Abstract
Context: Primary spinal primitive neuroectodermal tumor (PNET) of the central nervous system has a low incidence. The intraspinal case is very rare. Around 30 cases have been reported so far. We summarized the cases of primary spinal PNET available in the database of our institute, either intramedullary or extramedullary cases. Then we did literature review of the same disease.
Findings: There were eight cases of primary spinal PNET available in our database, with one intramedullary case and seven extramedullary cases. Surgical resection was performed. The histology diagnosis was PNET. Peri-operative image examinations of the whole central nervous system (CNS) were performed to exclude tumors other than spinal cord origin. Then during literature review, 33 reports of the disease were included. The pre-operative diagnosis rate was low. The disease had a high recurrence rate and poor prognosis given available treatment.
Conclusion: Primary spinal primitive neuroectodermal tumor is of high malignancy. Little is known due to its quite low incidence. The prognosis is poor due to lacking of effective treatment strategy. Present treatment strategy is referred to other common CNS malignancies like glioma. Further investigation of the disease is necessary.
Keywords: Spinal, Intramedullary, Extramedullary, Primitive neuroectodermal tumor (PNET), Primary
Introduction
Primitive neuroectodermal tumor (PNET) is a heterogeneous group of central nervous system (CNS) tumors composed of undifferentiated or poorly differentiated neuroepithelial cells. It can involve the whole CNS. The spinal PNET cases are very rare. Around 30 cases have been reported so far. It can locate either intramedullary or extramedullary. We added eight cases of histology confirmed primary spinal PNET of our institute with one intramedullary case and seven extra medullary ones. The results were compared with literatures.
Case series
There were eight cases of spinal PNET with histology confirmation in the histology database of our institute. Four of them were under 18 years old, two were 18, and two of them were adults. The mean age was 17 years old, range 7–43. Most lesions (5) located at lumber to sacral levels. Two were at the cervical level. One case had multiple foci. Only 1 of them was intramedullary. The other seven were extramedullary and had the tendency of invade out of the spinal canal through interspinal foramen. The symptoms depended on the exact spinal segment involved. Motor dysfunctions and pain were main symptoms.
Neuro-radiology examinations always showed iso- to hypo-intense on T1-weighted image and iso- to hyper-intense on T2-weighted image, with enhancement. In those cases extending out through the interspinal foramen a typical dumbbell shape could be observed. Pre-operative diagnosis was not easy due to its rare incidence. None of our cases were suspected as PNET before surgery. The differentiated diagnosis included both malignancies and non-malignant neoplasms. When located intramedullary it might be considered as glioma, lymphoma, sarcoma, or ependymoma, etc. When located extramedullary with interspinal foramen invasion, most were suspected as Schwannoma or neurofibroma, much more commonly seen that had typical dumbbell shape. The whole CNS image examination involving the brain and whole spinal cord were performed for these eight patients, either before or after surgery, to exclude secondary spinal cord lesion.
Surgical resection with lamiectomy was first-line treatment for all patients. The operation strategy depended on the exact location of the tumor. For the multiple case that all lesions could not be resected through a single approach, the lesion with most prominent symptom was resected first. The extent of resection was defined according to both intra-operative evaluation and post-operative MR scan. Gross total resection was reached for seven extra medullary cases. For the intramedullary case, the entity of the tumor was similar to glioma and extensive resection was not applicable for the protection of the spinal cord functions. Subtotal resection was performed for this case. All specimen were sent for pathological examination and were confirmed as PNET. Adjuvant radiotherapy was recommended for all patients. Chemotherapy should also be considered although no standard guideline existed yet for chemotherapy of spinal PNET. However, the compliance of patients varied. Three patients were lost to follow-up, aged 7, 18, and 43 at the admission. For the rest five patients, including three under 18, one 18 years old, and one above 18, were all died during five years of follow-up. One pediatric patient went on to take chemotherapy and died from recurrence nine months later. One pediatric patient took both radiotherapy and chemotherapy and died from recurrence 12 months later. One pediatric and one adult patient did not receive any adjuvant therapy and died without exact time of death. One patient of 18 years old was already a recurrent lesion at the first admission to our institute. After the operation, she received radiotherapy and died 16 months later. All the adjuvant radiotherapy and chemotherapy were offered by other institutes and no detailed strategy or regimen was available (Figs. 1 and 2) (Table 1).
