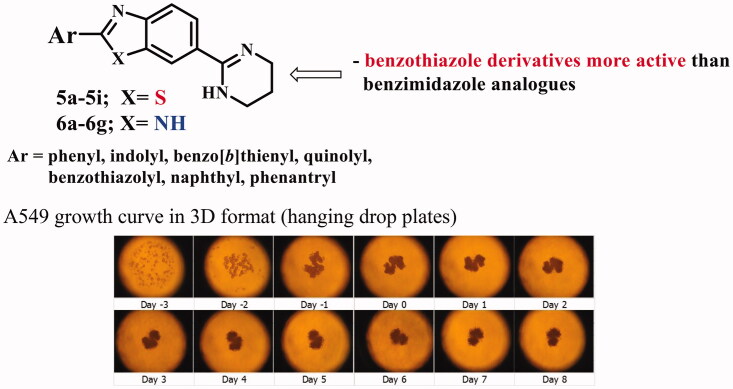
Abstract
Newly synthesised benzimidazole/benzotiazole derivatives bearing amidino, namely 3,4,5,6-tetrahydropyrimidin-1-ium chloride, substituents have been evaluated for their potential antitumor activity in vitro. Compounds and standard drugs (doxorubicin, staurosporine and vandetanib) were tested on three human lung cancer cell lines A549, HCC827 and NCI-H358. We tested compounds in MTS citotoxicity assay and in BrdU proliferative assay performed on 2 D and 3 D assay format. Because benzmidazole scaffold is similar to natural purines, we tested the most active compounds for ability to induce cell apoptosis of A549 by binding to DNA in comparison with doxorubicin and saturosporine. Additionally, the ADME properties of the most active benzothiazole/benzimidazole and non-active compounds were determined to see if the different ADME properties are the cause of different activity in 2 D and 3 D assays, as well as to see if the tested active compounds have drug like properties and potency for further profilation. ADME characterisation included solubility, lipophilicity, permeability, metabolic stability and binding to plasma proteins. In general, the benzothiazole derivatives were more active in comparison to their benzimidazole analogues. The exception was 2-phenyl substituted benzimidazole 6a being active with very pronounced activity especially towards HCC827 cells. All active compounds have similar mode of action on A549 cell line as standard compound doxorubicin, which binds to nucleic acids with the DNA double helix. Tested active benzothiazole compounds were characterised by moderate to good solubility, good metabolic stability, low permeability and high binding to plasma proteins. One tested active benzimidazole derivative showed ADME properties, but lower lipophilicity resulted in low PPB and higher metabolic instability. In addition, no significant difference was observed in ADME profile between active and non-active compounds.
Keywords: Amidines, benzimidazoles, benzotiazoles, 2D and 3D in vitro cytotoxicity assay, apoptotic activity, ADME
Graphical Abstract
1. Introduction
Lung cancer is the second most common cancer (about 14%) of new diagnosted cancers1. Although new drugs and approaches to treating lung cancer have been discovered in the last five years, chemotherapeutics are still the first line of therapy. Despite good patient response, there is still a medical need for new chemotherapeutics, primarily due to cancer drug resistance and toxicity, which are the main therapy limitations of the efficacy and clinical outcomes.
Taking into account the great biological importance of benzimidazole and benzothiazole derived natural, semisynthetic or synthetic derivatives as well as their versatile pharmacological features, these nitrogen scaffolds become unavoidable structural motifs in the rational design of novel drugs2–6. Nowadays there is still an increasing interest in medicinal and pharmaceutical chemistry for incorporation of benzimidazole/benzothiazole highly-privileged building substructures in order to developed novel heterocycles with possible pharmacological, chemical or industrial applications7,8. Suchlike derivatives display a broad spectrum of different biological features such as anticancer, antiviral, antioxidant, antibacterial, antifungal, antihistaminic, anti-inflammatory, etc9–11. Furthermore, the structural similarity of benzimidazole scaffold with naturally occurring purines is of great importance for studying the role of prepared derivatives in the function of many biologically important molecules like DNA, RNA or different proteins in living organisms12,13.
Additionally, the literature review revealed that amidines are structural parts of numerous biologically active compounds like many important medical and biochemical agents14. Recently, we have published several papers regarding the amidino substituted benzimidazole/benzotiazole derivatives with amidine group as positively charged substituent, placed at the end of the heteroaromatic substructures15. We have proven that within designing suchlike derivatives, the biological activity could be significantly improved while many of derivatives showed interaction with an electronegatively charged biological molecule such as DNA. The synthesised derivatives displayed antiproliferative, antibacterial, antifungal and antioxidative activity with several active compounds which were chosen as lead compounds for further optimisation and modification to get more active, selective and less cyctotoxic derivatives with improved physico-chemical properties16. Recently, we have published several papers describing the antiproliferative activity of various benzothiazole and benzimidazole derivatives substituted with either carboxamido, amino, halogeno, cyano, amidino, amino or nitro groups placed at different positions on the mentioned scaffold17–20. The most significant biological importance was observed with amidino substituted benzazoles bearing different types of amidine substituents suchlike unsubstituted, isopropyl, morpholinyl or imidazolinyl. Obtained results revealed that among all synthesised benzazole derivatives, cyclic amidino substituent, namely 2-imidazolinyl group showed the most significant influence and the enhancement of the antiproliferative activity in vitro with with IC50 values in submicromolar range of concentrations14,21. Very recently, we present the design and synthesis of amidino substituted 2-phenylbenzothiazole and benzimidazole derivatives with the variable number of hydroxy and methoxy groups attached to the phenyl ring and explore their antiproliferative and antioxidative activity in vitro21. Particularly, we were interested in synthesis and antiproliferative activity screening of cationic diamidino-substituted derivatives of phenylbenzothiazolyl and dibenzothiazolyl furans and thiophenes15, bisbenzothiazolyl-pyridines and pyrazine22, phenylene-bisbenzothiazoles23, amidino substituted 2-arylbenzothiazole hydrochloride24 and mesylate25.
Within this work, as a continuation of our previous scientific work, herein we present the synthesis and preclinical screening in vitro on human lung cancer cells of novel amidino substituted benzimidazole/benzothiazole derivatives substituted with different aryl moieties in position 2 of benzazole scaffold.
As we have describe previously26, two-dimensional (2 D) cell cultures are not able to imitate complex tumour structure as three-dimensional (3 D) cell cultures. Also, 3 D techniques have already great impact in screening of active new chemical entities (NCE) with potential antitumor activity. Among various 3 D methods we developed screening on 3 D spheroids because is more likely to tumour growth and physiology. Cytotoxicity and proliferation assays reads out give as two distinct characteristics of cells27, therefore we tested compounds in MTS cytotoxicity assay and in BrdU proliferative assay performed on 2 D and 3 D assay format. For 2 D cell assay format, we used a classic two-dimensional in vitro assay28 and as 3 D assay we used a hanging drop proliferation cell assay, previosuly described29.
Mechanism of action of most active compounds in antiproliferative assay was tested measuring apoptosis (anexin V staining) by FACS analysis in comparison with doxorubicin and staurosporine.
ADME properties are dependent of structural characteristics of newly synthesised molecules. Different modifications are undertaken and tested during early drug discovery in order to achieve better and improved drug-like properties30. ADME characterisation represents an important step in the drug discovery process and it includes several in vitro assays covering physicochemical and biochemical properties such as solubility, lipophilicity, permeability, metabolic stability and binding to plasma proteins31. Here, we have evaluated major ADME properties to see if compound’s activity, obtained in 3 D cell cultures, is the consequence of different physicochemical and biochemical properties in comparison with non-active compounds, as well as to see if compounds have drug-like properties and potential for further profiling in in vivo models.
Compounds selected for ADME characterisation (Table 3) included five active derivatives from benzothiazole series 5c–5e, 5 g, 5 h (Scheme 1) and one active benzimidazole 6a (Scheme 1), respectively. To test pannel, we also added 7 non-active benzimidazole/benzothiazole derivatives.
Table 3.
