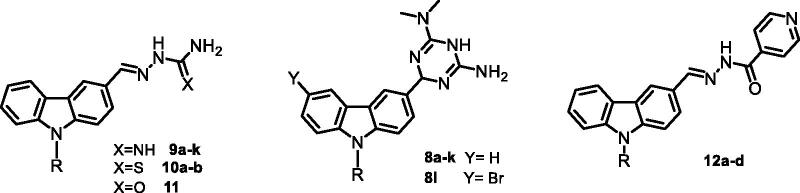
Abstract
Five series of novel carbazole derivatives containing an aminoguanidine, dihydrotriazine, thiosemicarbazide, semicarbazide or isonicotinic moiety were designed, synthesised and evaluated for their antimicrobial activities. Most of the compounds exhibited potent inhibitory activities towards different bacterial strains (including one multidrug-resistant clinical isolate) and one fungal strain with minimum inhibitory concentrations (MICs) between 0.5 and 16 µg/ml. Compounds 8f and 9d showed the most potent inhibitory activities (MICs of 0.5–2 µg/ml). Furthermore, compounds 8b, 8d, 8f, 8k, 9b and 9e with antimicrobial activities were not cytotoxic to human gastric cancer cell lines (SGC-7901 and AGS) or a normal human liver cell line (L-02). Structure–activity relationship analyses and docking studies implicated the dihydrotriazine group in increasing the antimicrobial potency and reducing the toxicity of the carbazole compounds. In vitro enzyme activity assays suggested that compound 8f binding to dihydrofolate reductase might account for the antimicrobial effect.
Keywords: Carbazole, antibacterial activities, antifungal activities, structure–activity relationship, cytotoxicity
1. Introduction
The steady increase of microbial pathogens that do not respond to conventional treatments presents a significant threat to global public health. Fungal and bacterial infections are becoming progressively more resistant towards currently available antimicrobial medicines, such as antibiotics. Therefore, there is an urgent requirement for new drugs that target these pathogens. Without effective antimicrobials for prevention and treatment of infections, medical procedures such as organ transplantation, chemotherapy, diabetes management and routine surgery (e.g. caesarean sections or hip replacements) have a significantly higher risk of morbidity and mortality1. The scale of the threat of antimicrobial resistance has led to the development of numerous strategies to conserve and make more effective use of existing antibiotics and to promote the development of novel antimicrobials2. In 2010, the Infectious Diseases Society of America (IDSA) launched the 10 × 20 initiative that called for the development of 10 novel, safe and effective systemic antibiotics by 20203.
Carbazole alkaloids have attracted considerable attention in medicinal chemistry because they exhibit a broad spectrum of biological and pharmacological activities, including antibacterial, antituberculous, antitumour, antioxidant and anti-inflammatory properties4–13. In our previous studies, guanidines exhibited significant antibacterial activity and triazine ring-containing compounds displayed hypoglycaemic, antitumour and antibacterial activities14,15. Dihydrotriazine compounds have a higher hydrophobicity compared with guanidine compounds and several molecules containing this scaffold have been reported as antibacterial agents16–18. Recent studies have identified dihydrotriazine-containing compounds as inhibitors of dihydrofolate reductase (DHFR), a ubiquitous enzyme that catalyses the conversion of 7,8-dihydrofolate to 5,6,7,8-tetrahydrofolate, which is involved in metabolic reactions such as purine and thymidine nucleotide biosynthesis19,20. DHFR is required by all organisms to grow and multiply; however, selective inhibitors of microbial enzymes have been utilised as therapeutic agents.
Here, we report the design and synthesis of five series of novel carbazole derivatives (Figure 1), totaling 30 compounds and the subsequent in vitro evaluations of their antibacterial and antifungal activities. Several different substituents were systematically introduced onto the carbazole ring and their effects on the overall antimicrobial activity were investigated.
Figure 1.
The structures of the target compounds.
2. Experimental
2.1. Chemistry
All reagents were obtained commercially and were used without further purification. Solvents were dried according to standard procedures. Reactions were monitored by thin-layer chromatography (TLC) on silica gel plates. Melting points were determined in open capillary tubes and were uncorrected. 1H NMR and 13C NMR spectra were measured on an AV-300 (Bruker, Switzerland), and all chemical shifts were given in ppm relative to TMS. Mass spectra were measured on a HP1100LC (Agilent Technologies). High-resolution mass spectra were measured on an MALDI-TOF/TOF mass spectrometer (Bruker Daltonik, Germany).
2.1.1. Procedure to synthesise compound 2a
To a solution of carbazole (1.03 g, 6.16 mmol) in DMC (10 ml), DABCO (0.069 g, 0.62 mmol) was added and the resulting solution was heated to 90–95 °C for 24 h to give compound 2a21.
2.1.2. Procedure to synthesise compound 2 b
A solution of potassium hydroxide (1.21 g, 21.56 mmol) and bromoethane (1.01 g, 9.24 mmol) in acetone (20 ml) was added to carbazole (1.03 g, 6.16 mmol) and the mixture was stirred at room temperature for 2 h to give compound 2b22.
2.1.3. General procedure to synthesise compounds 2c–k
To a solution of carbazole (1.67 g, 9.00 mmol) in DMF (20 ml) followed by addition of NaH (400 mg, 16.50 mmol) in small portions at 0 °C, over 5 min, benzyl halide (9.90 mmol) was added drop wise at 0 °C, over 5 min, the mixture was stirred at room temperature for 8–18 h to give compounds 2c–k23.
2.1.4. General procedure to synthesise compounds 4a–l
In a three-necked round-bottomed flask, POCl3 (700 mg, 4.56 mmol) was added dropwise to DMF (400 mg, 5.42 mmol) while stirring at 0 °C. The mixture was then stirred at room temperature for 1 h. A solution of 9-substituted carbazole (1.93 mmol) was slowly added to this mixture over 5 min at 45 °C, and then the temperature was raised to 95 °C for 8–18 h to yield compounds 4a–l23.
2.1.5. General procedure to synthesise compounds 8a–l
A mixture of the required compound 4 (2 mmol) and metformin hydrochloride (2 mmol) in glacial acetic acid (20 ml) was heated under reflux at 120 °C for 4–8 h (completion of the reaction was monitored by TLC). The solvent was then removed under reduced pressure. The crude products were purified by silica gel column chromatography using dichloromethane:methanol (20:1) as the eluent.
2.1.5.1. N2,N2-Dimethyl-6-(9-methyl-9H-carbazol-3-yl)-3,6-dihydro-1,3,5-triazine-2,4-diamine (8a)
White powder; yield 52.3%; m.p. 267.3–268.9 °C. IR (KBr) cm−1: 3300, 3134 (NH2, NH), 1606 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.88 (br s, 2H, NH2), 8.26–8.11 (m, 2H, Ar-H and NH), 7.76–7.46 (m, 5H, Ar-H), 7.25 (t, 1H, J = 7.6 Hz, Ar-H), 5.99 (s, 1H, CH), 3.90 (s, 3H, CH3), 3.08 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 157.59, 155.85, 141.05, 140.75, 131.06, 126.01, 123.96, 121.75, 121.63, 120.13, 119.01, 118.02, 109.39 (2 C), 62.77, 36.93 (2 C), 29.12. HRMS (MALDI) calcd for C18H20N6 [M + H]+: 321.1822, found: 321.1806.
2.1.5.2. 6-(9-Ethyl-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8 b)
White powder; yield 61.6%; m.p. 256.9–259.8 °C. IR (KBr) cm−1: 3356, 3132 (NH2, NH), 1598 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.80 (br s, 2H, NH2), 8.25–8.12 (m, 2H, Ar-H), 7.68 (dd, 2H, J = 15.0, 8.4 Hz, Ar-H), 7.51 (ddd, 2H, J = 16.2, 8.3, 1.5 Hz, Ar-H), 7.24 (t, 1H, J = 7.4 Hz, Ar-H), 5.98 (s, 1H, CH), 5.76 (s, 1H, NH), 4.48 (q, 2H, J = 7.1 Hz, CH2), 3.08 (s, 6H, CH3), 1.31 (t, 3H, J = 7.0 Hz, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 157.66, 155.83, 139.95, 139.66, 131.06, 126.02, 123.98, 121.95, 121.82, 120.27, 118.98, 118.21, 109.34 (2 C), 62.76, 37.06, 36.94 (2 C), 13.67. HRMS (MALDI) calcd for C19H22N6 [M + H]+: 335.1979, found: 335.1967.
2.1.5.3. 6-(9-Benzyl-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8c)
White powder; yield 46.1%; m.p. 237.1–238.8 °C. IR (KBr) cm−1: 3440, 3240 (NH2, NH), 1606 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.83 (br s, 2H, NH2), 8.27–8.16 (m, 2H, Ar-H), 7.70 (dd, 2H, J = 11.5, 8.4 Hz, Ar-H), 7.55–7.44 (m, 2H, Ar-H), 7.25 (dtd, 4H, J = 8.1, 6.5, 4.4 Hz, Ar-H), 7.16 (dd, 2H, J = 8.0, 1.7 Hz, Ar-H), 5.97 (s, 1H, CH), 5.76 (s, 1H, NH), 5.70 (s, 2H, CH2), 3.07 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 157.62, 155.85, 140.60, 140.29, 137.65, 131.37, 128.59 (2 C), 127.28 (2 C), 126.71, 126.20, 124.18, 122.01, 121.92, 120.30, 119.35, 118.29, 109.84 (2 C), 62.76, 45.67, 36.96, 33.9. HRMS (MALDI) calcd for C24H24N6 [M + H]+: 397.2135, found: 397.2134.