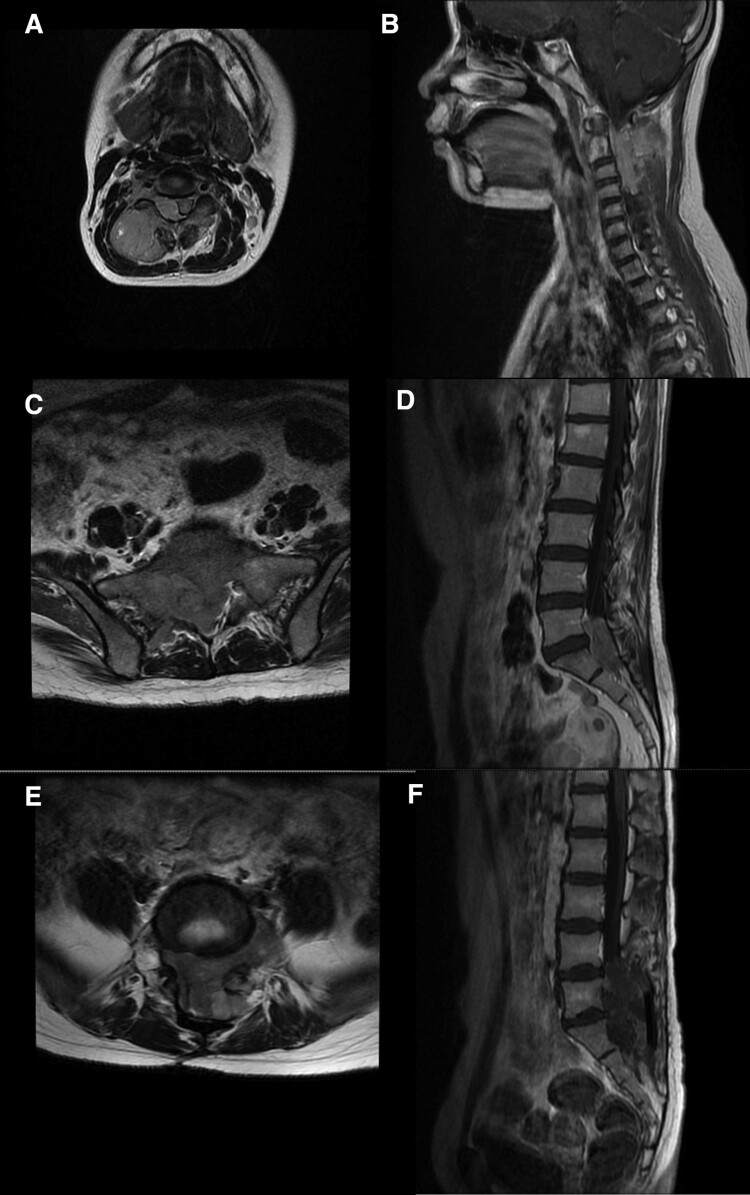
Figure 1.
Pre-operative MR scan of case 1, 6, and 7. (A,B: case 1, T2-weighted traverse and T1 enhancement saggital. The lesion located at C2-4, extramedullary, extend extraspinal through interspinal foramen. C,D: case 6, T2-weighted traverse and T1 enhancement saggital. The lesion located at L5-S1, extramedullary, extend extraspinal through interspinal foramen. E,F: case 7, T2-weighted traverse and T1 enhancement saggital. Multiple lesions at L5-S1, extramedullary, extend extraspinal through interspinal foramen.).