Summary of ADME properties of active compounds.
| 5c | 5d | 5e | 5g | 5h | 6a | |
|---|---|---|---|---|---|---|
| Kinetic solubility range afer 2 h (µM) | >100 | 30–100 | 30–100 | 30–100 | 10–30 | >100 |
| Chrom logD | 3.31 | 2.87 | 3.19 | 2.71 | 3.31 | 0.92 |
| Microsomes (1 µM) Predicted in vivo hep CL (%LBF) |
||||||
| Mouse | <30 | <30 | 63 | <30 | <30 | 41 |
| Human | <30 | <30 | <30 | <30 | <30 | 56 |
| PPB % bound (recovery) |
||||||
| Mouse | 97.5 (86) | 96.0 (82) | 98.7 (82) | 96.0 (85) | 97.3 (85) | 62.9 (79) |
| Human | 97.0 (89) | 94.7 (76) | 97.9 (79) | 95.1 (94) | 97.6 (80) | 59.7 (75) |
| Plasma stability (%remaining at 4 h) | ||||||
| Mouse | 94 | 85 | 77 | 81 | 85 | 79 |
| Human | 83 | 81 | 82 | 86 | 74 | 73 |
| MDCKII-MDR1 | ||||||
| Papp(A2B), | <0.1 – >6.6 | <0.1 – >6.5 | <0.1 – >2.0 | <0.1 – >3.9 | <0.1 – >0.7 | 0.4 – >0.7 |
| Papp(B2A), | 21.5 – >5.8 | 15.8 – >5.6 | 7.6 – >1.8 | 16.5 – >5.7 | 7.7 – >1.8 | 1.4 – >0.9 |
| Efflux ratio (w/o-> w/ P-gp inhibitor) |
>200 – >0.9 | >158 – >0.9 | >78 – >1.0 | >17 – >2.0 | >77 – >3.5 | 3.7 – >1.5 |
Scheme 1.
Synthesis of amidino-substituted benzazoles.
2. Experimental part
2.1. Chemistry
Melting points were determined by means of Original Kofler Mikroheitztisch apparatus (Reichert, Wien). The 1H NMR and the 13 C NMR spectra were recorded with the Bruker Avance DPX-300 or Bruker AV-600 using TMS as internal standard. Chemical shifts are reported in parts per million (ppm) relative to TMS. Elemental analyses for carbon, hydrogen and nitrogen were performed on Perkin-Elmer 2400 elemental analyser. Analyses are indicated as symbols of elements, analytical results obtained are within 0.4% of the theoretical value. All compounds were routinely checked by TLC using Merck silica gel 60 F-254 glass plates.
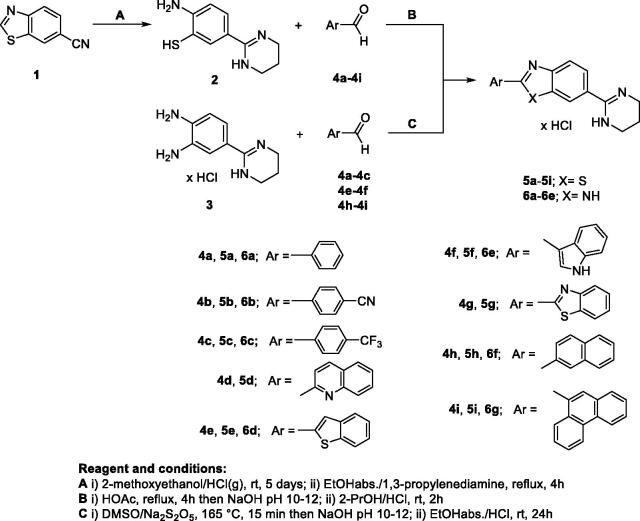
Synthesis of 6-cyanobenzothiazole (1) was carried out according to the literature32. Synthesis of 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride (3) was carried out according to the literature14.
2.1.1. Synthesis of 2-amino-5–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzenethiolate 2
A suspension of 6-cyanobenzothiazole 1 (4.0 g, 25 mmol) in 50 ml of dry 2-methoxyethanol was cooled to 5 °C and saturated with dry gaseous HCl. The flask was stoppered and stirred at room temperature for 4 days. Excess of HCl was removed from the suspension with a stream of nitrogen and the reaction mixture was poured into diethyl-ether. The resulting solid was filtered off, washed with diethyl-ether and dried under reduced pressure over KOH. The intermediate imidoyl-ether dihydrochloride was suspended in 100 ml of abs ethanol and 1,3-propylenediamine (10.5 ml, 125 mmol) added under nitrogen atmosphere.
The reaction mixture was refluxed for 4 h, cooled under nitrogen to 5 °C, and the obtained solid was filtered off and washed with dry ether. The solid mixture was suspended and heated to boiling in 70 ml of deoxygenated water, cooled under nitrogen at 5 °C for 2 h, and the resulting precipitate was filtered off and dried under reduced pressure over KOH. Yield of pure compound 2 as pale yellow solid was 4.26 g (83.2%) mp = 284–288 °C. 1H NMR (300 MHz, TFA-d1) δ 8.19 (bs, 2H), 7.97 (d, J = 1.8 Hz, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.70 (dd, J = 2.0 Hz, J = 8.4 Hz, 1H), 3.69 (t, J = 5.6 Hz, 4H), 2.19 (m, 2H); 13 C NMR (75 MHz, HOAc-d4) δ 159.1, 152.6, 133.1, 127.7, 116.3, 114.3, 112.1, 39.1, 18.2; Analysis calcd for C10H13N3S: C, 57.94; H, 6.32; N, 20.27% Found: C, 57.72; H, 6.54; N, 20.31%.
2.1.2. General method for preparation of compounds 5a–5i
To a stirred solution of 2-amino-5–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzenethiolate 2 (0.104 g, 0.5 mmol) in glacial acetic acid (5 ml), a corresponding carbaldehyde 4a–4i (0.5 mmol) was added under nitrogen atmosphere and heated to reflux for 4 h. The reaction mixture was poured onto ice and made alkaline with 20% NaOH. The resulting free base was filtered off, washed with water, and dried. The free base was suspended in 2-propanole and concd HCl (84 µl, 1.0 mmol) was added. The reaction mixture was stirred at room temperature for 1–2 h and cooled in refrigerator overnight. The resulting precipitate was filtered off, washed with diethyl-ether, and dried at 75 °C.
2.1.2.1. 2-Phenyl-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5a
Compound 5a was prepared from benzaldehyde 4a (0.053 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.064 g (38.8%) of white powder; m.p. = 272–275 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.23 (s, 2H), 8.63 (d, J = 1.6 Hz, 1H), 8.28 (d, J = 8.6 Hz, 1H), 8.20 − 8.13 (m, 2H), 7.88 (dd, J = 1.8 Hz, J = 8.6, Hz, 1H), 7.68 − 7.58 (m, 3H), 3.54 (m, 4H), 2.01 (m, 2H); 13 C NMR (151 MHz, DMSO-d6) δ 171.2, 158.8, 156.1, 134.6, 132.3, 132.2, 129.5 (2 C), 127.5 (2 C), 126.0, 125.3, 123.0, 122.8, 38.8 (2 C), 17.7; Analysis calcd for C17H16ClN3S: C, 61.90; H, 4.89; N, 12.74%; Found: C, 61.73; H, 5.11; N, 12.90%.
2.1.2.2. 2–(4-Cyanophenyl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5 b
Compound 5 b was prepared from 4-cyanobenzaldehyde 4 b (0.067 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.101 g (57.1%) of coloreless powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.27 (s, 2H), 8.69 (d, J = 1.6 Hz, 1H), 8.36–8.33 (m, 3H), 8.09 (d, J = 8.5 Hz, 2H), 7.92 (dd, J = 1.8 Hz, J = 8.6 Hz, 1H), 3.53 (m, 4H), 2.02 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 169.2, 158.7, 155.8, 136.0, 135.0, 133.4 (2 C), 128.2 (2 C), 126.3, 125.9, 123.5, 123.2, 118.2, 114.0, 38.8 (2 C), 17.7; Analysis calcd for C18H15ClN4S: C, 60.92; H, 4.26; N, 15.79%; Found: C, 60.81; H, 4.23; N, 15.88%.
2.1.2.3. 2–(4-Trifluoromethylphenyl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5c
Compound 5c was prepared from 4-trifluoromethylbenzaldehyde 4c (0.087 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.107 g (53.8%) of white powder; m.p. = 279–283 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.26 (s, 2H), 8.69 (d, J = 1.6 Hz, 1H), 8.38 (d, J = 8.1 Hz, 2H), 8.34 (d, J = 8.6 Hz, 1H), 7.99 (d, J = 8.3 Hz, 2H), 7.92 (dd, J = 1.9 Hz, J = 8.6 Hz, 1H), 3.54 (t, J = 5.6 Hz, 4H), 2.01 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 169.4, 158.7, 155.9, 135.8, 134.9, 131.5, 128.4 (2 C), 126.4 (2 C), 126.3, 125.8, 123.8, 123.4, 123.1, 38.4 (2 C), 17.7; Analysis calcd for C18H15ClF3N3S: C, 54.34; H, 3.80; N, 10.56%; Found: C, 54.22; H, 3.85; N, 10.66%.
