept still), type of MRI and part of body imaged, how success was measured, success rate (% of images that were usable), and scan time. Information was entered in a table and cross-checked by two reviewers.
2.3. Risk of bias in individual articles
The risk of bias in individual articles was assessed at the study level using a framework for critiquing health research developed by Caldwell, Henshaw and Taylor (2011). This tool consists of eight general questions; does the title reflect the content, are the authors credible, does the abstract summarize the key components, is the rationale for undertaking the research clearly outlined, is the literature review comprehensive and up-to-date, is the aim of the research clearly stated, are all ethical issues identified and addressed, and is the methodology identified and justified? There are six additional methodology questions for quantitative articles (all articles found in this review were quantitative); is the study design clearly identified and is the rationale for choice of design evident, is there an experimental hypothesis clearly stated and are the key variables clearly defined, is the population identified, is the sample adequately described and reflective of the population, is the method of data collection valid and reliable, is the method of data analysis valid and reliable? There were three final questions: are the results presented in a way that is appropriate and clear, are the results generalizable and is the conclusion compressive? For each study included in the systematic review, each item was answered yes, no or unsure to determine bias. Two authors independently assessed each article. Disagreements were resolved by discussion or by referral to a third author. Reviewers were not blinded to the bibliographic details of the papers, which included the author’s names and affiliations, which was required when using this framework. Regardless of risk of bias, all articles were included in data synthesis.
2.4. Summary measure
The success rate of obtaining usable MRI scans in infants with the sole use of non-pharmacological strategies was determined by dividing the number of usable images by the total number of images obtained.
2.5. Synthesis of results
For the systematic review, all results were combined, synthesized and presented narratively. For the meta-analysis, analyses were also stratified by design: (1) retrospective cross-sectional, (2) prospective cross-sectional and (3) prospective longitudinal. Random effects meta-analysis was conducted using restricted maximum likelihood (REML) estimation technique. Between-study variance, tau-squared (τ2), which reflects the amount of heterogeneity between articles in absolute scale was calculated. Furthermore, I2 statistics which is the ratio of between-study variance to the observed variance (i.e., the sum of between- and within-study variance) was also employed to quantify the magnitude of between-study heterogeneity. The Cochrane Q-test statistic was used to examine and formally test presence of study heterogeneity. Forest plots were used to visually display summary statistics. The significance level was established at p < 0.05. All meta-analyses were performed using STATA version 16.0 (StataCorp, College Station, TX, U.S.A.).
2.6. Risk of bias across articles
A funnel plot was created to assess publication bias. A symmetrical funnel plot suggests a reduced possibility of publication bias, while an asymmetrical funnel plot suggests an increased probability of publication bias (Higgins & Green, 2011).
3. Results
3.1. Study selection
The process for inclusion of articles identified during review and analysis is shown in Figure 1. Of the 521 non-duplicate articles found, 464 were excluded based on the titles and abstract. Of the 57 remaining articles, 22 were excluded after reviewing the full-text. Some reasons for exclusion were participants were older than 24 months of age, received sedation or anesthesia, could not determine if sedation or anesthesia was received, were not data-based, and the sample was duplicated. The references of the included full-text articles were reviewed, resulting in 23 additional articles resulting in a total of 58 articles included in this review. When combining all studies, the sole use of non-pharmacological strategies was successful in producing usable MRI scans in infants 4 – 100% of the time, with an average of 87.8%.
Figure 1.
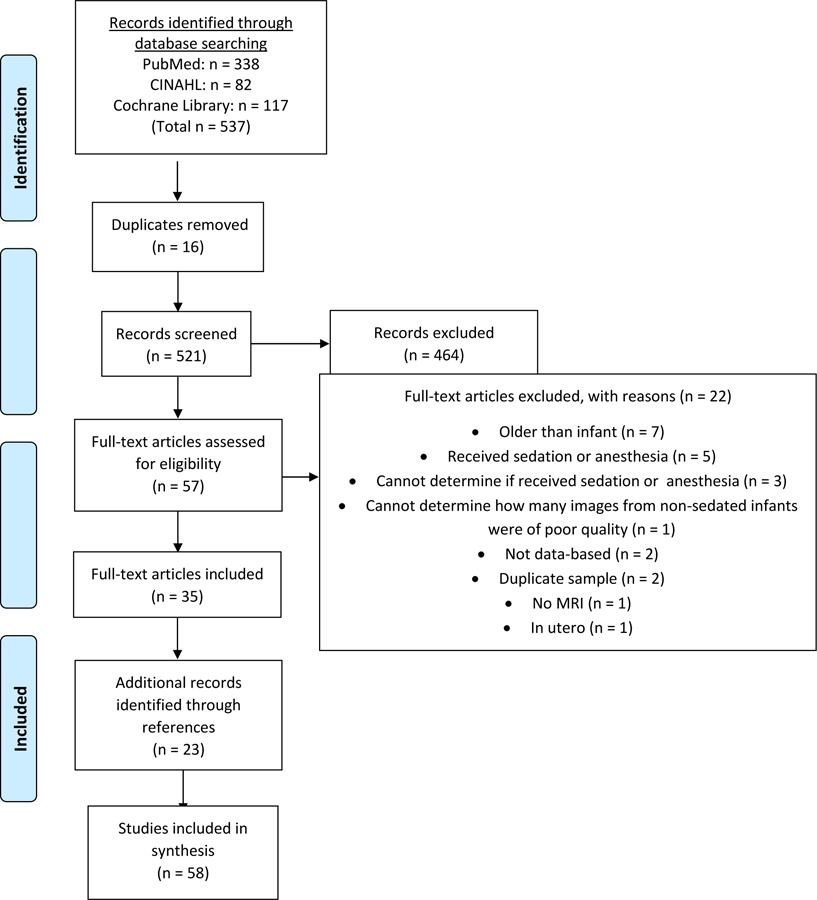
Flow Diagram according to PRISMA Statement (Liberati et al., 2009)
3.2. Study Characteristics
Sixteen of the articles were retrospective cross-sectional (Ashley et al., 2005; Dean et al., 2017; Fogel et al., 2011; Gale et al., 2013; Gould et al., 2012; Hansen, 2009; Iskandar, Sansone, Medow, & Rowley, 2004; O’Regan et al., 2012; Rabattu et al., 2014; Reilly, Byrne, & Ely, 2012; Rozovsky et al., 2013; Ryan, Jaju, Ciolino, & Alden, 2016; Sirin et al., 2013; Tsiflikas et al., 2019; Windram et al., 2012; Young, Duhaime, Caruso, & Rincon, 2016), thirty-two were prospective cross-sectional (Arthurs et al., 2011; Bartha et al., 2007; Born et al. 2000, Chateil et al., 1999; Foran et al., 2007; Golan et al., 2011; Groves et al., 2011; Groves et al., 2012; Higano et al., 2017; Inder et al., 2005; Jaramillo, Villegas-Medina, Laor, Shapiro, & Millis, 1998; Laor et al., 2000; Lin et al., 2008; Liu, Flax, Guise, Sukul, & Benasich, 2008; Maas et al., 2004; McNally et al., 1997; Merchant et al., 2009; Miller et al., 2007; Modi et al., 2001; Neil et al., 1998; Neubauer et al., 2011; Rutherford et al., 2004; Sigmund et al., 1991; Siles et al., 2014; Spann et al., 2015; Thomeer et al., 2015; Tkach et al., 2014; Walkup et al., 2015; Wang et al., 2008; Whitby et al., 2003; Whitby et al., 2004; Woodward, Anderson, Austin, Howard, & Inder, 2006) and eight were prospective longitudinal (Damaraju et al., 2014; Dean et al., 2014; Knickmeyer et al., 2008; McKinstry, Mathur et al., 2002; McKinstry, Miller et al., 2002; Nossin-Manor et al., 2013; Partridge et al., 2004; Smyser et al., 2010; see Table 2). Two articles had both a retrospective and prospective cross-sectional design (Haney et al., 2010; Missios et al., 2008). When a range of mean success rates were reported (Gale et al., 2013; Groves et al., 2011; Neil et al., 1998; Whitby et al., 2004; Whitby et al., 2003), the median was obtained. The success rate of obtaining usable MRI scans in infants with the sole use of non-pharmacological strategies ranged from 4–100% for retrospective cross-sectional articles, 49–100% for prospective cross-sectional articles, and 42.6–100% for prospective longitudinal articles.
Table 2.
Summary of articles (n = 58)
| Citation | Sample | Intervention | Magnetic Resonance Imaging (MRI) | Results (success rate measured by percentage) |
|---|---|---|---|---|
|
Arthurs et al., 2011 Prospective cross-sectional, timeframe: not reported United Kingdom |
12 patients referred following first urinary tract infection or urinary tract abnormalities confirmed on postnatal ultrasound Inclusion criteria: under 1 year of age |
Infants wrapped in blanket and fed before or after MRI according to normal feeding schedule. Pediatric nursing support provided. |
Magnetic resonance voiding cystourethrography Radiologist blinded to diagnosis determined if images were of diagnostic quality. |
All examinations were diagnostically acceptable and completed within 60 min. (success rate = 100%). Scan time = 36.6±13.0 minutes (range 20 – 60) |
|
Ashley et al., 2005 Retrospective cross-sectional review of a radiology information system from Jan.–Nov. 2003 United States |
53 patients with hydrocephalus, age 1– 22 years, 14 (21%) underwent more than one MRI Exclusion: Images not available digitally |
Not reported | MRI of the brain Radiologists determined if they were able to visualize the catheter. |
In all cases, radiologists visualized ventricular catheter when present (success rate=100%). Scan time = 22.5 minutes |
|
Bartha et al., 2007 Prospective cross-sectional, stable inpatients scanned solely for research between July 2004 and October 2005 United States |
16 healthy term neonates prospectively enrolled Exclusion criteria: Younger than 36 gestational weeks; suspected or confirmed metabolic disorder, congenital infection or malformation; signs of perinatal depression |
Custom-built magnetic resonance compatible neonatal incubator and high-sensitivity neonatal head coil built to reduce patient motion, increase patient safety and comfort, and improve signal-to-noise ratio of the images. Infants scanned immediately after fed. |
Diffusion tensor imaging of the brain Two experienced neonatal neuroradiologists assessed each scan separately for the presence and location of any signal intensity abnormalities and scored them using a previously validated MR imaging scoring system. Consensus was reached on all findings with an overall κ value of 0.9. |
All images were assessable (success rate = 100%). Scan time = not reported |
|
Born et al., 2000 Prospective cross-sectional, time frame not reported Denmark |
7 infant patients born prematurely (n = 4), suspected of having visual functioning defects (n = 1), or had MRI for reasons unrelated to visual function (n = 2) did not receive sedation | MRI performed during spontaneous sleep after feeding. | Functional magnetic resonance imaging of visual system Images were excluded if gross motion was present on the parameter plots, when there was visible motion on the cine loop of the time series, or when excessive motion artefacts in the typical localization appeared on the statistical map of a motion-corrected image. |
8 of 31 (26%) acquired datasets were acceptable in the unsedated infants Scan time = 4 minutes |
|
Chateil et al., 1999 Prospective cross-sectional, timeframe: not reported Location: France |
30 newborn infant patients suspected of having birth or postnatal asphyxia and need for resuscitation after birth Inclusion criteria: All infants suspected of suffered birth or post-natal asphyxia |
MRI performed during natural sleep. | MRI of the Brain Images were determined to be invalid due to poor quality of the images and spectra due to head motion. |
Image quality good in 25 of 30 newborns (success rate = 83.3%). Not possible to perform MRI during first hours of life or during critical early period in most severely affected infants. MRI not easily performed during first hours of life in infants with assisted ventilation or if infant weighed less than 2 kg. To study critically ill infants, necessary to use specialized non-magnetic incubator equipped with mechanical ventilation and complete system for physiological monitoring Scan time = total imaging time was 20–90 minutes with an average of 1 hour |
|
Damaraju et al.,2014 Prospective longitudinal MRI at 4 & 9 months of age, no timeframe reported United States |
Infants recruited from general pediatric clinics and community advertisements. All study participants born via uncomplicated term delivery without known medical or developmental disorders. Exclusion criteria: genetic disorders, visual or hearing impairment |
Imaging during natural sleep. Research assistant remained at infant’s side throughout MRI scan to observe movement and be immediately available if subject woke up. | MRI of the brain Images were used from infants who did not wake up or move during the scan. |
At 4 months, of 84 infants imaged, 30 awoke prior to resting state MRI, leaving 54 completed MRI scans (success rate = 64.3%). At 9 months, 23 of these 54 infants did not wake up or move during scan (success rate = 42.6%). Total scan time = not reported |
|
Dean et al., 2014 Prospective longitudinal January 2010 to April 2013 United States |
Infants scanned at three 6-month intervals. Initial scan: 148 infants Follow-up scan #1: 83 infants Follow-up scan #2: 33 infants Follow-up scan #3: 10 Inclusion criteria: uncomplicated singleton birth between 37 and 42 weeks’ gestation with no physical MRI contraindications; no diagnosis of major psychiatric, depressive or learning disorders; no preexisting neurological conditions or major head trauma and no exposure to illicit drugs during pregnancy |
Vacuum immobilization placed under infant before asleep. Once asleep, infant secured in immobilizer and transferred to scanner suite. Infant’s head positioned into head coil, headphones placed over infant’s ears and secured using foam cushions. Research assistant remained inside scanning suite in case child woke up during scan. Parents invited to remain in imaging suite during scan. | MRI of the brain Scans were deemed unusable due to image artifacts, i.e. intra-scan motion, off-resonance artifacts. |
Success of scanning infants at initial visit = 95.9%. Success of scanning infants at follow-up visits: 100%. Scan time <30 minutes |
|
Dean et al., 2017 Prospective cross-sectional United States |
149 infants were recruited based on the following criteria for the mother: 18 and 40 years of age, expecting singleton birth, no diagnosis of psychotic illnesses (i.e., schizophrenia, bipolar disorder, borderline personality disorder), no pre-existing neurological conditions or major head trauma, no major autoimmune disease or infections during pregnancy, and uncomplicated childbirth. Exclusion criteria: any exposure to the neonatal intensive care unit (NICU) and if the infant did not go home with the mother at discharge. |
Imaging was scheduled to correspond with the infant’s daily nap schedule after the infant was fed and swaddled. Infants were swaddled with a vacuum immobilization bag and foam cushions were placed around their heads to reduce intra-scan motion. A foam insert was fitted to the inside of the scanner bore, utilizing both malleable ear plugs and noise-attenuating ear covers, and using electrodynamic headphones that played white noise during the image acquisition. | Diffusion tensor imaging; neurite orientation dispersion and density imaging Images were used from infants who did not wake up during the scan. |
33 infants woke up prior to and 13 infants woke up during the diffusion acquisition, resulting in a final study sample of 104 (success rate = 69.8%) Scan time: 10 minutes |
|
Fogel et al., 2011 Retrospective cross-sectional chart review from Jan. 1, 2005–Mar. 18, 2009 United States |
24 patients, <6 months old investigated for aortic arch abnormalities Exclusion criteria: tenuous respiratory status, more extensive imaging necessary, and parental instead of physician preference for type of imaging |
Families instructed not to feed infants 2–3 hours before MRI. Ear plugs and hat placed over ears. Infant moved to room with lights dimmed; infant in mother’s or nurse’s arms, swaddled with blanket, and fed. Rocking and singing performed as needed. Once asleep, infant transferred to scanner, placed in supine position within head coil with supporting cushions. | Angiography of the heart The only criteria was that the image quality was great enough to obtain the diagnostic information. |
One patient awoke during MRI. Examination of remaining 23 yielded a definitive diagnosis (success rate 95.8%). Scan time = 6.2 ± 3.1 minutes |
|
Foran et al., 2007 Prospective cross-sectional study, timeframe: not reported United Kingdom |
12 preterm infants, median gestational age 29 weeks (range: 26–33 weeks), median postconceptional age at the time of MRI was 33 weeks (range: 31–40 weeks) 2 infants had patent ductus arteriosus previously diagnosed, 10 believed to have structurally and functionally normal hearts Inclusion criteria: preterm infants undergoing intensive care |
Infants fed, allowed to fall asleep in quiet environment and laid in custom MRI-compatible cradle and placed in scanner with MRI-compatible physiologic monitoring and ear protection. | MRI of the heart Images had to allow for long-axis estimations |
10 of 12 allowed image analysis (83.3% success rate). Each scan lasted ~45 min. |
|
Gale et al., 2013 Retrospective cross-sectional review of whole body MRI research without the use of sedation over the past decade United Kingdom |