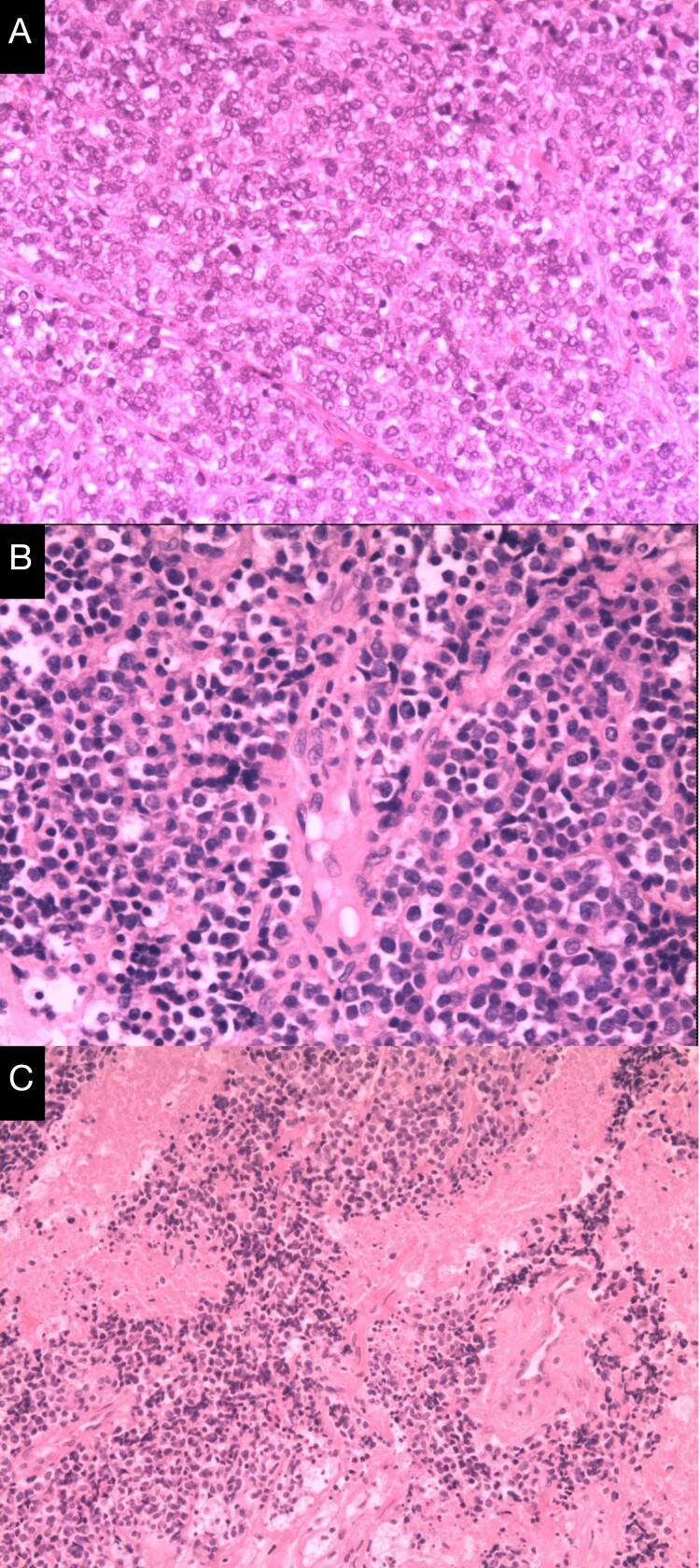
Figure 2.
Post-op histology slides of case 1, 6, and 7 with diagnosis of primitive neuroectodermal tumor. (Hematoxylin-eosin stain, original magnification×100. A-C: Case 1, 6, and 7 correspondingly).
Table 1. Summary of primary spinal primitive neuroectodermal tumor of our institute.
| Gender | Age (yr.) | Location | MR | Other signs on image examination | Other case information | Adjuvant therapy | Follow-up | |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 9 | C2–4 | T1-T2-, homogeneous enhancement. | Extramedullary, Extend extraspinal through interspinal foramen. | — | Chemotherapy | Recurrent and died 9 month later. |
| 2 | M | 9 | C2–7 | T1↓T2↑, prominent enhancement. | Intramedullary. | — | Radiotherapy & Chemotherapy | Recurrent and died 12 months later. |
| 3 | F | 18 | T11–S1 | T1-T2-↑, heterogeneous enhancement. | Extramedullary, Multiple. | A recurrence at admission. | Radiotherapy | Recurrent and died 16 months later. |
| 4 | F | 25 | L5–S1 | T1↓T2↑, prominent enhancement. | Extramedullary, Extend extraspinal through interspinal foramen. | — | No | Died (Family were reluctant to offer time of death). |
| 5 | F | 7 | L4 | T1-T2-, homogeneous enhancement. | Extramedullary, Extend extraspinal through interspinal foramen. | — | Not clear | Loss |
| 6 | F | 43 | L5–S1 | Heterogeneous T1/T2, prominent enhancement. | Extramedullary, Extend extraspinal through interspinal foramen. | — | Not clear | Loss |
| 7 | F | 18 | L5–S1 | T1-T2↑, slight enhancement. | Extramedullary, Extend extraspinal through interspinal foramen. | A recurrence at admission. | Not clear | Loss |
| 8 | M | 7 | S1–3 | T1-T2-, medium enhancement. | Extramedullary. | — | No | Died (Family were reluctant to offer time of death). |
M, male; F, female; yr., years old; -, iso-intensive; ↑, hyper-intensive; ↓, hypo-intensive.
