Abstract
Most patients with large cell lymphoma are cured with frontline chemoimmunotherapy. For individuals with refractory disease and those who relapse after conventional therapies, chimeric antigen receptor (CAR) T cells are an important treatment option and have led to remissions in otherwise refractory patients. In the pivotal trials, durable responses were achieved in approximately 40% to 50% of patients treated with axicabtagene ciloleucel, tisagenlecleucel, or lisocabtagene maraleucel, indicating that many patients will require subsequent treatment. Failure after CAR T cell therapy is caused by a variety of factors that can be divided into 3 broad categories: tumor intrinsic factors, other host factors, and inadequacies of the CAR T cells. Within this framework, this article reviews possible mechanisms of treatment failures and, based on the timing of relapse, considers potential salvage therapies and opportunities for future clinical studies.
INTRODUCTION
Most patients with diffuse large B cell lymphoma (DLBCL) are cured with frontline chemoimmunotherapy, but a significant proportion will relapse. Approximately half of patients who receive subsequent chemotherapy will fail to achieve a response sufficient to proceed to autologous hematopoietic cell transplantation (HCT). These individuals and those who relapse after autologous HCT face a poor prognosis with shortened overall survival (OS) [1–5].
Chimeric antigen receptor (CAR) T cell therapy in DLBCL and its variants provides a new treatment option for patients who relapse after 2 or more lines of therapy. Commercially available CAR T cells are produced by transducing autologous T cells with a gene encoding a hybrid receptor comprising an extracellular target binding domain, a transmembrane domain, and intracellular signaling domains that can eliminate CD19 expressing tumor via effector and cytolytic processes [6,7]. Second-generation CD19-directed CAR T cell therapies, those containing both a CD3-ζ signaling domain and a costimulatory domain, were evaluated in relapsed/refractory B cell lymphomas, and durable objective responses were observed in single-arm, multicenter, pivotal phase 2 trials. Important differences between the products may be responsible for the variability in outcomes reported across studies. Differences in cellular constructs (CD19CD3ζ/CD28 in ZUMA-1 versus CD19/CD3ζ/4–1BB in JULIET and TRANSCEND), conditioning therapy (fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2× 3 days [ZUMA-1] versus fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 × 3 days or bendamustine 90 mg/m2 × 2 days [JULIET] versus fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 × 3 days [TRANSCEND]), prevalence of refractory versus relapsed/refractory disease, prior autologous HCT, and other dissimilarities between groups may be responsible for the differences reported between studies. In ZUMA-1, treatment with axicabtagene ciloleucel (axi-cel) led to a 58% complete response (CR) rate and ongoing responses in 39% beyond 2 years ( NCT02348216) [8]. In JULIET, tisagenlecleucel showed an overall CR rate of 40% ( NCT02445248), and in the phase 1 portion of TRANSCEND, lisocabtagene maraleucel demonstrated an overall response rate (ORR) of 50% at the 6-month landmark ( NCT02631044) [9–12]. As a result of these studies, 2 CAR T cell products are approved for commercial use, and a third approval is anticipated [13,14].
The total number of patients with DLBCL treated with CAR T cell therapy is rapidly increasing because of the availability of commercial products and a growing number of clinical studies [15]. There are more than 750 cellular therapies in development, and approximately half of these are in clinical trials [16]. Randomized phase 3 trials using CD19-directed CAR T cell therapy earlier in the treatment course, including comparisons with second-line salvage chemotherapy and autologous HCT, are open and accruing patients [17]. Commercial CAR T cell therapy continues to grow, with financial earning reports estimating that almost 500 patients received axi-cel as a standard of care therapy in the first 3 quarters of 2018 [18]. Despite the activity of these agents, the optimal management of patients with DLBCL who relapse after CART cell therapy is an area of uncertainty.
Data to guide the management of patients relapsing after CD19 CART cell therapy are limited. However, there is increasing interest in the identification of optimal treatment strategies for these patients. In this article, we review patterns of responses and relapses, highlight the limited data and case reports that describe the management of these patients, and speculate on possible strategies that could be used and studied in the future. Future directions of CAR T cell therapy are well described in the medical literature and are not within the scope of this article.