2.5.1.4. N2,N2-Dimethyl-6-(9-(4-methylbenzyl)-9H-carbazol-3-yl)-3,6-dihydro-1,3,5-triazine-2,4-diamine (8d)
White powder; yield 58.8%; m.p. 237.2–239.8 °C. IR (KBr) cm−1: 3350, 3037 (NH2, NH), 1609 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.88 (s, 1H, NH2), 8.82 (s, 1H, NH2), 8.32–8.14 (m, 2H, Ar-H), 7.69 (dd, 2H, J = 12.8, 8.2 Hz, Ar-H), 7.49 (dd, 2H, J = 19.7, 8.4 Hz, Ar-H), 7.25 (t, 1H, J = 7.4 Hz, Ar-H), 7.07 (d, 4H, J = 4.7 Hz, Ar-H), 5.97 (s, 1H, CH), 5.77 (s, 1H, NH), 5.65 (s, 2H, CH2), 3.07 (s, 6H, CH3), 2.22 (s, 3H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 158.06, 156.35, 141.06, 140.77, 136.92, 135.05, 131.69, 129.58 (2 C), 127.20 (2 C), 126.63, 124.63, 122.47, 122.40, 120.75, 119.77, 118.79, 110.33 (2 C), 63.34, 55.44, 45.95, 37.41, 21.09. HRMS (MALDI) calcd for C25H26N6 [M + H]+: 411.2292, found: 411.2299.
2.5.1.5. 6-(9-(2-Fluorobenzyl)-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8e)
White powder; yield 40.1%; m.p. 201.2–205.0 °C. IR (KBr) cm−1: 3423, 3144 (NH2, NH), 1603 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.90 (s, 1H, NH2), 8.84 (s, 1H, NH2), 8.30–8.16 (m, 2H, Ar-H), 7.68 (dd, 2H, J = 13.2, 8.3 Hz, Ar-H), 7.56–7.43 (m, 2H, Ar-H), 7.33–7.22 (m, 3H, Ar-H), 7.03 (td, 1H, J = 7.4, 1.6 Hz, Ar-H), 6.88–6.78 (m, 1H, Ar-H), 5.97 (s, 1H, CH), 5.77 (s, 3H, NH and CH2), 3.07 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 161.69, 158.44, 157.61, 155.96, 140.57, 140.28, 131.43, 129.50 (d, 1 C, J = 8.1 Hz), 128.71 (d, 1 C, J = 4.2 Hz), 126.23, 124.57 (d, 1 C, J = 3.4 Hz), 124.22, 124.04, 122.08 (d, 1 C, J = 2.3 Hz), 120.28, 119.50, 118.34, 115.65, 115.37, 109.75, 62.92, 62.01, 56.01, 36.95. HRMS (MALDI) calcd for C24H23FN6 [M + H]+: 415.2041, found: 415.2037.
2.5.1.6. 6-(9-(2,4-Dichlorobenzyl)-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8f)
White powder; yield 40.1%; m.p. 255.2–257.1 °C. IR (KBr) cm−1: 3240, 3060 (NH2, NH), 1603 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.93–8.72 (br s, 2H, NH2), 8.35–8.18 (m, 2H, Ar-H), 7.75 (d, 1H, J = 2.1 Hz, Ar-H), 7.68–7.17 (m, 7H, Ar-H), 6.33 (d, 1H, J = 8.4 Hz, CH), 5.97 (s, 1H, NH), 5.76 (s, 2H, CH2), 3.08 (s, 6H, CH3). 13C NMR (75 MHz, DMSO-d6, ppm) δ 157.55, 155.97, 140.52, 140.25, 133.76, 132.77, 132.69, 131.74, 129.10, 128.50, 127.69, 126.43, 124.41, 122.17, 120.43 (2 C), 119.76, 118.48, 109.63 (2 C), 62.92, 45.32, 43.47, 36.95. HRMS (MALDI) calcd for C24H22Cl2N6 [M + H]+: 465.1356, found: 465.1359. Purity: 97.41% by HPLC (A: 0.1% FA in H2O; B: 0.1% FA in CH3CN, graded: 20–100%), tR 12.273 min, λ: 250 nm.
2.1.5.7. 6-(9-(2-Chlorobenzyl)-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8 g)
White powder; yield 37.6%; m.p. 240.7–243.2 °C. IR (KBr) cm−1: 3396, 3211 (NH2, NH), 1603 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.86 (s, 1H, NH2), 8.81 (s, 1H, NH2), 8.38–8.20 (m, 2H, Ar-H), 7.70–7.39 (m, 6H, Ar-H), 7.37–7.23 (m, 2H, Ar-H), 7.11 (dd, 1H, J = 8.8, 6.3 Hz, Ar-H), 6.36 (d, 1H, J = 8.0 Hz, CH), 5.98 (s, 1H, NH), 5.84–5.75 (m, 2H, CH2), 3.09 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 157.99, 156.37, 141.07, 140.80, 134.95, 132.24, 132.07, 130.10, 129.52, 127.97, 127.59, 126.86, 124.86, 122.57, 122.54, 120.90, 120.13, 118.95, 110.13 (2 C), 63.36, 55.43, 44.22, 37.42. HRMS (MALDI) calcd for C24H23ClN6 [M + H]+: 431.1745, found: 431.1733.
2.1.5.8. 6-(9-(3-Chlorobenzyl)-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8 h)
White powder; yield 54.9%; m.p. 207.0–209.2 °C. IR (KBr) cm−1: 3313, 3029 (NH2, NH), 1600 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 8.87 (s, 1H, NH2), 8.81 (s, 1H, NH2), 8.34–8.16 (m, 2H, Ar-H), 7.71 (dd, 2H, J = 13.6, 8.3 Hz, Ar-H), 7.59–7.45 (m, 2H, Ar-H), 7.39–7.20 (m, 4H, Ar-H), 7.06 (dd, 1H, J = 6.3, 2.9 Hz, Ar-H), 5.97 (s, 1H, CH), 5.77 (s, 1H, NH), 5.74 (s, 2H, CH2), 3.12 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 158.06, 156.36, 140.96, 140.74, 140.69, 133.67, 131.94, 131.03, 127.78, 127.03, 126.80, 125.77, 124.82, 122.52, 122.46, 120.85, 120.02, 118.91, 110.23 (2 C), 63.34, 55.44, 45.55, 37.42. HRMS (MALDI) calcd for C24H23ClN6 [M + H]+: 431.1745, found: 431.1749.
2.1.5.9. 6-(9-(4-Chlorobenzyl)-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8i)
White powder; yield 36.7%; m.p. 262.4–264.1 °C. IR (KBr) cm−1: 3373, 3059 (NH2, NH), 1603 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 9.00 (s, 1H, NH2), 8.94 (s, 1H, NH2), 8.36–8.14 (m, 2H, Ar-H), 7.69 (dd, 2H, J = 14.6, 8.2 Hz, Ar-H), 7.60–7.43 (m, 3H, Ar-H), 7.35 (dd, 2H, J = 8.6, 2.1 Hz, Ar-H), 7.29–7.13 (m, 3H, Ar-H and NH), 5.98 (s, 1H, CH), 5.72 (s, 2H, CH2), 3.07 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 158.02, 156.35, 140.95, 140.67, 137.15, 132.36, 131.92, 129.05 (4 C), 126.75, 124.73, 122.52, 122.46, 120.83, 119.96, 118.85, 110.26 (2 C), 63.32, 49.04, 45.47, 37.41. HRMS (MALDI) calcd for C24H23ClN6 [M + H]+: 431.1745, found: 431.1761.
2.1.5.10. 6-(9-(4-Bromobenzyl)-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8j)
White powder; yield 35.1%; m.p. 248.7–252.1 °C. IR (KBr) cm−1: 3311, 3054 (NH2, NH), 1603 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 9.06 (s, 1H, NH2), 8.99 (s, 1H, NH2), 8.34–8.13 (m, 2H, Ar-H), 7.68 (dd, 2H, J = 15.2, 8.3 Hz, Ar-H), 7.63–7.41 (m, 5H, Ar-H), 7.25 (t, 1H, J = 7.5 Hz, Ar-H), 7.17–7.06 (m, 2H, Ar-H and NH), 5.99 (s, 1H, CH), 5.69 (s, 2H, CH2), 3.07 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 157.53, 155.87, 140.47, 140.20, 137.10, 131.49(4 C), 128.91 (2 C), 126.28, 124.27, 122.04, 121.98, 120.37, 119.49, 118.39, 109.79 (2 C), 62.86, 45.04, 36.94 (2 C). HRMS (MALDI) calcd for C24H23BrN6 [M + H]+: 475.1240, found: 475.1230.
2.1.5.11. 4-((3-(4-Amino-6-(dimethylamino)-2,5-dihydro-1,3,5-triazin-2-yl)-9H-carbazol-9-yl)methyl)benzonitrile (8k)
White powder; yield 52.9%; m.p. 234.1–235.4 °C. IR (KBr) cm−1: 3306, 3061 (NH2, NH), 1605 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 9.06 (s, 1H, NH2), 9.01 (s, 1H, NH2), 8.35–8.15 (m, 2H, Ar-H), 7.78–7.62 (m, 4H, Ar-H), 7.56 (d, 1H, J = 8.4 Hz, Ar-H), 7.51–7.43 (m, 1H, Ar-H), 7.36–7.23 (m, 3H, Ar-H), 6.01 (s, 1H, CH), 5.83 (s, 2H, CH2), 5.77 (s, 1H, NH), 3.09 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 158.06, 156.33, 143.92, 140.95, 140.66, 133.04 (2 C), 132.15, 127.98 (2 C), 126.82, 124.80, 122.58, 122.54, 120.87, 120.10, 119.11, 118.88, 110.59, 110.18 (2 C), 63.28, 55.41, 49.03, 37.42. HRMS (MALDI) calcd for C25H23N7 [M + H]+: 422.2088, found: 422.2077.