Review of literature
The PubMed online database was searched in July 2018, with the query of “((spinal cord[Title/Abstract]) OR intramedullary[Title/Abstract]) AND primitive neuroectodermal tumor[Title/Abstract]”. Fifty-eight articles were generated. Four were not English. Twenty-one were not about spinal PNET, but focused on lesions of other histology or PNET of locations other than spinal region. Thirty-three primary spinal PNET were included, seven of which with no available full text (Table 2).
Table 2. Review of the literatures of primary spinal primitive neuroectodermal tumor.
| Author | Date of publication | Patient information (gender, age/yr.) | Spinal segment | Location | Extent of resection | Adjuvant therapy | Follow-up | Other |
|---|---|---|---|---|---|---|---|---|
| Wang G et al.1 | 2017 Dec | M, 26 | Lumbar | Intramedullary | GTR | Radiotherapy & Chemotherapy (TMZ) after the 2nd operation | Recurrence 9 months later, received 2nd surgery and adjuvant therapy. PFS for 5 more months. | — |
| Sharma P et al.2 | 2016 Apr-Jun | M, 11 | Cervical | Intramedullary | Near total removal | No details. | No details. | — |
| Harbhajanka A et al.3 | 2012 Jan | F, 18 | Thoracic-Lumbar | Intramedullary | Tumor decompression | Awaiting chemoradiotherapy. | Awaiting adjuvant therapy. | — |
| Alexiou GA et al.4 | 2013 Feb | F, 0 (2 months) | Cervical-Thoracic | Intramedullary | Radical resection (some small tumor remnants left) + VP-shunt | Chemotherapy (Head Start II protocol).*1 | No recurrence 9 months later. | Hydrocephalus since 33 weeks’ gestation. |
| Coumans JV et al.5 | 2012 Jan | M, 26 | Cervical-Thoracic | Intramedullary | GTR | Radiotherapy and chemotherapy (back-bone regimen).*2 | PFS after adjuvant therapy. | — |
| Mulholland CB et al.6 | 2011 Oct | M, 27 | Cervical | Intramedullary | 80% resected | Chemotherapy. | Died 3 months later. | Associated with NF1. |
| Fujisawa H et al.7 | 2011 Feb | M, 65 | Cervical-Thoracic | Intramedullary | Partial resection | Radiotherapy.*3 | Survive 6 months later. | 20 years after pineal tumor and hydrocephalus. |
| Tsutsumi S et al.8 | 2010 Apr | M, 39 | Thoracic-Lumbar | Intramedullary | Incomplete resection | Radioatherapy.*4 | Died 11 months later. | — |
| Otero-Rodríguez A et al.9 | 2009 Aug | M, 1 (17 months) | Thoracic | Intramedullary | Near total removal | Chemotherapy. | Recurrence 6 months later. | — |
| Kumar R et al.10* | 2007 | -, 8 | Thoracic | Intramedullary | Not available | Not available. | Not available. | — |
| -, 9 | Holocord | — | Not available | Not available. | Not available. | — | ||
| -, 18 | Cervical | Extramedullary | Not available | Not available. | Not available. | — | ||
| Jain A et al.11 | 2006 Jun | F, 54 | Cervical | Intramedullary | Partial excision | Radiotherapy. | Awaiting chemotherapy. | — |
| De Tommasi A et al.12 | 2006 Mar | M, 38 | Thoracic | Intramedullary | Biopsy | Chemotherapy.*5 | Died 18 months later. | — |
| Chen YC et al.13 | 2005 Aug | F, 19 | Cervical-Thoracic | Intramedullary | Biopsy | Chemotherapy & radiotherapy. *6 | Died 9 months later. | Concomitant pregnancy & IH. |
| Albrecht CF et al.14* | 2003 Jan | F, 29 | Thoracic | Intramedullary | No details (multifocal) | Radiotherapy and chemotherapy.*7 | Died 17 months later. | — |
| F, 49 | Lumbar | Extramedullary | GTR | Radiotherapy and chemotherapy.*7 | Died 23 months later. | — | ||
| Mawrin C et al.15 | 2002 Jan | M, 69 | Thoracic | Intramedullary | Partial removal | Radiotherapy. *8 | Died 12 weeks later. | — |