2.1.2.4. 2-(Quinoline-2-yl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5d
Compound 5d was prepared from quinoline-2-carbaldehyde 4d (0.079 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.121 g (63.7%) of coloreless powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.22 (s, 2H), 8.69–8.66 (m, 2H), 8.50 (d, J = 8.5 Hz, 1H), 8.38 (d, J = 8.6 Hz, 1H), 8.19–8.13 (m, 2H), 7.96 − 7.88 (m, 2H), 7.76 (m, 1H), 3.54 (t, J = 4.8 Hz, 4H), 2.02 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 172.8, 158.8, 158.7, 156.3, 149.9, 147.1, 138.2, 135.7, 131.0, 128.9, 128.3, 126.0, 123.7, 123.1, 118.0, 38.9 (2 C), 17.7; Analysis calcd for C20H17ClN4S: C, 63.07; H, 4.50; N, 14.71%; Found: C, 62.99; H, 4.51; N, 14.78%.
2.1.2.5. 2-(Benzo[b]thiophen-2-yl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5e
Compound 5e was prepared from benzo[b]thiophene-2-carbaldehyde 4e (0.081 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.120 g (62.2%) of white powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.23 (s, 2H), 8.62 (d, J = 1.6 Hz, 1H), 8.40 (s, 1H), 8.26 (d, J = 8.6 Hz, 1H), 8.12 − 7.99 (m, 2H), 7.87 (dd, J = 1.8 Hz, J = 8.6 Hz, 1H), 7.56 − 7.44 (m, 2H), 3.52 (t, J = 5.5 Hz, 4H), 2.00 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 164.8, 159.0, 155.6, 140.4, 139.3, 135.5, 134.9, 128.0, 127.1, 126.3, 125.8, 125.5, 125.3, 123.0, 122.9, 122.8, 38.9 (2 C), 17.7; Analysis calcd for C19H16ClN3S2: C, 59.13; H, 4.18; N, 10.89%; Found: C, 59.19; H, 4.06; N, 10.98%.
2.1.2.6. 2-(1H-Indol-3-yl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5f
Compound 5f was prepared from 1H-indole-3-carbaldehyde 4f (0.073 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.050 g (27.2%) of coloreless powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 12.25 (s, 1H), 10.23 (s, 2H), 8.51 (d, J = 1.7 Hz, 1H), 8.42 − 8.37 (m, 2H), 8.13 (d, J = 8.5 Hz, 1H), 7.83 (dd, J = 1.8 Hz, J = 8.6 Hz, 1H), 7.57 (m, 1H), 7.34 − 7.25 (m, 2H), 3.53 (t, J = 5.5 Hz, 4H), 2.02 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 166.7, 158.8, 156.7, 136.9, 133.3, 130.3, 125.6, 124.4, 123.7, 123.0, 122.0, 121.5, 121.4, 120.6, 112.6, 110.1, 38.8 (2 C), 17.8; Analysis calcd for C19H17ClN4S: C, 61.86; H, 4.65; N, 15.19%; Found: C, 61.97; H, 4.69; N, 15.02%.
2.1.2.7. 2-(Benzothiazole-2-yl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5 g
Compound 5 g was prepared from Benzothiazole-2-carbaldehyde 4 g (0.082 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.069 g (35.8%) of white powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.28 (s, 2H), 8.71 (s, 1H), 8.42 (d, J = 8.6 Hz, 1H), 8.30 (d, J = 7.3 Hz, 1H), 8.23 (d, J = 7.6 Hz 1H), 7.94 (d, J = 9.2 Hz, 1H), 7.72 − 7.59 (m, 2H), 3.55 (m, 4H), 2.02 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 158.8, 155.2, 152.7, 135.2, 135.0, 127.2, 127.1, 126.5, 126.2, 123.6, 123.0, 122.7, 38.7 (2 C), 17.5; Analysis calcd for C18H15ClN4S2: C, 55.88; H, 3.91; N, 14.48%; Found: C, 55.99; H, 3.78; N, 14.56%.
2.1.2.8. 2-(Naphthalene-2-yl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5 h
Compound 5 h was prepared from 2-naphthaldehyde 4 h (0.078 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.078 g (41.1%) of pale yellow powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.25 (s, 2H), 8.79 (s, 1H), 8.67 (s, 1H), 8.35 − 8.18 (m, 3H), 8.14 (d, J = 8.6 Hz, 1H), 8.05 (m, 1H), 7.90 (m, 1H), 7.72 − 7.62 (m, 2H), 3.55 (t, J = 4.8 Hz, 4H), 2.02 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 158.6, 156.0, 134.6, 134.3, 132.5, 129.5, 128.9, 128.7, 127.9, 127.8, 127.5, 127.0, 125.8, 125.1, 123.6, 122.7, 122.5, 38.7 (2 C), 17.8; Analysis calcd for C21H18ClN3S: C, 66.39; H, 4.78; N, 11.06%; Found: C, 66.28; H, 4.90; N, 11.09%.
2.1.2.9. 2-(Phenanthrene-9-yl)-6–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzothiazole chloride 5i
Compound 5i was prepared from phenanthrene-9-carbaldehyde 4i (0.103 g, 0.5 mmol). The resulting free base was converted into salt as described below to obtained 0.097 g (45.1%) of colourless powder; m.p. 196–200 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.34 (s, 2H), 9.05 − 8.98 (m, 2H), 8.95 (d, J = 8.3 Hz, 1H), 8.74 (d, J = 1.7 Hz, 1H), 8.54 (s, 1H), 8.42 (d, J = 8.6 Hz, 1H), 8.24 (d, J = 7.8 Hz, 1H), 7.97 (dd, J = 1.8 Hz, J = 8.6 Hz, 1H), 7.89 − 7.74 (m, 4H), 3.57 (m, 4H), 2.04 (m, 2H); 13 C NMR (151 MHz, DMSO-d6) δ 170.9, 158.8, 156.1, 134.7, 131.9, 130.8, 130.3, 130.1, 129.7, 129.1, 128.2, 128.0, 127.8, 127.7, 127.6, 126.2, 126.0, 125.5, 123.5, 123.3, 123.0, 122.6, 38.8 (2 C), 17.7; Analysis calcd for C25H20ClN3S: C, 69.84; H, 4.69; N, 9.77%; Found: C, 69.84; H, 4.69; N, 9.77%.
2.1.3. General method for preparation of compounds 6a–6g
Solution of equimolar amounts of aldehydes 4a–4i, 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3, and sodium metabisulfite as oxidising reagents in dimethyl sulfoxide, was heated for 15 min at 160 °C. After the reaction mixture was cooled, water was added and reaction mixture was made alkaline with 20% NaOH. The resulting free base was filtered off, washed with water and dried. The free base was suspended in absolute ethanol and concd HCl was added. The reaction mixture was stirred at room temperature for 24 h and the resulting precipitate was filtered off.
2.1.3.1. 2-Phenyl-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6a
Compound 6a was prepared from benzaldehyde 4a (0.047 g, 0.44 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.100 g, 0.44 mmol), and sodium metabisulfite (0.084 g, 0.44 mmol) in dimethyl sulfoxide (0,8 ml). The resulting free base was converted into salt as described below to obtained 0.040 g (29.0%) of beige powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.22 (s, 2H), 8.47 − 8.45 (m, 2H), 8.19 (s, 1H), 7.94 (d, J = 8.4 Hz, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.69 (s, 3H), 3.53 (m, 4H), 2.02 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 159.3, 152.7, 132.2, 129.3, 127.7, 123.8, 123.2, 115.0, 38.8, 17.7; Analysis calcd for C17H17ClN4: C, 65.28; H, 5.48; N, 17.91%; Found: C, 65.14; H, 5.61; N, 17.99%.
2.1.3.2. 2–(4-Cyanophenyl)-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6 b
Compound 6 b was prepared from 4-cyanobenzaldehyde 4 b (0.058 g, 0.44 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.100 g, 0.44 mmol), and sodium metabisulfite (0.084 g, 0.44 mmol) in dimethyl sulfoxide (1 ml). The resulting free base was converted into salt as described below to obtained 0.075 g (50.3%) of beige powder; m.p. >300 °C; 1H NMR (600 MHz, DMSO-d6) δ 14.20 (bs, 1H), 10.04 (s, 2H), 8.47 (d, J = 7.7 Hz, 2H), 8.11 (s, 1H), 8.06 (d, J = 7.8 Hz, 2H), 7.83 (d, J = 7.8 Hz, 1H), 7.62 (d, J = 8.0 Hz, 1H), 3.52 (m, 4H), 2.01 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 160.1, 152.8, 134.1, 133.5, 128.0, 123.0, 122.4, 119.0, 113.0, 39.3, 18.4; Analysis calcd for C18H16ClN5: C, 64.00; H, 4.77; N, 20.73%; Found: C, 64.03; H, 4.74; N, 20.71%.
