Abstract
In this study, the complete mitogenome sequence of massive coral, Porites lutea (Cnidaria: Poritidae), has been sequenced by next-generation sequencing method. The overall base composition of Porites lutea mitogenome is 26.0% for A, 13.3% for C, 23.0% for G and 37.8% for T and have high AT content of 63.7%. The assembled mitogenome, consisting of 18 646 bp, has unique 13 protein-coding genes (PCGs), seven transfer RNAs and two ribosomal RNAs genes. The Porites lutea mitogenome has the common mitogenome gene organization and feature of scleractinian coral. Among 13 PCGs, ND5 and COX1 genes are interrupted by group I intron (11 130 and 971 bp, respectively). There are 13 genes embedded in ND5 group I intron (tRNA-Glu, ND1, CYTB, tRNA-Met, ND2, ND6, ATP6, ND4, 12S rRNA, COX3, COX2, ND4L and ND3), and two genes embedded in COX1 group I intron (tRNA-Ile and tRNA-Pro). The complete mitogenome provides essential and important DNA molecular data for further phylogenetic and evolutionary analysis for stony coral.
Keywords: Group I intron, massive coral, mitogenome, next-generation sequencing, Porites lutea
The massive coral (Porites lutea) is widely distributed throughout the Indo-Pacific, from the Red Sea and east Africa to French Polynesia and east Pacific (Veron 2000). The colonies are hemispherical or helmet-shaped and may be over four metres across. The surface is usually smooth. The colour is usually cream or yellow but may be bright colour in shallow waters. The massive coral is a dominant species in back reef margins, lagoons and some fringing reefs, lagoons and some fringing reefs are inhibited. The first establishment of P. lutea mitogenome is important for further evolutionary and phylogenetic analyses for stony coral.
Samples (voucher no. 466) of P. lutea were collected from Hainan Island, China. The methods for genomic DNA extraction, library construction and next-generation sequencing were followed by our previous publication (Shen et al. 2014). Initially, the raw next-generation sequencing reads generated from MiSeq (Illumina, San Diego, CA). About 0.2% raw reads (8948 out of 4 372 520) were de novo assembly by using commercial software (Geneious V8, Auckland, New Zealand) to produce a single, circular form of complete mitogenome with about an average 161 X coverage.
The complete mitochondrial genome of P. lutea was 18 646 bp in size (GenBank KU159432) and its overall base composition is 26.0% for A, 13.3% for C, 23.0% for G and 37.8% for T, showing 99% identities to the complete mitogenome of P. rus (GenBank: LN864762). The protein coding, rRNA and tRNA genes of P. lutea mitogenome were predicted by using DOGMA (Wyman et al. 2004), ARWEN (Laslett & Canback 2008), MITOS (Bernt et al. 2013) tools and manually inspected. The complete mitogenome of P. lutea includes unique 13 protein-coding genes (PCGs), seven transfer RNA genes and two ribosomal RNA genes. All PCGs, tRNA and rRNA genes were encoded on H-strand.
It is important to note that all PCGs are started with ATG codon expect for ND1 (GTG), ND6 (TTG), ND3/ND5 (GTG) and ND4L (ATA). Seven of 13 PCGs is inferred to terminate with TAA (ND4, CYTB, ND2, ND1, ND6, ND4L and ATP8) and others with TAG (COX3, COX2, ATP6, ND3, ND5 and COX1) stop codon. The longest one is ND5 gene (1839 bp) in all PCGs, whereas the shortest is ATP8 gene (216 bp). The size of small ribosomal RNA (12S rRNA) and large ribosomal RNA (16S rRNA) genes are 1107 bp and 1437 bp, respectively. Among 13 PCGs, ND5 and COX1 genes are interrupted by group I intron (11 130 and 971 bp, respectively). There are 13 genes embedded in ND5 group I intron (tRNA-Glu, ND1, CYTB, tRNA-Met, ND2, ND6, ATP6, ND4, 12S rRNA, COX3, COX2, ND4L and ND3) and two genes embedded in COX1 group I intron (tRNA-Ile and tRNA-Pro). Similar mitogenomic organization has been reported in previous studies in scleractinian coral (Medina et al. 2006; Fukami et al. 2007).