| Meltzer CC et al.16 | 1998 Jul | M, 30 | Cervical-Conus medullaris. | Intramedullary | No details | Radiotherapy and chemotherapy.*9 | Died 1 year later. | FDG PET investigation in a recurrent case. |
| Deme S et al.17* | 1997 Dec | F, 22 | Thoracic-Lumbar | Intramedullary | GTR | Radiotherapy and chemotherapy. | Recurrence 10 weeks later. | — |
| Kwon OK et al.18* | 1996 Oct | F, 0 (3 months) | Thoracic | Intramedullary | Biopsy | Chemotherapy. | Died 21 days later. | With intracranial seeding. |
| Brunberg JA et al.19 | 1991 Nov | Pediatric | — | Intramedullary | No details | No details. | No details. | MR and sonography studies in 11 pediatric intramedullary lesions, including one PNET case. |
| Ogasawara H et al.20* | 1992 Apr | - | — | Intramedullary | Not available | Radiotherapy and chemotherapy. | Not available | With intracranial seeding. |
| Freyer DR et al.21* | 1989 | Pediatric | — | Intramedullary | Not available | Not available. | Not available. | — |
| Eghbal K et al.22 | 2017 Mar 9 | F, 38 | Lumbar | Extramedullary | Total resection | No details. | Survive 6 months later. | — |
| Rege SV et al.23 | 2016 Oct-Dec | F, 8 | Thoracic | Extramedullary | Complete excision | Radiotherapy. *10 | Survive after adjuvant therapy. | — |
| Meng XT et al.24 | 2015 Apr | F, 60 | Lumbar | Extramedullary | Complete removal | Radiotherapy. *11 | Recurrence 4 months later, died 8 months later. | — |
| Chan SH et al.25 | 2015 Feb | M, 7 | Lumbar-Sacral | Extramedullary | Biopsy (twice) | Chemotherapy & radiotherapy. | Died 2.5 years later. | — |
| Venkataraman S et al.26 | 2013 Apr | F, 19 | Thoracic-Lumbar | Extramedullary | GTR | Chemotherapy. | Awaiting chemotherapy. | — |
| Hung PC et al.27 | 2007 Oct | F, 16 | Thoracic-Lumbar | Extramedullary | No details | Chemotherapy & local radiotherapy. | No details. | — |
| Nutman A et al.28* | 2007 Jan-Feb | F, 19 | Thoracic-Lumbar | Extramedullary | No details | Radiotherapy (entire cranio-spinal axis irradiation) & chemotherapy with autologous stem cell rescue. | PFS 24 months later. | — |
| Aydin MV et al.29 | 2004 Oct | M, 16 | Thoracic | Extramedullary | Totally excision | Radiotherapy and chemotherapy.*12 | PFS 7 months later. | — |
| Fabre E et al.30 | 2006 Jul | M, 70 | Cauda equina | Extramedullary | Partial resection | Radiotherapy and chemotherapy.*13 | Survive 1 year later. | — |
| Isotalo PA et al.31 | 2000 Aug | M, 52 | Cauda equina | Extramedullary | Debulking | Radiotherapy. *14 | PFS 1 year later. | — |
| Akai T et al.32 | 1998 Aug | M, 4 | Thoracic | Extramedullary | GTR | Radiotherapy and chemotherapy.*15 | PFS 6 years 4 months later. | — |
| Papadatos D et al.33 | 1998 Apr | F, 22 | Thoracic | Extramedullary | Debulking | Radiotherapy and chemotherapy.*16 | PFS 1 year later. | — |
yr., years old; -, details not available due to no full text available; GTR, gross total resection; TMZ, temozolomide; PFS, progression-free survival; NF1, neurofibromatosis type I; IH, intracranial hypertension.
*No full text available.
*1–*16 Refer to supplement table 1 for details of available adjuvant therapy.
Discussion
PNET is a heterogeneous group of tumors composed of undifferentiated or poorly differentiated neuroepithelial cells, WHO grade IV. According to differentiations of cell types the tumor is further classified into neuroblastomas with only neuronal differentiation, ganglioneuroblastomas if ganglion cells are also present, medulloepitheliomas with features of neural tube formation, and ependymoblastomas with ependymoblastic rosettes formation.