2.1.3.3. 2–(4-Trifluoromethylphenyl)-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6c
Compound 6c was prepared from 4-trifluoromethylbenzaldehyde 4c (0.077 g, 0.44 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.100 g, 0.44 mmol), and sodium metabisulfite (0.084 g, 0.44 mmol) in dimethyl sulfoxide (0.8 ml). The resulting free base was converted into salt as described below to obtained 0.101 g (60.1%) of beige powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 14.13 (bs, 1H), 10.05 (s, 2H), 8.51 (d, J = 8.1 Hz, 2H), 8.12 (s, 1H), 7.97 (d, J = 8.3 Hz, 2H), 7.84 (d, J = 8.1 Hz, 1H), 7.62 (d, J = 8.3 Hz, 1H), 3.53 (t, J = 5.5 Hz, 4H), 2.01 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 160.1, 133.7, 131.0, 130.5, 128.1, 126.5, 126.5, 126.3, 123.0, 122.7, 39.3, 18.4; Analysis calcd for C18H16ClF3N4: C, 56.77; H, 4.24; N, 14.71%; Found: C, 56.89; H, 4.21; N, 14.60%.
2.1.3.4. 2-(Benzo[b]thiophen-2-yl)-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6d
Compound 6d was prepared from benzo[b]thiophene-2-carbaldehyde 4e (0.054 g, 0.33 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.075 g, 0.33 mmol), and sodium metabisulfite (0.063 g, 0.33 mmol) in dimethyl sulfoxide (0.6 ml). The resulting free base was converted into salt as described below to obtained 0.053 g (43.1%) of beige powder; m.p. >300 °C; 1H NMR (600 MHz, DMSO-d6) δ 10.16 (s, 2H), 8.23 (s, 2H), 8.00 (s, 1H), 8.00 (d, J = 6.6 Hz, 1H), 7.91 (d, J = 6.8 Hz, 1H), 7.65 (d, J = 8.3 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.43 − 7.36 (m, 2H), 3.51 (t, J = 5.3 Hz, 4H), 1.99 (m, 2H); 13 C NMR (75 MHz, DMF-d7) δ 160.7, 140.8, 140.6, 126.0, 125.6, 125.0, 123.4, 120.5, 116.3, 39.8, 19.1; Analysis calcd for C19H17ClN4S: C, 61.86; H, 4.65; N, 15.19%; Found: C, 61.88; H, 4.62; N, 15.21%.
2.1.3.5. 2-(1H-Indol-3-yl)-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6e
Compound 6e was prepared from 1H-indole-3-carbaldehyde 4f (0.064 g, 0.44 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.100 g, 0.44 mmol), and sodium metabisulfite (0.084 g, 0.44 mmol) in dimethyl sulfoxide (0.8 ml). The resulting free base was converted into salt as described below to obtained 0.033 g (21.3%) of light brown powder; m.p. >300 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.82 (s, 1H), 10.16 (s, 2H), 8.51 (d, J = 7.2 Hz, 1H), 8.35 (s, 1H), 7.97 (s, 1H), 7.70 (d, J = 8.1 Hz, 1H), 7.52 (d, J = 8.0 Hz, 2H), 7.24 (t, J = 6.8 Hz, 1H), 7.21 (t, J = 6.8 Hz, 1H), 3.52 (t, J = 5.5 Hz, 4H), 1.99 (m, 2H); 13 C NMR (151 MHz, DMSO-d6) δ 159.5, 136.6, 127.4, 125.2, 122.3, 121.4, 121.3, 120.5, 120.5, 112.0, 105.9, 39.0, 18.7; Analysis calcd for C19H18ClN5: C, 64.86; H, 5.16; N, 19.91%; Found: C, 64.83; H, 5.17; N, 19.90%.
2.1.3.6. 2-(Naphthalene-2-yl)-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6f
Compound 6f was prepared from 2-naphthaldehyde 4 h (0.069 g, 0.44 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.100 g, 0.44 mmol), and sodium metabisulfite (0.084 g, 0.44 mmol) in dimethyl sulfoxide (0.8 ml). The resulting free base was converted into salt as described below to obtained 0.081 g (50.6%) of beige powder; m.p. >300 °C; 1H NMR (600 MHz, DMSO-d6) δ 10.25 (s, 2H), 8.80 (d, J = 7.2 Hz, 1H), 8.27 (s, 1H), 8.24 (d, J = 8.6 Hz, 1H), 8.17 (d, J = 7.2 Hz, 1H), 8.13 (d, J = 7.5 Hz, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.83 − 7.74 (m, 2H), 7.73 − 7.65 (m, 2H), 3.55 (m, 4H), 2.03 (m, 2H); 13 C NMR (151 MHz, DMSO-d6) δ 159.4, 153.3, 133.4, 131.7, 130.1, 129.3, 128.6, 127.7, 126.7, 125.5, 125.3, 124.7, 123.2, 122.6, 115.5, 115.0, 38.9, 17.8
Analysis calcd for C21H19ClN4: C, 69.51; H, 5.28; N, 15.44%; Found: C, 69.49; H, 5.30; N, 15.48%.
2.1.3.7. 2-(Phenanthrene-9-yl)-5(6)–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzimidazole chloride 6 g
Compound 6 g was prepared from phenanthrene-9-carbaldehyde 4i (0.091 g, 0.44 mmol), 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3 (0.100 g, 0.44 mmol), and sodium metabisulfite (0.084 g, 0.44 mmol) in dimethyl sulfoxide (0.8 ml). The resulting free base was converted into salt as described below to obtained 0.077 g (42.3%) of beige powder; m.p. >300 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.27 (s, 2H), 9.02 (d, J = 8.1 Hz, 1H), 8.97 (d, J = 8.2 Hz, 1H), 8.88 (d, J = 8.0 Hz, 1H), 8.56 (s, 1H), 8.29 (s, 1H), 8.16 (d, J = 7.4 Hz, 1H), 7.97 (d, J = 8.5 Hz, 1H), 7.90 − 7.74 (m, 5H), 3.55 (m, 4H), 2.03 (m, 2H); 13 C NMR (75 MHz, DMSO-d6) δ 159.8, 153.8, 131.5, 131.1, 130.7, 130.5, 130.0, 129.4, 129.0, 128.1, 128.0, 127.0, 124.0, 123.6, 123.5, 123.0, 39.3, 18.3; Analysis calcd for C25H21ClN4: C, 72.72; H, 5.13; N, 13.57%; Found: C, 72.76; H, 5.10; N, 13.52%.
2.2. Biology
2.2.1. Antitumor activity in 2 D and 3 D assays
2.2.1.1. Material and methods
2.2.1.1.1. Test compounds
Doxorubicin was purchased from Apollo (BID0120; Opelika, AL), staurosporine was purchased from Biotrend (BS0188; Zurich, SUI) and vandetanib was purchased from Selleckchem (S1046, Houston, TX, USA). Test compounds were synthesised by research group at Department of Organic Chemistry, Faculty of Chemical Engineering and Technology, University of Zagreb.
Mother plates (96-well-V plates, polypropylene, Greiner Bio-one, Cat. 651201) with serial dilutions of compounds in pure DMSO are prepared from 10 mM DMSO stock solutions on Janus automatic pipetting workstation (Perkin-Elmer). Compounds are diluted 1:3. 500 nL (for 2 D cell culture) or 400 nL (for 3 D cell culture) of compound were transferred from mother plate to test plate by using Mosquito (TTP labtech). DMSO percentage in test concentrations was 0.5 − 1.0%. Starting concentration of the test compounds and standard compound doxorubicin was 50 µM (2 D cell culture) or 100 µM (3 D cell culture).
Starting concentration of staurosporine was 10 µM in both, 2 D and 3 D cell culture assays, while starting concentration of vandetanib was 25 µM (2 D cell culture) or 50 µM (3 D cell culture).
2.2.1.1.2. Cell cultures
Human lung cancer cell lines A549 (ATCC CCL-185), HCC827 (ATCC CRL-2868) and NCI-H358 (ATCC CRL-5807) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA).