Outpatient MRI of 457 infants (386 neonates, 71 infants ages 6–12 weeks) conducted for research purposes Exclusion criteria: imaging solely the brain |
MRI performed after infants fed, swaddled, placed in quiet, comfortable, private room with dim lighting, noise shielding (dental putty to ears before covering with earmuffs held in place with hat), and foam padding on infant’s head. Parents sat with research team. | MRI of the whole body Success rate was determined by obtaining good quality images free of movement artefact. |
Achieved 94.0% success rate in obtaining diagnostic quality MRI scans. Scan time = 9.26 – 17.42 with a median of 14 minutes |
|
Golan et al., 2011 Prospective cross-sectional, all term patients who underwent imaging procedures during a non-specified 12 month period Israel |
40 patients examined, representing all imaging performed in 12 month period of data collection. Gestational age at birth was 27–40 weeks, examinations performed at ages ranging from delivery to 6 months old | Infants fed, diaper changed, & placed in immobilizing device. Pacifier with or without sucrose given if needed. Parents strongly encouraged to join neonatal team to assist with cuddling. | 15 CT scans, 25 MRIs of the brain, 1 bone scan Images had to be considered qualitatively appropriate for interpretation by the radiologist on service. |
100% success rate Scan time = not reported |
|
Gould et al. 2012 Retrospective cross-sectional review of MRIs performed over a 28-month period United States |
34 MRI studies performed on 24 patients with developmental hip dysplasia. Exclusion criteria: No patient was scanned if underwent open reduction with internal fixation of the hip. |
Not reported | MRI of the hip Review of all sequences was independently performed by 2 pediatric radiologists experienced in musculoskeletal MRI. Three criteria were used to evaluate the quality of the imaging: motion, tissue contrast, and spatial resolution. Scores were assigned on an agreed-upon scale (0, 1, 2), in which 0 was best and 2 was worst. For every sequence, average scores for each criterion and overall scores were calculated for each evaluator. If individual studies contained 1 or more attempts at a given type of sequence, the best of those repeated sequences was scored. Aggregate scores for each sequence type were also calculated. |
33 of 34 MRIs were of diagnostic quality (97.1% success rate) Scan time < 30 minutes |
|
Groves et al., 2011 Prospective cross-sectional study of stable inpatients scanned solely for research, time frame not reported United Kingdom |
108 infants; 53 males, 74 on neonatal unit, 34 on postpartum unit, scanned at 26–42 weeks gestation | Infants fed and allowed to fall into natural sleep, laid in MRI-compatible cradle. | MRI of the Heart Where possible scans were repeated using the initial imaging geometry (scan–rescan repeatability) after 5–15 min. Repeatability of analysis was also assessed from a single set of images by the same (intra-) and a different (inter-) observer. In all cases, observers were blinded to prior data when analyzing flow volumes. |
100% success rate Scan time = 45–60 minutes |
|
Groves et al., 2012 Prospective cross-sectional study of stable inpatients scanned solely for research, timeframe not reported United Kingdom |
14 newborns No inclusion/exclusion criteria reported. |
Infants fed and allowed to fall into natural sleep, laid in MRI-compatible cradle. | MRI of the Heart The magnitude and phase images of each acquisition were assessed for image quality by a single observer using a subjective scale where 0 = unsuitable for analysis; 1 = acceptable for analysis, significant artifacts present; 2 = good image quality, almost no artifacts; and 3 = excellent image quality, no artifacts. |
13 newborns had MRI scans of sufficient quality for analysis (success rate: 92.9%). Scan time = not reported |
|
Haney et al., 2010 Design: Quality Improvement Project Retrospective cross-sectional chart review of NICU patients undergoing MRI at one hospital Jan.-Dec. 2007 Prospective examination of NICU patients in MRI studies after implementation of new protocol designed to decrease or eliminate sedation with MRIs in Mar. 2009-Feb. 2010 United States |
Retrospective: 154 patients born between 23–42 weeks Prospective: 155 patients born between 23–42 weeks No exclusion criteria reported |
Retrospective:Prepared infant in radiology suite. Prospective: Prepared infant in NICU and encouraged parental participation. Infants were swaddled, comforted and those tolerating enteral nutrition fed. Swaddled infants placed in vacuum immobilizer and taken to MRI. |
MRI of the brain 91% of the time. Additional body sites imaged included the spine, abdomen, chest, pelvis and multiple body sites. Image quality was determined to be excellent, acceptable, poor, or unacceptable by the MRI technician at the time the imaging study was completed. |
Retrospective: Achieved 3.9% success rate (6/154 = 3.9%) in retrospective design. Prospective: Achieved 94.2% (146/155) success rate in prospective design. Scan time = non-sedated patients were away from the NICU for 48 minutes |
| Hansen et al., 2009 Retrospective cross-sectional chart review of patients between Jan. 2005 to April 2006 Australia |
36 patients age 3 days to 39 weeks Inclusion criteria: pediatric patients scheduled for MRI exam without use of general anesthetic at one institution |
Patients scheduled to arrive half an hour prior to scan after fasting for 4 hours. Infant fed until satisfied and nursed by family member or guardian to calm down. Infant escorted into scan room and positioned in beanbag. Air sucked out of beanbag and shaped to child’s body, maintaining good immobilization. Family member or guardian stayed in scan room for duration of MRI to monitor the infant. |
MRI of the brain Image quality was assessed using three categories; good (no motion artefacts present), diagnostic (slight motion artefacts were seen but the radiologist was still able to answer the referring clinician’s questions based on the acquired images) or unsuccessful (substantial movement artefacts on the images or if the child could not be settled and immobilized in the beanbag). |
32 out of 36 infants had good or diagnostic image quality (success rate = 88.9%). Majority of scan times were < 15 minutes Four patients did not complete scan: (3) did not settle after feeding (1) too large for immobilizer |
|
Higano et al., 2017 Prospective cross-sectional, no time frame reported United States |
Five neonatal patients recruited from the neonatal intensive care unit (NICU) and primarily diagnosed with seizures or gastrointestinal issues, but with no suspected lung disease. Five NICU patients with various pulmonary morbidities |
Not reported | Ultrashort Echo time MRI of lung parenchyma Images from all five diseased subjects were evaluated by a radiologist with 22 years of experience and included classification of radiological findings within the ROI and degree of confidence in radiological assessment. All images were anonymized and evaluated in random order. (There was no mention of how the scans of patients without lung disease were evaluated.) |
Success rate for patients with no suspected lung disease = 100% (Excluding one who received sedation for clinical purposes.) Success rate for patients with lung disease = 80% (One received sedation specifically for the scan.) Scan times 15–90 minutes. |
|
Inder et al., 2005 Prospective cross-sectional study, between November 1998 to December 2000 New Zealand and Australia |
129 consecutive premature infants with birth weight <1500g and gestation ≤ 32 weeks postconceptional age had MRI scan at term equivalent 21 random healthy term-born infants from same geographical sites |
Infants fed and wrapped in bean bag. | MRI of the brain 10 images could not be processed due to motion artifact (n=5), sequence errors (n=3), or registration difficulties (n=2) |
119 (92.2%) infants had usable scans Scan time = Not reported |
|
Iskandar et al., 2004 Retrospective cross-sectional chart review of all patients who received brain MRI April 2002–April 2003 United States |
Seventy-two patients, birth to 62 years old (most children, median age 3.46 years) initially presented to emergency department or neurosurgery clinic with symptoms of shunt malfunction, total of 76 quick-brain MRIs acquired. Inclusion criteria: MR imaging performed for symptomatic workup or asymptomatic follow up of shunt-treated hydrocephalus |
Not reported. | MRIs of the brain Had to be able to see ventricular anatomy. |
No image had movement artifact sufficient to obscure ventricular anatomy (success rate=100%). Quick-brain MRI studies required a mean of 3.4 minutes to complete. |
|
Jaramillo et al. 1998 Prospective cross-sectional study during an 18 Month period United States |
18 consecutive infant patients Inclusion criteria: within 24 hours of hip reduction and spica cast placement |
Imaged after discharge from recovery room from hip reduction and spica cast placement. Spica cast taped to imaging table to minimize motion artifact. | MRI of the hip One pediatric radiologist evaluated the images according to the following parameters: location of the hip, obstacles to reduction, gadolinium enhancement, and image quality. For initial evaluation, the observer was unaware of the clinical information and all other imaging studies. In 14 infants, the MR findings were correlated with arthrograms obtained during reduction. On the images obtained after administration of gadolinium, 2 pediatric radiologists independently evaluated the pattern of enhancement and compared it with the angle of femoral abduction shown on the images. |
Although some studies were degraded by motion artifact, enhancement and reduction could be assessed in all (success rate = 100%). Scan time = 15 minutes |
|
Knickmeyer et al., 2008 Prospective longitudinal, infants recruited during second trimester of pregnancy from outpatient clinic and scanned for research purposes shortly after birth, and at ages 1 and 2 years No time frame reported United States |
153 infants initially scanned at 2–4 weeks 86 infants scanned at follow-up at age 1 67 infants scanned at next follow-up at age 2 Exclusion criteria: Presence of abnormalities on fetal ultrasound or major medical or psychotic illness in the mother |
Infants at 1 and 2 years mildly sleep deprived (i.e., parents asked to wake child 1 hour early and skip nap). Scans scheduled to coincide with child’s normal naptime or bedtime. Once asleep, fitted with earplugs or earphones and placed in MRI scanner with head in immobilization device and additional foam padding to diminish sounds of scanner. Neonatal scans performed with neonatal nurse present. For older infants, member of research team remained in scanner room to monitor child throughout scan. | MRI of the brain All scans were reviewed by an anatomical expert to determine if the results of the tissue segmentation were accurate. Segmentations for 5 of the 35 scans collected at age 1 were deemed poor quality. These scans were not included in the analysis of tissue volumes but are included in the analysis of ventricle, hippocampal, and caudate volumes. A single rater performed all segmentations for a specific structure. For the lateral ventricles, intrarater reliability was 0.99; for the caudate, intrarater reliability was 0.93; and for the hippocampus, intrarater reliability was 0.95. All reliabilities are intraclass correlations |
Success of scanning at initial visit: 61.4%, follow-up 48.9%, next follow-up 52.2% Total scan time = not reported |
|
Laor et al., 2000 Prospective cross-sectional study between January 1999 to October 1999 United States |
10 infants age 4–20 months referred for cross-sectional examination after intraoperative reduction for developmental dysplasia of hip and spica casting or recasting were performed. | Each child imaged supine and feet first in MRI within 4 hours of surgery. Infant scanned in between routinely scheduled patients. All children restrained enough in spica cast to allow imaging up to several hours after reduction without additional sedation. Most fully awake, and several were crying. Parents or guardians were present in scanning room in all cases. |
MRI of hip Diagnostic images were deemed successful. |
3 images moderately degraded by motion but all MRIs diagnostic and none had to be repeated (success rate = 100%) Mean imaging time was ~ 3 minutes. |
|
Lin et al., 2008 Design: Prospective cross-sectional study, no timeframe reported United States |
85 children: 38 neonates (2–4 weeks of age), 26 one-year-olds, and 21 two-year-olds Inclusion: Birth between the gestational ages of 35 and 42 weeks, weight appropriate for gestational age Exclusion criteria: maternal pre-eclampsia, placental abruption, neonatal hypoxia, or any neonatal illness requiring >1-day stay in NICU; mother with HIV; any mother actively using illegal drugs/narcotics during pregnancy; or any chromosomal or major congenital abnormality |
All subjects imaged asleep. | Functional MRI of the brain Images were excluded due to motion artefact. |
14 infants met exclusion criteria (2 neonates, 6 one-year olds, & 6 two-year olds) and were excluded from final data analysis (85– 14=71) Motion artifacts were observed in 32 additional infants; 18 neonates, 6 one-year-old and 8 two-year-olds. As a result, 39 infants (18 neonates, 14 one-year-olds, and 7 two-year-olds) were included for final data analysis (39/71=54.9% success rate). Scan time = Not reported |
|
Liu et al., 2008 Design: Prospective cross-sectional study, part of a larger longitudinal study, participants recruited 2002–2005 United States |
11 full-term, normally developing, healthy infants (mean age 12.8 months) Inclusion criteria: Drawn from larger study, 59 scans acquired after 94 attempts (63%). Of 59 scans, 17 included both structural and functional data. Of 17, 11 full-term, normally developing, healthy infants. |
Infants scanned in evening near normal bedtime. Parents encouraged to omit afternoon naps. Scanning suite setup with crib, rocking chair, lullaby music and lights dimmed. Mothers nursed or fed infant. After infant asleep, placed on scanner bed. | MRI of the brain Seven subjects had movements of less than 0.1 mm; three subjects had head motions greater than 0.1 mm but less than 1 mm; and one subject showed head motion greater than 2 mm but less than 3 mm. The motion correction was applied to all the data and for the subjects with >1 mm head movement, data subsets were removed within the run to correct for the motion artifacts. After the motion correction, all data contained only movements that were under 0.1 mm. |
After motion-correction, all scans were usable (success rate=100%). Scan time = 5 minutes |
|
Maas et al., 2004 Prospective cross-sectional study, timeframe not reported United States |
2 extremely premature infants born at estimated gestational ages of 24 and 25 menstrual weeks. Estimated ages of patients at time of scanning were 25 and 27 menstrual weeks, or 23 and 25 post-ovulatory weeks, respectively. | Not described. | Diffusion tensor imaging of the brain How success was defined was not reported. |
100% success Scan time = not reported |
|
McKinstry, Mathur et al. 2002 Prospective longitudinal study with preterm patients recruited from NICU and special care nursery, no time frame reported United States |
38 scans total 24 patients imaged within first 36 hours after birth 14 scanned again prior to discharge from hospital Inclusion criteria: Gestational age ranged from 26 to 41 weeks and was average of mother’s last menstrual period, patient’s Ballard score and fetal ultrasound if available. Infants whose MR scan prior to discharge included if no known complications during hospital course that would cause cortical injury. Exclusion criteria: Gestational age estimates did not agree within 1 week, evidence of drug exposure in utero, brain injury, significant hypoxia, severe respiratory distress, congenital malformations, or infants on continuous positive airway pressure. |