2.1.5.12. 6-(6-Bromo-9-phenyl-9H-carbazol-3-yl)-N2,N2-dimethyl-3,6-dihydro-1,3,5-triazine-2,4-diamine (8 l)
White powder; yield 41.4%; m.p. 221.5–224.8 °C. IR (KBr) cm−1: 3383, 3070 (NH2, NH), 1600 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 9.19 (s, 2H, NH2), 8.44 (d, 2H, J = 19.4 Hz, Ar-H), 7.64 (dd, 4H, J = 15.7, 8.1 Hz, Ar-H), 7.55 (d, 3H, J = 7.6 Hz, Ar-H), 7.39 (d, 1H, J = 8.4 Hz, Ar-H), 7.29 (t, 1H, J = 6.9 Hz, Ar-H), 6.05 (s, 1H, CH), 5.76 (s, 1H, NH), 3.08 (s, 6H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 158.01, 156.25, 141.08, 139.79, 136.62, 133.51, 130.71 (2 C), 129.41, 128.52, 127.02 (2 C), 125.85, 124.88, 123.47, 121.88, 119.35, 112.82, 112.18, 110.60, 63.04, 55.41, 49.03. HRMS (MALDI) calcd for C23H21BrN6 [M + H]+: 461.1084, found: 461.1066. Purity: 98.69% by HPLC (A: 0.1% FA in H2O; B: 0.1% FA in CH3CN, graded: 20–100%), tR 11.660 min, λ: 250 nm.
2.1.6. General procedure to synthesise compounds 9a–k
A mixture of the required compound 4 (2.3 mmol) and aminoguanidine hydrochloride (2 mmol) in ethanol (20 ml) in the presence of 5 drops of concentrated hydrochloric acid was stirred at 50–60 °C for 8–12 h. The solvent was then removed under reduced pressure. The crude products were purified by silica gel column chromatography using dichloromethane:methanol (10:1) as the eluent.
2.1.6.1. (E)-2-((9-Methyl-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9a)
White powder; yield 52.3%; m.p. 199.1-200.8 °C. IR (KBr) cm−1: 3330, 3147 (NH2, NH), 1629 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.89 (br s, 1H, NH), 8.63 (s, 1H, CH = N), 8.33 (s, 1H, Ar-H), 8.18 (d, 1H, J = 8.0 Hz, Ar-H), 8.05 (d, 1H, J = 8.4 Hz, Ar-H), 7.91–7.46 (m, 6H, Ar-H and NH), 7.29 (d, 1H, J = 7.5 Hz, Ar-H), 3.94 (s, 3H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.41, 147.77, 141.81, 141.07, 126.20, 125.14, 124.36, 122.08, 121.96, 120.59, 120.36, 119.41, 109.53, 109.40, 29.16. HRMS (MALDI) calcd for C15H15N5 [M + H]+: 266.1400, found: 266.1405.
2.1.6.2. (E)-2-((9-Ethyl-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9b)
White powder; yield 59.2%; m.p. 199.4–200.5 °C. IR (KBr) cm−1: 3354, 3120 (NH2, NH), 1628 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.85 (br s, 1H, NH), 8.63 (s, 1H, CH = N), 8.33 (d, 1H, J = 1.9 Hz, Ar-H), 8.18 (d, 1H, J = 7.7 Hz, Ar-H), 8.03 (d, 1H, J = 8.6 Hz, Ar-H), 7.77–7.46 (m, 6H, Ar-H and NH), 7.27 (t, 1H, J = 7.3 Hz, Ar-H), 4.49 (q, 2H, J = 6.9 Hz, CH2), 1.33 (t, 3H, J = 7.1 Hz, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.42, 147.76, 140.83, 140.04, 131.54, 128.68, 126.26, 125.23, 124.44, 122.31, 120.72, 120.53, 119.42, 109.56, 109.40, 37.18, 18.68. HRMS (MALDI) calcd for C16H17N5 [M + H]+: 280.1557, found: 280.1548.
2.1.6.3. (E)-2-((9-Benzyl-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9c)
White powder; yield 63.1%; m.p. 271.0–272.4 °C. IR (KBr) cm−1: 3319, 3154 (NH2, NH), 1600 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.91 (br s, 1H, NH), 8.67 (s, 1H, CH = N), 8.34 (s, 1H, Ar-H), 8.21 (d, 1H, J = 7.7 Hz, Ar-H), 8.01 (d, 1H, J = 8.5 Hz, Ar-H), 7.84–7.65 (m, 5H, Ar-H and NH), 7.47 (t, 1H, J = 7.7 Hz, Ar-H), 7.24 (dt, 6H, J = 18.8, 7.5 Hz, Ar-H), 5.71 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.82, 148.14, 141.94, 141.09, 137.95, 132.14, 131.97, 129.05, 127.80, 127.21, 126.85, 125.85, 125.26, 122.87, 122.73, 121.10, 120.99, 120.21, 110.50, 110.33, 46.20. HRMS (MALDI) calcd for C21H19N5 [M + H]+: 342.1713, found: 342.1701.
2.1.6.4. (E)-2-((9-(4-Methylbenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9d)
White powder; yield 45.0%; m.p. 295.5–298.0 °C. IR (KBr) cm−1: 3382, 3134 (NH2, NH), 1629 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.82 (br s, 1H, NH), 8.59 (s, 1H, CH = N), 8.25 (s, 1H, Ar-H), 8.13 (d, 1H, J = 7.7 Hz, Ar-H), 7.94 (dd, 1H, J = 8.7, 1.6 Hz, Ar-H), 7.75–7.58 (m, 4H, Ar-H and NH), 7.40 (t, 2H, J = 7.7 Hz, Ar-H), 7.20 (t, 1H, J = 7.4 Hz, Ar-H), 7.01 (s, 4H, Ar-H), 5.59 (s, 2H, CH2), 2.14 (s, 3H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.46, 147.63, 141.47, 140.63, 136.53, 134.46, 129.14 (2 C), 126.81 (2 C), 126.37, 125.39, 124.78, 122.43, 122.30, 120.65, 120.54, 119.71, 110.09, 109.91, 45.56, 20.63. HRMS (MALDI) calcd for C22H21N5 [M + H]+: 356.1869, found: 356.1854. Purity: 96.74% by HPLC (A: 0.1% FA in H2O; B: 0.1% FA in CH3CN, graded: 20–100%), tR 11.533 min, λ: 250 nm.
2.1.6.5. (E)-2-((9-(2-Fluorobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9e)
White powder; yield 39.3%; m.p. 248.3–249.9 °C. IR (KBr) cm−1: 3356, 3163 (NH2, NH), 1637 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.88 (s, 1H, NH2), 8.67 (s, 1H, CH = N), 8.32 (s, 1H, Ar-H), 8.21 (d, 1H, J = 7.8 Hz, Ar-H), 8.02 (dd, 1H, J = 8.7, 1.6 Hz, Ar-H), 7.89–7.41 (m, 6H, Ar-H and NH), 7.37–7.19 (m, 3H, Ar-H), 7.04 (td, 1H, J = 7.4, 1.4 Hz, Ar-H), 6.89 (td, 1H, J = 7.7, 1.7 Hz, Ar-H), 5.77 (d, 2H, J = 2.2 Hz, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 161.68, 158.43, 155.40, 147.52, 141.38, 140.51, 128.75 (d, 1 C, J = 4.1 Hz), 126.40, 125.36, 124.93, 124.57 (d, 1 C, J = 3.3 Hz), 124.12, 123.92, 122.43, 122.30, 120.58 (d, 1 C, J = 8.3 Hz), 119.85, 115.67, 115.39, 109.89 (d, 1 C, J = 8.6 Hz), 65.01. HRMS (MALDI) calcd for C21H18FN5 [M + H]+: 360.1619, found: 360.1611.
2.1.6.6. (E)-2-((9-(2,4-Dichlorobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9f)
White powder; yield 58.2%; m.p. 240.7–242.8 °C. IR (KBr) cm−1: 3397, 3146 (NH2, NH), 1629 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 12.12 (br s, 1H, NH), 8.71 (s, 1H, CH = N), 8.35 (s, 1H, Ar-H), 8.24 (d, 1H, J = 7.7 Hz, Ar-H), 8.11–7.66 (m, 5H, Ar-H and NH), 7.59 (d, 1H, J = 8.6 Hz, Ar-H), 7.54–7.41 (m, 2H, Ar-H), 7.29 (t, 1H, J = 7.3 Hz, Ar-H), 7.19 (dd, 1H, J = 8.3, 2.3 Hz, Ar-H), 6.41 (d, 1H, J = 8.4 Hz, Ar-H), 5.75 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.52, 147.63, 141.45, 140.55, 133.66, 132.83, 132.76, 129.13, 128.66, 127.72, 126.61, 125.56, 125.24, 122.63, 122.46, 120.74, 120.68, 120.12, 109.83, 109.73, 43.59. HRMS (MALDI) calcd for C21H17Cl2N5 [M + H]+: 410.0934, found: 410.0932.