HYPOTHESIZED MECHANISMS OF CAR T CELL TREATMENT FAILURES
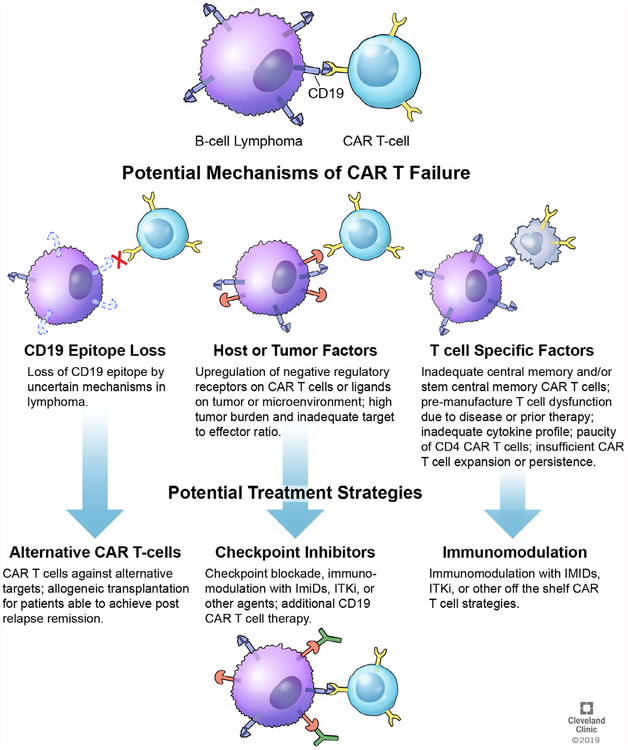
More than half of patients with high-grade lymphomas will progress and require additional therapy after C CAR T cell therapy. The exact mechanisms causing tumor escape remain unknown, and we conceptualize that these could be divided into 3 broad categories: tumor intrinsic factors, other host factors, and inadequacies of the CAR T cell that lead to these failures (Figure 1).
Figure 1.
Tumor Intrinsic Factors
CD19 CAR T cell therapy for lymphoma depends on binding to its target: CD19 on the cell surface of tumor. A well-characterized mechanism of resistance in patients with acute lymphoblastic leukemia (ALL) is the loss of tumor expression of the extracellular CD19 epitope, which the CAR binds. This appears to affect 10% to 20% of patients with ALL and can occur through several mechanisms, including the development of CD19 exon splice variants, which leads to expression of CD19 protein lacking the extracellular domain; lineage switch to the myeloid phenotype (associated with the mixed-lineage leukemia or MLL gene fusion); and through alterations in CD19 secondary to acquired mutations and loss of heterozygosity [19–24]. In patients with DLBCL treated on ZUMA-1, loss of tumor CD19 as evaluated by immunohistochemistry or flow cytometry by local pathologic evaluation was described in 3 of 11 (27%) with biopsies at relapse after CART cell therapy [10].
To remain effective, CAR T cells need to proliferate and remain active in vivo. Although CD19-expressing circulating B cells can recover in patients in CR without heralding relapse, the duration of persistence necessary remains unclear.
T cell surface inhibitory receptors, including programmed death 1 (PD-1), cytotoxic T lymphocyte-associated protein 4, Tim-3, and LAG-3 act as negative regulators of the immune response during infections and inflammation [25,26]. Following antigen engagement under physiologic conditions, T cells naturally upregulate these coinhibitory signals to tightly govern immune activation and prevent untoward autoimmunity [27]. The degree to which these negative regulatory signals may contribute to CAR T cell resistance is unclear. CAR T cells may express these ligands in an immunosuppressive environment, leading to inhibited CAR T cell activation and suppressed product expansion [28]. Solid tumor data suggest that malignant tissue also expresses and can upregulate inhibitory signals that suppress T cell activation and reduce immunogenicity [29,30]. Similarly, in patients with high-grade lymphoma, low levels of immune checkpoint ligands are present in chemoresponsive disease, whereas elevated soluble PD-L1 is linked with poor clinical outcomes [31,32].
In DLBCL, changes in the tumor major histocompatibility complex (MHC) represent another mechanism by which the tumor can evade immune surveillance and destruction. Data from lymphoma cell lines show reduced MHC-II, whereas a large series with patient samples reported the presence of HLA class I in 53 of 117 (45.3%) cases and class II expression in 78 of 117 (66.7%) [33,34]. HLA loss poses a challenge for antigen presentation, a key component of the mechanism of action for checkpoint inhibitors, and is linked with decreased tumor surveillance and poor clinical outcomes [35].
Although an advantage of CAR T cells are their ability to recognize and eliminate tumor cells independent of MHC, subtle alterations in CD19 may result in resistance to CAR T cell therapy. The role of epitope spreading of immune responses against different epitopes within CD19 (intramolecular) or alternate tumor-associated antigens (intermolecular) following CAR T cell therapy remains unknown. Whether epitope spreading is necessary to maintain durable remissions in DLBCL and overcome the variability that arises from tumor-driven immunoediting is not yet known and should be a focus of investigation. Furthermore, inhibitory signals, such as PD-L1, may reduce epitope spreading and increase the risk of CAR T failure driven by subtle changes in tumor antigen [36].
Finally, a high tumor burden has been linked with CAR T cell therapy failure in ALL. Recent data from patients with <5% blasts demonstrated a superior OS compared with those with a higher disease burden [37–39]. Alternatively, in chronic lymphocytic leukemia (CLL), there was no correlation between tumor burden and response [40]. Early data from ZUMA-1 suggest that high tumor volumes are associated with inferior durable responses in DLBCL [41].