Infants swaddled in warm sheets/blankets, placed on scanner table on MR-compatible chemical heating pad and heads restrained with soft cushions. | Diffusion tensor imaging of the brain All ROI placements were verified by a CAQ-certified neuroradiologist and a board certified pediatric neurologist. Images had to be sufficiently free of movement artifact to allow for analysis. |
28/38 had usable scans (no mention of whether these were the first or second scan) Success rate = 73.7% Scan time = 40 minutes |
|
McKinstry, Miller et al. 2002 Prospective longitudinal study, newborns imaged day 1, 3 & 7 of life between November 1997 to October 2001 United States |
12 newborns recruited from NICU. Inclusion criteria: Term infants following uncomplicated pregnancies with clear-cut event near birth that could be timed accurately and likely to cause brain injury, such as tight nuchal cord at delivery, shoulder dystocia, skull fracture, and uterine rupture with placental abruption. All infants had depressed level of consciousness and a disturbance of muscle tone (typically hypotonia), indicating encephalopathy. Exclusion critera: Infant too unstable to transport to scanner or identified after first day of life. |
Infant swaddled in warm blankets, and knit cap placed on head. | Diffusion Tensor Imaging of Brain Each ROI placement was verified by a CAQ-certified neuroradiologist (who was blinded to the patient’s history, all the imaging studies obtained earlier, and the diffusion scans) and a board-certified pediatric neurologist. Images were considered successful if the quality was sufficient to allow for analysis. |
One patient excluded due to excess motion artifact. Since it was not stated if the patient was excluded at day 1, 3 or 7, an assumption is made that patient was excluded at day 1. Day 1: 11/12 infants had usable scans: 91.7% success rate Day 2: 11/11. 100% Day 3: 11/11, 100% Scan time = 40 minutes |
|
McNally et al., 1997 Prospective cross-sectional,13 consecutive patients over a 20 month period (specific timeframe not reported) United Kingdom |
13 patients Inclusion criteria: after reduction of developmental dysplasia of the hip |
Hip held in place with spica cast. | MRI of the hip Definition of success not reported. |
All scans adequate, although 4 required a 2nd sequence. (100% success rate) Scan time = not reported |
|
Merchant et al., 2009 Prospective cross-sectional over a 20 month time period between 2007–2008 United Kingdom |
72 preterm infants weighing less than 1500 g were recruited over a 20 month period (2007–2008). Exclusion criteria: Attending neonatologist judged infants too unstable to transfer to imaging suite. |
Infants given milk feeding prior to scanning. Infants receiving parenteral nutrition receive equivalent volume given as 10% glucose with additives as appropriate for the duration of the scan. Infants placed in two layers of clothing and plastic wrap prior to transport to MRI scanner room, swaddled with prewarmed sheets encouraged sleep, and reduce movement. Moldable dental putty applied to ears and covered with neonatal ear muffs. Hat applied over ear muffs. Vacuum bag covered with muslin cloth and wrapped around the baby’s head. Foam insertions between the vacuum pack and coil. | MRI of brain The definition of successful image acquisition was not reported. |
70 underwent successful image acquisition (success rate = 97.2%). Median scan time = 55 minutes |
|
Miller et al., 2007 Design: Prospective cross-sectional between September 2001 to July 2005 United States |
41 term newborns with congenital heart disease Exclusion criteria: gestational age at birth < 36 weeks or if suspected congenital infection or genetic malformation syndrome 16 control newborns of similar gestational age Inclusion criteria: no signs of perinatal illness or major malformations (e.g., congenital heart disease) |
Infants imaged in MRI compatible neonatal incubator | MRI, magnetic resonance spectroscopy and diffusion tensor imaging of the brain A neuroradiologist who was unaware of all clinical information except for age and cardiac diagnosis scored each MRI scan for acquired focal, multifocal, or global changes. |
100% success rate Scan time = Not reported |
|
Missios et al., 2008 Design: Retrospective cross-sectional chart review of 1146 Brain MRIs from Feb 2003–Dec 2007 and prospective review of 457 “quick brain” MRI studies (no time frame reported). United States |
457 imaging scans done in 346 patients ages 1 day to 78 years old (mean age 4.1 years, median age 21.9 months) Inclusion: Macrocephaly, Chiari malformation, intracranial cyst, screening prior to lumbar puncture for intracranial pathological conditions associated with increased intracranial pressure, screening for congenital anomalies, trauma, and suspected intracranial pathological conditions in the presence of neurological symptoms such as seizures Exclusion: Patients undergoing imaging for known hydrocephalus or shunt follow-up |
Parents/family members of infants or staff assisted by holding infant’s head in place during MRI | MRI of the brain Success rate not defined. |
Success rate = 100% Scan time < 2.5 minutes |
|
Modi et al., 2001 Prospective cross-sectional study with control group Timeframe not reported United Kingdom |
16 patients were imaged as soon after delivery as possible. 10 patients exposed to repeated antenatal glucocorticoid therapy born at or close to term 6 patients who were not exposed to antenatal glucocorticoid therapy born at term |
Infants imaged in normal quiet sleep, as soon after delivery as possible within 1 week of birth. | MRI of the brain All analyses were performed blinded to patient group. All 16 infants showed normal brain anatomy. |
All MRIs were usable (success rate=100%). Scan time = Not reported |
|
Neil et al., 1998 Prospective cross-sectional study, timeframe not reported United States |
28 healthy newborn patients 31–41 weeks gestational ages Inclusion criteria: unremarkable pregnancies Exclusion criteria: movement artifact, questionable gestational age, seizures |
Some given bottle 30– 45 min. before imaging, all swaddled with head restrained cushions | Diffusion tensor imaging of the brain within the first 36 hours of life Some movement artifact was tolerated as long as anatomic structures could be reliably identified. |
22 images usable (12 term and 10 preterm, success rate = 78.6%) Scan time = 30–40 minutes per patient |
|
Neubauer et al., 2011 Prospective cross-sectional study between October 2007 and May 2010 Austria |
56 preterm infants born before 32 weeks gestational age & admitted to the NICU Inclusion: Patients born at one hospital before 32 weeks gestational age and admitted to the neonatal intensive care unit |
Infants fed 20 to 30 min before scan, fitted with earmuffs, swaddled and laid on vacuum cushion. Parents accompanied infant and rocked infant to sleep if necessary. Pacifier & orally administered glucose solution used if necessary. | MRI of brain around term-equivalent age The radiologist in charge of the study was available to evaluate the quality of the scan during the examination. In the case of poor-quality images because of movement artefacts, the MR examination was interrupted at any time. |
52 of 56 MRI scans completed successfully (success rate=92.9%) 39 of 56 (69.6%) accomplished without delay. 17 (30.4%) interrupted because infant agitation or crying; these included 4 (40%) interruptions in in-patient and 13 (28.3%) in out-patient infants. Of these 17 infants, 13 (76.5%) calmed during acceptable time and high-quality images still achieved. Mean duration of all sessions = 36 ± 14 min. MRI session in out-patient infants concluded within 32 ± 12 min, while mean duration with in-patients was 54 ± 10 min (p < 0.01, n = 46 and 10). |
|
Nossin-Manor et al., 2013 Prospective longitudinal MRIs acquired between March 2008 and April 2010 as part of broader cohort of prospective longitudinal study. Canada |
54 preterm neonates born 24–32 weeks gestational age scanned within 2 weeks of birth and 31 scanned for second time at term equivalent age between 36 and 45 weeks gestational age Exclusion criteria: Grade III and IV intraventricular hemorrhage and ventriculomegaly |
Not mentioned | MRI of the brain Images free of motion artifacts were retained for analysis. |
44 of 54 preterm scans were usable (success rate = 81.5%) 17 of 31 term equivalent age scans were usable (success rate = 54.8%) Total scan time = 28 minutes |
|
O’Regan et al. 2012 Design: Retrospective cross-sectional chart review of brain MRI during 2 year period: December 2006–December 2008 Ireland |
49 term and preterm neonates Group A: Imaged prior to introduction of MR-compatible incubator using standard MR sequences and equipment (knee coil) Group B: Imaged using MR-compatible incubator and standard MR sequences Group C: Imaged using MR-compatible incubator and modified MR sequences Inclusion criteria: term or preterm neonates who received MRI at one institution during a 2 year time period |
Neonates imaged after feeding with attention to swaddling and stabilization with head coil. | MRI of the brain Subjective and objective measures of image quality were assessed by two radiologists who were blinded to patient details and to whether the patient was scanned using standard equipment or using the MR-compatible incubator. First, overall image quality was assessed on a 3-point rating system based on image sharpness and clarity of the grey–white matter interfaces. Second, it was documented whether motion artefact was present or absent on the examination in any of the sequences. |
10 patients excluded for incomplete examinations and non-standard MR parameters # of patients with usable scans: Group A: 8 out of 15 (53.3%) Group B: 10 out of 15 (66.7%) Group C: 8 out of 9 (88.9%) Mean scan time: Group A: 14 min Group B: 15 min Group C: 17.5 min |
|
Partridge et al. 2004 Prospective longitudinal study of over 28-month duration (between November 8, 2001 and March 5, 2003), 50 premature neonates imaged. United States |
15 premature neonates met inclusion criteria. Patients first imaged between 28 and 39 weeks gestational age (median, 33 weeks), and 8 received second imaging at or near term age or just before discharge from hospital Inclusion criteria: Neonates born at gestational ages of 24 to 36 weeks with no evidence of white matter injury on conventional MR imaging. Exclusion criteria: Greater than Grade 1 hemorrhage (i.e., small intraventricular bleed confined to the subependymal region), congenital infection, brain malformation, or a multiple congenital anomaly syndrome. |
Cotton ear muffs, comfortable contoured padding, warm environment, double-walled construction, and acoustic and vibration damping all tend to facilitate undisturbed sleep and/or calm resting during scans. | Diffusion tensor imaging (DTI) of the brain Images were excluded if motion artifacts substantially degraded the DTI acquisition. |
2 scans excluded from one patient due to motion artifact 14/15 at time 1 = 93.3% 7/8 at time 2 = 87.5% Total scan time = not reported |
|
Rabattu et al., 2014 Retrospective cross-sectional chart review, no timeframe reported France |
9 patients required MRI for lower limb disorder Inclusion criteria: requiring spica cast immobilization |
Temporary spica cast immobilization, parents stood close to their child during the whole examination | MRI of lower limb All MRI were examined by a consultant radiologist to determine whether the diagnosis could be reached or if the rate of movement artifacts were too numerous, hampering clinical scan interpretation. |
Although 2 of 9 infant MRIs had artifact, diagnosis achieved in all cases = 100% success rate. Scan time = not reported |
|
Reilly et al., 2012 Retrospective cross-sectional chart review, descriptive, comparative study conducted through auditing neonate and infant charts with completed MRI brain with sedation or with the use of an immobilizer between 1/1/2007 – 9/30/2010 United States |
36 patients did not receive sedation Inclusion criteria: less than or equal to 90 days of age, weighing at least 2 kg, required MRI brain scan that was predicted to take no more than 60 min to complete Exclusion criteria: patients weighing less than 2 kg with a post-conceptual age greater than 3 months, artificial airway or mechanical ventilator support, umbilical catheter, or were unable to be fed orally or enterally |
Non-sedated patients fed and placed in infant immobilizer prior to MRI. | MRI of the brain Final radiology reports were obtained from the patient’s medical record. Reports indicated diagnostic quality of the images obtained based on interpretation by the radiologist. |
Images considered diagnostic in 94.4% of non-sedated infants (34 out of 36) The scans were ≤ 60 min. |
|
Rozovsky et al., 2013 Retrospective cross-sectional radiology chart review between January 2008 and August 2010 Israel |
30 patients age 1 day to 5 years (mean: 18 months) Inclusion criteria: Scan performed to assess shunt position, size and configuration of fluid-filled structures Exclusion criteria: MRI performed in patients older than 5 years, those performed under general anesthesia or sedation at any age, and studies performed for clinical indications other than assessment of the ventricular system and extra-axial cerebrospinal fluid paces |
Not reported. | MRI of the brain Images were reviewed in detail by a clinical fellow with 5 years’ experience in pediatric radiology and a pediatric neuroradiologist with 10 years’ experience in pediatric radiology & 4 years’ experience in neuroradiology. Radiologists had knowledge of the clinical indications for each study but were blinded to previously reported findings. Their interpretations were subsequently compared to findings reported in Picture Archiving and Communication System. All studies were compared to the most recent MRI or CT study, which served as a reference standard. Disagreements between readers and between the new interpretation and original report were resolved in consensus. |
All brain MRIs provided satisfactory answers to the clinical question (100% success rate). Scan time = not reported |
|
Rutherford et al., 2004 Prospective cross-sectional study of infants delivered at >36 weeks’ gestation and presented with seizures in first 72 hours after delivery. Group 1: Infants with neonatal encephalopathy Group 2: Infants who did not have criteria for neonatal encephalopathy as outlined above. Control Subjects Fifteen infants with normal brain imaging and normal neurologic examination Timeframe: Not reported United Kingdom |
63 patients in group 1 14 patients in group 2 15 infants in control group Inclusion criteria for groups 1 & 2: Delivered at >36 weeks gestation and presentation of seizures within 48 hours post-delivery. Additional inclusion for group 1: Neonatal encephalopathy, abnormal tone patterns, feeding difficulties, and altered alertness and at least 3 of the following: 1) late decelerations on fetal monitoring or meconium staining, 2) delayed onset of respiration, 3) arterial cord blood pH <7.1, 4) Apgar scores <7 at 5 minutes, and 5) multi organ failure. Additional inclusion criteria for group 2: Seizures within 72 hours of birth but no neonatal encephalopathy Additional inclusion criteria for control group: No resuscitation at birth, normal Apgar scores, and no seizures or other clinical neurologic symptoms Exclusion criteria: evidence of metabolic disease, congenital infection, major malformations, alcohol or drug embryopathies, hydrops, chromosome abnormalities, brain damage on initial scan or evidence of developmental abnormalities |
Groups 1 & 2: Infants examined during natural sleep or after feed Control group: Infants examined during natural sleep |
Diffusion weighted imaging of the brain Analysis of the DWI was undertaken by 1 experienced researcher. |
All scans usuable (success rate=100%). Scan time = not reported |
|
Ryan et al., 2016 Retrospective cross-sectional chart review from 2009 through 2013 United States |