2.1.6.7. (E)-2-((9-(2-Chlorobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9 g)
White powder; yield 45.4%; m.p. 249.7–250.4 °C. IR (KBr) cm−1: 3386, 3159 (NH2, NH), 1629 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.88 (br s, 1H, NH), 8.70 (s, 1H, CH = N), 8.33 (s, 1H, Ar-H), 8.24 (d, 1H, J = 7.6 Hz, Ar-H), 8.00 (dd, J = 8.6, 1H, 1.7 Hz, Ar-H), 7.96–7.43 (m, 7H, Ar-H and NH), 7.37–7.24 (m, 2H, Ar-H), 7.18–7.06 (m, 1H, Ar-H), 6.51–6.40 (m, 1H, Ar-H), 5.77 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.88, 147.98, 141.96, 141.05, 134.82, 132.26, 130.10, 129.55, 127.95, 127.71, 126.99, 125.94, 125.55, 122.97, 122.83, 121.20, 121.10, 120.45, 110.29, 110.18, 44.30. HRMS (MALDI) calcd for C21H18ClN5 [M + H]+: 376.1326, found: 376.1322.
2.1.6.8. (E)-2-((9-(3-Chlorobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9 h)
White powder; yield 41.5%; m.p. 256.1–257.5 °C. IR (KBr) cm−1: 3389, 3137 (NH2, NH), 1634 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.93 (s, 1H, NH), 8.68 (s, 1H, CH = N), 8.33 (s, 1H, Ar-H), 8.22 (d, 1H, J = 7.7 Hz, Ar-H), 8.09–7.58 (m, 6H, Ar-H and NH), 7.49 (t, 1H, J = 7.7 Hz, Ar-H), 7.29 (t, 4H, J = 6.0 Hz, Ar-H), 7.08 (dd, 1H, J = 6.6, 3.1 Hz, Ar-H), 5.75 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.89, 148.00, 141.82, 140.97, 140.57, 133.66, 131.02, 127.81, 127.07, 126.96, 125.94, 125.83, 125.46, 122.91, 122.77, 121.19, 121.07, 120.38, 110.43, 110.28, 45.59. HRMS (MALDI) calcd for C21H18ClN5 [M + H]+: 376.1326, found: 376.1319.
2.1.6.9. (E)-2-((9-(4-Chlorobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9i)
White powder; yield 38.7%; m.p. 239.5–240.8 °C. IR (KBr) cm−1: 3266, 3057 (NH2, NH), 1629 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 12.07 (br s, 1H, NH), 8.68 (s, 1H, CH = N), 8.35 (d, 1H, J = 5.4 Hz, Ar-H), 8.21 (d, 1H, J = 7.7 Hz, Ar-H), 8.01 (d, 1H, J = 8.3 Hz, Ar-H), 7.68 (m, 5H, Ar-H and NH), 7.47 (t, 1H, J = 7.7 Hz, Ar-H), 7.38–7.24 (m, 3H, Ar-H), 7.18 (d, 2H, J = 8.7 Hz, Ar-H), 5.72 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.44, 147.58, 141.38, 140.53, 136.56, 131.96, 128.66 (2 C), 128.62 (2 C), 126.48, 125.47, 124.96, 122.49, 122.35, 120.72, 120.62, 119.88, 110.02, 109.86, 45.08. HRMS (MALDI) calcd for C21H18ClN5 [M + H]+: 376.1326, found: 376.1329.
2.1.6.10. (E)-2-((9-(4-Bromobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9j)
White powder; yield 41.1%; m.p. 239.5–240.7 °C. IR (KBr) cm−1: 3377, 3161 (NH2, NH), 1629 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.86 (s, 1H, NH), 8.67 (s, 1H, CH = N), 8.32 (s, 1H, Ar-H), 8.21 (d, 1H, J = 7.7 Hz, Ar-H), 8.09–7.98 (m, 1H, Ar-H), 7.97–7.56 (m, 5H, Ar-H and NH), 7.48 (dt, 3H, J = 7.0, 3.5 Hz, Ar-H), 7.29 (t, 1H, J = 7.5 Hz, Ar-H), 7.13 (d, 2H, J = 8.5 Hz, Ar-H), 5.72 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.43, 147.62, 141.39, 140.54, 136.98, 131.54 (2 C), 129.00 (2 C), 126.49, 125.48, 124.96, 122.49, 122.35, 120.71, 120.61, 120.46, 119.89, 110.02, 109.85, 45.16. HRMS (MALDI) calcd for C21H18BrN5 [M + H]+: 420.0818, found: 420.0829.
2.1.6.11. (E)-2-((9-(4-Cyanobenzyl)-9H-carbazol-3-yl)methylene)hydrazine-1-carboximidamide (9k)
White powder; yield 45.7%; m.p. 240.6–243.9 °C. IR (KBr) cm−1: 3300, 3123 (NH2, NH), 1631 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.92 (br s, 1H, NH), 8.68 (s, 1H, CH = N), 8.33 (s, 1H, Ar-H), 8.22 (d, 1H, J = 7.7 Hz, Ar-H), 8.17–7.55 (m, 8H, Ar-H and NH), 7.48 (t, 1H, J = 7.7 Hz, Ar-H), 7.30 (dd, 3H, J = 7.9, 2.2 Hz, Ar-H), 5.85 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 155.31, 147.68, 143.30, 141.37, 140.50, 132.61 (2 C), 127.56 (2 C), 126.60, 125.57, 125.09, 122.54, 122.35, 120.71 (2 C), 120.05, 118.65, 110.15, 109.91, 109.79, 45.43. HRMS (MALDI) calcd for C22H18N6 [M + H]+: 367.1666, found: 367.1656.
2.1.7. General procedure to synthesise compounds 10a–b and 11
A mixture of the required compound 4 (2 mmol) and either thiosemicarbazide hydrochloride or semicarbazide hydrochloride(2 mmol) in ethanol (20 ml) was stirred at 50–60 °C for 8–12 h in the presence of five drops of concentrated hydrochloric acid. The solution was evaporated to dryness under reduced pressure, and the residue was purified by silica gel column chromatography using dichloromethane:methanol (80:1) as the eluent.
2.1.7.1. (E)-2-((9-Methyl-9H-carbazol-3-yl)methylene)hydrazine-1-carbothioamide (10a)
White solid; yield 60.5%; m.p. 222.4–223.8 °C. IR (KBr) cm−1: 3238, 3026 (NH2, NH), 1630 (C = N), 1120 (C = S). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.42 (s, 1H, NH), 8.59 (s, 1H, CH = N), 8.21 (d, 3H, J = 7.8 Hz, Ar-H), 8.08–7.89 (m, 2H, NH2), 7.62 (d, 2H, J = 8.6 Hz, Ar-H), 7.50 (t, 1H, J = 7.6 Hz, Ar-H), 7.25 (t, 1H, J = 7.4 Hz, Ar-H), 3.90 (s, 3H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 177.45, 143.65, 141.54, 141.02, 126.11, 125.11, 125.02, 122.19, 122.00, 120.47, 120.19, 119.30, 109.41, 109.36, 29.09. HRMS (MALDI) calcd for C15H14N4S [M + H]+: 283.1012, found: 283.1020.
2.1.7.2. (E)-2-((9-Ethyl-9H-carbazol-3-yl)methylene)hydrazine-1-carbothioamide (10 b)
White solid; yield 64.6%; m.p. 199.8–202.0 °C. IR (KBr) cm−1: 3280, 3043 (NH2, NH), 1594 (C = N), 1200 (C = S). 1H NMR (300 MHz, DMSO-d6, ppm) δ 11.40 (s, 1H, NH), 8.58 (s, 1H, CH = N), 8.27–8.13 (m, 3H, Ar-H), 8.04–7.91 (m, 2H, Ar-H), 7.64 (s, 1H, NH2), 7.61 (s, 1H, NH2), 7.53–7.44 (m, 1H, Ar-H), 7.24 (t, 1H, J = 7.4 Hz, Ar-H), 4.45 (q, 2H, J = 6.9 Hz, CH2), 1.32 (t, 3H, J = 6.8 Hz, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 177.45, 143.56, 140.52, 139.96, 126.12, 125.13, 125.08, 122.39, 122.21, 120.61, 120.24, 119.25, 109.39, 109.29, 37.10, 18.64. HRMS (MALDI) calcd for C16H16N4S [M + H]+: 297.1168, found: 297.1161.
2.1.7.3. (E)-2-((9-Ethyl-9H-carbazol-3-yl)methylene)hydrazine-1-carboxamide (11)
White solid; yield 42.3%; m.p. 211.1–212.0 °C. IR (KBr) cm−1: 3343, 3049 (NH2, NH), 1691 (C = O), 1595 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 10.17 (s, 1H, NH), 8.51 (s, 1H, CH = N), 8.20 (d, 1H, J = 7.7 Hz, Ar-H), 8.01 (s, 1H, Ar-H), 7.86 (d, 1H, J = 8.5 Hz, Ar-H), 7.61 (dd, 2H, J = 8.4, 3.7 Hz, Ar-H), 7.47 (t, 1H, J = 7.7 Hz, Ar-H), 7.23 (t, 1H, J = 7.4 Hz, Ar-H), 6.52 (br s, 2H, NH2), 4.46 (q, 2H, J = 7.0 Hz, CH2), 1.32 (t, 3H, J = 6.9 Hz, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 157.03, 140.48, 140.05, 139.93, 126.01, 125.89, 124.46, 122.33, 122.22, 120.58, 119.22, 119.08, 109.31, 109.19, 37.06, 13.72. HRMS (MALDI) calcd for C16H16N4O [M + H]+: 281.1397, found: 281.1392.
2.1.8. General procedure for the synthesis of compounds 12a–12d
The intermediate 4 (2 mmol) reacted with isonicotinic acid hydrazide (2 mmol) in the presence of catalytic amounts of hydrochloric acid (5 drops) in ethanol (20 ml) at 70 °C for 5 h. The solution was evaporated to dryness under reduced pressure, and the residue was purified by silica gel column chromatography with (dichloromethane: methanol = 60:1).