Host Factors
A recent analysis of patients from ZUMA-1 showed no significant differences between patients based on the number of prior lines of therapy received [41]. The limited number of patients with DLBCL treated to date precludes the detection of subtle differences in patients who received immunotherapy before CAR T cell therapy. Further, none of the DLBCL patients were allog-rafted before enrollment (these patients were excluded from ZUMA-1 and JULIET), but the ALL literature indicates that CAR T cell therapy after allogeneic HCT is feasible and safe [37,42]. Similarly, response rates were consistent across prognostic sub-groups, including prior ASCT and double-hit lymphoma in JULIET [43]. Collectively, these data support the potent impact of CAR T cell therapy in otherwise refractory disease.
Incomplete T cell depletion may also negatively affect treatment outcomes. Fludarabine/cyclophosphamide conditioning chemotherapy increased levels of homeostatic cytokines and increased CART cell expansion and function [44,45]. Data comparing fludarabine/cyclophosphamide with cyclophosphamide conditioning chemotherapy showed that the latter was associated with increased CAR T cell expansion, persistence, and improved response rates, indicating that lymphodepletion is an important driver of response [46].
After infusion, CAR T cells rapidly expand in vivo, engage CD19+ cells, and lead to tumor destruction. In ZUMA-1, the kinetics of this expansion correlated with treatment response, with a 5.4-fold higher number of CAR T cells seen in responders within the first month [10]. In JULIET, there were no significant differences between transgene levels in responders versus nonresponders [12]. Separate data from ALL and CLL show that high levels of tisa-cel in the peripheral blood, greater maximum serum concentration, and area under the curve values are seen in responders [47]. Products that fall short of specifications approved by the US Food and Drug Administration may be released through expanded access programs (ie, Kite: ZUMA-9, Novartis Managed Access Program). The efficacy and safety of these products are not reported in the medical literature, but given the poor prognosis with conventional therapies for these patients, we encourage efforts to administer the so-called out-of-spec cells.
Regulatory T cells and myeloid-derived suppressor cells may suppress CART cell proliferation and cytokine production, leading to a dampening of the antitumor response and ultimately treatment failures.
The extent to which CAR T cell persistence correlates with remission durability is also an area of uncertainty in DLBCL. Long-term follow-up from ZUMA-1 demonstrates that 75% of patients with ongoing responses had B cell recovery, and data from JULIET showed no link between expansion, T cell concentration, and clinical outcomes [8,12]. These data, which are in line with others, seem to indicate that persistence of the CAR T cell product is not required to maintain a durable remission [48].
Inadequacy of CAR T Cell Therapy
Qualitative features of the CAR T cell product are also important to clinical outcomes. “Exhausted” CAR T cells are less proliferative, have a higher number of inhibitor receptors (ie, PD-1), and are less potent/cytotoxic than nonexhausted T cells. As a result, these cells have poor effector cell function and reduced efficacy [49,50]. CAR T cell exhaustion may arise from prior chemotherapy, alterations in the tumor microenvironment, or contributions from circulating cells, or they may be related to variations in the manufacturing process [51].
Recent translational studies have shown that the T cell composition of the infused product can also affect responses. For instance, in CLL, the analysis of CAR T cell transcription profiles from responding patients shows enrichment in memory-related genes such as IL-6/STAT3. In contrast, T cells from nonresponders are more likely to show upregulation of T cell effector genes as well as exhaustion and apoptosis [52]. Another study from the National Cancer Institute demonstrated that DLBCL responses to the same CAR construct used in axi-cel correlate with a T cell polyfunctionality strength index, a measure of the ability of each individual product CAR T cell to secrete more than 1 cytokine [53].
DLBCL RESPONSE TO CAR T CELL THERAPY
CD19 CAR T cell therapy can lead to remarkable responses in otherwise refractory patients. In ZUMA-1 and JULIET, responders experienced a significantly longer progression-free survival and OS compared with nonresponders [10,32,54]. Importantly, CAR T cell responses are dynamic, especially in the first 3 months, and a limited number of patients may initially achieve a PR that does deepen into a CR until over 1 year postinfusion [8].
Surveillance in Responders (CR/PR)
In DLBCL, durable remission following frontline or autologous HCT can reliably be defined as 2-year relapse-free survival. CR rates with CAR T cell in DLBCL range from 40% to 58%, and early data from pivotal trials suggest that 30% to 40% of patients maintain responses beyond 1 year [8–10,55,56]. Recently, the long-term follow-up from ZUMA-1 showed 39% of patients had ongoing responses at a median of 27 months of follow-up, and the median duration of response for patients in CR was not reached [8]. A similar proportion of patients treated with tisagenlecleucel in the JULIET study had durable responses. In patients with ≥3 months of follow-up, the best ORR was 52%, and patients who achieved a remission had an 81% probability of remaining in remission at 12 months [12].