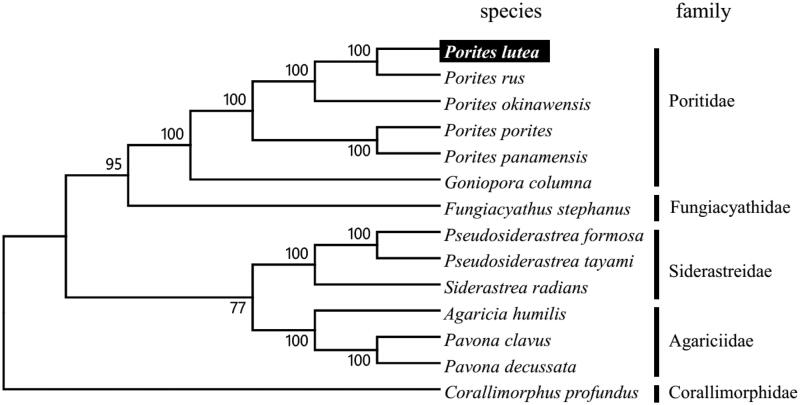
To validate the phylogenetic position of P. lutea, we used MEGA6 software (Tamura et al. 2013) to construct a maximum-likelihood tree (with 500 bootstrap replicates) containing complete mitogenomes of 13 species derived from four different families in Scleractinia. Corallimorphus profundus derived from Corallimorphidae was used as out group for tree rooting. Result shows P. lutea can be unambiguously grouped in Poritidae showing close related to P. rus with high bootstrap value supported (Figure 1). In conclusion, the complete mitogenome of the P. lutea deduced in this study provides essential and important DNA molecular data for further phylogenetic and evolutionary analysis for stony coral phylogeny.
Figure 1.
Molecular phylogeny of Porites lutea and other related species in Scleractinia based on complete mitogenome. The complete mitogenomes is downloaded from GenBank and the phylogenic tree is constructed by maximum-likelihood method with 500 bootstrap replicates. The gene's accession number for tree construction is listed as follows: Porites lutea (KU159432), Porites rus (NC_027526), Porites okinawensis (NC_015644), Porites porites (NC_008166), Porites panamensis (NC_024182), Goniopora columna (NC_015643), Fungiacyathus stephanus (NC_015640), Pseudosiderastrea formosa (NC_026530), Pseudosiderastrea tayami (NC_026531), Siderastrea radians (NC_008167), Agaricia humilis (NC_008160), Pavona clavus (NC_008165), Pavona decussata (NC_026527) and Corallimorphus profundus (NC_027105)
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding information
This research was supported by the grants from Basic Science Research Fund of the Third Institute of Oceanography, SOA (No. 2015035), National Natural Science Foundation of China (No. 41406161) and the Ocean Welfare Scientific Research Project of China (No. 201105012).
References
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Putz J, Middendorf M, Stadler PF.. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319. [DOI] [PubMed] [Google Scholar]
- Fukami H, Chen CA, Chiou CY, Knowlton N.. 2007. Novel group I introns encoding a putative homing endonuclease in the mitochondrial cox1 gene of Scleractinian corals. J Mol Evol 64:591–600. [DOI] [PubMed] [Google Scholar]
- Laslett D, Canback B.. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175. [DOI] [PubMed] [Google Scholar]
- Medina M, Collins AG, Takaoka TL, Kuehl JV, Boore JL.. 2006. Naked corals: skeleton loss in Scleractinia. Proc Natl Acad Sci USA. 103:9096–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KN, Yen TC, Chen CH, Li HY, Chen PL, Hsiao CD.. 2014. Next generation sequencing yields the complete mitochondrial genome of the flathead mullet, Mugil cephalus cryptic species NWP2 (Teleostei: Mugilidae). Mitochondrial DNA. 1–2. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S.. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron JEN. 2000. Corals of the world. Vol 1-3. Stafford-Smith M. (Ed.) Australian Institute of Marine Science, Townsville, Australia. 1382 p. [Google Scholar]
- Wyman SK, Jansen RK, Boore JL.. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255. [DOI] [PubMed] [Google Scholar]