2.1.8.1. (E)-N'-((9-Benzyl-9H-carbazol-3-yl)methylene)isonicotinohydrazide (12a)
Yellow solid; yield 80.0%; m.p. 198.0–199.5 °C. IR (KBr) cm−1: 3250, 3046 (NH), 1646 (C = O), 1601 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 12.02 (s, 1H, NH), 8.90–8.73 (m, 2H, Ar-H), 8.65 (s, 1H, CH = N), 8.55 (s, 1H, Ar-H), 8.31 (dd, 1H, J = 9.6, 6.5 Hz, Ar-H), 7.97–7.63 (m, 5H, Ar-H and pyridine-H), 7.48 (dd, 1H, J = 10.7, 4.7 Hz, Ar-H), 7.38–7.06 (m, 6H, Ar-H), 5.73 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 161.44, 150.29 (2 C), 141.50, 140.77, 140.70, 137.43, 128.59 (2 C), 127.32 (2 C), 126.70 (2 C), 126.40, 125.41, 125.04, 122.60, 122.25, 121.54 (2 C), 120.69, 120.29, 119.74, 110.03, 109.92, 45.81. HRMS (MALDI) calcd for C26H20N4O [M + H]+: 405.1710, found: 405.1719.
2.1.8.2. (E)-N'-((9-(4-Methylbenzyl)-9H-carbazol-3-yl)methylene)isonicotinohydrazide (12 b)
Yellow solid; yield 74.7%; m.p. 196.0–197.8 °C. IR (KBr) cm−1: 3320, 3060 (NH), 1647 (C = O), 1595 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 12.02 (s, 1H, NH), 8.80 (d, 2H, J = 5.0 Hz, Ar-H), 8.63 (s, 1H, CH = N), 8.54 (s, 1H, pyridine–H), 8.29 (d, 1H, J = 7.4 Hz, pyridine–H), 7.95–7.81 (m, 3H, Ar-H), 7.71 (dd, 2H, J = 23.9, 8.3 Hz, pyridine–H), 7.48 (t, 1H, J = 7.6 Hz, Ar-H), 7.27 (t, 1H, J = 7.4 Hz, Ar-H), 7.09 (s, 4H, Ar-H), 5.66 (s, 2H, CH2), 2.22 (s, 3H, CH3). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 161.40, 150.29 (2 C), 150.21, 141.43, 140.72, 140.63, 136.52, 134.40, 129.13 (2 C), 126.73 (2 C), 126.37, 125.28, 124.95, 122.52, 122.19, 121.57, 120.69, 120.33, 119.68, 110.08, 109.97, 45.53, 20.58. HRMS (MALDI) calcd for C27H22N4O [M + H]+: 419.1866, found: 419.1854.
2.1.8.3. (E)-N'-((9-(2-Fluorobenzyl)-9H-carbazol-3-yl)methylene)isonicotinohydrazide (12c)
Yellow solid; yield 73.8%; m.p. 220.3–221.5 °C. IR (KBr) cm−1: 3366, 3050 (NH), 1651 (C = O), 1589 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 12.07 (s, 1H, NH), 8.82 (s, 2H, Ar-H), 8.68 (s, 1H, CH = N), 8.57 (s, 1H, pyridine–H), 8.26 (s, 1H, pyridine–H), 8.00–7.83 (m, 3H, Ar-H), 7.66 (dd, 2H, J = 26.4, 8.4 Hz, pyridine–H), 7.46 (t, 1H, J = 7.6 Hz, Ar-H), 7.35–7.16 (m, 3H, Ar-H), 7.00 (t, 1H, J = 7.2 Hz, Ar-H), 6.89 (t, 1H, J = 7.7 Hz, Ar-H), 5.74 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 161.68, 161.43, 150.29 (2 C), 150.14, 141.38, 140.70, 140.55, 129.53 (d, 1 C, J = 8.1 Hz), 128.66 (d, 1 C, J = 4.3 Hz), 126.44, 125.48, 125.05, 124.58 (d, 1 C, J = 3.4 Hz), 122.59, 122.24, 121.56 (2 C), 120.71, 120.28, 119.86, 115.67, 115.39, 110.03, 109.86, 40.23. HRMS (MALDI) calcd for C26H19FN4O [M + H]+: 423.1616, found: 423.1612.
2.1.8.4. (E)-N'-((9-(2,4-Dichlorobenzyl)-9H-carbazol-3-yl)methylene)isonicotinohydrazide (12d)
Yellow solid; yield 64.2%; m.p. 209.1–210.3 °C. IR (KBr) cm−1: 3400, 3137 (NH), 1657 (C = O), 1597 (C = N). 1H NMR (300 MHz, DMSO-d6, ppm) δ 12.04 (s, 1H, NH), 8.88–8.73 (m, 2H, Ar-H), 8.64 (s, 1H, CH = N), 8.58 (d, 1H, J = 1.6 Hz, pyridine–H), 8.33 (d, 1H, J = 7.7 Hz, pyridine–H), 7.96–7.69 (m, 4H, Ar-H and pyridine–H), 7.68–7.17 (m, 5H, Ar-H), 6.42 (d, 1H, J = 8.4 Hz, Ar-H), 5.76 (s, 2H, CH2). 13 C NMR (75 MHz, DMSO-d6, ppm) δ 161.38, 150.28 (2 C), 150.01, 141.34, 140.67, 140.50, 133.60, 132.73, 132.67, 129.09, 128.41, 127.68, 126.60, 125.74, 125.18, 122.67, 122.31, 121.54 (2 C), 120.84, 120.34, 120.07, 109.92, 109.73, 54.91. HRMS (MALDI) calcd for C26H18Cl2N4O [M + H]+: 473.0930, found: 473.0941.
2.2. Evaluation of antibacterial activity in vitro
The in vitro antimicrobial and antifungal activities of the synthesised compounds were evaluated to obtain the minimum inhibitory concentrations (MICs), using a 96-well microtiter plate and a serial dilution method. Gatifloxacin and moxifloxacin were used as positive controls and DMSO as a negative control. The micro-organisms used in the present study were two Gram-positive strains (Staphylococcus aureus 4220, Streptococcus mutans 3289), one clinical isolate of multidrug-resistant Gram-positive bacterial strain (Methicillin-resistant Staphylococcus aureus CCARM 3167), one Gram-negative strain (Escherichia coli 1924) and one fungus (Candida albicans 7535). The bacteria were grown to mid-log phase in Mueller-Hinton broth and diluted 1000-fold in the same medium. Stock solutions of the test compounds in dimethyl sulfoxide were prepared and then poured into 96-well plates. The final concentration of 0.5–64 µg/mL underwent a twofold serial dilution14,24. The bacteria were suspended and contained approximately 105 CFU/mL. These were applied to 96-well plates with a serial dilution and incubated at 37 °C for 24 h. The bacteria growth was measured from the turbidity at 630 nm using a microplate reader. All experiments were carried out in triplicate. Their MBC and MFC values were also determined (only for the compounds with MIC values of < 256 μg/mL).
2.3. MTT assay
Cell viability has been determined using the 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium Bromide (MTT, Sigma–Aldrich, Milan, Italy) assay. Cells have been seeded on 48 well plates and grown in complete medium. Before being treated, cells have been starved in serum-free medium for 24 h for allowing cell cycle synchronisation. 72 h after treatments, fresh MTT, re-suspended in PBS, was added to each well (final concentration (0.5 mg/mL). After 2 h incubation at 37 °C, cells have been lysed with DMSO, and then optical density was measured at 570 nm using a microplate reader. At least six doses of the studied compounds, solubilised in DMSO (0.1% final concentration), have been evaluated and each experiment has been performed in triplicate. The absorbance values have been used to determine the IC50 for each cell line using GraphPad Prism 5 Software (GraphPad Inc., San Diego, CA).
2.4. Docking study
Preliminary docking was performed to evaluate whether binding to DHFR might account for the bactericidal effect of the compounds. All docking studies were carried out using Discovery Studio 2017 (Accelrys, San Diego, CA). Docking studies were performed according to our previous report25. The crystal structure data were obtained from the protein data bank (E. coli DHFR PDB_ID: 1RX7). Enzyme structures were checked for missing atoms, bonds and contacts. Hydrogen atoms were added to the enzyme structure. Water molecules and bound ligands were manually deleted. Then the protein was refined with CHARMm. The structures of compounds were sketched in 2D and converted into 3D using the DS molecule editor. Automated docking studies were carried out to investigate the binding mode of compounds 8b and 8f utilizing DS-CDOCKER protocol. The pose with the top CDOCKER_INTERACTION_ENERGY was chosen for analysing the binding features of compounds 8b and 8f with DHFR.
2.5. Inhibition of DHFR activities in vitro
The inhibition of DHFR activities of compound 8f was measured using ELISA kits (Mlbio, Shanghai, China) under different concentrations (0, 0.1, 0.3, 1, 3, 10 µmol/L), according to the manufacturer’s instructions.