The 3-month disease response status serves as a suitable proxy for durable response in these patients. In ZUMA-1 and JULIET, most patients in remission at 3 months remained in remission at 1 year [12,55]. In JULIET, 79% of patients had a CR, and 65% of all responders are expected to remain relapse free at 12 months [12]. Many of these 1-month and 3-month PRs deepened to a CR without further intervention. In JULIET, 13 of 24 patients (54%) with an initial PR response converted to a CR, and in ZUMA-1, 11 of 35 (31%) deepened their response from PR to CR up to 15 months after infusion [10,11].
Current evidence does not support the use of maintenance therapy or autologous or allogeneic HCT for patients responding to CAR T cell therapy because many patients will achieve long-term remissions without further intervention. On the basis of these observations, we recommend close observation without administration of additional therapy until there is evidence of disease progression in patients with DLBCL with a PR or CR following CAR T cell therapy. Carefully designed studies may identify predictors for progression (eg, minimal residual disease [MRD] monitoring) in patients who initially achieve a PR, with interventions planned to improve upon these outcomes [57,58].
Management of Stable Disease
A minority of patients in JULIET and ZUMA-1 did not achieve any disease response. Most patients responding to CAR T cell therapy will have a rapid and measurable decrease in lymphoma burden, but a minority of patients may have stable disease (SD) by objective criteria at first disease assessment, and these patients may experience continued shrinkage of tumor over time. In ZUMA-1, 48% of patients (12/25) with SD deepened their response without further intervention, and several JULIET patients with SD subsequently achieved a CR [10,55]. However, ZUMA-1 patients with SD at 3 months had a 2-year progression-free survival of 22.2%. Persistent SD at 3 months identifies a group at high risk for later progression, and carefully designed trials are necessary to evaluate the impact of an intervention for these patients.
There is no evidence to support routine administration of chemotherapy or maintenance therapy in the setting of SD after CAR T cell therapy. This is particularly important before 3 months, and even some patients with SD at 3 months will later improve and go on to experience durable responses without further intervention [8,55].
Like the management of responding patients, we recommend watchful waiting for patients with SD immediately after CAR T cell therapy. Regular follow-up with standard interval imaging is warranted as less than 50% of these patients will develop subsequent remissions. Determining the optimal management of this patient population through well-designed studies should be a priority for our field. Allogeneic HCT has been considered in some carefully selected SD patients based on disease bulk, donor availability, recovery from CAR T cell therapy, comorbidities, organ function, and other factors. Prospective trials or retrospective review of data might determine if HCT provides prolonged disease-free survival compared with watchful waiting, maintenance therapy, or alternative interventions.
PATTERNS OF CAR T CELL TREATMENT FAILURE AND MANAGEMENT RECOMMENDATIONS
Most CAR T cell therapy patients treated in early clinical studies relapsed and required subsequent therapy. What this optimal treatment is, and how it should be aligned with other therapies, is not firmly established. At 6 months, the median number of measurable CAR T cells had fallen to approximately 50% of peak values [10]. Based on the timing of relapse, we subdivide patients into primary resistance (primary refractory), patients with early relapse (<3 months of infusion), and patients with late relapse (≥3 months after infusion).
Primary Resistance
In ZUMA-1, 12% of patients had no response to axi-cel and experienced a short OS [10]. Separately, data from 51 patients with lymphoma with PD after CAR T cell therapy confirmed these patients had inferior outcomes relative to those with ≥SD on initial assessment [59]. Collectively, these data underscore the poor prognosis associated with patients not responding to CAR T cell therapy. Well-designed studies that identify and intervene in this high-risk population may improve patient selection and ultimately outcomes. The degree to which each of the proposed resistance mechanisms contributes to phenomena is unknown.
For patients progressing after CAR T cell therapy, additional treatment with cytotoxic chemotherapy may provide short-term disease control and improve survival, but this benefit is unlikely to be robust [59]. It must be recognized that patients evaluated in the pivotal trials had significant prior lines of therapy before CAR T cell therapy, and as the treatment is moved to earlier in the disease course, it is possible that nonresponding patients may have a higher degree of chemosensitivity.
In summary, we recommend patients with progressive disease be considered for clinical trial enrollment, and in patients with many prior lines of therapy, supportive care may be appropriate. Patients who achieve a remission and are suitable candidates should be considered for allogeneic HCT.
Early Relapse (<3 Months of Infusion)
Patients who progress soon after CAR T cell therapy and meet the eligibility criteria for well-designed clinical trials should be considered for enrollment. Standard lymphoma salvage strategies, including immunomodulatory agents, radiation, and chemotherapy, may be considered, but there are no data to confirm efficacy in this specific patient population.
Off-label use of the PD-1 blocking antibodies, pembrolizumab and nivolumab, has resulted in clinical responses with evidence for enhanced CAR T cell function and antitumor activity through the re-expansion and restored antitumor activity of exhausted CART cells.