Precise incidence is not clearly known. Intraspinal case is very rare comparing to intracranial location. Around 30 cases have been reported so far. We reported eight cases. Only one of them was intramedullary. Then 33 literatures were identified with the keywords of spinal cord or intraspinal and primitive neuroectodermal tumor, with 21 reports of intramedullary PNET of the spinal cord with 24 cases in all and 12 cases of extramedullary PNET, either intradural or extradural. The earliest one was a pediatric intramedullary case reported by Freyer DR et al.21 dating back to 1989. Most were sporadic cases. Mulholland CB et al.6 reported a case with concomitant NF1 disease in 2011. Features common to all CNS PNET include early onset and aggressive clinical behavior. For all of our eight cases, the mean age at diagnosis was 17 years old. There was no age information of three intramedullary cases from the 33 literatures; however, 2 of them were pediatric cases. The mean age of the rest 33 cases in literatures were 26.9, with 26.6 for intramedullary cases and 27.6 for extramedullary cases respectively. The ratio of M:F was 2:6 of our cases and was 15:15 of the 30 cases in literatures with information available. Signs and symptoms are related to the site of origin of the tumor. Lumbar-sacral region was most common, including conus medullaris and cauda equina. However, the pan spinal level could be involved. In our series, five involved lumbar-sacral region, two involved cervical segment, and two invaded extremely long segments from thoracic to sacral level. In the 36 cases reviewed, four were cervical, four were cervical-thoracic, 10 were thoracic, six were thoracic-lumbar, and five were lumbar-sacral lesions. Two invaded the whole spinal cord segments. Three cases were lacking in location details. The whole CNS could be affected, especially for those intramedullary cases. Both Kwon OK et al.18 and Ogasawara H et al.20 reported a intramedullary case each with intracranial seeding. Alexiou GA et al.4 reported a two-month-old female of cervical-thoracic intramedullary PNET with concomitant hydrocephalus. Chen YC et al.13 also reported a intramedullary case with intracranial hypertension as an initial manifestation. The image examination results were similar to any other CNS malignancies originated from the neural stem cells, like glioma, lymphoma, etc. and pre-operative diagnosis rate was quite low due to its rare incidence. The differentiation diagnosis included other common spinal malignancies such as glioma, ependymoma, and lymphoma when located intramedullary and from those neoplasms such as Schwannoma and neurofibroma when located extramedullary. Meltzer CC et al.16 discussed the PET characteristic of a recurrent case. Brunberg JA et al.19 also mentioned one pediatric intramedullary PNET in a study of a MR and sonography results investigation of 11 intramedullary lesions, but the details of the case was not offered. Surgical resection with laminectomy is recommended as early. Sometimes the aggressive resection is not applicable due to its close relationship with spinal cord, especially for those intramedullary cases. Adjuvant radiotherapy and chemotherapy are also necessary. However, no standard guideline existed yet for radiochemotherapy of spinal PNET. We’re lacking in datas of detailed adjuvant therapy. In the literature review, some oncologists referred to glioma treatment strategies, and some referred to medulloblastoma. With lacking of standard and effective therapies the prognosis for all CNS PNET is bleaker especially for infants less than two years old at the time of diagnosis. The recurrence and mortality rate is high. Of our cases with available follow-up data, all patients died and three of them died around one year after operation. According to literatures, most patients die within two years given all available treatment methods for now. Only one exception was observed in Akai T et al.’s report32 about a four-year-old male who survived more than six years after total resection and adjuvant radiochemotherapy. After the surgery, the patient underwent focal irradiation (36 Gy) and combination chemotherapy with cyclophosphamide, pirarubicin, cisplatin, and etoposide postoperatively. Chemotherapy was performed 14 times during three years. There is still a long way to go for the understanding of the disease.
Conclusion
Spinal PNET is a quite rare group of tumors composed of heterogeneous undifferentiated or poorly differentiated neuroepithelial cells. It’s highly malignant. The pre-operative diagnosis based on image examinations was a challenge and should be differentiated from other CNS malignancies like glioma, lymphoma, and ependymoma when located intramedullary and from those common CNS neoplasms like Schwannoma and neurofibroma when located extramedullary. The medical history was important for clinical evaluation. The tumor tends to have an early onset and aggressive clinical behavior. Positive interventions should be considered as early for suspected cases. However, the prognosis is quite poor and there’s still a lot of unknown areas of spinal PNET. Multi-center cooperation may be a way to perform further investigations of this rare disease.
Disclaimer statements
Consent The study is approved by Institutional Review Board of Beijing Tiantan Hospital affiliated to Capital Medical University. Written informed consent was obtained from each patient for the publication of this study including case presentation and images. Copies of the written consents are available.
Conflict of interests The authors declare no conflict of interest concerning the materials or the methods used in this study or the findings specified in this paper.
Supplementary Material
Funding Statement
Jun Ma is supported by the Funding Project for Development Program for Beijing Superior Talent. Granting organization: Organization Department of Beijng Municipal Committee. Program No. 2015000021469G223. Wang Jia is supported by National Natural Science Funding. Granting organization: National Natural Science Foundation of China. Program No. 81471229.