61 patients (range 1 day to 22 years, mean age 2.4 years) with a prior CT, either at this institution or another institution, loaded onto a digital picture archiving and communications system workstation within 48 h of rapid MRI Patients were excluded in cases of interval neurosurgical procedure, inadequate CT imaging, or no identifiable hemorrhage by CT. |
None reported | MRI of the brain Imaged were anonymized, and reviewed by two board certified pediatric neuroradiologists. Motion degradation scored from none (0) to severe (3). |
Success rate = 100% Scan time = not reported |
|
Sigmund et al.,1991 Prospective cross-sectional study between Sept. 1989 to Nov. 1990 Germany |
9 infant patients Inclusion criteria: suspected morphologic pathology of kidneys and urinary tract |
Infants fed. | MRI of urinary tract The quality of the images were subjectively classified as excellent (no motion artifacts & a high signal-to-noise ratio), satisfactory (motion artifacts & considerable noise but images were still diagnostic) and poor (non-diagnostic). |
All images diagnostic (success rate = 100%). Scan time = not reported |
|
Siles et al., 2014 Prospective cross-sectional study between December 2008 to October 2010 France |
16 full-term neonates and infants younger than 3 months and without any clinical or biological anomaly suggesting cholestasis. | Eight of the 11 were scanned after their usual milk feeding and gentle swaddling, which spontaneously rendered them calm or asleep; the other three were examined after 3–4 h fasting, gentle swaddling, and rocked to sleep. All were restrained in a plastic cast with arms resting against their sides. | MRI of the brain, orbit or face Images were read jointly by two experienced pediatric radiologists (6–15 years of practice) and a junior radiologist with 6 months of training in Pediatric Radiology. Case inconsistencies were discussed during the readings in order to reach a consensus. |
Performed 11 out of 16 exams without sedation (success rate = 68.8%). The acquisitions’ duration ranged from 2 minutes 30 seconds to 8 minutes 15 seconds (mean 5 minutes 20 seconds, median 4 minutes 50 seconds). |
|
Sirin et al., 2013 Retrospective cross-sectional chart review between February 2011 to May 2012 Germany |
8 patients were scanned without sedation Inclusion criteria: All infants who received a MRI of the brain on a 3 Tesla MR scanner |
All infants fed prior to MRI. Mini muffs and molded ear plugs from silicone-based putty used for noise reduction. | MRI of the brain Images that had motion artifacts with relevant impairment of image quality were considered unusable. |
6 out of 8 scans without sedation were usable (success rate = 75%) Mean scan time 34 min (standard deviation 20 min, range 12–71 min). |
|
Smyser et al. 2010 Prospective longitudinal study of preterm infants aged 26 weeks postmenstrual age (PMA) through term equivalent PMA recruited from well-baby nursery and NICU as part of ongoing study. Initial image acquisitions performed within first 2 weeks of life (as early as 26 weeks PMA). Based on clinical status and gestational age at time of delivery, serial data sets for each infant collected ~ every 4– 5 weeks (30–31, 34–35, and 38–40 weeks PMA). Data from 10 term control infants (4 males) collected within 2–3 days of birth. No time frame reported United States |
90 data sets collected from 53 preterm infants | Infants studied during natural sleep or resting quietly in scanner. NICU staff member present in scanner room throughout each acquisition. | MRI of the brain Images with excessive motion artifact were removed. |
10 datasets excluded due to excessive motion at varied stages of acquisition. Success rate = 80/90 = 88.9% Total scan time = 50 minutes |
|
Spann et al., 2015 Prospective cross-sectional study between 2005–2009 United States |
37 healthy infants Inclusion criteria: maternal age at conception of 18– 45 years, no major prenatal or delivery complications, gestational age ≥ 37 weeks, birth weight >10th percentile relative to the national standards, no major congenital anomalies, and an uncomplicated neonatal nursery course. Exclusion criteria: maternal history of a chronic medical disease, used drugs of abuse, smoked cigarettes, or drank more than 1 ounce of alcohol during any trimester. |
Infants were fed, swaddled, and given time to fall asleep. Foam ear plugs along with ear shields were applied to dampen scanner noise. Infants were acclimated to the scanner environment and noise before the start of scanning. | MRI of brain Operators were blinded to infant characteristics. The operator interrater reliability was assessed on 10 scans with intraclass correlation coefficients > 0.95. |
Success rate = 100%. However, these infants were a subset of participants in a larger study. Scan time = not reported |
|
Thomeer, et al.,2015 Prospective cross-sectional study between 2008 and the first half of 2014, all patients born in one institution Netherlands |
36 patients admitted for anorectal malformation in postnatal period Inclusion criteria: neonatal period up to 4 months after birth. Exclusion criteria: 3 excluded for only one examination performed (either MRI or colostography/fistulography) |
Infant patients fed and wrapped prior to imaging. The majority of infants sleeping during imaging. | MRI of pelvic floor The MRI studies were prospectively and independently analysed by two readers (two experienced staff members of paediatric radiology and abdominal radiology), blinded to the results of surgery. |
One MRI unsuccessful yielding success rate of 32/33= 97.0%. Scan time = not reported |
|
Tkach et al., 2014 Prospective cross-sectional study, time frame not reported United States |
15 neonates Inclusion criteria: medically and thermally stable (not recruited on the basis of a suspected or known clinical need) |
Fed 1 hour prior to MRI. Fussy infants received 25% sucrose solution immediately before and, if needed, during the imaging. | 15 MRIs of the brain, 7 MRIs of the abdomen, 6 MRIs of the chest, 2 MRIs of the heart The images obtained of the brain, chest, and abdomen were reviewed by board certified pediatric radiologists with additional expertise in neuroradiology and body MRI, respectively. The cardiac images were evaluated by a pediatric cardiologist experienced in cardiac MRI. In all cases, the evaluation was subjective. Each dataset was assessed for overall study quality (limited, adequate, or good), motion (none, moderate, or significant), spatial resolution (poor, moderate, or excellent), SNR (poor, moderate, or excellent), and contrast (poor, moderate, or excellent) as compared with similar neonatal examinations performed on conventional MR scanners in same institution. |
All infants participated in single session without sedation (success rate = 100%). No imaging session ended prematurely. 7 of 15 subjects had significant motion during imaging necessitating temporary suspension of scanning to calm infant by re-swaddling, administering 24% sucrose solution, or both. MRI was resumed and successfully completed after each intervention. For infants not responding to feed-and-swaddle method, fast imaging techniques used, proving successful in providing diagnostic information even in presence of significant intermittent motion. Total scan time = limited to 60 minutes |
|
Tsiflikas et al. (2019) Retrospective cross-sectional review of all functional MRI urographies performed in infants younger than 1 year in a radiology database at one institution between 2010 and 2017 United States |
42 infants younger than 1 year No additional inclusion or exclusion criteria noted |
Infants scheduled for a feed-and-sleep examination were deprived of sleep and were fasted for 4 h before MRI scan. Each child was fed just before the scan | Functional MRI urographies Evaluated by a pediatric radiologist and by a pediatric surgeon (both with more than 10 years of experience in MRurography) in consensus using a 3-point scale for motion artifacts (1: no artifacts visible; 2: moderate artifacts without hampering the diagnostic quality; 3: marked artifacts hampering the diagnostic quality). |
In the feed-and-sleep group, 38 of 42 examinations (90%) were completed successfully. Scan time = mean of 28 minutes |
|
Walkup et al., 2015 Prospective cross-sectional study, timeframe not reported United States |
18 infants were divided into three subgroups: Full-term control group (n = 6), defined as NICU patients born at greater than or equal to 36 weeks gestational age without major, suspected pulmonary complications Premature non-bronchopulmonary dysplasia group (n = 6), defined as birth at less than 36 weeks gestational age Premature infants with clinical diagnosis of bronchopulmonary dysplasia (n = 6) |
Infants fed, swaddled, and equipped with ear protection before imaging. | MRI of lung parenchyma MRIs were clinically scored by a radiologist with no prior knowledge of clinical diagnosis using a modified Ochiai scoring system with a higher score reflective of more severe findings (range, 0–14); the radiologist also scored the diagnostic quality of the images (0–2, with 0 considered nondiagnostic quality). |
All 18 infants participated in single imaging session without sedation (success rate = 100%). Scan time = 1 hour for preparation and scanning. 30 minutes for localization and acquisition |
|
Wang et al., 2008 Prospective cross-sectional study, time frame not reported United States |
Normal infants, 19 infants imaged at 7 or 13 months of age studied at single site No additional inclusion or exclusion criteria noted |
Infants fed and allowed to fall asleep. Foam padding restricted head movement and provided sound attenuation. | Arterial spin labeled perfusion MRI of brain CBF maps showing spuriously low values (<20 ml/100 g/min for whole brain) suggested ineffective labeling and were discarded. |
Acceptable cerebral blood flow data obtained from 8 infants aged 6.9 ± 0.2 months and 8 infants aged 12.7±0.2 months, corresponding to a success rate of 84%. Total Scan time = Not reported |
|
Whitby et al., 2004 Prospective cross-sectional study, time frame not reported United Kingdom |
7 stable neonate patients born from 24 weeks gestation to term were imaged at age 2 days to 4 months in custom-built incubator before discharge home from unit Exclusion criteria: none reported |
None mentioned. | Diffusion weighted imaging of the brain The images were scored for quality by 2 neuroradiologists and 1 neonatal radiologist, and a radiologic diagnosis was reached by consensus in each case. |
100% success rate Scan time = 10–21 minutes |
|
Whitby et al., 2003 Prospective cross-sectional double blind trial, time frame not reported United Kingdom |
134 neonatal patients Group 1 (control): 89 neonates with no known neurological symptoms and not expected to have intracranial disease (40 preterm & 49 term) Group 2: 43 neonates with known or suspected intracranial pathology on clinical grounds (23 preterm & 20 term) Exclusion criteria: none noted |
Neonates swaddled in blanket | MRI of brain Images were independently reviewed for pathology by two experienced radiologists who were blinded to the ultrasound results. Results are expressed according to whether they agreed with the ultrasound findings and whether this altered clinical management of the infant. |
Group 1: 87/89=97.8% success rate for neonates with no known neurological symptoms 43 of the MRIs from neonates with known or suspected intracranial pathology were reportable (success rate = 100%). Typical time in the scanner was 30–40 minutes. |
|
Windram et al.,2012 Retrospective cross-sectional review of 20 consecutive patient charts between January 2010 to January 2011 Canada |
20 patients younger than 6 months requiring cardiovascular MRI who received no sedation Inclusion criteria: <2 years old |
“Feed and sleep” method: fasting infant for 4 hours prior to scan and feeding just prior to scan, earmuffs, immobilizer, dim lighting, extra feed if infant awoke | MRI of the heart Images had to be of sufficient quality to provide a diagnosis. |
Achieved 100% success rate. The median time was 46.5 min (range 20 to 66 min). |
|
Woodward et al. 2006 Prospective cross-sectional study of 1 hospital in Christchurch, New Zealand (November 1998 to December 2000) and 1 hospital in Melbourne, Australia (July 2001 and May 2002) |
167 very premature infant patients born ≤30 weeks gestation Exclusion criteria: 1 blind infant and 2 infants with incomplete data |
Infant fed, wrapped and placed in beanbag. | MRI of brain All scans were scored independently by one of the authors and by a pediatric neuroradiologist (Christchurch) or neonatologist (Melbourne). Raters were unaware of the infants’ perinatal history and ultrasonographic findings. A standardized scoring system developed in this study was used, consisting of eight 3-point scales. |
All usable scans (100% success rate). Total scan time = not reported |
|
Young et al., 2016 Retrospective cross-sectional chart review of emergency department at one institution between January 2010 and May 2015 United States |
33 patients age ≤ 6 years with head trauma and received a rapid non-sedated MRI within 24 hours of injury | Parent or caregiver accompanied child into the MRI room and laid with the child on the scanner table so that the caregiver could provide reassurance to child throughout the scan. The caregiver held the child’s head between her or his hands for additional tactile reassurance and to aid in control of head motion while the technologist placed the coil over the child’s head. The child and parent/caregiver were moved into the scanner bore together and the scan was performed. | MRI of the brain The scans were de-identified and reviewed independently by two board-certified neuroradiologists. |
All usable scans (100% success rate). Total scan time for the four core sequence protocol was 1.5 to 2 min. Additional sequences were obtained if requested by the radiologist or treating physician and if tolerated by the child (scan times not reported). |
Articles included in this review represent data collected in twelve countries (see Table 2): Thirty-one in the United States (Ashley et al., 2005; Bartha et al., 2007; Damaraju et al., 2014; Dean et al., 2014; Dean et al., 2017; Fogel et al., 2011; Gould et al., 2012; Haney et al., 2010; Higano et al., 2017; Iskandar et al., 2004; Jaramillo et al., 1998; Knickmeyer et al., 2008; Laor et al., 2000; Lin et al., 2008; Liu et al., 2008; Maas et al., 2004; McKinstry, Mathur et al., 2002; McKinstry, Miller et al., 2002; Miller et al., 2007; Missios et al., 2008; Neil et al., 1998; Partridge et al., 2004; Reilly et al., 2012; Ryan et al., 2016; Smyser et al., 2010; Spann et al., 2015; Tkach et al., 2014; Tsiflikas et al., 2019; Walkup et al., 2015; Wang et al., 2008; Young et al., 2016); eleven in the United Kingdom (Arthurs et al., 2011; Foran et al., 2007; Gale et al., 2013; Groves et al., 2011; Groves et al., 2012; McNally et al., 1997; Merchant et al., 2009; Modi et al., 2001; Rutherford et al., 2004; Whitby et al., 2003; Whitby et al., 2004); three in Australia (Hansen, 2009; Inder, Warfield, Wang, Hüppi, & Volpe, 2005; Woodward et al., 2006) and France (Chateil et al., 1999; Rabattu et al., 2014; Siles et al., 2014); two in Canada (Nossin-Manor et al., 2013; Windram et al., 2012), Germany (Sigmund et al., 1991; Sirin et al., 2013), Israel (Golan et al., 2011; Rozovsky et al., 2013), and New Zealand (Inder et al., 2005; Woodward et al., 2006); and one in Austria (Neubauer et al., 2011), Denmark (Born et al. 2000), Ireland (O’Regan et al., 2012) and the Netherlands (Thomeer et al., 2015). The success rate of obtaining usable MRI scans in infants with the sole use of non-pharmacological strategies ranged from 4–100% in the United States, 76.5–100% in the United Kingdom, 88.9–100% in Australia, 68.8–100% in France, 54.8–100% in Canada, 75–100% in Germany, 100% in Israel, 92–100% in New Zealand, 92.9% in Austria, 100% in Denmark, 69.6% in Ireland, and 97.0% in the Netherlands.