In the first reported case, a man with progressive primary mediastinal B cell lymphoma after CAR T cell infusion received pembrolizumab every 3 weeks from day 26. CAR T cells expanded, and he achieved a CR [60]. In the second report, a patient with rapidly progressive DLBCL received nivolumab on day 11 with rapid re-expansion of CAR T cells and regression of his lymphadenopathy, leading to a PR. Nivolumab administration was associated with elevation in LDH, grade 3 cytokine release syndrome, and grade 1 neurotoxicity consistent with re-expansion of CART cells and a second peak in inflammatory cytokines [61]. Importantly, in both cases, PD-1 inhibitors were initiated within the first 30 days after CAR T cell therapy in the setting of refractory disease that rapidly progressed. It is unknown whether equivalent outcomes can be expected in patients who initially respond and then relapse or if the potential combinatorial effects will apply if these agents are initiated later in the disease course.
In a separate case series, patients who progressed after CD19 CAR T cell therapy were treated with pembrolizumab. Of 11 evaluable patients, there were 1 CR and 2 PRs with an ORR of 27%, which is in line with data from single-agent PD-1 inhibition in refractory DLBCL in the absence of prior CAR T cell therapy [62,63]. The end of phase 1 results of ZUMA-6, a study of axi-cel in combination with atezolizumab, and anti-PD-L1 therapy, administered on day +1 after axi-cel and then every 3 weeks × 4 doses, indicate that the combination is safe and that cytokine release syndrome and neurotoxicity may not be increased significantly, despite a suggestion for greater CAR T cell expansion (measured as area under the curve) compared with ZUMA-1 [64]. The efficacy of the combination was similar to that described in ZUMA-1, with effectiveness in 9 of 10 patients with an ORR of 90%. Of the 10 patients, 6 of 10 entered CR and 3 achieved a PR, and 2 of 3 PRs later deepened to CR [64].
For patients relapsing within 3 months after CAR T cell therapy, standard salvage strategies should be considered. Treatment with PD-1/PD-L1 blocking antibodies may be considered for patients who have no response to CAR T cell therapy, but this should be carefully considered given the paucity of data supporting their use in lymphoma and in this setting. The optimal approach for these patients remains uncertain. Robust interrogation of historical data on the efficacy of different salvage approaches for these patients and well-designed prospective clinical trials are needed.
Late Relapse (≥3 Months after Infusion)
Late relapses occur in patients who achieve a suitable response to CAR T cell therapy but lose this response several months later. A repeat biopsy should be considered to assess for persistence of the CD19 target and to guide clinical management. In ZUMA-1, 9 patients with an initial response for >3 months and CD19-proven relapse were retreated with axi-cel at disease progression, resulting in 5 responses (2 CRs and 3 PRs) [10]. Additional studies are required to assess whether retreatment with CAR T cell therapy, using the same or an alternate product, represents a viable option for these patients. Clinical trials testing retreatment in combination with PD-1-directed therapy, or other immunomodulating agents, should be considered, but currently there are limited data to support this approach for patients with late relapses.
Other treatment options include ibrutinib, immunomodulatory (IMiD) therapy, and radiation therapy depending on the location, number of lesions, and tumor size (Table 1). The evidence supporting these approaches is largely preclinical and anecdotal. Most data supporting the use of ibrutinib as an adjuvant to CAR T cell therapy come from studies of CLL in which the combination or sequential therapy appeared safe [65]. Patients who received ibrutinib before CD19+ CAR T cell therapy showed improved CAR T cell expansion and had superior clinical outcomes compared with untreated patients. In human xenograft models, ibrutinib improved tumor clearance and survival, suggesting it may augment CAR T cell function [65]. Treatment with lenalidomide, or other IMiD therapy, is generally well tolerated in the relapsed setting. In murine models, lenalidomide directly enhanced the antitumor responses of CAR T cells in DLBCL with limited direct tumor effect [66]. Finally, small but growing data suggest that radiation therapy may improve CAR T cell targeting through remodeling the tumor environment and amplifying the immune response [67]. This has been described in cell lines and solid tumors. Recently, the outcomes of 8 patients who were treated with radiation therapy while waiting for CAR T cell manufacturing showed that the approach is a safe and effective method of obtaining local disease control, suggesting that post-CAR T radiation similarly might be safe [68]. Data supporting the role of radiation therapy in hematologic malignancies before CAR T cell therapy are limited. Radiation may be impractical for patients with large tumor volumes [69,70]. These studies are summarized in Table 1.