References
- 1.Wang G, Guo F.. Primary intramedullary primitive neuroectodermal tumor: a case report and review of the literature. Medicine (Baltimore). 2017 Dec;96(49):e9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Das KK, Mehrotra A, Srivastava AK, Sahu RN, Jaiswal A, et al. Cervicomedullary intramedullary peripheral primitive neuroectodermal tumor with intratumoral bleed: report of one case and review of literature. J Craniovertebr Junction Spine. 2016 Apr-Jun;7(2):111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orguc S, Arkun R.. Primary tumors of the spine. Semin Musculoskelet Radiol. 2014 Jul;18(3):280–99. Epub 2014 Jun 4. [DOI] [PubMed] [Google Scholar]
- 4.Alexiou GA, Siozos G, Stefanaki K, Moschovi M, Prodromou N.. Intramedullary spinal cord primitive neuroectodermal tumor presenting with hydrocephalus. J Child Neurol. 2013 Feb;28(2):246–50. Epub 2012 Apr 24. [DOI] [PubMed] [Google Scholar]
- 5.Coumans JV, Walcott BP, Nahed BV, Oh KS, Chi AS.. Multimodal therapy of an intramedullary cervical primitive neuroectodermal tumor in an adult. J Clin Oncol. 2012 Jan 10;30(2):e15–8. Epub 2011 Dec 5. [DOI] [PubMed] [Google Scholar]
- 6.Mulholland CB, Barkhoudarian G, Cornford ME, McBride DQ.. Intraspinal primitive neuroectodermal tumor in a man with neurofibromatosis type 1: case report and review of the literature. Surg Neurol Int. 2011;2:155. Epub 2011 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa H, Kaneko T, Tohma Y, Kida S, Kaizaki Y.. Central nervous system primitive neuroectodermal tumor of spinal cord developing 20 years after curative treatment of pineal tumor. Neurol Med Chir (Tokyo). 2011;51(8):596–9. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsumi S, Nonaka Y, Abe Y, Yasumoto Y, Nakazato Y, Ito M.. Intramedullary primitive neuroectodermal tumor presenting with rapidly-progressive cauda equina syndrome. Neurol Med Chir (Tokyo). 2010;50(11):1031–5. [DOI] [PubMed] [Google Scholar]
- 9.Otero-Rodríguez A, Hinojosa J, Esparza J, Muñoz MJ, Iglesias S, Rodríguez-Gil Y, et al. Purely intramedullary spinal cord primitive neuroectodermal tumor: case report and review of the literature. Neurocirugia (Astur). 2009 Aug;20(4):381–6; discussion 386–7. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Reddy SJ, Wani AA, Pal L.. Primary spinal primitive neuroectodermal tumor: case series and review of the literature. Pediatr Neurosurg. 2007;43(1):1–6. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Jalali R, Nadkarni TD, Sharma S.. Primary intramedullary primitive neuroectodermal tumor of the cervical spinal cord. Case report. J Neurosurg Spine. 2006 Jun;4(6):497–502. [DOI] [PubMed] [Google Scholar]
- 12.De Tommasi A, De Tommasi C, Occhiogrosso G, Cimmino A, Parisi M, Sanguedolce F, et al. Primary intramedullary primitive neuroectodermal tumor (PNET)–case report and review of the literature. Eur J Neurol. 2006 Mar;13(3):240–3. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Tang LM, Chen CJ, Jung SM, Chen ST.. Intracranial hypertension as an initial manifestation of spinal neuroectodermal tumor. Clin Neurol Neurosurg. 2005 Aug;107(5):408–11. [DOI] [PubMed] [Google Scholar]
- 14.Albrecht CF, Weiss E, Schulz-Schaeffer WJ, Albrecht T, Fauser S, Wickboldt J, et al. Primary intraspinal primitive neuroectodermal tumor: report of two cases and review of the literature. J Neurooncol. 2003 Jan;61(2):113–20. [DOI] [PubMed] [Google Scholar]
- 15.Mawrin C, Synowitz HJ, Kirches E, Kutz E, Dietzmann K, Weis S.. Primary primitive neuroectodermal tumor of the spinal cord: case report and review of the literature. Clin Neurol Neurosurg. 2002 Jan;104(1):36–40. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer CC, Townsend DW, Kottapally S, Jadali F.. FDG imaging of spinal cord primitive neuroectodermal tumor. J Nucl Med. 1998 Jul;39(7):1207–9. [PubMed] [Google Scholar]