The articles that reported sample sizes had sample sizes ranging from 2–457 with success rates of obtaining usable MRI scans 4–100% (, see Table 2). Some articles did not state how many in the sample were infants (Ashley et al., 2005; Missios et al., 2008; Rozovsky et al., 2013; Ryan et al., 2016); they all had success rates of 100%. Some articles based their success rate on the number of scans rather than the sample size (Ashley et al., 2005; Iskandar et al., 2004; Missios et al., 2008; Smyser et al., 2010) with success rates of obtaining usable MRI scans 90–100% (, see Table 2).
Forty-eight percent of the articles were comprised of infants who received imaging for clinical purposes (Arthurs et al., 2011; Ashley et al., 2005; Born et al. 2000; Chateil et al., 1999; Fogel et al., 2011; Foran et al., 2007; Golan et al., 2011; Gould et al., 2012; Haney et al., 2010 [retrospective arm]; Hansen, 2009; Iskandar et al., 2004; Jaramillo et al., 1998; Laor et al., 2000; Maas et al., 2004; McNally et al., 1997; Missios et al., 2008; O’Regan et al., 2012; Rabattu et al., 2014; Reilly et al., 2012; Rozovsky et al., 2013; Ryan et al., 2016; Siles et al., 2014; Sirin et al., 2013; Thomeer et al., 2015; Tsiflikas et al., 2019; Windram et al., 2012; Woodward et al., 2006; Young et al., 2016), with a success rate of obtaining usable MRI scans ranging from 4 – 100%. The rest of the articles comprised infants who received imaging for research purposes with success rates of obtaining usable MRI scans ranging from 48.8 – 100%.
Most of the articles (68.5%) were comprised of at-risk infants with various health conditions (see Table 2). Success rates of obtaining usable MRI scans from these at-risk infants ranged from 4 – 100%. Infants born prematurely were imaged the most (Born et al. 2000; Inder et al., 2005; Maas et al., 2004; McKinstry Mathur et al., 2002; McKinstry Miller et al., 2002; Merchant et al., 2009; Neubauer et al., 2011; Nossin-Manor et al., 2013; O’Regan et al., 2012; Patridge et al., 2004; Rutherford et al., 2004), with success rates at 53–100%. Cardiac abnormalities were the most common conditions imaged (Fogel et al., 2011; Foran et al., 2007; Miller et al., 2007; Rozovsky et al., 2013; Windram et al., 2012) with the success rates between 83–100%, followed by hip dysplasia (Gould et al., 2012; Jaramillo et al., 1998; Laor et al., 2000; McNally et al., 1997) with a success rates of 76–100%. By comparison, articles with healthy infants had success rates of 48.9 – 100%.
The majority of articles (75.9%) acquired structural MRIs with success rates for obtaining usable MRI scans of 4–100% (, see Table 2). Two articles acquired functional MRIs with an average success rate of 90% (Born et al., 2000; Tsiflikas et al., 2019). Another article used MRI or computed tomography angiography with a success rate of 96% (Fogel et al., 2011). A minority of articles used diffusion tensor imaging (Bartha et al., 2007; Dean et al., 2017; Maas et al., 2004; Neil et al., 1998; McKinstry, Mathur et al., 2002; McKinstry, Miller et al., 2002; Partridge et al., 2004) and obtained usable scans 74–100% of the time. Two articles used diffusion weighted images (Rutherford et al., 2004; Whitby et al., 2004) and obtained usable scans 100% of the time. One article used arterial spin labeling with an 84% success rate (Wang et al., 2008). One article used magnetic resonance voiding cystourethrography with a 100% success rate (Arthurs et al., 2011).
The brain was often imaged (65.5%) with a 43–100% success rate in obtaining usable MRI scans (see Table 2). Five articles (10%) imaged the heart (Fogel et al., 2011; Foran et al., 2007; Groves et al., 2012, Groves et al., 2011; Windram et al., 2012) with a 83–100% success rate. Four (8.0%) imaged the hip (Gould et al., 2012; Jaramillo et al., 1998; Laor et al., 2000; McNally et al., 1997) with a 76.4–100% success rate. When other parts of the body were imaged, the success rates in of obtaining usable MRI scans was 94% for the whole body (Gale et al., 2013), 95% for the lung (Higano et al., 2017; Walkup et al., 2015), 97% for the urinary tract (Arthurs et al., 2011; Sigmund et al., 1991; Tsiflikas et al., 2019) and pelvic floor (Thomeer et al., 2015), and 100% for the visual system (Born et al., 2000), and lower limb (Rabattu et al., 2014).
Scan times were reported in 47% of the articles, with scan times ranging from 2.5 to 60 minutes and success rates of obtaining usable MRI scans 43–100% (Arthurs et al., 2011; Ashley et al., 2005; Born et al. 2000; Chateil et al., 1999; Dean et al., 2014; Dean et al., 2017; Fogel et al., 2011; Foran et al., 2007; Gould et al., 2012; Hansen, 2009; Iskandar et al., 2004; Jaramillo et al., 1998; Laor et al., 2000; Liu et al., 2008; McKinstry, Mathur et al., 2002; McKinstry, Miller et al., 2002; Missios et al., 2008; Neubauer et al., 2011; Nossin-Manor et al., 2013; O’Regan et al., 2012; Reilly et al., 2012; Siles et al., 2014; Sirin et al., 2013; Smyser et al., 2010; Tkach et al., 2014; Tsiflikas et al., 2019; Walkup et al., 2015). Three percent of articles that reported the median scan time (Merchant et al., 2009; Windram et al., 2012) averaged 51 minutes and a success rate of 98.6%. When a range of scan times was reported (Gale et al., 2013; Groves et al., 2011; Higano et al., 2017; Neil et al., 1998; Whitby et al., 2004; Whitby et al., 2003), the median was obtained; the average of these median scan times was 34 minutes, with a success rate of 79–100%. One article reported time away from the NICU, with a mean scan time of 54 minutes and a success rate of 49% (Haney et al., 2010). Scan times were not reported in 38% of articles with success rates ranging from 43–100% (Bartha et al., 2007; Damaraju et al., 2014; Golan et al., 2011; Groves et al., 2012; Inder et al., 2005; Knickmeyer et al., 2008; Lin et al., 2008; Maas et al., 2004; McNally et al., 1997; Miller et al., 2007; Modi et al., 2001; Partridge et al., 2004; Rabattu et al., 2014; Rozovsky et al., 2013; Rutherford et al., 2004; Sigmund et al., 1991; Thomeer, et al., 2015; Wang et al., 2008; Woodward et al. 2006; Young et al., 2016). See Table 2.
Authors varied in reporting how they determined if a scan was successfully imaged or not. Many authors described the expertise of the person evaluating the images, such as radiologists (Arthurs et al., 2011; Ashley et al., 2005; Golan et al., 2011; Hansen et al., 2009; Higano et al., 2017; Neubauer et al., 2011; O’Regan et al., 2012; Rabattu et al., 2014; Reilly et al., 2012; Walkup et al., 2015), pediatric radiologists (Gould et al., 2012; Jaramillo et al., 1998; Rozovsky et al., 2013; Ryan et al., 2016; Tkach et al., 2014; Tsiflikas et al., 2019), neuroradiologists (Miller et al., 2007; McKinstry, Mathur et al., 2002; McKinstry, Miller et al., 2002; Siles et al., 2014; Whitby et al., 2004; Young et al., 2016), neonatal radiologists (Whitby et al., 2004), neonatal neuroradiologists (Bartha et al., 2007), pediatric neuroradiologists (Rozovsky et al., 2013; Woodward et al., 2006), pediatric neurologists (McKinstry, Mathur et al., 2002), pediatric cardiologist (Tkach et al., 2014), pediatric surgeon (Tsiflikas et al., 2019), neonatologist (Woodward et al., 2006), researchers (Rutherford et al., 2004), anatomical expert (Knickmeyer et al., 2008), staff members from pediatric and abdominal radiology (Thomeer et al., 2015), and MRI technician (Haney et al., 2010). Authors varied in the number of individuals who reviewed images. Images were reviewed by one individual (Groves et al., 2012; Haney et al., 2010; Higano et al., 2017; Jaramillo et al., 1998; Knickmeyer et al., 2008; McKinstry, Miller et al., 2002; Miller et al., 2007; Neubauer et al., 2011; Rabattu et al., 2014; Reilly et al., 2012; Rozovsky et al., 2013; Rutherford et al., 2004; Ryan et al., 2016; Thomeer et al., 2015; Walkup et al., 2015), two individuals (Bartha et al., 2007; Gale et al., 2013; McKinstry, Mathur et al., 2002; O’Regan et al., 2012; Tsiflikas et al., 2019; Whitby et al., 2003; Young et al., 2016) and three individuals (Whitby et al., 2004), or whichever radiologist was on service at the time (Golan et al., 2011). Some were junior reviewers with less experience (Siles et al., 2014) while other had up to 22 years of experience (Higano et al., 2017). Many were blinded to certain participants’ details (Arthurs et al., 2011; Higano et al., 2017; Jaramillo et al., 1998; McKinstry, Miller et al., 2002; Miller et al., 2007; Modi et al., 2001; O’Regan et al., 2012; Rozovsky et al., 2013; Spann et al., 2015; Thomeer et al., 2015; Walkup et al., 2015; Whitby et al., 2003; Woodward et al., 2006).
Regardless of whether the expertise of those assessing the images was reported, some articles described the criteria for a successful image, such as the quality of the images had to be sufficient to allow for analysis (McKinstry, Mathur et al., 2002; McKinstry, Miller et al., 2002) and to obtain a diagnosis (Arthurs et al., 2011; Fogel et al., 2011; Golan et al., 2011; Laor et al., 2000; Reilly et al., 2012; Ryan et al., 2016; Sigmund et al., 1991; Walkup et al., 2015; Windram et al., 2012). Others used images from infants who did not wake up during the scan (Dean et al., 2017; Damaraju et al., 2014). Many described criteria related to motion (Born et al., 2000; Chateil et al., 1999; Gale et al., 2013; Gould et al., 2012; Hansen et al., 2009; Inder et al., 2005; Lin et al., 2008; Liu et al., 2008; McKinstry, Mathur et al., 2002; Neil et al., 1998; Neubauer et al., 2011; Nossin-Manor et al., 2013; O’Regan et al., 2012; Partridge et al., 2004; Rabattu et al., 2014; Sigmund et al., 1991; Smyser et al., 2010; Tkach et al., 2014; Tsiflikas et al., 2019), artifacts such as intra-scan motion and off-resonance (Dean et al., 2014) or flow visualization, magnitude and phase images (Groves et al., 2012), tissue (Gould et al., 2012), contrast (Gould et al., 2012; Tkach et al., 2014), registration difficulties (Inder et al., 2005), sequence errors (Inder et al., 2005), signal intensity abnormalities (Bartha et al., 2007), spatial resolution (Gould et al., 2012; Tkach et al., 2014), signal to noise (Tkach et al., 2014) and/or image sharpness and clarity (O’Regan et al., 2012). Some were based on specific imaging information, such as being able to visualize the catheter (Ashley et al., 2005), see ventricular anatomy (Iskandar et al., 2004), allow for long-axis estimations (Foran et al., 2007). Some authors created a three (Gould et al., 2012; O’Regan et al., 2012; Sigmund et al., 1991; Tsiflikas et al., 2019; Walkup et al., 2015) or four-point scoring system (Groves et al., 2012; Haney et al., 2010; Ryan et al., 2016; Woodward et al., 2006) and provided a numerical value based on how many of the criteria the scans met. Finally, some articles did not report how an image was determined to be successful (Maas et al., 2004; McNally et al., 1997; Merchant et al., 2009; Missios et al., 2008).
