Abstract
Here we present two incomplete mitochondrial genome sequences of Hirudo medicinalis and Hirudo verbana (Annelida, Hirudinea). The corresponding sequences are 14,729 and 14,604 base pairs in length. They contain all mitochondrial genes (13 protein-coding genes, 22 tRNAs and two rRNAs) but lack the non-coding region. Nevertheless, the robust reconstruction of their phylogenetic relationships presented here reveals distinct separation of both leeches from other annelids and at the same time relatively high dissimilarity between each other.
Keywords: Hirudo medicinalis, Hirudo verbana, mitochondrial genome
Hirudo medicinalis, one of the species more commonly known as medicinal leech, has been used in various medical practices for centuries. Nowadays being approved by US FDA as prescription medical device in 2004, it has several clinical applications such as reconstructive surgery (Porshinsky et al. 2011), at the same time, attracting research as widely used model organism in fields like neurobiology (Kristan et al. 2005) . In 2007 Sidall et al. (2007) demonstrated that commercially available leeches marked as H. medicinalis were actually H. verbana, so closely related species that there are concerns as to whether they are in fact different species (Hildebrandt & Lemke 2011). Since morphology, geographical distribution and incomplete reproductive isolation (Petrauskienė et al. 2009) are too controversial to elucidate the phylogenetic relationships of these leeches, mitochondrial DNA (mtDNA) sequences might be invaluable to this task. In this study, we report two draft mitochondrial genomes of H. medicinalis and H. verbana lacking only one non-coding region each.
The specimens were provided by HIRUD I.N. Ltd. (Balakovo, Saratov Region, Russia). The collection of H. medicinalis took place at the pond near Volkovo, Saratov region, Russia (51°91′03″, 47°34′90″) and H. verbana – at the lake Manych, Stavropol Krai, Russia (46°01′09″, 43°48′21″). Total genomic DNA was isolated from muscle tissue, and amplicons corresponding to mtDNA were generated by PCR and sequenced using Ion Proton (Life Technologies, Carlsbad, CA). Primary assembly was conducted by Newbler 2.6 (Life Technologies, Carlsbad, CA) and gaps were filled via Sanger sequencing using ABI Prism Genetic Analyzer 3730XL (Applied Biosystems, Waltham, MA). The annotation was performed by web-based tool MITOS (Bernt et al. 2013) and then manually corrected by comparison with complete mitochondrial genomes of other annelids.
The sequences of mtDNA of H. medicinalis (14,729 bp) and H. verbana (14,604 bp) reported here were deposited in GenBank under the accession numbers KU672396 and KU672397, respectively. Each of them comprised 13 protein-coding genes, 22 tRNAs and two rRNAs all encoded on the same strand. The gene order shown in Table 1 was consistent with other members of Hirudinea subclass. However, the non-coding regions of both leeches located between tRNA-Arg and tRNA-His appeared to be longer and more complex compared with those of close relatives, hence were only partially assembled and included in the sequences resulting in two almost complete mitogenomes. The annotation of the latter is represented in Table 1 along with predicted start and stop codon usage which was found to be identical between H. medicinalis and H. verbana. They employed three different start codons: ATG (ND1, ND2, ND3, ND4, ND6, COX1, CYTB, ATP6 and ATP8), GTG (ND5, ND4L and COX2) and TTG (COX3). Stop codon of choice for these mitochondria was TAA, although six genes (ND2, ND4, ND6, COX1, COX3 and ATP8) utilized termination codon T–, completed via polyadenylation.
Table 1.