Table 1.
| Setting | Agent | Study | Outcome/Conclusion | Reference |
|---|---|---|---|---|
| Preclinical | Lenalidomide | Cell lines/xenograft models | Lenalidomide augments CAR-T function | [66] |
| Preclinical | Radiation therapy | Cell lines (leukemia) | Radiation sensitizes cell lines to CAR-T-mediated death | [69] |
| Preclinical | Radiation therapy | Cell lines (solid tumor) | Radiation enhances CAR-T penetration and expansion | [67,70] |
| Pre-CAR-T | Radiation therapy | Retrospective | Pre-CAR-T radiation is safe and led to effective local disease control | [68] |
| Pre-CAR-T | Ibrutinib | Retrospective | Ibrutinib before CAR-T therapy in CLL may improve function | [65] |
| Upfront | Atezolizumab (PD-L1) | Phase 1 | Combination atezolizumab + axi-cel is safe, higher level of expansion | [64] |
| Progressive disease | Pembrolizumab (PD-1) | Case report | Disease regression | [60] |
| Progressive disease | Nivolumab (PD-1) | Case report | Re-expansion of CAR-T product, disease regression, CRS/CRES | [61] |
| Progressive disease | Pembrolizumab (PD-1) | Prospective study | Combination pembrolizumab + CART19 in R//R led to responses in 3 of 11 patients. 1CR, 2 PRs. | [62] |
CRS, cytokine release syndrome; CRES, XXX.
In summary, there is no standard of care for patients who progress after initially responding to CAR T cell therapy. Enrollment in well-designed clinical trials should be prioritized for these patients. In the event that a study is not available, treatment with PD-1 inhibitors, ibrutinib, IMiDs, and/or radiation therapy may be considered. These patients are at risk for future treatment failures, and allogeneic HCT with the best available donor can be considered if these patients achieve a remission.
Future Directions
There are limited data to guide the optimal management of patients with LBCL who relapse after CAR T cell therapy. Although the number of these patients is growing, differences between products and treatment indications make standardized recommendations challenging. Furthermore, the profile of patients who relapse after CAR T cell therapy is likely to change as CAR T cell move into earlier lines of therapy. Where to stage conventional therapies, including autologous HCT and novel therapies, after CAR T cell failure will require future study. The benefit of second CART cell infusions directed at the same antigen is also largely unknown. A minority of ZUMA-1 patients received second infusions, but the optimal population and timing for this are not yet well understood. Subsequent CAR T cell infusions, possibly in combination with adjunctive immunotherapy, in carefully selected CD19+ patients may be beneficial and worthy of future study. Finally, carefully characterizing patterns of failure, identifying patients who are likely to relapse after CAR T cell therapy, and developing pre-emptive treatment strategies is another important, unmet need.
Future generations of CAR T cells are expected to target antigens outside of CD19 and incorporate dual B cell antigens. Early experiences with alternative targets are reported primarily in ALL with encouraging results. The phase 1 data of a CD22-targeted CAR in 21 children/adults, including 17 previously treated with CD19-directed CARs, showed CRs in 73% of patients (11/15) who were dosed at ≥1 × 106 CD22-CAR T cells/kg [71,72]. Early dual-target CAR T cells that target CD19 and CD22 are primarily focused in ALL and also have reported favorable results. In the AUTO3 study, 6 of 8 patients treated achieved an MRD-negative CR after receiving a CD19/CD22 CAR [73]. Results from the Stanford group reported that 1 of 5 patients with DLBCL and 1 of 2 patients with ALL achieved a CR with CD19/CD22 CARs; MRD-negative CRs were reported in 3 of 4 pediatric and young adult patients with ALL [74,75]. Finally, the development of “armored CARs,” or CAR T cells administered in combination with anti-PD-1 therapy, also demonstrated early efficacy [76,77]. The Memorial Sloan Kettering group recently reported the outcomes of 24 evaluable patients (6 DLBCLs), with CRs seen in 16 patients (67%) [77].
CONCLUSION
The development of CAR T cell therapy has led to deep, durable responses in patients with otherwise refractory lymphoma and limited life expectancy. Most patients with DLBCL treated with CAR T cell therapy will respond to treatment and should be observed in the immediate postinfusion period as responses may deepen without further intervention. Approximately half of ZUMA-1 patients who initially responded and 21% to 35% of JULIET patients will relapse and require further therapy, but there are currently no biomarkers to identify these patients before relapse. The optimal management of these patients is not yet known. Providing standardized treatment recommendations for this patient population is not practical for the reasons previously cited.
Rebiopsy with CD19 assessment is recommended, and clinical trial enrollment should be considered for all patients with progressive disease after CAR T cell therapy. In patients with early relapses who remain CD19+, PD-1 inhibitor therapy can be considered to attempt to stimulate the residual T cells, although prospective trials testing this strategy must confirm the efficacy of the approach. Patients with late relapse can be considered for other salvage modalities, including reinfusion of the CAR T cells, ibrutinib, immunomodulatory therapy, or, if the tumor is localized, radiation therapy. Further study is needed to provide more clinical data to justify these approaches, and well-designed clinical trials are necessary to refine management strategies.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Byrne: None
Oluwole: Consultancy with Bayer, significant financial interest in Pfizer/Gilead
Hill: Kite/Gilead research funding/consulting, Novartis consulting, Juno consulting
Savani: None
Majhail: Consultancy with Anthem, advisory board for NIKARTA and Incyte, speaker for Mallincrodt
Locke: Scientific advisor for Kite/Gilead and GammaDelta T cell Therapeutics, consultant to Cellular BioMedicine Group, Inc.