Cells were maintained in appropriated, recommended by supplier medium, supplemented with 10% heat inactivated foetal bovine serum and penicillin/streptomycin, in incubator (INCO2, Memmert) in a humidified atmosphere of 5% CO2 and 95% O2 at 37 °C.
2.2.1.2. High-throughput 2 D and 3 D drug screening setup
2.2.2.2.1. 2 D cell culture assay
Cells were grown in 96 well cell star polystyrene plates. 10,000 cells/well were seeded in wells 4 h prior the treatment with different compounds concentrations and incubated for 72 h. A cell viability assay was performed according to the manufacturer’s instruction, using CellTiter 96 aqueous solution, MTS kit (Promega, Madison, WI, USA). After 0.5–2 h of cell incubation with MTS in 2 D cell culture, the plates were read using PE EnVision absorbance at 490 nm. The results for each of tested compounds are reported as growth percentages from two independent concentrations curves compared with the untreated control cells after drug exposure. Since we prefer homogeneous assays (mix and read procedures) and therefore MTS is more practical (one step less than MTT). MTS is more sensitive and accurate in comparison with MTT assay.
2.2.2.2.2. 3 D cell culture assay
Cells were grown in 96 well Perfecta 3 D hanging drop plates 5000 cell/well for 4 days until spheres were formed (and checked under the microscope). After sphere formation cells were treated with compounds followed by 72 h incubation. Cell viability assay was performed according to the manufacturer’s instruction, using CellTiter GLO 3 D for 3 D cell culture (Promega); cell incubation was 5 min on the shaker followed by 25 min incubation in the dark. Plates were read using PE EnVision luminescence. The results for each of tested compounds are reported as growth percentages from two independent concentrations curves compared with the untreated control cells after drug exposure.
2.2.1.3. BrdU proliferation assay
Proliferation assay was performed using Cell Proliferation ELISA, BrdU (Sigma, St. Louis, MO, USA). Briefly, 48 h hours after compound addition, 10 µM of BrdU was added to each well and incubated for following 24 h. After incubation, single cell suspension was made for 3 D cell culture in the new 96 well plate. Plates were centrifuged and supernatants were removed from each plate (for 2 D and 3 D cell culture) followed by 60 min incubation at 60° C. The rest of the proliferation assay was performed according to the manufacturer instructions.
2.2.1.4. Annexin V assay – apoptotic changes in plasma membrane
Under physiological conditions, choline phospholipids (phosphatidylcholine, sphingomyelin) are exposed on the external leaflet while aminophospholipids (phosphatidylserine, phosphatidylethanolamine) are exclusively located on the cytoplasmic surface of the lipid bilayer. This asymmetry is scrambled during apoptosis when phosphatidylserine becomes exposed on the extracellular side of the membrane33.
Phosphatidylserine is detected by anticoagulant protein Annexin V (tagged with fluorochrome) that reversibly binds to phosphatidylserine residues on the extracellular side of the membrane. Annexin V apoptotic assay was performed using Annexin V Alexa Fluor 488 conjugate (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer instruction. Briefly, 1 × 105 cells were seeded on 24 well plate and treated with IC50 concentration of chosen compounds from amidine series for 36 h. Cells suspensions were collected after incubation into 5 ml Falcon tubes, washed and centrifuged 2 times (once with PBS, followed by Annexin V Binding Buffer (AVBB)) for 5 min, 400 g at room temperature (RT). 100 µL of AVBB was added to each tube followed by addition of 2 µL of Annexin V Alexa Fluor 488 conjugate and incubated for 15 min at RT. Cells were washed in AVBB for 5 min, 400 g at RT. SYTOX™ AADvanced™ Dead Cell Stain Kit (Thermo Fisher Scientific) was used for detection of late apoptotic and necrotic cells. 1 µL of SYTOX AADvanced was added to each tube and incubated at RT for 5 min in dark. 500 µL of AVBB was added and samples were kept on ice until analysed. The results for each of tested compounds are reported as percentage of positive cells (live cells (Annexin V−/SytoxAAD−), apoptotic cells (Annexin V+/SytoxAAD−), late apoptotic/necrotic cells (Annexin V+/SytoxAAD+) and necrotic cells (Annexin V−/SytoxAAD+)).
2.2.1.5. Statistical analysis
Calculation of IC50 data, curves and QC analysis is made by using Excel tools and GraphPadPrism software (La Jolla, CA), v. 5.03. In brief, individual concentration–effect curves are generated by plotting the logarithm of the tested concentration of tested compounds (X) versus corresponding percent inhibition values (Y) using least squares (ordinary) fit. Best fit IC50 values are calculated using Log(inhibitor) versus normalised response – Variable slope equation, where Y = 100/(1 + 10((LogIC50 – X) * HillSlope)). QC criteria parameters (Z', S:B, R2, HillSlope) were checked for every IC50 curve.
2.2.2. Dmpk in vitro analyis
2.2.2.1. Materials
Dimethyl sulfoxide (DMSO), phosphate-buffered saline (PBS), nicotinamide adenine dinucleotide phosphate (NADP), glucose-6-phosphate, glucose-6-phosphate dehydrogenase, magnesium chloride, Dulbecco’s phosphate-buffered saline (D-PBS), Dulbecco’s Modified Eagles medium (DMEM), fetalbovine serum (FBS), glutamax, nonessential amino acids (NEAA), EDTA, 0.05% Trypsin-EDTA, ammonium acetate, sulfaphenazole, α–naphtoflavone, propranolol, caffeine, acebutolol, verapamil, nicardipine warfarin sodium, benfluorex hydrochloride, eucatropine hydrochloride and diclofenac were purchased from Sigma Aldrich (St. Louis, MO, USA). Ammonia solution, min. 25% p.a. was obtained from Kemika (Zagreb, Croatia). Lucifer yellow was purchased from EndoTherm (Saarland University, Germany), elacridar from International Laboratory (South San Francisco, CA, USA) and amprenavir form AKSci (Union City, CA, USA). Acetonitrile, methanol, sodium hydrogen phosphate, potassium dihydrogen phosphate ethanol and sodium chloride were obtained from Merck (Darmstadt, Germany) and acetonitrile ultra gradient grade HPLC analysed from J.T. Baker, Fisher Scientific (Pittsburgh, PA, USA). BCA Protein Assay Kit was purchased from Thermo Scientific (Waltham, MA, USA), 4-androsten-17b-ol-3-one (testosterone) from Steraloids (Newport, RI, USA) and human and mouse liver microsomes from Corning (New York, NY, USA). MDCKII-hMDR1 cells were obtained from Solvo Biotechnology (Szeged, Hungary), antibiotic/antimycotic from Gibco, Thermo Scientific (Waltham, MA, USA) and physiological solution, 0.9% NaCl from Croatian Institute for Transfusion Medicine (Zagreb, Croatia). Human and mouse plasma were purchased from BioIVT/Seralab (Sussex, United Kingdom).
2.2.2.2. Kinetic solubility
Test compounds and assay controls (sulfaphenazole and α-naphtoflavone) were serially diluted in DMSO by factor 3.3x, 3x, 3.3x and 3x, resulting in a total of 5 different concentrations. Phosphate buffer (50 mM, pH 7.4) was spiked with diluted test compounds and controls with final concentration range in the assay as follows: 100, 30, 10, 3 and 1 µM (1% DMSO). Plate was then incubated by gentle shaking (200–300 rpm) for 1 h and 45 min at 37 °C. After additional15 min on room temperature (without shaking), plate absorbance at 620 nm was measured with microplate reader Tecan, Infinite F500 (total incubation time = 2 h).
2.2.2.3. Chromatographic lipophilicity study
In order to determine the lipophilicity range, Chromatographic Hydrophobicity Index was measured by gradient reverse-phase HPLC at physiological pH. Sample working solutions were prepared from 10 mM DMSO stock solutions by dilution with acetonitrile to a final concentration of 1.25 mM and analysed on Agilent 1100 Series liquid chromatography system with HPLC diode-array detector (DAD) coupled with Micromass Quattro micro API mass spectrometer. Samples were injected onto an HPLC column (Phenomenex Luna C18, 50 × 3 mm, 5 µm) and eluted with a gradient at room temperature (sample temperature 15 °C). The mobile phase was composed of 50 mM Ammonium acetate, pH = 7.4 and acetonitrille. A total run time was 5 min, with the flow rate of 1 ml/min (under gradient conditions). The HPLC system was initially calibrated using the calibration set of 10 compounds with literature CHI values. The experimentally determined gradient retention times were plotted against the CHI7.4 values published in the literature resulting in the equation obtained from linear regression analysis. This equation was further used for the calculation of CHI7.4 for test compounds from determined retention times of the main chromatographic peak from UV chromatogram for each compound. The obtained CHI7.4 values were further converted to Chrom log D7.4 as follows: CHI7.4 × 0.0857 − 2.