The annotation of mtDNA sequences of H. medicinalis and H. verbana.
|
Hirudo medicinalis |
Hirudo verbana |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Location, bp | Size, bp | Location, bp | Size, bp | Anticodon | Start codon | Stop codon |
| tRNA-His | 93–153 | 61 | 183–243 | 61 | GTG | ||
| ND5 | 154–1857 | 1704 | 244–1953 | 1710 | GTG | TAA | |
| tRNA-Phe | 1857–1918 | 62 | 1953–2013 | 61 | GAA | ||
| tRNA-Glu | 1919–1972 | 54 | 2022–2068 | 47 | TTC | ||
| tRNA-Pro | 1977–2037 | 61 | 2072–2132 | 61 | TGG | ||
| tRNA-Thr | 2039–2097 | 59 | 2134–2192 | 59 | TGT | ||
| ND4L | 2098–2385 | 288 | 2193–2480 | 288 | GTG | TAA | |
| ND4 | 2379–3711 | 1333 | 2474–3806 | 1333 | ATG | T– | |
| tRNA-Cys | 3721–3780 | 60 | 3816–3875 | 60 | GCA | ||
| tRNA-Met | 3781–3843 | 63 | 3876–3939 | 64 | CAT | ||
| s-rRNA | 3844–4583 | 740 | 3940–4677 | 738 | |||
| tRNA-Val | 4584–4642 | 59 | 4678–4736 | 59 | TAC | ||
| l-rRNA | 4643–5782 | 1140 | 4737–5880 | 1144 | |||
| tRNA-Leu | 5783–5842 | 60 | 5881–5940 | 60 | TAG | ||
| tRNA-Ser | 5843–5909 | 67 | 5941–6007 | 67 | TGA | ||
| tRNA-Ala | 5910–5971 | 62 | 6008–6069 | 62 | TGC | ||
| tRNA-Leu | 5972–6032 | 61 | 6070–6130 | 61 | TAA | ||
| ND1 | 6033–6953 | 921 | 6131–7051 | 921 | ATG | TAA | |
| tRNA-Ile | 6952–7013 | 62 | 7050–7111 | 62 | GAT | ||
| tRNA-Lys | 7014–7075 | 62 | 7112–7173 | 62 | TTT | ||
| ND3 | 7076–7420 | 345 | 7174–7518 | 345 | ATG | TAA | |
| tRNA-Ser | 7419–7475 | 57 | 7517–7573 | 57 | TCT | ||
| ND2 | 7476–8460 | 985 | 7574–8561 | 988 | ATG | T– | |
| COX1 | 8461–9994 | 1534 | 8562–10,095 | 1534 | ATG | T– | |
| tRNA-Asn | 9995–10,057 | 63 | 10,096–10,159 | 64 | GTT | ||
| COX2 | 10,058–10,741 | 684 | 10,160–10,843 | 684 | GTG | TAA | |
| tRNA-Asp | 10,740–10,802 | 63 | 10,842–10,904 | 63 | GTC | ||
| ATP8 | 10,803–10,953 | 151 | 10,905–11,055 | 151 | ATG | T– | |
| tRNA-Gly | 10,954–11,011 | 58 | 11,056–11,114 | 59 | TCC | ||
| tRNA-Tyr | 11,012–11,071 | 60 | 11,114–11,172 | 59 | GTA | ||
| COX3 | 11,089–11,869 | 781 | 11,189–11,969 | 781 | TTG | T– | |
| tRNA-Gln | 11,870–11,938 | 69 | 11,970–12,038 | 69 | TTG | ||
| ND6 | 11,939–12,398 | 460 | 12,039–12,498 | 460 | ATG | T– | |
| CYTB | 12,399–13,541 | 1143 | 12,499–13,641 | 1143 | ATG | TAA | |
| tRNA-Trp | 13,543–13,604 | 62 | 13,643–13,704 | 62 | TCA | ||
| ATP6 | 13,663–14,388 | 726 | 13,763–14,467 | 705 | ATG | TAA | |
| tRNA-Arg | 14,380–14,427 | 48 | 14,477–14,524 | 48 | TCG | ||
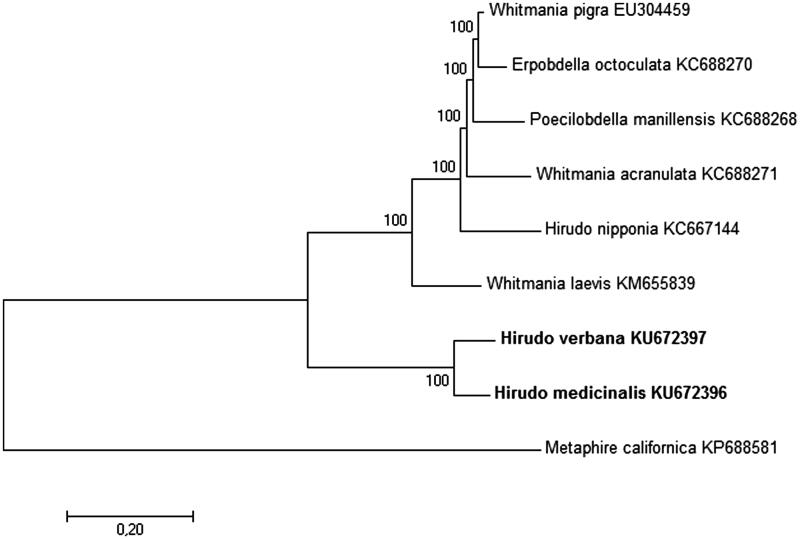