3. Results and discussion
3.1. Chemistry
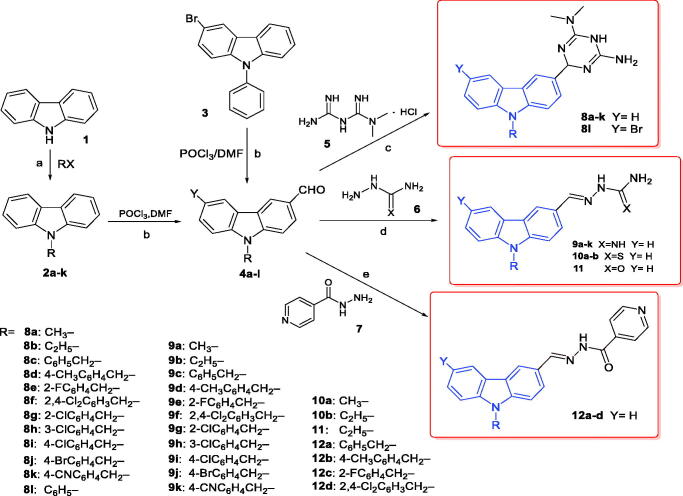
The synthetic routes used for the construction of target compounds 8a–l, 9a–k, 10a–b, 11 and 12a–d are outlined in Scheme 1. A series of intermediates (2) was obtained from carbazole (1) via N-alkylation or N-arylation. Next, we performed a formylation reaction (Vilsmayer-Hack) on the intermediates (2) and commercially available compound 3 in the presence of POCl3 and DMF to obtain 9-substituted carbazole-3-carbaldehyde analogues 4 in good yields. Compounds 8a–l were prepared by the reaction of each of the series 4 compounds with metformin hydrochloride in acetic acid. Compounds 9a–k were synthesised via the reactions of each of the series 4 compounds with aminoguanidine hydrochloride in ethanol with a catalytic amount of concentrated hydrochloric acid. Compounds 10a–b and 11 were prepared by reacting compounds 4 with thiosemicarbazide and semicarbazide, respectively, in the presence of acetic acid in methanol under reflux. Next, we reacted compounds 4 with isonicotinic in the presence of acetic acid in alcohol to obtain compounds 12a–d.
Scheme 1.
Synthetic scheme for the synthesis of the target compounds. Reagents and conditions: (a) DMC, DABCO, 95 °C, 24 h (2a); KOH, 25 °C, 2 h (2b); NaH, DMF, 25 °C, 16 h (2c–k) (b) POCl3, DMF, 90 °C, 8–18 h (c) AcOH, 120 °C, 4–8 h (d) CH2CH3OH, HCl, 40 °C, 6 h (9a–k); CH3OH, AcOH, 68 °C, 4 h (10a–b,11) (e) CH2CH3OH, AcOH, 70 °C, 5 h.
3.2. Evaluation of in vitro antimicrobial activities
The antimicrobial activities of the synthesised compounds were then tested against a series of pathogenic bacterial and fungal strains. Several different bacterial strains and one fungal strain were susceptible to most of the compounds tested with MICs in the range of 1–64 µg/ml. N-aryl carbazole derivatives containing an aminoguanidine or 1,4-dihydro-1,3,5-triazine moiety (8c–l and 9c–k) exhibited potent antibacterial activities with MICs ranging from 0.5 to 16 µg/ml. These compounds also exhibited antifungal activity against Candida albicans 7535 with MICs ranging from 1 to 32 µg/ml.
Compounds 8f, 8l, 9d and 9e exhibited strong antibacterial activity against two Gram-positive strains (methicillin-resistant Staphylococcus aureus (MRSA CCARM 3167) and Staphylococcus aureus RN4220) and one Gram-negative strain (Escherichia coli 1924) with MIC values of 0.5 or 1 µg/ml. Furthermore, the antibacterial activities of compound 8f against Gram-positive bacterial strains (S. aureus RN4220 and Streptococcus mutans 3289) were comparable to the positive control drugs gatifloxacin and moxifloxacin. In addition, compound 8f displayed considerable potency against MRSA CCARM 3167 (MIC of 0.5 μg/ml) that was two- and four-fold greater compared with moxifloxacin (MIC of 1 μg/ml) and gatifloxacin (MIC of 2 μg/ml) respectively. In addition, compound 8f demonstrated a strong inhibitory activity (MIC of 0.5 μg/ml) against E. coli 1924, which was four-fold greater than the activities of moxifloxacin (MIC of 2 μg/ml) and gatifloxacin (MIC of 2 μg/ml), respectively. In contrast, compound 8f showed a weaker activity against the fungus C. albicans 7535 compared with the positive controls.
Moreover, we determined the minimum bactericidal concentrations (MBCs) and minimum fungicidal concentrations (MFCs) for compounds whose MIC value less than 256 µg/ml against strains. MBCs and MFCs are defined as the lowest bactericidal or fungicidal concentration required to kill a particular bacterial strain or the fungal strain over a fixed incubation time. Most of the series 8 compounds displayed MBCs at 1–2 fold higher than their MICs against the strains tested. Compounds 9i and 9k also had four-fold higher MIC values compared with their MBCs against S. aureus RN4220 and MRSA CCARM 3167, respectively. The MBC/MIC ratio of compounds 9 g and 9j were ≥4 for S. mutans 3289. Compounds 9c, 9e, 9h and 9i demonstrated MFC values that were four-fold higher than their MICs against Candida albicans 7535. Notably, bacteria appeared to be less tolerant to the series 8 compounds compared with series 9 compounds. When we altered the phenyl ring substituents of compounds 8a–l, we observed a significant effect on the potency of their antimicrobial activities. The order of antibacterial activities were as follows: phenyl group > 2,4-dichloro-substitutions > 4-CH3(for compounds bearing an electron-donating group) > halogen substitutions > benzyl group > 4-CN (for compounds bearing an electron-withdrawing group) > alkyl group. Furthermore, bromo- and chloro-substitutions on the phenyl ring in compounds 8a–l were observed to improve their antifungal activity against C. albicans 7535. The antibacterial activity order of compounds 9a–k was similar to compounds 8a–l. Compounds in series 10a–b, 11 and 12a–d generally showed weak activity against all the strains tested in this study, with MICs greater than 256 µg/ml. As shown in Table 1, most of the compounds exhibited equivalent or higher potency (with MIC values in the range of 0.5 to 16 µg/ml) towards MRSA CCARM 3167 compared with the positive controls gatifloxacin and moxifloxacin (MICs of 2 and 1 µg/ml, respectively).
Table 1.
Inhibitory activities (MIC/MBC or MFC, μg/mL) of compounds 8a–l, 9a–k, 10a–b, 11 and 12a–d against various bacteria and fungi.
| Compd | R |
Gram-positive strains |
Gram-negative strain | Fungus | ||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | MRSA CCARM | S. mutans | E. coli | C. albicans | ||||
| Y | R | X | 4220a | 3167b | 3289c | 1924d | 7535e | |
| 8a | H | CH3 | — | 16/16 | 16/16 | 64/64 | 8/8 | 128/128 |
| 8b | H | C2H5 | — | 64/64 | 8/8 | 32/128 | 8/16 | 64/128 |
| 8c | H | benzyl | — | 4/4 | 0.5/1 | 2/4 | 0.5/1 | 32/32 |
| 8d | H | 4-CH3C6H4CH2- | — | 1/1 | 0.5/0.5 | 2/2 | 0.5/0.5 | 4/8 |
| 8e | H | 2-FC6H4CH2- | — | 4/4 | 0.5/0.5 | 0.5/4 | 0.5/1 | 32/32 |
| 8f | H | 2,4-2ClC6H3CH2- | — | 0.5/0.5 | 0.5/0.5 | 0.5/1 | 0.5/0.5 | 2/2 |
| 8g | H | 2-ClC6H4CH2- | — | 2/2 | 0.5/1 | 2/4 | 1/1 | 1/1 |
| 8h | H | 3-ClC6H4CH2- | — | 4/4 | 0.5/1 | 2/8 | 1/1 | 2/2 |
| 8i | H | 4-ClC6H4CH2- | — | 2/2 | 0.5/0.5 | 2/2 | 1/1 | 1/1 |
| 8j | H | 4-BrC6H4CH2- | — | 2/2 | 0.5/1 | 2/2 | 1/1 | 2/2 |
| 8k | H | 4-CNC6H4CH2- | — | 16/16 | 16/32 | 16/16 | 8/8 | 8/8 |
| 8l | Br | C6H5- | — | 1/1 | 0.5/0.5 | 1/2 | 0.5/1 | 1/1 |
| 9a | — | CH3 | NH | 2/4 | 2/2 | 4/4 | 2/2 | 4/8 |
| 9b | — | C2H5 | NH | 4/4 | 8/8 | 8/16 | 4/4 | 4/8 |
| 9c | — | benzyl | NH | 2/2 | 1/2 | 1/2 | 1/2 | 1/4 |
| 9d | — | 4-CH3C6H4CH2- | NH | 1/2 | 0.5/0.5 | 0.5/1 | 0.5/1 | 1/2 |
| 9e | — | 2-FC6H4CH2- | NH | 2/4 | 0.5/0.5 | 1/1 | 1/1 | 1/4 |
| 9f | — | 2,4-2ClC6H3CH2- | NH | 2/4 | 1/2 | 2/2 | 2/2 | 16/16 |
| 9g | — | 2-ClC6H4CH2- | NH | 1/2 | 1/1 | 1/4 | 1/1 | 2/4 |
| 9h | — | 3-ClC6H4CH2- | NH | 1/2 | 0.5/0.5 | 1/2 | 2/2 | 2/8 |
| 9i | — | 4-ClC6H4CH2- | NH | 1/4 | 0.5/0.5 | 1/1 | 2/2 | 2/8 |
| 9j | — | 4-BrC6H4CH2- | NH | 2/2 | 0.5/1 | 2/16 | 2/2 | 4/8 |
| 9k | — | 4-CNC6H4CH2- | NH | 2/4 | 2/8 | 2/4 | 2/2 | 2/4 |
| 10a | — | CH3 | S | 256/256 | >256 | >256 | >256 | >256 |
| 10b | — | C2H5 | S | >256 | >256 | >256 | >256 | >256 |
| 11 | — | C2H5 | O | >256 | >256 | >256 | >256 | >256 |
| 12a | — | benzyl | — | >256 | >256 | >256 | >256 | >256 |
| 12b | — | 4-CH3C6H4CH2- | — | >256 | >256 | >256 | >256 | >256 |
| 12c | — | 2-FC6H4CH2- | — | >256 | >256 | >256 | >256 | >256 |
| 12d | — | 2,4-2ClC6H3CH2- | — | >256 | >256 | >256 | >256 | >256 |
| Gatifloxacin | 0.25/0.25 | 2/2 | 0.25/n.t.f | 2/2 | 0.5/0.5 | |||
| Moxifloxacin | 0.25/0.25 | 1/2 | 0.25/n.t. | 2/2 | 0.5/0.5 | |||
CCARM: Culture Collection Antimicrobial Resistant Microbes; KCTC: Korean Collection for Type Cultures.
aStaphylococcus aureus RN 4220.
bMethicillin-resistant S. aureus CCARM 3167.
cStreptococcus mutans 3289. dEscherichia coli KCTC 1924. eCandida albicans 7535.
fn.t.: Not test.