2.2.2.4. Metabolic stability in liver microsomes
Tested compounds (final concentration of 1 µM, 0.1% DMSO), as well as testosterone and propranolol as positive controls and caffeine as negative control were incubated in phosphate buffer (50 mM, pH 7.4) for 60 min at 37 °C with liver microsomes (human and mouse) in the absence and presence of the NADPH cofactor. The NADPH generating system was prepared in phosphate buffer and consisted of nicotinamide adenine dinucleotide phosphate (NADP, 0.38 mM), glucose-6-phosphate (1.49 mM), glucose-6-phosphate dehydrogenase (1.5 U) and magnesium chloride (0.1 mM). Aliquots were taken at different time points (0, 10, 20, 30, 45 and 60 min) and reaction was terminated by addition of a MeCN/MeOH (2:1) mixture, containing diclofenac as internal standard. Samples were then centrifuged (at 4500 rpm, at 4 °C, for 30 min) and resulting supernatants were subjected to LC-MS/MS analysis. The in vitro half life (t½) was determined from the slope of the linear regression of ln % parent compound remaining versus incubation time. In vitro intrinsic clearance, expressed as µl/min/mg liver, was determined from in vitro half life (t½) and normalised for the protein concentration in the incubation mixture. Predicted in vivo hepatic clearance was determined from in vitro intrinsic clearance assuming 52.5 mg of protein/g of liver and using constant values for liver weight/body weight [g/kg] (25.7 for human, 87.5 for mouse) and liver blood flow (LBF) [ml/min/kg] (21 for human and 131 for mouse).
2.2.2.5. Plasma protein binding
The extent of binding to plasma proteins was assessed using equilibrium dialysis method. Plasma (human and mouse) was spiked with test compounds and three controls (nicardipine and verapamil in both species, acebutolol in human and caffeine in mouse plasma) to obtain final concentration of 5 µM (0.5%DMSO). Hydrated membranes were inserted into equilibrium dialysis unit (HT Dialysis) according to manufacturer’s instructions. The dialysate side was then loaded with appropriate volume of buffer, while the same volume of spiked plasma was added into sample side of the well. Incubation lasted 4 h at 37 °C with gentle shaking. Afterwards, protein precipitation was done by mixing matrix matched aliquot of plasma or buffer with 3 volumes of MeCN/MeOH (2:1) mixture, containing internal standard (diclofenac). After centrifugation (at 4500 rpm, at 4 °C, for 30 min) resulting supernatants were subjected to LC-MS/MS.
2.2.2.7. Stability in mouse and human plasma
Tested compounds (final concentration of 5 µM, 0.5% DMSO) were incubated for 4 h at 37 °C in human and mouse plasma, respectively. Aliquots were taken at different time points (0, 30, 120 and 240 min) and reaction was terminated by addition of a MeCN/MeOH (2:1) mixture, containing internal standard (diclofenac). Samples were then centrifuged (at 4500 rpm, at 4 °C, for 30 min) and resulting supernatants were subjected to LC-MS/MS analysis. The same procedure was followed for controls used in the assay: propranolol (both species), benfluorex (mouse) and eucatropine (human), respectively.
2.2.2.8. MDCKII-MDR1 permeability assay
Permeability and P-glycoprotein substrate assessment was done on MDCKII-hMDR1, Madin-Darby canine epithelial cells over-expressing human MDR1 gene, coding for P-glycoprotein.
Cells were prepared for transport studies by seeding on 96-well cell culture inserts (Millipore, MA, USA) in a concentration of 0.25 × 106 cells per ml. The cells were fed with fresh medium 24 h post seeding and cultured to confluence for 3 days before use. On the day of experiment, the cell monolayers were washed and equilibrated with transport medium (DPBS, pH 7.4 containing 1% DMSO) with or without P-gp specific inhibitor, elacridar (2 µM) for 45 min (37 °C, 5% CO2, 95% humidity). Test compound solution consisted of test substance (10 μM) in D-PBS medium containing lucifer yellow (100 μM) and 1% DMSO. Transport assays were conducted in apical to basolateral (A2B) and basolateral to apical (B2A) directions, respectively. Monolayers were incubated with the compound solution for 60 min at 37 °C under gentle agitation. Apical and basolateral compartments were sampled at the end of the transport experiment, while donor solutions were also sampled at the beginning of the experiment in order to determine initial concentration. Test substance concentrations in both compartments were determined by LC-MS/MS. There were several controls used in the assay: 1) amprenavir (0.5 μM) served as a low permeable control, being also a P-gp substrate; 2) diclofenac (10 μM) was used as a high permeable control; 3) Lucifer yellow, a fluorescent marker for the paracellular membrane transport, was used as a control of cell monolayer integrity.
2.2.2.9. LC-MS/MS analysis
All ADME samples were analysed on a Sciex API 4000 or Sciex API4500 Triple Quadrupole Mass Spectrometer (Sciex, Framingham, MA, USA) coupled to a UHPLC System (Shimadzu Nexera X2; Shimadzu, Kyoto, Japan). Samples were injected onto an UPLC column (HALO2 C18, 2.1 × 20 mm, 2 µm or Phenomenex Luna Omega Polar C18, 30 × 2.1 mm, 1.6 µm) and eluted with a gradient at temperature of 50 °C. The mobile phase was composed of acetonitrile (with 0.1% formic acid) and 0.1% formic acid in deionised water. A total run time was 1 or 1.5 min, with the flow rate of 0.7 ml/min (under gradient conditions). A positive ion mode with turbo spray, an ion source temperature of 500 °C and a dwell time of 150 ms were utilised for mass spectrometric detection. Multiple reaction monitoring (MRM) was used at the specific transitions for each compound/control tested: compound 5c: 361.9 → 304.9; compound 5d: 344.8 → 287.9; compound 5e: 349.8 → 292.9; compound 5 g: 350.8 → 293.9; compound 5 h: 344.0 → 286.9; compound 6a: 276.9 → 220.0; compound 5 b: 319.0 → 262.0; compound 6 b: 302.1 → 245.1; compound 6c: 344.9 → 288.0; compound 6d: 333.0 → 276.0; compound 6e: 316.1 → 259.0; compound 6f: 327.1 → 270.0; compound 6 g: 377.1 → 320.1; testosterone: 289.3→ 97.1; propranolol: 260.1 → 182.8; caffeine: 195.2 → 138.1; acebutolol: 337.2 → 116.2, nicardipine: 480.2 → 315.0; verapamil: 455.4 → 165.1; amprenavir: 506.2 → 245.4; diclofenac: 296.1 → 213.7 or 296.2 → 215.3; eucatropine: 292.2 → 109.1; benfluorex: 352.1 → 230.3, and warfarin: 309.2 → 163.2
3. Results and discussion
3.1. Chemistry
The targeted amidino substituted benzothiazoles 5a–5i and benzimidazoles 6a–6g were synthesised according to the procedure shown in Scheme 1 by using conventional methods for cyclocondenastion to fused benzazole derivatives. Within the cyclocondensation in refluxing acetic acid between commercially aryl aldehydes 4a–4i and amidino substituted benzenethiolate 2, followed by quenching with hydrochloric acid, benzothiazoles 5a–5i as hydrochloride salts obtained in moderate to good reaction yields. This method has been optimised in order to perform direct condensation of aldehydes with amidino substituted 2-aminothiophenoles without using any catalyst or oxidant. The precursor 2-amino-5–(3,4,5,6-tetrahydropyrimidin-1-ium-2-yl)benzenethiolate 2 in the form of zwitterion was prepared from 6-cyanobenzothiazole by Pinner reaction according to our previously described and well developed method34.
Amidino substituted benzimidazole derivatives 6a–6g (Scheme 1) were prepared following the experimental protocol shown in the Scheme 1. Within the reaction of cyclocondensation, from substituted aryl aldehydes 4a–4i and 2–(3,4-diaminophenyl)-3,4,5,6-tetrahydropyrimidin-1-ium chloride 3, by using sodium metabisulfite as oxidising reagents, corresponding 2-aryl substituted benzimidazoles 6a–6g as hydrochloride salts were prepared in moderate reaction yields. Cyclocondesation with 2-quinolinyl-carboxaldehyde 4d and 2-benzothiazolylcarboxaldehyde 4 g failed and desired product was not isolated successfully. Amidino substituted intermediar 3 obtained in the acidic Pinner reaction from corresponding cyano substituted precursors according to the previously published procedures14.