In order to infer the phylogenetic relationships of these leeches, seven complete mitochondrial DNA sequences of other annelids were downloaded from NCBI GenBank, six of which represented Hirudinea subclass (Hirudo nipponia, Whitmania laevis, Whitmania pigra, Whitmania acranulata, Erpobdella octoculata and Poecilobdella manillensis) and one (Metaphire californica) belonged to Oligochaeta subclass and was used as the outgroup. Circular mitogenomes were linearized to start with tRNA-His and end with tRNA-Arg for alignment and following phylogenetic analysis. The resulting tree drawn to scale is shown in Figure 1. Interestingly, H. medicinalis and H. verbana form a separate clade from all other species of Hirudinea subclass. It is also worth pointing out that corresponding branch lengths within this clade suggest enough divergence for these two leeches to be classified as different species.
Figure 1.
The phylogenetic relationships of H. medicinalis and H. verbana (both shown in bold). The analysis was performed using Maximum Likelihood method based on the Tamura 3-parameter model (Tamura 1992) in MEGA7 (Kumar et al. 2012). Metaphire californica was used as an outgroup. The robustness of each node is represented by a bootstrap value obtained by 500 steps. The scale bar corresponds to a number of substitutions per site. Each NCBI accession number of the sequence used to construct the tree is shown next to corresponding taxa name.
Acknowledgments
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding information
This work was supported by the Russian Science Foundation (Project 14-14-00696).
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF.. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69: 313–319. [DOI] [PubMed] [Google Scholar]
- Hildebrandt JP, Lemke S.. 2011. Small bite, large impact-saliva and salivary molecules in the medicinal leech, Hirudo medicinalis. Naturwissenschaften. 98:995–1008. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Calabrese RL, Friesen WO.. 2005. Neuronal control of leech behavior. Prog Neurobiol. 76:279–327. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Peterson D, Tamura K.. 2012. MEGA-CC: computing core of molecular evolutionary genetics analysis program for automated and iterative data analysis. Bioinformatics. 28:2685–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrauskienė L, Utevska O, Utevsky S.. 2009. Can different species of medicinal leeches (Hirudo spp.) interbreed? Invertebr Biol. 128:324–331. [Google Scholar]
- Porshinsky BS, Saha S, Grossman MD, Beery IiPR, Stawicki SPA.. 2011. Clinical uses of the medicinal leech: a practical review. J Postgrad Med. 57:65–71. [DOI] [PubMed] [Google Scholar]
- Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS.. 2007. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc Biol Sci. 274:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol Biol Evol. 9:678–687. [DOI] [PubMed] [Google Scholar]