Most articles reported how they kept infants still during imaging (87.9%) and used a combination of feeding and/or swaddling, an immobilizer to reduce body movement, ear muffs for noise reduction, and imaging the infant while sleeping with a success rate ranging from 4 – 100% in obtaining usable MRI scans (see Table 2). Some also had a parent or assistant in the MRI with the infant during the acquisition (Dean et al., 2014; Missios et al., 2008; Rabattu et al., 2014; Smyser et al., 2010; Young et al., 2016), with an average success rate of 98.1%. Occasionally an infant received a pacifier, sometimes with glucose/sucrose (Golan et al., 2011; Neubauer et al., 2011; Tkach et al., 2014), with an average success rate of 97.6%. Several articles imaged infants in a spica cast (Gould et al., 2012; Jaramillo et al. 1998; McNally et al., 1997; Rabattu et al., 2014) and had an average success rate of 94.7%. Notably, one article described imaging crying infants in a spica cast (Laor et al., 2000). Seven articles (12.1%) did not report how they kept infants still during imaging (Ashley et al., 2005; Higano et al., 2017; Maas et al., 2004; Nossin-Manor et al., 2013; Rozovsky et al., 2013; Ryan et al., 2016; Whitby et al., 2004) and had success rates ranging from 54.8% – 100%.
3.3. Risk of Bias within Articles
There were three areas where a risk of bias was found (see Supplementary Table 1). The first area was not identifying how the rights of infants were protected, failing to report if informed consent was obtained, and failing to report if the local IRB approved their project. Six articles did not report this information (Golan, Marco, Raz, & Shany, 2011; Haney et al., 2010; Laor, Roy, & Mehlman, 2000; McNally, Tasker, & Benson, 1997; O’Regan, Filan, Pandit, Maher, & Fanning, 2012; Sigmund et al., 1991). All of these articles were quality improvement projects. Two examined the utility of an infant immobilizer newly implemented at their institution (Golan et al., 2011; Haney et al., 2010), Two examined the utility of a MRI with reduced imaging time (Laor et al., 2000; Sigmund et al., 1991). One examined the usefulness of a dedicated magnetic resonance-compatible incubator with integrated radiofrequency coils (O’Regan et al., 2012). One examined the utility of MRI after developmental dysplasia of the hip reduction (McNally et al., 1997).
A second area where a risk of bias was found was in the methodology where thirteen articles did not clearly state an experimental hypothesis (Dean et al., 2014; Dean et al., 2017; Gale et al., 2013; Haney et al., 2010; McNally et al., 1997; Rozovsky, Ventureyra, & Miller, 2013; Ryan et al., 2016; Siles et al., 2014; Tkach et al., 2014; Tsiflikas et al., 2019; Whitby et al., 2003; Whitby et al., 2004; Young et al., 2016). Two of these articles appear to be quality improvement projects (Haney et al., 2010; McNally et al., 1997). Seven articles were descriptive (Dean et al., 2014; Dean et al., 2017; Gale et al., 2013; Rozovsky et al., 2013; Tkach et al., 2014; Whitby et al., 2003; Whitby et al., 2004). Four were exploratory (Ryan et al., 2016; Siles et al., 2014; Tsiflikas et al., 2019; Young et al., 2016).
A third area where a risk of bias was found was with generalizability. All articles had selection bias, limiting generalizability. Even studies and quality improvement projects that included all patients during a particular time frame were limited to those who presented at a particular health care facility for a specific reason.
3.4. Meta-analysis Results
Five articles were excluded from the meta-analysis because we could not determine how many of the participants or patients were infants (Ashley et al., 2005; Iskandar et al., 2004; Missios et al., 2008; Rozovsky et al., 2013; Ryan et al., 2016). (These articles were included in the systematic review because all the MRI scans were usable.) Meta-analysis performed on 53 articles comprising 3,410 infants found the success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies was 87% (95% CI 0.83, 0.91). The I2 =98.30% (95% CI 97.6, 98.8) suggests considerable heterogeneity (Higgins & Green, 2011).
Heterogeneity was explored with sub-group analyses (Higgins & Green, 2011) based on the design of articles; retrospective cross-sectional, prospective cross-sectional, and prospective longitudinal. The success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies in 1,031 infants in retrospective cross-sectional was 81% (95% CI 0.68, 0.94, p<.001) the I2 = 98.63, suggested considerable heterogeneity (See Figure 2). The success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies in 1,428 infants in prospective cross-sectional articles was 95% (95% CI 0.92, 97). The I2 = 87.71, suggesting substantial heterogeneity (See Figure 3). The success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies in 951 infants in prospective longitudinal was 79% (95% CI 0.70, 0.88) The I2 = 97.39 (See Figure 4).
Figure 2.
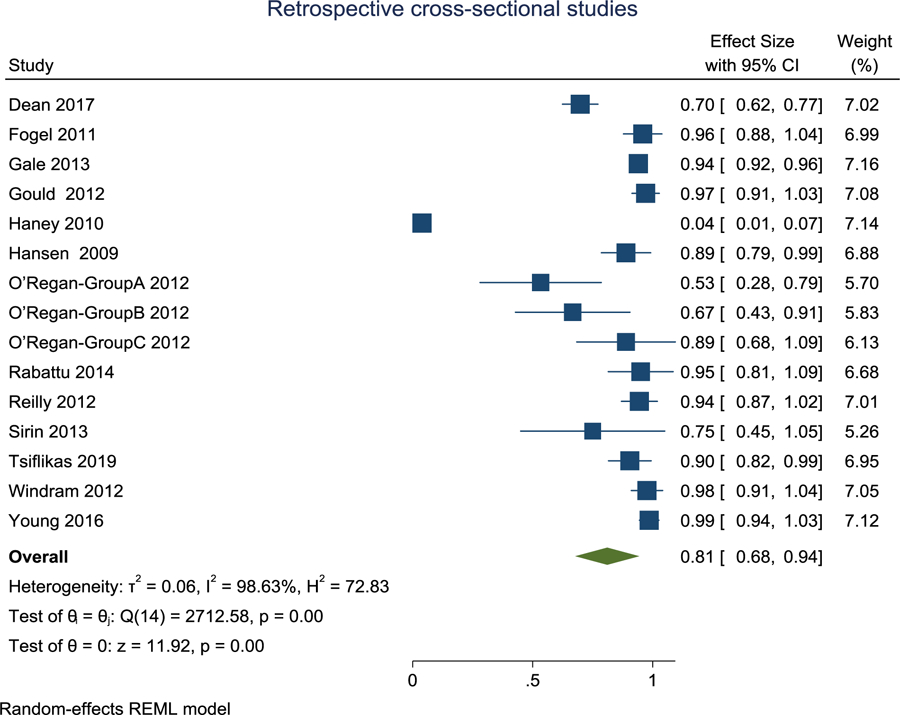
Forest plot of retrospective cross-sectional articles. The success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies was 81%.
Figure 3.
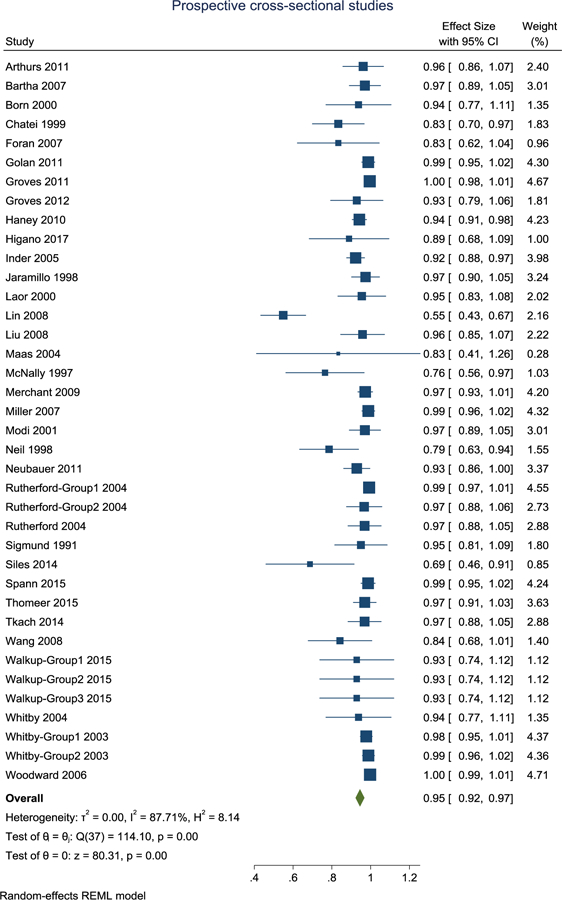
Forest plot of prospective cross-sectional articles. The success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies was 95%.
Figure 4.
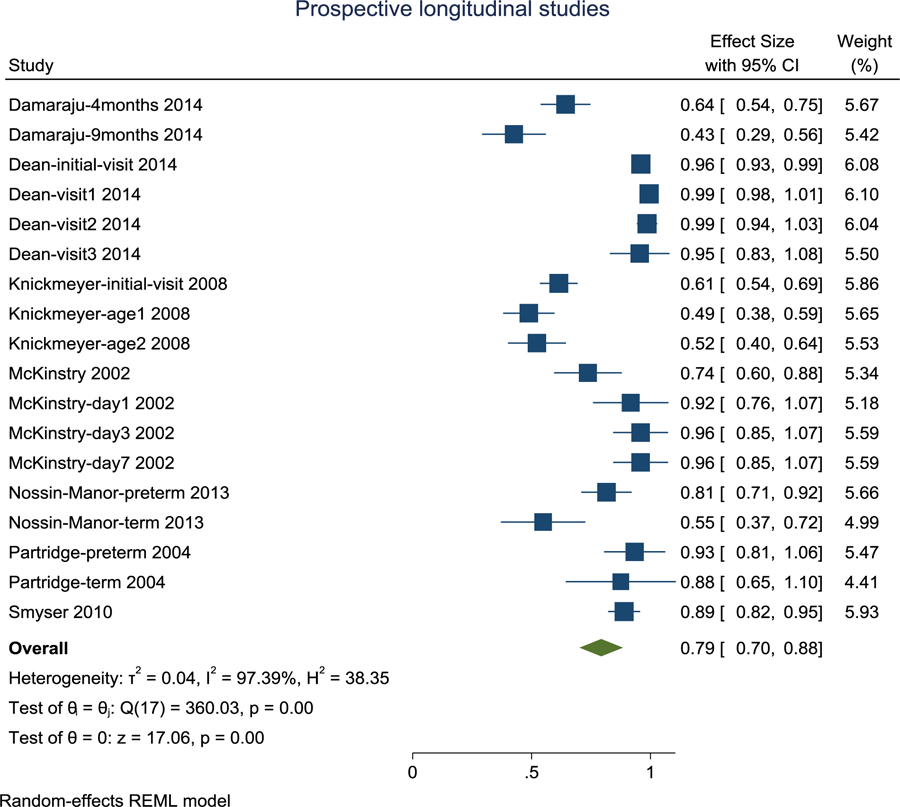
Forest plot of prospective longitudinal articles. The success rate of obtaining usable MRI scans with the sole use of non-pharmacological strategies was 79%.
3.5. Risk of Bias across Articles
Based on regression test for funnel plot asymmetry, while there was no evidence of publication bias in the retrospective cross-sectional (z = −.40, p = .7) and prospective longitudinal (z = −1.36, p = .17) articles, the funnel plot for the prospective cross-sectional articles was asymmetrical (z = −7.27, p < .0001), suggesting presence of considerable publication bias (Figure 5).
Figure 5.
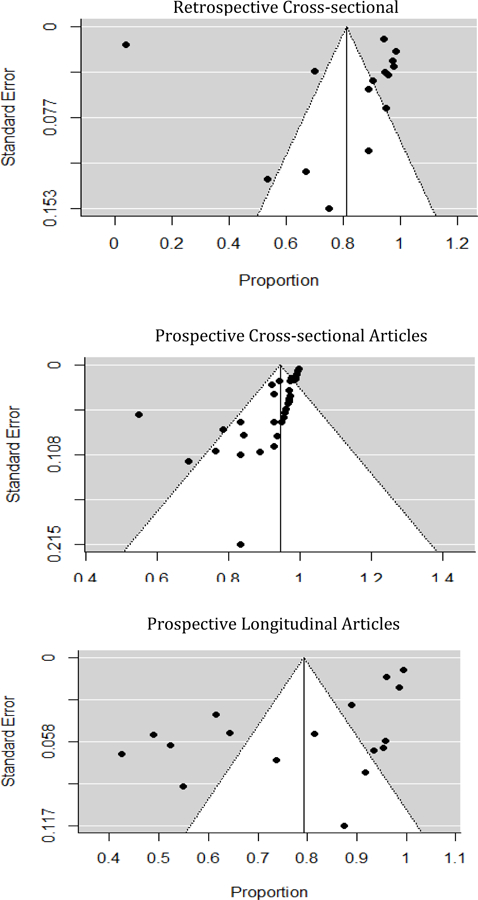
Funnel plots by sub-group. While there was no evidence of publication bias in the retrospective cross-sectional and prospective longitudinal articles, the funnel plot for the prospective cross-sectional articles was asymmetrical, suggesting publication bias.
4. Discussion
The purpose of this study was to determine the success rate of obtaining usable MRI scans in infants with the sole use of non-pharmacological strategies. This systematic review and meta-analysis provided evidence that non-pharmacological techniques are valuable in acquiring images of usable quality from non-sedated infants, producing images of usable quality 88% of the time in an average of 30 minutes.
There were numerous sources of heterogeneity. The authors of each article determined whether an image was usable. There was no standard grading scale used across the articles to determine if an image was usable or not; what was determined unusable in one article could be determined usable in another article. Success rates varied by country, for example 4–100% in the United States compared to 76–100% in the United Kingdom. Success rates also varied by whether infants received imaging for clinical (4–100%) or research purposes (42–100%) and were healthy (48.9 – 100%) or at risk (4 – 100%). Structural MRIs had the most varied success rates of 4–100%, while all other forms of MRIs had success rates of 70–100%. The brain had the most varied success rates of 4–100%, while all other areas of the body had success rates of 76–100%. While some articles reported scan times, others reported median scan time or time away from the NICU, and 34% did not report scan times. Most articles reported a variety of measures to image infants while they slept. However, a few articles did not report how they kept infants still, and notably, one article reported imaging infants while crying (Laor et al., 2000). All of the articles had limited generalizability, and evidence of publication bias was found in the prospective cross-sectional articles.