The structures of all newly prepared amidino substituted benzimidazole/benzothiazole derivatives were confirmed by means of 1H and 13 C NMR spectroscopy. NMR analysis based on the values of chemical shifts and H–H coupling constants in the 1H spectra confirmed the structures of compounds. Furthermore, 13 C NMR chemical shifts were consistent with the suggested structures. Also, IR spectroscopy was used for the monitoring of Pinner reaction due to the synthesis of main precursors 2 and 3.
3.2. Antiproliferative activity in vitro
We have tested compounds in MTS cytotoxicity assay and in BrdU proliferative assay. MTS assay give us information of living cells number, in this case, metabolically active cells, whereas BrdU proliferation assay give as a measure of cell population in active division phase.
To avoid that some prominent compounds can be discarded in early screening phase and to give a chance to further profiling only to compounds active on 2 D classical format assays, we tested compounds in both format assays 2 D and 3 D. Both assays were performed on 2 D and 3 D assay format. For 2 D cell assay format, we used a classic two-dimensional in vitro assay and as 3 D assay, we used a hanging drop proliferation cell assay previously described.
We have tested the antiproliferative activity on 2 D and 3 D cell culture assays in vitro of newly synthesised amidino substituted benzimidazole/benzothiazole derivatives 5a–5i and 6a–6g on three human lung cancer cell lines A549, HCC827 and NCI-H358. As standard drugs, doxorubicin, staurosporine and vandetanib were used. Doxorubicin interact with DNA by intercalation and inhibits synthesis of biomoleculas, staurosporin is protein kinase C (PKC) inhibitor and vandetanib multikinase inhibitor (VGFRs, EGFR and RET kinase) is antitumor drug with potential use in a broad range of tumours types, especially thyroid and lung. For 2 D cell assay we used a classic two-dimensional in vitro cancer cell line proliferation assay and as 3 D assay we used a hanging drop proliferation cell assay. Antiproliferative activity for each compound is presented as an IC50 value that was calculated using the program GraphPadPrism software (La Jolla, CA), v. 5.03., and average values from three independent experiments. The results for each of tested compounds are reported as growth percentages from two independent concentrations curves compared with the untreated control cells after drug exposure. In both assay formats, inhibition of proliferation was measured by MTS viability assay. Obtained IC50 inhibitory concentrations in 2 D and 3 D cell culture system for three human lung cancer cell lines are depicted in Table 1.
Table 1.
Antitumor activity of prepared compounds in 2 D and 3 D cell cultures.
| Compound | IC50 (µM)±SD; N = 2 |
|||||
|---|---|---|---|---|---|---|
|
A549
|
HCC827
|
NCI-H358
|
||||
| 2D | 3D | 2D | 3D | 2D | 3D | |
| 5a | >50 | >100 | >50 | >100 | >50 | >100 |
| 5b | >50 | 60 ± 1.44 | >50 | >100 | >50 | >100 |
| 5c | 34 ± 8.65 | 16 ± 0.65 | 7 ± 0.45 | 12 ± 0.16 | 23 ± 0.79 | 34 ± 0.70 |
| 5d | >50 | 16 ± 1.3 | 14 ± 2.09 | >100 | 20 ± 2.53 | 40 ± 4.89 |
| 5e | 36 ± 6.37 | 23 ± 6.24 | 7 ± 0.12 | 22 ± 9.45 | 16 ± 0.98 | 34 ± 0.16 |
| 5f | >50 | >100 | >50 | >100 | >50 | >100 |
| 5g | 41 ± 0.54 | 15 ± 1.35 | 19 ± 2.21 | 17 ± 0.93 | 26 ± 0.30 | 31 ± 0.05 |
| 5h | 38 ± 3.68 | 14 ± 0.38 | 6 ± 0.05 | 12 ± 0.26 | 10 ± 1.00 | 31 ± 0.33 |
| 5i | >50 | >100 | >50 | >100 | >50 | >100 |
| 6a | 15 ± 1.70 | 13 ± 1.46 | 6 ± 0.5 | 9 ± 3.05 | 13 ± 0.28 | 17 ± 1.94 |
| 6b | >50 | >100 | >50 | >100 | >50 | >100 |
| 6c | >50 | >100 | >50 | >100 | >50 | >100 |
| 6d | >50 | >100 | >50 | >100 | >50 | >100 |
| 6e | >50 | >100 | >50 | >100 | >50 | >100 |
| 6f | >50 | >100 | >50 | >100 | >50 | >100 |
| 6g | >50 | >100 | >50 | >100 | >50 | >100 |
| Doxorubicin | 2 ± 0.23 | 5 ± 0.26 | 0.39 ± 0.05 | 0.78 ± 0.08 | 0.11 ± 0.01 | 0.25 ± 0.03 |
| Staurosporine | 1 ± 0.07 | 0.16 | 0.15 ± 0.01 | 0.02 ± 0.00 | 0.15 ± 0.02 | 0.12 ± 0.0 |
| Vandetanib | >25 | >50 | 0.81 ± 0.05 | 2 ± 0.11 | 3 ± 0.85 | 0.30 ± 0.02 |
As presented in Table 1, several compounds, mosty benzothiazole derivatives (5c–e, 5 g, 5 h) and one benzimidazole derivative 6a, displayed antitumor activity in 2 D as well as in 3 D assays against all three cancer cells. Benzothiazole derivatives 5c and 5d show same or slightly lower activity in 2 D MTS assay format in comparison to 3 D format. Benzothiazole derivatives 5e, 5 g, 5 h activity was also same or lower in 2 D format in comparison with IC values on 3 D assay format with exception for A549 cell line (Tabel 1) where copmpounds showed higher activity in 3 D format. In proliferative (BrdU) assay, activity of benzothiazole derivatives 5d, 5e, 5 g were same or lower on 2 D format in comparison to 3 D (Table 2) while 5c and 5 h showed same activity pattern prevous compounds with exception for A549 and NCI-H358 cell lines where activity were opposite: higher on 3 D assay format. The most potent compound was benzimidazole derivative 6a substituted with phenyl ring at position 2 with the lowest IC50 values on both assays, MTS and BrdU, and in format assay, 2 D and 3 D.
Table 2.
Proliferation activity of prepared compounds in 2 D and 3 D cell cultures.
| Compound | IC50 (µM)±SD; N = 2 |
|||||
|---|---|---|---|---|---|---|
|
A549
|
HCC827
|
NCI-H358
|
||||
| 2D | 3D | 2D | 3D | 2D | 3D | |
| 5a | >50 | >100 | >50 | >100 | >50 | >100 |
| 5b | >50 | 87 ± 0.11 | >50 | >100 | >50 | >100 |
| 5c | 48 ± 0.73 | 13 ± 0.78 | 15 ± 2.18 | 21 ± 1.41 | 16 ± 4.14 | 4.31 |
| 5d | 1 | 16 ± 1.30 | 11 ± 0.17 | 32 ± 11.68 | 9 ± 0.31 | 13 ± 1.65 |
| 5e | 16 ± 0.39 | 23 ± 6.24 | 10 ± 1.21 | 24 ± 7.63 | 9 ± 0.65 | 5 ± 0.81 |
| 5f | >50 | >100 | >50 | >100 | >50 | >100 |
| 5g | 7 ± 2.31 | 14 ± 3.07 | 18 ± 0.76 | 17 ± 0.93 | 13 ± 0.45 | 7 ± 2.33 |
| 5h | 10 ± 0.78 | 14 ± 0.06 | 9 ± 0.83 | 12 ± 0.26 | 7 ± 0.07 | 1 ± 0.81 |
| 5i | >50 | >100 | >50 | >100 | >50 | >100 |
| 6a | 11 ± 5.56 | 12 ± 0.28 | 7 ± 0.35 | 12 ± 0.25 | 7 ± 0.3 | 3 ± 1.41 |
| 6b | >50 | >100 | >50 | >100 | >50 | >100 |
| 6c | >50 | >100 | >50 | >100 | >50 | >100 |
| 6d | >50 | >100 | >50 | >100 | >50 | >100 |
| 6e | >50 | >100 | >50 | >100 | >50 | >100 |
| 6f | >50 | >100 | >50 | >100 | >50 | >100 |
| 6g | >50 | >100 | >50 | >100 | >50 | >100 |
| Doxorubicin | 0.06 ± 0.01 | 0.39 ± 0.02 | 0.02 ± 0.00 | 1.03 ± 0.71 | 0.04 ± 0.00 | 0.34 ± 0.36 |
| Staurosporine | 0.23 ± 0.05 | 0.16 ± 0.06 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.15 ± 0.02 | 0.05 ± 0.04 |
| Vandetanib | >25 | 1.25 ± 2.82 | 2.82 ± 1.98 | 0.87 ± 1.15 | 1 ± 0.28 | 1.91 ± 0.09 |
Other tested compounds showed very low activity or were not active at all. As we mention before, viability of cell (MTS assay) is a measure of the living cells whereas proliferation (BrdU) test is a measure of cell division (or proliferation rate). All active compounds (5c–e, 5 g, 5 h and 6a) showed strong activity in BrdU assay on NCI-H358 cell line, in comparison with MTS assay for the same cell line, meaning that compounds have promising antiproliferative effect with lower cytotoxicity potency. As we describe previoslly, cell grown in a 3 D environment support their natural 3 D physical shape and viable cells in the proliferating stage are mainly on the outer layer due to higher exposition to the medium35. We assume that low IC50 values in 3 D format assays for some active compounds are consequence of more exposed outer layer of proliferative cells on spheroids. Nevertheless, the proliferating rates cells in sferoids is depend on cell types, number of cells in inoculum, conditions in which cells are cultured but above of all is depend of cell line sensitivity and suscetability to antitumor drugs.