To summarise, our findings revealed that the compounds in series 8 exhibited significantly higher antimicrobial activities compared with the compounds in the other four series. This suggests that the presence of a dihydrotriazine moiety is critical to the potency of these carbazole derivatives. These results therefore provide further evidence to suggest that aryl groups and the dihydrotriazine moiety play critical roles in the activity of these carbazole compounds.
3.3. Cell viability
To determine whether the synthesised compounds were selectively toxic towards microbes and not human cells, we evaluated the cytotoxicity of six compounds in two gastric cancer cell lines (SGC-7901 and AGS) and a normal human liver cell line (L-02) using a standard technique. As shown in Table 2, the cytotoxicities of compounds 8b, 8d, 8f and 8k were lower than that of compounds 9b and 9e. Compounds 8b, 8d, 8f and 8k had no effect on human liver cell viability at their MICs and, in addition, their IC50 values were significantly higher than their MIC values. However, the IC50 values of the series 9 compounds were close to their MIC values, which make it likely that the promising antibacterial activity of these compounds is due to their cytotoxic properties.
Table 2.
Cytotoxic activity (IC50a, µg/mL) of compounds 8b, 8d, 8f, 8k, 9b and 9e against human cell lines.
| Compd |
In vitro cytotoxicity IC50a (μg/mL) |
||
|---|---|---|---|
| SGC-7901b | AGSb | L-02c | |
| 8b | >33.4 | >33.4 | >33.4 |
| 8d | 7.1 | 9.9 | 10.1 |
| 8f | 10.0 | 7.8 | 9.4 |
| 8k | >42.1 | >42.1 | >42.1 |
| 9b | 1.8 | 1.2 | 3.8 |
| 9e | 1.5 | 0.4 | 1.1 |
aIC50 is the concentration required to inhibit the cell growth by 50%. Data represent the average of three independent experiments running in triplicate. Variation was generally between 5 and 10%.
bHuman gastric cancer cells.
cHuman normal hepatic cells.
Compounds in series 8a–l, containing a dihydro-1,3,5-triazine ring moiety, were generally found to have more potent antimicrobial effects but less cytotoxicity compared with the corresponding series 9a–l compounds that comprised an aminoguanidine moiety. Interestingly, our results also showed that compound 9 b was selectively more toxic to human cancer cells compared with normal human cells. The IC50 value is about three times higher in normal cells than AGS cell lines. Further studies are required to investigate if this compound has any potential as a new anticancer agent.
3.4. Docking analysis
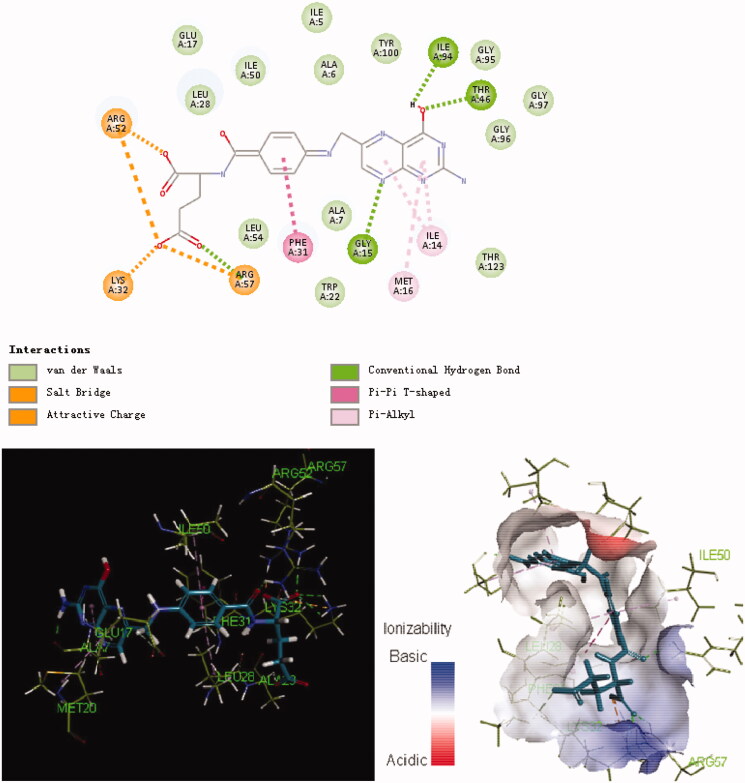
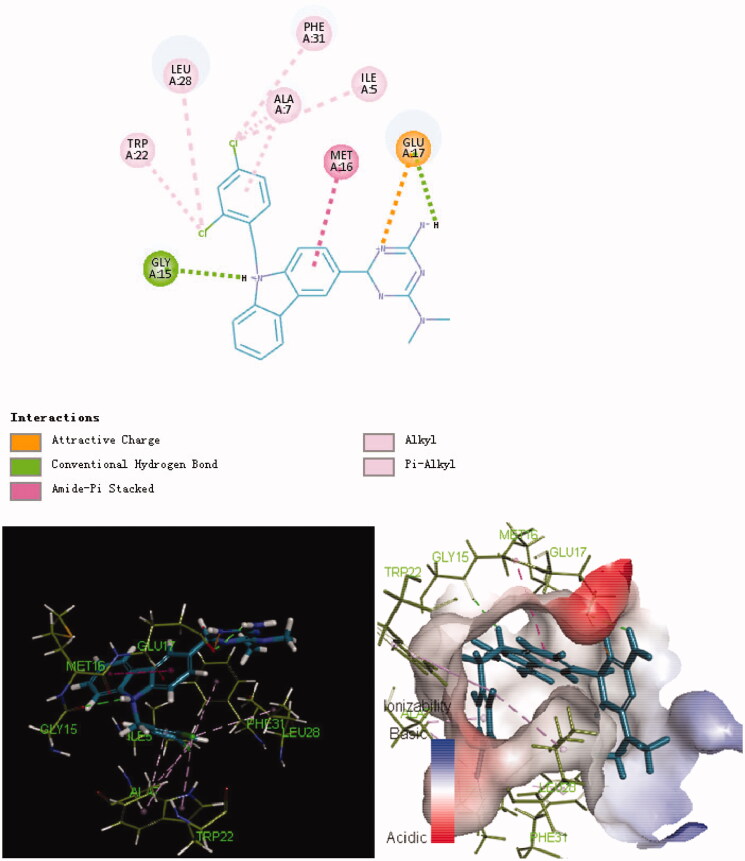
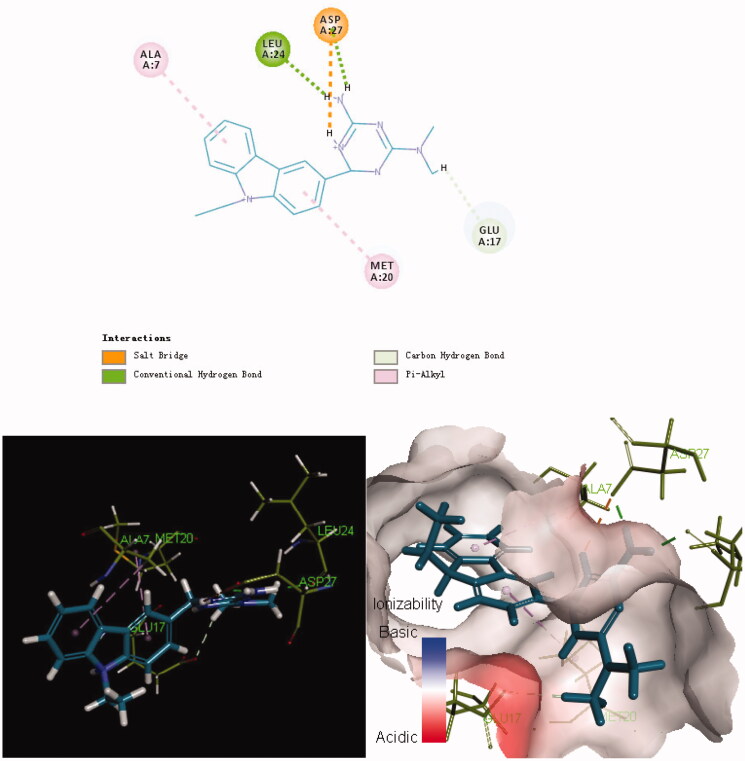
The potent and selective antimicrobial activities of the series 8 compounds prompted us to study the binding of these derivatives to their potential target, E. coli DHFR. In 3D binding mode, alkyl chains (ethyl and methylene) at the 9-position of carbazole possess considerable flexibility (Figures 2–4). As shown in Figure 3, the aryl group at the 9-position of carbazole in compound 8f inserted deeply into the active pocket of DHFR (composed of Ala7, Trp22, Leu28 and Phe31). Moreover, both the folic acid substrate and compound 8f have a nitrogen atom in their heterocyclic rings that forms a hydrogen bond with the E. coli DHFR residue Gly15. The hydrophobic interactions between compound 8f and residues in the DHFR active pocket were enhanced due to the presence of the carbazole moiety. In addition, the primary amine group at the N-3 position of the carbazole ring plays an important role in binding to the active site of E. coli DHFR. A salt bridge was formed between the primary amine and Glu17. To summarise, docking results suggested that 8f, the compound with the most therapeutic potential, has interacted with the critical active-site residues of E. coli DHFR.