- 17.Deme S, Ang LC, Skaf G, Rowed DW.. Primary intramedullary primitive neuroectodermal tumor of the spinal cord: case report and review of the literature. Neurosurgery. 1997 Dec;41(6):1417–20. [DOI] [PubMed] [Google Scholar]
- 18.Kwon OK, Wang KC, Kim CJ, Kim IO, Chi JG, Cho BK.. Primary intramedullary spinal cord primitive neuroectodermal tumor with intracranial seeding in an infant. Childs Nerv Syst. 1996 Oct;12(10):633–6. [DOI] [PubMed] [Google Scholar]
- 19.Brunberg JA, DiPietro MA, Venes JL, Dauser RC, Muraszko KM, Berkey GS, et al. Intramedullary lesions of the pediatric spinal cord: correlation of findings from MR imaging, intraoperative sonography, surgery, and histologic study. Radiology. 1991 Nov;181(2):573–9. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara H, Kiya K, Kurisu K, Muttaqin Z, Uozumi T, Sugiyama K, et al. Intracranial metastasis from a spinal cord primitive neuroectodermal tumor: case report. Surg Neurol. 1992 Apr;37(4):307–12. [DOI] [PubMed] [Google Scholar]
- 21.Freyer DR, Hutchinson RJ, McKeever PE.. Primary primitive neuroectodermal tumor of the spinal cord associated with neural tube defect. Pediatr Neurosci. 1989;15(4):181–7. [DOI] [PubMed] [Google Scholar]
- 22.Eghbal K, Dehghanian AR, Ghaffarpasand F. Lumbosacral epidural primitive neuroectodermal tumor (PNET): case report and literature review. Turk Neurosurg. 2017 Mar 9. [Epub ahead of print] [DOI] [PubMed]
- 23.Rege SV, Tadghare J, Patil H, Narayan S.. Primary intraspinal extradural primitive neuroectodermal tumor: A rare case. J Pediatr Neurosci. 2016 Oct-Dec;11(4):351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng XT, He SS.. Primitive neuroectodermal tumor in the spinal canal: a case report. Oncol Lett. 2015 Apr;9(4):1934–6. Epub 2015 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan SH, Tsang DS, Wong VC, Chan GC.. Spinal primitive neuroectodermal tumor mimicking as chronic inflammatory demyelination polyneuropathy: a case report and review of literature. J Child Neurol. 2015 Feb;30(2):254–8. Epub 2014 Mar 20. [DOI] [PubMed] [Google Scholar]
- 26.Venkataraman S, Pandian C, Kumar SA.. Primary spinal primitive neuroectodermal tumour – a case report. Ann Neurosci. 2013 Apr;20(2):80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung PC, Fan KT, Lai HC, Shen CH, Luk HN.. Postoperative paraplegia as a result of undiagnosed primitive neuroectodermal tumor, not epidural analgesia. J Chin Med Assoc. 2007 Oct;70(10):456–9. [DOI] [PubMed] [Google Scholar]
- 28.Nutman A, Postovsky S, Zaidman I, Elhasid R, Vlodavsky E, Kreiss Y, et al. Primary intraspinal primitive neuroectodermal tumor treated with autologous stem cell transplantation: case report and review of the literature. Pediatr Hematol Oncol. 2007 Jan–Feb;24(1):53–61. [DOI] [PubMed] [Google Scholar]
- 29.Aydin MV, Sen O, Ozel S, Kayaselcuk F, Caner H, Altinors N.. Primary primitive neuroectodermal tumor within the spinal epidural space: report of a case and review of the literature. Neurol Res. 2004 Oct;26(7):774–7. [DOI] [PubMed] [Google Scholar]
- 30.Fabre E, Guillevin R, Chretien F, Le Guerinel C, Duffau H.. Peripheral primitive neuroectodermal tumor of the cauda equina in an elderly patient. Case report. J Neurosurg Spine. 2006 Jul;5(1):68–71. [DOI] [PubMed] [Google Scholar]
- 31.Isotalo PA, Agbi C, Davidson B, Girard A, Verma S, Robertson SJ.. Primary primitive neuroectodermal tumor of the cauda equina. Hum Pathol. 2000 Aug;31(8):999–1001. [DOI] [PubMed] [Google Scholar]
- 32.Akai T, Iizuka H, Kadoya S, Nojima T, Kohno M.. Primitive neuroectodermal tumor in the spinal epidural space–case report. Neurol Med Chir (Tokyo). 1998 Aug;38(8):508–11. [DOI] [PubMed] [Google Scholar]
- 33.Papadatos D, Albrecht S, Mohr G, del Carpio-O'Donovan R. Exophytic primitive neuroectodermal tumor of the spinal cord. AJNR Am J Neuroradiol. 1998 Apr;19(4):787–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Chi SN, Gardner SL, Levy AS, et al. . Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22:4881–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.