Given the variety of non-pharmacological techniques available to decrease infant movement and enhance the quality of images, a conceptual framework would be helpful to guide clinicians and researchers when imaging infants. The Neonatal Integrative Developmental Care Model proposes seven neuroprotective interventions to be used in the NICU (Altimier & Phillips, 2013), which also has relevance for acquiring quality images: 1) providing a healing environment such as imaging infants in a warm room with dim lighting (Windram et al., 2012), double-walled construction, and acoustic and vibration damping facilitating undisturbed sleep and/or calm resting during the scans (Partridge et al., 2004); 2) partnering with families such as joining the neonatal team in cuddling (Golan et al., 2011), rocking the infant to sleep if necessary (Neubauer et al., 2011) and standing close to their infant during the entire scan (Rabattu et al., 2014); 3) positioning and handling such as swaddling and placing the infant in foam padding (Wang et al., 2008), bean bag (Inder et al., 2005; Woodward et al., 2006), spica cast (Gould et al., 2012; Jaramillo et al. 1998; Laor et al., 2000; McNally et al., 1997; Rabattu et al., 2014), MRI-compatible cradle (Groves et al., 2011; Groves et al., 2012) or immobilizing device (Golan et al., 2011; Merchant et al., 2009; Reilly et al, 2012); 4) safeguarding sleep such as imaging the infant during natural sleep (Born et al., 2000; Chateil et al., 1999; Foran et al., 2007; Groves et al., 2011; Groves et al., 2012; Modi et al., 2001); 5) minimizing stress and pain such as using a pacifier with or without oral glucose/sucrose if necessary (Golan et al., 2011; Neubauer et al., 2011); 6) optimizing nutrition such as feeding infants right before the scan (Arthurs et al., 2011; Bartha et al., 2007; Born et al., 2000; Fogel et al., 2011; Foran et al., 2007; Gale et al., 2013; Golan et al., 2011; Groves et al., 2011; Groves et al., 2012; Haney et al., 2010; Hansen, 2009; Inder et al., 2005; Liu et al., 2008; Merchant et al., 2009; Neil et al., 1998; Neubauer et al., 2011; O’Regan et al., 2012; Reilly et al., 2012; Rutherford et al., 2004; Thomeer et al., 2015; Tkach et al., 2014; Wang et al., 2008; Walkup et al., 2015; Windram et al., 2012; Woodward et al., 2006).
In addition to non-pharmacological techniques to decrease infant movement and enhance the quality of images, technical MRI solutions are promising. Complementary strategies have been proposed involving the use of hardware or algorithmic solutions such as slice to volume reconstructions (Jiang et al., 2007), motion probes (Paley et al., 2017), generalized reconstructions (Cordero-Grande et al., 2018), or simple rejection of outlier slices (Sairanen, Leemans & Tax, 2018). A variety of technical solutions have been discussed in existing surveys (Dong, Zhu & Bulas, 2019; Jaimes & Gee, 2016; Jaimes, Kirsch & Gee, 2018; Malamateniou et al., 2013, Zaitsev, Maclaren & Herbst, 2015). For example, strategies to compensate for bulk motion artifacts on MRIs include prevention, minimization, detection and correction (Malamateniou et al., 2013).
There are limitations to this study. Only articles written in English were included. Most of the articles used structural magnetic resonance imaging, which may limit the generalizability to other forms of imaging. Moreover, the majority of the articles imaged the brain, limiting generalizability to other areas of the body. However, there are also several strengths to this review. There were a large number of articles found. Articles were included from twelve countries with sample sizes ranged from 2–457. About half of the articles comprised infants who received imaging for clinical purposes, extending generalizability beyond research studies to clinical settings.
In conclusion, the success rate of obtaining usable MRI scans in infants with the sole use of non-pharmacological strategies to minimize motion was 87% with an average of 30 minutes. Non-pharmacological techniques such as feeding and swaddling infants before imaging encourage infants to sleep during the scan. However, it was more difficult to obtain usable images if the infants were not healthy and a structural MRI of the brain was required. Non-pharmacological techniques can decrease the need for sedation or anesthesia and its associated side-effects and costs in many but not all infants requiring a MRI.
Supplementary Material
What is already known about the topic:
Sedation is commonly practiced to keep infants still while receiving magnetic resonance imaging.
What this paper adds:
There are several effective non-pharmacological techniques for obtaining usable magnetic resonance imaging scans in infants without the use of sedation.
Acknowledgement
The authors would like to acknowledge the following individuals for their contribution to the conception of this review: Wanda Meeteer, Katryn Remler, Shannon Renick, Emily Schumacher and Christina Trunzo. Dr. Torres was supported by the Clinical and Translational Science Award program through the NIH National Center for Advancing Translational Sciences [UL1TR000427 & KL2TR000428] and the Mississippi Center for Clinical and Translational Research [5U54GM115428]. Dr. Dean is supported by a career development award through the National Institutes of Mental Health [R00 MH110596]. The funding sources had no role in the study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit for publication. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest: none
References
- Alamo L, Gudinchet F, & Meuli R (2015). Imaging findings in fetal diaphragmatic abnormalities. Pediatric Radiology, 45, 1887–1900. 10.1007/s00247-015-3418-5 [DOI] [PubMed] [Google Scholar]
- Altimier L, & Phillips RM (2013). The Neonatal Integrative Developmental Care Model: Seven neuroprotective core measures for family-centered developmental care. Newborn & Infant Nursing Reviews, 13(1), 9–22. 10.1053/j.nainr.2012.12.002 [DOI] [Google Scholar]
- Ameis SH, & Catani M (2015). Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex, 62, 158–181. 10.1016/j.cortex.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Edwards AD, Joubert I, Graves MJ, Set PA, & Lomas DJ (2011). Interactive magnetic resonance voiding cystourethrography (iMRVC) for vesicoureteric reflux (VUR) in unsedated infants: A feasibility study. European Radiology, 21, 1874–1881. 10.1007/s00330-011-2124-4 [DOI] [PubMed] [Google Scholar]
- Ashley WW, McKinstry RC, Leonard JR, Smyth MD, Lee BC, & Park TS (2005). Use of rapid-sequence magnetic resonance imaging for evaluation of hydrocephalus in children. Journal of Neurosurgery: Pediatrics, 103, 124–130. 10.3171/ped.2005.103.2.0124 [DOI] [PubMed] [Google Scholar]
- Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, & Tardieu M (2007). Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurology, 6(10), 887–902. 10.1016/S1474-4422(07)70242-9 [DOI] [PubMed] [Google Scholar]
- Bartha AI, Yap KR, Miller SP, Jeremy RJ, Nishimoto M, Vigneron DB, … Ferriero DM (2007). The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. American Journal of Neuroradiology, 28, 1015–1021. 10.3174/ajnr.A0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born AP, Miranda MJ, Rostrup E, Toft PB, Peitersen B, Larsson HB, & Lou HC (2000). Functional magnetic resonance imaging of the normal and abnormal visual system in early life. Neuropediatrics, 31(1), 24–32. 10.1055/s-2000-15402 [DOI] [PubMed] [Google Scholar]
- Boxer RA, Singh S, LaCorte MA, Goldman M, & Stein HL (1986). Cardiac magnetic resonance imaging in children with congenital heart disease. Journal of Pediatrics, 109, 460–464. [DOI] [PubMed] [Google Scholar]
- Caldwell K, Henshaw L, & Taylor G (2011). Developing a framework for critiquing health research: An early evaluation. Nurse Education Today, 31, e1–e7. 10.1016/j.nedt.2010.11.025 [DOI] [PubMed] [Google Scholar]
- Callen DJ, Shroff MM, Branson HM, Lotze T, Li DK, Stephens D, & Banwell BL (2009). MRI in the diagnosis of pediatric multiple sclerosis. Neurology, 72, 961–967. 10.1212/01.wnl.0000338629.01627.54 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, & Durston S (2005). Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Science, 9, 104–110. 10.1016/j.tics.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Chateil JF, Quesson B, Brun M, Thiaudière E, Sarlangue J, Delalande C, … Diard F (1999). Localised proton magnetic resonance spectroscopy of the brain after perinatal hypoxia: A preliminary report. Pediatric Radiology, 29, 199–205. 10.1007/s002470050572 [DOI] [PubMed] [Google Scholar]
- Cordero-Grande L, Hughes EJ, Hutter J, Price AN, & Hajnal JV (2018). Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging. Magnetic Resonance in Medicine, 79, 1365–1376. 10.1002/mrm.26796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, & Phillips JP (2014). Functional connectivity in the developing brain: A longitudinal study from 4 to 9 months of age. Neuroimage, 84, 169–180. 10.1016/j.neuroimage.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darge K, Anupindi SA, & Jaramillo D (2011). MR imaging of the abdomen and pelvis in infants, children, and adolescents. Radiology, 261, 12–29. 10.1148/radiol.11101922 [DOI] [PubMed] [Google Scholar]
- Dean DC, Dirks H, O’Muircheartaigh J, Walker L, Jerskey BA, Lehman K, … Deoni SC (2014). Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatric Radiology, 44, 64–72. 10.1007/s00247-013-2752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, Planalp EM, Wooten W, Adluru N, Kecskemeti SR, Frye C, … Alexander AL (2017). Mapping white matter microstructure in the one month human brain. Scientific Reports, 7, 9759 10.1038/s41598-017-09915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong SZ, Zhu M, & Bulas D (2019). Techniques for minimizing sedation in pediatric MRI. Journal of Magnetic Resonance Imaging, 50, 1047–1054. 10.1002/jmri.26703 [DOI] [PubMed] [Google Scholar]
- Durston S, & Casey BJ (2006). What have we learned about cognitive development from neuroimaging? Neuropsychologia, 44, 2149–2157. 10.1016/j.neuropsychologia.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Edwards AD, & Arthurs OJ (2011). Paediatric MRI under sedation: Is it necessary? What is the evidence for the alternatives? Pediatric Radiology, 41, 1353–1364. 10.1007/s00247-011-2147-7. [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, … Warner DO (2011). Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics, 128, e1053–1061. 10.1542/peds.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel MA, Pawlowski TW, Harris MA, Whitehead KK, Keller MS, Wilson J, … Harris C (2011). Comparison and usefulness of cardiac magnetic resonance versus computed tomography in infants six months of age or younger with aortic arch anomalies without deep sedation or anesthesia. American Journal of Cardiology, 108, 120–125. 10.1016/j.amjcard.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Foran AM, Fitzpatrick JA, Allsop J, Schmitz S, Franklin J, Pamboucas C, … Edwards AD (2007). Three-tesla cardiac magnetic resonance imaging for preterm infants. Pediatrics, 120, 78–83. 10.1542/peds.2006-3305 [DOI] [PubMed] [Google Scholar]
- Gale C, Jeffries S, Logan KM, Chappell KE, Uthaya SN, & Modi N (2013). Avoiding sedation in research MRI and spectroscopy in infants: Our approach, success rate and prevalence of incidental findings. Archives of Disease in Childhood - Fetal and Neonatal Edition, 98(3), F267–F268. 10.1136/archdischild-2012-302536 [DOI] [PubMed] [Google Scholar]
- Giedd JN, & Rapoport JL (2010). Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron, 67, 728–734. 10.1016/j.neuron.2010.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan A, Marco R, Raz H, & Shany E (2011). Imaging in the newborn: infant immobilizer obviates the need for anesthesia. Israel Medical Association Journal, 13), 663–665. [PubMed] [Google Scholar]
- Gould SW, Grissom LE, Niedzielski A, Kecskemethy HH, Bowen JR, & Harcke HT (2012). Protocol for MRI of the hips after spica cast placement. Journal of Pediatric Orthopedics, 32, 504–509. 10.1097/BPO.0b013e31825a23e4 [DOI] [PubMed] [Google Scholar]
- Govaert P, Ramenghi L, Taal R, de Vries L, & Deveber G (2009). Diagnosis of perinatal stroke I: Definitions, differential diagnosis and registration. Acta Paediatrica, 98, 1556–1567. 10.1111/j.1651-2227.2009.01461.x. [DOI] [PubMed] [Google Scholar]
- Groves AM, Chiesa G, Durighel G, Goldring ST, Fitzpatrick JA, Uribe S, … Edwards AD (2011). Functional cardiac MRI in preterm and term newborns. Archives of Disease in Childhood: Fetal and Neonatal Edition, 96, F86–91. 10.1136/adc.2010.189142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AM, Durighel G, Finnemore A, Tusor N, Merchant N, Razavi R, … Edwards AD (2012). Disruption of intracardiac flow patterns in the newborn infant. Pediatric Research, 71, 380–385. 10.1038/pr.2011.77 [DOI] [PubMed] [Google Scholar]
- Haney B, Reavey D, Atchison L, Poull J, Dryer L, Anderson B, & Pallotto E (2010). Magnetic resonance imaging studies without sedation in the neonatal intensive care unit: Safe and efficient. Journal of Perinatal and Neonatal Nursing, 24, 256–266. 10.1097/JPN.0b013e3181e8d566 [DOI] [PubMed] [Google Scholar]
- Hansen S (2009). Feed-and-sleep: a non-invasive and safe alternative to general anaesthesia when imaging very young children. Radiographer, 56, 5–8. [Google Scholar]
- Hendren RL, De Backer I, & Pandina GJ (2000). Review of neuroimaging studies of child and adolescent psychiatric disorders from the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 815–828. 10.1097/00004583-200007000-00010 [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, … Magnotta V (2002). The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia, 43, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Higano NS, Fleck RJ, Spielberg DR, Walkup LL, Hahn AD, Thomen RP, … Woods JC (2017). Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. Journal of Magnetic Resonance Imaging, 46, 992–1000 10.1002/jmri.25643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, & Green S (2011). Cochrane handbook for systematic reviews of interventions. Retrieved from www.handbook.cochrane.org