REFERENCES
- 1.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituimab era. J Clin Oncol. 2010;28:4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. [DOI] [PubMed] [Google Scholar]
- 3.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001;19:406–413. [DOI] [PubMed] [Google Scholar]
- 4.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. [DOI] [PubMed] [Google Scholar]
- 5.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51:51–57. [DOI] [PubMed] [Google Scholar]
- 6.Daniyan AF, Brentjens RJ. At the bench: chimeric antigen receptor (CAR) T cell therapy for the treatment of B cell malignancies. J Leukoc Biol. 2016;100:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadelain M CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramson JS, Palomba L, Gordon LI, Lunning M, Arnason J, A F-T. Transcend NHL 001: immunotherapy with the CD19-directed CAR T-cell product JCAR017 results in high complete response rates in relapsed or refractory B-cell non-Hodgkin lymphoma. Paper presented at: ASH Annual Meeting and Exposition San Diego: CA; 2016. [Google Scholar]
- 10.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. YESCARTA (axicabtagene ciloleucel). Vol 20182018: FDA Approval Notice.
- 14.Food and Drug Administration. Supplement Approval. 2018. Silver Spring, MD: FDA; 2018. [Google Scholar]
- 15.Nastoupil L, Jain M, Spiegel J, Ghobadi A, Lin Y. Axicabtagene ciloleucel (axi-cel) CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: real world eperience. Paper presented at: ASH Annual Meeting San Diego: CA; 2018. [Google Scholar]
- 16.Tang J, Hubbard-Lucey VM, Pearce L, O’Donnell-Tormey J, Shalabi A. The global landscape of cancer cell therapy. Nat Rev Drug Discov. 2018;17:465–466. [DOI] [PubMed] [Google Scholar]
- 17.Oluwole O ZUMA-7: a phase 3 randomized trial of axicabtagene ciloleucel (axi-cel) versus standard-of-care (SOC) therapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL). Paper presented at: ASCO Annual Meeting 2018 Chicago: IL; 2018. [Google Scholar]
- 18.Gilead. Quarterly Earnings. 2019.
- 19.Lee DW III, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C. Long-term outcomes following CD19 CAR T cell therapy for B-ALL are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Paper presented at: ASH Annual Meeting San Diego: CA; 2016. [Google Scholar]
- 20.Gardner RA, Finney O, Smithers H, Leger K, Annesley CE. Prolonged functional persistence of CD19 CAR t cell products of defined CD4:CD8 composition and transgene expression determines durability of MRD-negative ALL remission. Paper presented at: ASOC Annual Meeting Chicago: IL; 2017. [Google Scholar]
- 21.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24:1504–1506. [DOI] [PubMed] [Google Scholar]
- 25.Bucktrout SL, Bluestone JA, Ramsdell F. Recent advances in immunotherapies: from infection and autoimmunity, to cancer, and back again. Genome Med. 2018;10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. [DOI] [PubMed] [Google Scholar]
- 27.Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty GL, Moon EK. Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology. 2014;3: e970027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGray AJ, Hallett R, Bernard D, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther. 2014;22:206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T (regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossille D, Azzaoui I, Feldman AL, et al. Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: a replication study and combined analysis of 508 patients. Leukemia. 2017;31:988–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cycon KA, Rimsza LM, Murphy SP. Alterations in CIITA constitute a common mechanism accounting for downregulation of MHC class II expression in diffuse large B-cell lymphoma (DLBCL). Exp Hematol. 2009;37:184–194. [DOI] [PubMed] [Google Scholar]
- 34.Nijland M, Veenstra RN, Visser L, et al. HLA dependent immune escape mechanisms in B-cell lymphomas: implications for immune checkpoint inhibitor therapy? Oncoimmunology. 2017;6:e1295202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood. 2004;103:4251–4258. [DOI] [PubMed] [Google Scholar]
- 36.Jackson HJ, Brentjens RJ. Overcoming antigen escape with CAR T-cell therapy. Cancer Discov. 2015;5:1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locke F, Ghobadi A, Lekakis L, Miklos D, Jacobson C. Outcomes by prior lines of therapy (LoT) in ZUMA-1, the pivotal phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients (Its) with refractory large B cell lymphoma. Paper presented at: ASCO Chicago: IL; 2018. [Google Scholar]
- 42.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borchmann P, Tam C, Jager U, McGuirk J, Holte H. An updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Paper presented at: European Hematology Association Stockholm: Sweden; 2018. [Google Scholar]
- 44.Heczey A, Louis CU, Savoldo B, et al. CAR T Cells Administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. 2017;25:2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8:355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller KT, Maude S, Porder DL, Frey N, Wood P. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Paper presented at: ASH Annual Meeting. Atlanta: GA; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25:2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer. 2018;17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. [DOI] [PubMed] [Google Scholar]