Figure 2.
Binding mode of folate inside the E. coli DHFR active site.
Figure 3.
Binding mode of 8f inside the E. coli DHFR active site.
Figure 4.
Binding mode of 8b inside the E. coli DHFR active site.
3.5. Inhibition studies of compound 8f with DHFR
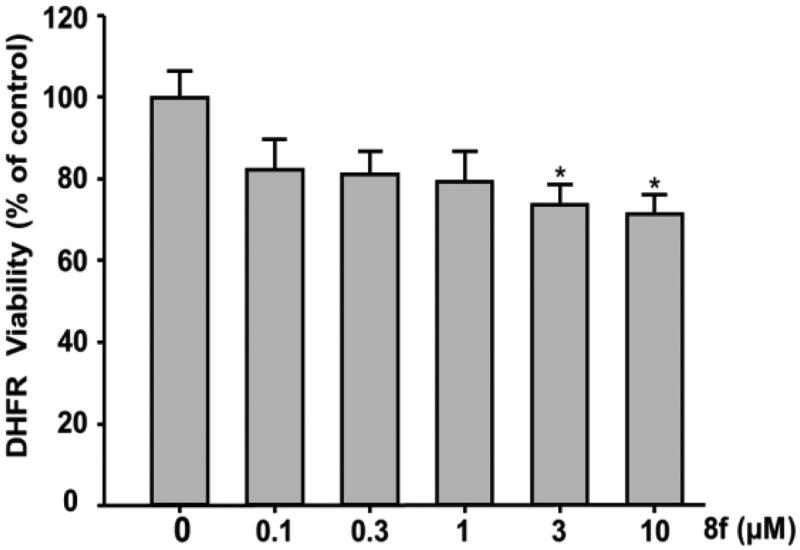
To investigate whether compound 8f can bind to and block the active site of DHFR, we performed in vitro enzyme assays to test the inhibitory effect of compound 8f (MIC of 0.5 μg/ml) on DHFR activity (Figure 5). At concentrations of 3 and 10 μmol/L, compound 8f decreased DHFR activity by 71% compared with the negative control. The results indicated that compound 8f exerts its antibacterial activity via binding to DHFR, which however might not be the only mechanism of action.
Figure 5.
Inhibition of DHFR of compound 8f.
4. Conclusions
We have designed, synthesised and evaluated the antibacterial and antifungal activities of five series of novel carbazole derivatives. Compound 8f showed the most potential as a therapeutic agent, with an MIC of 0.5–2 µg/ml against selected bacterial strains. Furthermore, compound 8f also was the least cytotoxic to SGC-7901, AGS and L-02 cells. Compound 9d also exhibited strong antibacterial activity with the cancer therapeutic potential. Therefore, the clinical potential of carbazole derivatives 8f and 9d could be explored further for future applications in antitumour and antimicrobial therapies.
Docking simulation and in vitro enzyme activity assays suggested that binding to DHFR might account for the antimicrobial activity of the compounds. Further studies of the mechanisms of action of these compounds are currently underway in our laboratories and will be reported in due course.
Funding Statement
This work was supported by the National Natural Science Foundation of China [Grant numbers 81260468 and 81360473].
Disclosure statement
We declare that we have no conflict of interest with respect to this study.
References
- 1.WHO. Antimicrobial resistance. 2018. Available from: http://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance.
- 2.Paphitou NI. Antimicrobial resistance: action to combat the rising microbial challenges. Int J Antimicrob Agents 2013;42:S25–S8. [DOI] [PubMed] [Google Scholar]
- 3.Infectious Diseases Society of America. The 10×’20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis 2010;50:1081–3. [DOI] [PubMed] [Google Scholar]
- 4.Salih N, Salimon J, Yousif E.. Synthesis and antimicrobial activities of 9H-carbazole derivatives. Arab J Chem 2016;9:S781–S6. [Google Scholar]
- 5.Gu W, Hao Y, Zhang G, et al. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg Med Chem Lett 2015;25:554–7. [DOI] [PubMed] [Google Scholar]
- 6.Indumathi T, Ahamed VSJ, Moon SS, et al. l-Proline anchored multicomponent synthesis of novel pyrido[2,3-a]carbazoles; investigation of in vitro antimicrobial, antioxidant, cytotoxicity and structure activity relationship studies. Eur J Med Chem 2011;46:5580–90. [DOI] [PubMed] [Google Scholar]
- 7.Guillonneau C, Nault A, Raimbaud E, et al. Cytotoxic and antitumoral properties in a series of new, ring D modified, olivacine analogues. Bioorg Med Chem 2005;13:175–84. [DOI] [PubMed] [Google Scholar]
- 8.Al-Trawneh SA, Zahra JA, Kamal MR, et al. Synthesis and biological evaluation of tetracyclic fluoroquinolones as antibacterial and anticancer agents. Bioorg Med Chem 2010;18:5873–84. [DOI] [PubMed] [Google Scholar]
- 9.Zhang FF, Gan LL, Zhou CH.. Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorg Med Chem Lett 2010;20:1881–4. [DOI] [PubMed] [Google Scholar]
- 10.Ruan B, Tian Y, Zhou H, et al. Synthesis, crystal structure and in vitro antibacterial activity of two novel silver(I) complexes. J Organomet Chem 2009;694:2883–7. [Google Scholar]
- 11.Saturnino C, Palladino C, Napoli M, et al. Synthesis and biological evaluation of new N-alkylcarbazole derivatives as STAT3 inhibitors: preliminary study. Eur J Med Chem 2013;60:112–9. [DOI] [PubMed] [Google Scholar]
- 12.Bandgar BP, Adsul LK, Chavan HV, et al. Synthesis, biological evaluation, and docking studies of 3-(substituted)-aryl-5-(9-methyl-3-carbazole)-1H-2-pyrazolines as potent anti-inflammatory and antioxidant agents. Bioorg Med Chem Lett 2012;22:5839–44. [DOI] [PubMed] [Google Scholar]
- 13.Hieda Y, Anraku M, Choshi T, et al. Antioxidant effects of the highly-substituted carbazole alkaloids and their related carbazoles. Bioorg Med Chem Lett 2014;24:3530–3. [DOI] [PubMed] [Google Scholar]
- 14.Wei ZY, Chi KQ, Yu ZK, et al. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg Med Chem Lett 2016;26:5920–5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang TY, Li C, Li YR, et al. Synthesis and antimicrobial evaluation of aminoguanidine and 3-amino-1,2,4-triazole derivatives as potential antibacterial agents. Lett Drug Des Discov 2016;13:1063–75. [Google Scholar]
- 16.Lebreton S, Newcombe N, Bradley M.. Antibacterial single-bead screening. Tetrahedron 2003;59:10213–22. [Google Scholar]
- 17.Lubbers T, Angehrn P, Gmunder H, et al. Design, synthesis, and structure-activity relationship studies of ATP analogues as DNA gyrase inhibitors. Bioorg Med Chem Lett 2000;10:821–6. [DOI] [PubMed] [Google Scholar]
- 18.Tsitsa P, Antoniadou-Vyza E, Hamodrakas SJ, et al. Synthesis, crystal structure and biological properties of a new series of lipophilic s-triazines, dihydrofo-late reduetase inhibitors. Eur J Med Chem 1993;28:149–58. [Google Scholar]
- 19.Kumar AA, Mangum JH, Blankenship DT, et al. Affinity labeling of chicken liver dihydrofolate reductase by a substituted 4,6-diaminodihydrotriazine bearing a terminal sulfonyl fluoride. J Biol Chem 1981;256:8970–6. [PubMed] [Google Scholar]
- 20.Srinivasan B, Tonddast-Navaei S, Skolnick J.. Ligand binding studies, preliminary structure-activity relationship and detailed mechanistic characterization of 1-phenyl-6,6-dimethyl-1,3,5-triazine-2,4-diamine derivatives as inhibitors of Escherichia coli dihydrofolate reductase. Eur J Med Chem 2015;103:600–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dell S, Lozanov ME, Shieh WC. N-alkyation of indole derivatives. US20040059131, USA; 2004.
- 22.Li Z, Li J, Qin JG.. A simple approach to the synthesis of N-ethylcarbazole. Huaxue Shiji 2001;23:297. [Google Scholar]
- 23.Kahraman M, Borchardt AJ, Davis RL, et al. Preparation of carbazole compounds as inhibitors of histamine receptors. US8080566, USA; 2011.
- 24.Li YR, Li C, Liu JC, et al. Synthesis and biological evaluation of 1,3-diaryl pyrazole derivatives as potential antibacterial and anti-inflammatory agents. Bioorg Med Chem Lett 2015;25:5052–7. [DOI] [PubMed] [Google Scholar]
- 25.Zhao LM, Guo Z, Xue YJ, et al. Synthesis and evaluation of 3-substituted-4-(quinoxalin-6-yl) pyrazoles as TGF-type I receptor kinase inhibitors. Molecules 2018;23:3369–89. [DOI] [PMC free article] [PubMed] [Google Scholar]