- Inder TE, Warfield SK, Wang H, Hüppi PS, & Volpe JJ (2005). Abnormal cerebral structure is present at term in premature infants. Pediatrics, 115, 286–294. 10.1542/peds.2004-0326 [DOI] [PubMed] [Google Scholar]
- Iskandar BJ, Sansone JM, Medow J, & Rowley HA (2004). The use of quick-brain magnetic resonance imaging in the evaluation of shunt-treated hydrocephalus. Journal of Neurosurgery, 101(2 Suppl), 147–151. 10.3171/ped.2004.101.2.0147 [DOI] [PubMed] [Google Scholar]
- Jaimes C, & Gee MS (2016). Strategies to minimize sedation in pediatric body magnetic resonance imaging. Pediatric Radiology, 46, 916–927. 10.1007/s00247-016-3613-z [DOI] [PubMed] [Google Scholar]
- Jaimes C, Kirsch JE, & Gee MS (2018). Fast, free-breathing and motion-minimized techniques for pediatric body magnetic resonance imaging. Pediatric Radiology, 48, 1197–1208. 10.1007/s00247-018-4116-x [DOI] [PubMed] [Google Scholar]
- Jaramillo D, Villegas-Medina O, Laor T, Shapiro F, & Millis MB (1998). Gadolinium-enhanced MR imaging of pediatric patients after reduction of dysplastic hips: Assessment of femoral head position, factors impeding reduction, and femoral head ischemia. American Journal of Roentgenology, 170, 1633–1637. 10.2214/ajr.170.6.9609187 [DOI] [PubMed] [Google Scholar]
- Jiang S, Xue H, Glover A, Rutherford M, Rueckert D, & Hajnal JV (2007). MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): Application to fetal, neonatal, and adult brain studies. IEEE Transactions on Medical Imaging, 26, 967–80. 10.1109/TMI.2007.895456 [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, … Gilmore JH (2008). A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience, 28, 12176–12182. 10.1523/JNEUROSCI.3479-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy R (2008). Pediatric cardiac MRI: Anatomy and function. Pediatric Radiology, 38 (Supplement 2), S192–199. 10.1007/s00247-008-0786-0 [DOI] [PubMed] [Google Scholar]
- Laor T, Roy DR, & Mehlman CT (2000). Limited magnetic resonance imaging examination after surgical reduction of developmental dysplasia of the hip. Journal of Pediatric Orthopedics, 20, 572–574. [DOI] [PubMed] [Google Scholar]
- Lebel C, & Deoni S (2018). The development of brain white matter microstructure. Neuroimage, 182, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Poupon C, Amadon A, & Lethimonnier F (2006). Artifacts and pitfalls in diffusion MRI. Journal of Magnetic Resonance Imaging, 24, 478–488. 10.1002/jmri.20683 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, … Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6(7), e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, … Gilmore JH (2008). Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. American Journal of Neuroradiology, 29, 1883–1889. 10.3174/ajnr.A1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Flax JF, Guise KG, Sukul V, & Benasich AA (2008). Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Research, 1223, 42–49. 10.1016/j.brainres.2008.05.054 [DOI] [PubMed] [Google Scholar]
- Maas LC, Mukherjee P, Carballido-Gamio J, Veeraraghavan S, Miller SP, Partridge SC, … Vigneron DB (2004). Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage, 22, 1134–1140. 10.1016/j.neuroimage.2004.02.035 [DOI] [PubMed] [Google Scholar]
- Malamateniou C, Malik SJ, Counsell SJ, Allsop JM, McGuinness AK, Hayat T, … Rutherford MA (2013). Motion-compensation techniques in neonatal and fetal MR imaging. American Journal of Neuroradiology, 34, 1124–36. 10.3174/ajnr.A3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, & Rigamonti D (2005). Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery, 57, 699–705. [DOI] [PubMed] [Google Scholar]
- McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, … Neil JJ (2002). Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cerebral Cortex, 12, 1237–1243. [DOI] [PubMed] [Google Scholar]
- McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, … Neil JJ (2002). A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology, 59, 824–833. [DOI] [PubMed] [Google Scholar]
- McNally EG, Tasker A, & Benson MK (1997). MRI after operative reduction for developmental dysplasia of the hip. Journal of Bone and Joint Surgery: British Volume, 79, 724–726. [DOI] [PubMed] [Google Scholar]
- Merchant N, Groves A, Larkman DJ, Counsell SJ, Thomson MA, Doria V, … Boardman JP (2009). A patient care system for early 3.0 Tesla magnetic resonance imaging of very low birth weight infants. Early Human Development, 85, 779–783. 10.1016/j.earlhumdev.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, … Vigneron DB (2007). Abnormal brain development in newborns with congenital heart disease. New England Journal of Medicine, 357, 1928–1938. 10.1056/NEJMoa067393 [DOI] [PubMed] [Google Scholar]
- Missios S, Quebada PB, Forero JA, Durham SR, Pekala JS, Eskey CJ, & Duhaime AC (2008). Quick-brain magnetic resonance imaging for nonhydrocephalus indications. Journal of Neurosurgery: Pediatrics, 2, 438–444. 10.3171/PED.2008.2.12.438 [DOI] [PubMed] [Google Scholar]
- Modi N, Lewis H, Al-Naqeeb N, Ajayi-Obe M, Doré CJ, & Rutherford M (2001). The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatric Research, 50, 581–585. 10.1203/00006450-200111000-00008 [DOI] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, … Conturo TE (1998). Normal brain in human newborns: Apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology, 209, 57–66. 10.1148/radiology.209.1.9769812 [DOI] [PubMed] [Google Scholar]
- Neubauer V, Griesmaier E, Baumgartner K, Mallouhi A, Keller M, & Kiechl-Kohlendorfer U (2011). Feasibility of cerebral MRI in non-sedated preterm-born infants at term-equivalent age: Report of a single centre. Acta Paediatrica, 100, 1544–1547. 10.1111/j.1651-2227.2011.02388.x [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R, Card D, Morris D, Noormohamed S, Shroff MM, Whyte HE, … Sled JG (2013). Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T1 imaging. Neuroimage, 64, 505–516. 10.1016/j.neuroimage.2012.08.086 [DOI] [PubMed] [Google Scholar]
- O’Regan K, Filan P, Pandit N, Maher M, & Fanning N (2012). Image quality associated with the use of an MR-compatible incubator in neonatal neuroimaging. British Journal of Radiology, 85, 363–367. 10.1259/bjr/66148265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley M, Reynolds S, Ismail N, Herigstad M, Jarvis D, & Griffiths P (2017). Wireless accelerometer for neonatal MRI motion artifact correction. Technologies, 5(1), 6 10.3390/technologies5010006 [DOI] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, … Vigneron DB (2004). Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage, 22, 1302–1314. 10.1016/j.neuroimage.2004.02.038 [DOI] [PubMed] [Google Scholar]
- Prada CE, Hufnagel RB, Hummel TR, Lovell AM, Hopkin RJ, Saal HM, & Schorry EK (2015). The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type 1. Journal of Pediatrics, 167, 851–856. 10.1016/j.jpeds.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabattu PY, Courvoisier A, Bourgeois E, Eid A, Durand C, & Griffet J (2014). Spica cast as an alternative to general anesthesia for lower limb MRI in young children. Journal of Orthopaedics and Traumatology, 15(1), 55–58. 10.1007/s10195-013-0251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy V, Miller SP, Barkovich AJ, Partridge JC, & Ferriero DM (2004). Perinatal stroke in term infants with neonatal encephalopathy. Neurology, 62, 2088–2091. 10.1212/01.WNL.0000129909.77753.C4 [DOI] [PubMed] [Google Scholar]
- Reilly L, Byrne AH, & Ely E (2012). Does the use of an immobilizer provide a quality MR image of the brain in infants? Journal of Radiology Nursing, 31, 91–96. 10.1016/j.jradnu.2012.04.002 [DOI] [Google Scholar]
- Rozovsky K, Ventureyra EC, & Miller E (2013). Fast-brain MRI in children is quick, without sedation, and radiation-free, but beware of limitations. Journal of Clinical Neuroscience, 20, 400–405. 10.1016/j.jocn.2012.02.048 [DOI] [PubMed] [Google Scholar]
- Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, … Cowan F (2004). Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: A comparison with site of lesion and time from birth. Pediatrics, 114, 1004–1014. 10.1542/peds.2004-0222 [DOI] [PubMed] [Google Scholar]
- Ryan ME, Jaju A, Ciolino JD, & Alden T (2016). Rapid MRI evaluation of acute intracranial hemorrhage in pediatric head trauma. Neurology, 58, 793–799. 10.1007/s00234-016-1686-x [DOI] [PubMed] [Google Scholar]
- Sairanen V, Leemans A, & Tax CM (2018). Fast and accurate Slicewise OutLIer Detection (SOLID) with informed model estimation for diffusion MRI data. NeuroImage, 181, 331–346. 10.1016/j.neuroimage.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Sigmund G, Stoever B, Zimmerhackl LB, Frankenschmidt A, Nitzsche E, Leititis JU, … Hennig J (1991). RARE-MR-urography in the diagnosis of upper urinary tract abnormalities in children. Pediatric Radiology, 21, 416–420. [DOI] [PubMed] [Google Scholar]
- Siles P, Aschero A, Gorincour G, Bourliere-Najean B, Roquelaure B, Delarue A, & Petit P (2014). A prospective pilot study: Can the biliary tree be visualized in children younger than 3 months on magnetic resonance cholangiopancreatography? Pediatric Radiology, 44, 1077–1084. 10.1007/s00247-014-2953-9. [DOI] [PubMed] [Google Scholar]
- Sirin S, Goericke SL, Huening BM, Stein A, Kinner S, Felderhoff-Mueser U, & Schweiger B (2013). Evaluation of 100 brain examinations using a 3 Tesla MR-compatible incubator-safety, handling, and image quality. Neuroradiology, 55, 1241–1249. 10.1007/s00234-013-1241-y [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, & Neil JJ (2010). Longitudinal analysis of neural network development in preterm infants. Cerebral Cortex, 20, 2852–2862. 10.1093/cercor/bhq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spader HS, Ellermeier A, O’Muircheartaigh J, Dean DC, Dirks H, Boxerman JL, … Deoni SC (2013). Advances in myelin imaging with potential clinical application to pediatric imaging. Neurosurgical Focus, 34(4), E9 10.3171/2013.1.FOCUS12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann MN, Serino D, Bansal R, Hao X, Nati G, Toth Z, … Peterson BS (2015). Morphological features of the neonatal brain following exposure to regional anesthesia during labor and delivery. Magnetic Resonance Imaging, 33, 213–221. 10.1016/j.mri.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomeer MG, Devos A, Lequin M, De Graaf N, Meeussen CJ, Meradji M, … Sloots CE (2015). High resolution MRI for preoperative work-up of neonates with an anorectal malformation: A direct comparison with distal pressure colostography/fistulography. European Radiology, 25, 3472–3479. 10.1007/s00330-015-3786-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach JA, Merhar SL, Kline-Fath BM, Pratt RG, Loew WM, Daniels BR, … Dumoulin CL (2014). MRI in the neonatal ICU: Initial experience using a small-footprint 1.5-T system. American Journal of Roentgenology, 202, W95–W105. 10.2214/AJR.13.10613 [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp DP, Destiche D, Doran S, … Alexander AL (2012). Diffusion tensor imaging in autism spectrum disorder: A review. Autism Research, 5, 289–313. 10.1002/aur.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiflikas I, Obermayr F, Werner S, Teufel M, Fuchs J, & Schafer JF (2019). Functional magnetic resonance urography in infants: Feasiblity of a feed-and-sleep technique. Pediatric Radiology, 49, 351–357. 10.1007/s00247-018-4307-5. [DOI] [PubMed] [Google Scholar]
- Vanneste JA (2000). Diagnosis and management of normal-pressure hydrocephalus. Journal of Neurology, 247, 5–14. [DOI] [PubMed] [Google Scholar]
- Walkup LL, Tkach JA, Higano NS, Thomen RP, Fain SB, Merhar SL, … Woods JC (2015). Quantitative magnetic resonance imaging of bronchopulmonary dysplasia in the neonatal intensive care unit environment. American Journal of Respiratory and Critical Care Medicine, 192, 1215–1222. 10.1164/rccm.201503-0552OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fernández-Seara M, Alsop DC, Liu WC, Flax JF, Benasich AA, & Detre JA (2008). Assessment of functional development in normal infant brain using arterial spin labeled perfusion MRI. Neuroimage, 39, 973–978. 10.1016/j.neuroimage.2007.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby EH, Griffiths PD, Lonneker-Lammers T, Srinivasan R, Connolly DJ, Capener D, & Paley MN (2004). Ultrafast magnetic resonance imaging of the neonate in a magnetic resonance-compatible incubator with a built-in coil. Pediatrics, 113(2), e150–152. [DOI] [PubMed] [Google Scholar]
- Whitby EH, Paley MN, Smith MF, Sprigg A, Woodhouse N, & Griffiths PD (2003). Low field strength magnetic resonance imaging of the neonatal brain. Archives of Disease in Childhood, Fetal and Neonatal Edition, 88, F203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windram J, Grosse-Wortmann L, Shariat M, Greer ML, Crawford MW, & Yoo SJ (2012). Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatric Radiology, 42, 183–187. 10.1007/s00247-011-2219-8 [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, & Inder TE (2006). Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine, 355, 685–694. 10.1056/NEJMoa053792 [DOI] [PubMed] [Google Scholar]
- Woodthorpe C, Trigg A, Alison G, & Sury M (2007). Nurse led sedation for paediatric MRI: Progress and issues. Paediatric Nursing, 19(2), 14–18. [PubMed] [Google Scholar]
- Young JY, Duhaime AC, Caruso PA, & Rincon SP (2016). Comparison of non-sedated brain MRI and CT for the detection of acute traumatic injury in children 6 years of age or less. Emergency Radiology, 23, 325–331. 10.1007/s10140-016-1392-3 [DOI] [PubMed] [Google Scholar]
- Zaitsev M, Maclaren J, & Herbst M (2015). Motion artifacts in MRI: A complex problem with many partial solutions. Journal of Magnetic Resonance Imaging, 42, 887–901. 10.1002/jmri.24850 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