- 51.Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. 2018;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galon J, Rossi J, Turcan S, et al. Characterization of anti-CD19 chimeric antigen receptor (CAR) T cell-mediated tumor microenvironment immune gene profile in a multicenter trial (ZUMA-1) with axicabtagene ciloleucel (axi-cel, KTE-C19). Paper presented at: American Society of Clinical Oncology Chicago: IL; 2017. [Google Scholar]
- 53.Rossi J, Paczkowski P, Shen YW, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018;132:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abramson JS, LI G, Palomba MI, Lunning MA, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. Paper presented at: ASCO 2018. [Google Scholar]
- 55.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 56.Abramson JS, Gordon LJ, Palomba ML, Lunning MA, Arnason JE, Forero-Torres A. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. Paper presented at: ASCO Chicago: IL; 2018. [Google Scholar]
- 57.Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol. 2018;36:2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank M, Hossain N, Bukhari A, Dean E, Spiegel J. Monitoring ctDNA in r/r DLBCL patients following the CAR T-cell therapy axicabtagene ciloleucel: day 28 landmark analysis. Paper presented at: ASCO Annual Meeting Chicago: IL; 2019. [Google Scholar]
- 59.Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Shadman M. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Paper presented at: ASH Annual Meeting San Diego: CA; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129:1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill B, Roberts Z, Rossi J, Smith M. Marked re-expansion of chimeric antigen receptor (CAR) T cells and tumor regression following nivolumab treatment in a patient treated with axicabtagene ciloleucel (axi-cel; KTE-C-19) for refractory diffuse large B cell lymphoma (DLBCL). Paper presented at: American Society of Hematology Annual Meeting & Exposition Atlanta: GA;2017. [Google Scholar]
- 62.Chong EA, Svoboda J, Nasta SD, Landsburg DJ, Winchell N. Sequential anti-CD19 directed chimeric antigen receptor modified T-cell therapy (CART19) and PD-1 blockade with pembrolizumab in patients with relapsed or refractory B-cell non-Hodgkin lymphomas. Paper presented at: ASH Annual Meeting San Diego: CA; 2018. [Google Scholar]
- 63.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobsen CA, Locke FL, Miklos DB, Herrera AF, Westin JR, Lee J. End of phase 1 results from Zuma-6: axicabtagene ciloleucel (axi-cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. Paper presented at: ASH Annual Meeting San Diego: CA; 2018. [Google Scholar]
- 65.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otáhal P, Pr ková D, Král V, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5:e1115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flynn JP, O’Hara MH, Gandhi SJ. Preclinical rationale for combining radiation therapy and immunotherapy beyond checkpoint inhibitors (i.e., CART). Transl Lung Cancer Res. 2017;6:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sim AJ, Jain MD, Figura N, et al. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-cell lymphoma [e-pub ahead of print] Int J Radiat Oncol Biol Phys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeSelm C, Hamieh M, Sadelain M. Radiation sensitizes tumor cells to CAR T cell immunotherapy. Paper presented at: ASTRO Boston: MA; 2016. [Google Scholar]
- 70.Cortez M, Korngold A, Valdecanas D, et al. Using radiation therapy to improve CAR T-cell targeting of solid tumors. Paper presented at: ASTRO Boston: MA; 2016. [Google Scholar]
- 71.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.PJ A, Wynn R, Hough R, et al. Simultaneous targeting of CD19 and CD22: phase I study of AUTO3, a bicistronic chimeric antigen receptor (CAR) T-cell therapy, in pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL): Amelia study. Paper presented at: American Society of Hematology Annual Meeting San Diego: CA; 2018. [Google Scholar]
- 74.Hossain N, Sahaf B, Abramian M, Spiegel J, Kong K, Kim S. Phase I experience with a bi-specific CAR targeting CD19 and CD22 in adults with B-cell malignancies. Paper presented at: American Society of Hematology Annual Meeting San Diego: CA; 2018. [Google Scholar]
- 75.Schultz L, Davis K, Baggot C, Chaudry C, Marcy A, Mavroukakis S. Phase 1 study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with B cell acute lymphoblastic leukemia (ALL). Paper presented at: American Society of Hematology Annual Meeting San Diego: CA; 2018. [Google Scholar]
- 76.Chen T, Yuan Y, Huang L, Pu C, Ding T, Xiao X. Dominant-negative PD1-armored CART cells induce remission in refractory diffuse large B-cell lymphoma (DLBCL) patients. Paper presented at: ASCO Annual Meeting Chicago: IL; 2019. [Google Scholar]
- 77.Park J, Palomba M, Batlevi C, et al. A phase I first-in-human clinical trial of CD19-targeted 19–28z/4–1BBL “armored” CAR T cells in patients with relapsed or refractory NHL and CLL including Richter’s transformation. Paper presented at: American Society of Hematology Annual Meeting San Diego: CA; 2018. [Google Scholar]