It is know that A49 cell line is less susceptible in comparison to other two cell lines and therefore IC50s values are higher. When compared to standard drugs, all derivatives were significantly less active except several compounds (5c–e, 5 g, 5 h and 6a) which were more active against A549 cell line in comparison to vandetanib. Reagarding the obtained results of proliferation activity (Table 2), the smiliar results are obtained with the compounds 5c–e, 5 g, 5 h and 6a being the most active ones.
3.3. Annexin V assay – apoptotic changes in plasma membrane
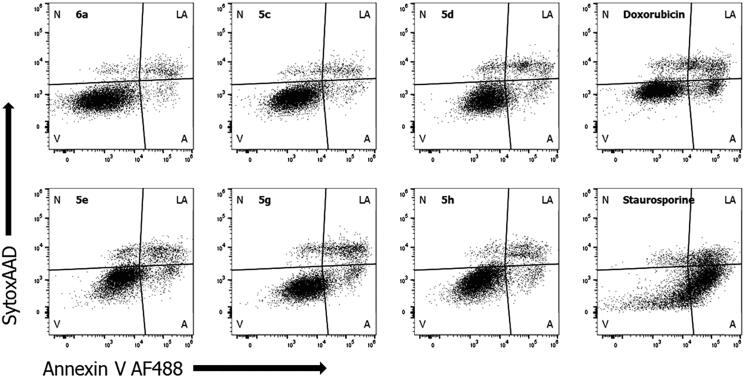
Mechanism of action of most active compounds in antiproliferative assay, benzothiazole derivatives 5c–e, 5 g, 5 h and one benzimidazole derivative 6a, was tested. As shown on Figure 1, all active compounds have similar mode of action on A549 cell line as standard compound doxorubicin, which is a cytotoxic anthracycline antibiotic that binds to nucleic acids by specific intercalation of the planar anthracycline nucleus with the DNA double helix. These results are in line with proposed chemical structure of novel amidino substituted benzimidazole/benzothiazole derivatives that suggest possible intercalation to DNA because of the structural similarity of benzimidazole scaffold with naturally occurring purines. Similar results were obtained for other cell lines tested (not shown).
Figure 1.
Annexin V staining to measure apoptosis. A549 cells were treated with active compounds (6a, 5c–e, 5 g and 5 h) or standard compounds (doxorubicin and staurosporine) at determined IC50 value for 36 h to induce apoptosis in 2 D cell culture (V: viable cells; A: apoptotic; LA: late apoptotic; N: necrotic).
3.4. Dmpk in vitro analysis
ADME results are presented in Table 3 (active compounds) and Table 4 (non-active compounds). To avoid mistake in our conclusion, that some compounds are active in 3 D because such cell cultures are more similar to in vivo conditions and that this is advantage in implementation of 3 D assay, we should excluded possibility that active compounds are active due to better ADME properties (especially solubility, and lipophilicity) in comparison to non-active compounds. Therefore, we profiled active and non-active compounds and see that ADME properties are the same for all tested compounds. In general, no significant difference was observed in ADME profile for these two set of compounds.
Table 4.
Summary of ADME properties of non-nactive compounds.
| 5b | 6b | 6c | 6d | 6e | 6f | 6g | |
|---|---|---|---|---|---|---|---|
| Kinetic solubility range afer 2 h (µM) | >100 | >100 | >100 | 10–30 | 10–30 | 30–100 | 30–100 |
| Chrom logD | 2.18 | 1.17 | 2.60 | 2.43 | 1.38 | 2.18 | 3.27 |
| Microsomes (1 µM) Predicted in vivo hep CL (% LBF) |
|||||||
| Mouse | <30 | <30 | <30 | 71 | 78 | 77 | 31 |
| Human | <30 | <30 | <30 | <30 | <30 | <30 | <30 |
| PPB % bound (recovery) |
|||||||
| Mouse | 75.1 (98) | 63.5 (87) | 87.0 (117) | 93.9 (89) | 74.1 (91) | 88.0 (102) | 99.7 (95) |
| Human | 66.6 (93) | 54.3 (91) | 85.6 (97) | 92.6 (86) | 65.1 (81) | 81.0 (91) | 98.5 (96) |
| Plasma stability (% remaining at 4 h) | |||||||
| Mouse | 95 | 119 | 94 | 92 | 84 | NA | 81 |
| Human | 86 | 98 | 96 | 95 | 98 | 106 | 85 |
| MDCKII-MDR1 | |||||||
| Papp(A2B) | 0.59 | 0.92 | 0.69 | 0.11 | 0.57 | 0.78 | 0.02 |
| Papp(B2A) | 20.5 | 1.02 | 1.93 | 0.86 | 2.17 | 2.49 | 0.2 |
| Efflux ratio | 35.4 | 1.13 | 2.82 | 7.75 | 3.89 | 3.23 | 6.6 |
3.4.1. Kinetic solubility
Majority of compounds showed good (>100 µM) or moderate (30–100 µM) solubility after 2 h incubation with exception of low soluble (10–30 µM) compounds 5 h, 6d and 6e.
3.4.2. Chromatographic lipophilicity study
Majority of both active and inactive compounds have chromlogD7.4 values in the range 1–3.3 and in general, higher values are obtained for a set of active compounds. Exceptions to this are active compound 6a with chromlogD7.4 value of 0.92, and inactive compound 6 g that seems to be the one of the most lipophilic compounds with chromlogD7.4 value 3.27.
3.4.3. Metabolic stability in liver microsomes
The in vitro metabolic stability, expressed as predicted in vivo hepatic clearance (% of liver blood flow, LBF), of selected compounds was investigated in human and mouse liver microsomes. Following incubation in liver microsomes majority of compounds are classified as stable molecules in both species and have low predicted in vivo clearance values (<30% LBF). As exceptions to this, compounds 5e and 6 g (in mouse liver microsomes) and 6a (in both species) are characterised by moderate clearance (30–70% LBF). Compounds 6d, 6e and 6f are characterised by high clearance (>70% LBF) in mouse liver microsomes.
3.4.4. Plasma protein binding and stability in human and mouse plasma
Test compounds showed different range of binding to proteins of human and mouse plasma. In general, lower binding is observed in the group of inactive compounds, what could probably be explained by their lower lipophilicity (with exception of 6 g). 6a, 6 b, 5 b and 6e are characterised by low binding in both species, with fraction bound (% Fb) between 54.3 and 75.1%. Compounds 6c, 6d and 6f, are characterised by moderate binding in both species, with fraction bound (% Fb) between 80 and 95%. 5d showed moderate binding in human plasma, with fraction bound of 94.7% and high binding in mouse plasma (% Fb= 96.0). On contrary, remaining compounds 5c, 5e, 5 g and 5 h in both species and 6 g in human plasma are characterised by high binding, with fraction bound between 95 and 99%. In addition, 6 g, one of the most lipophilic compounds, shows even higher binding, i.e. very high binding in mouse plasma, with fraction bound of 99.7%. All test compounds are stable in both human and mouse plasma (>70% after 4 h incubation). These results are in accordance with recovery values obtained in PPB experiment.
3.4.5. Mdckii-MDR1 permeability assay
Both active and non-active compounds display low permeability, with Papp(A2B) values below 2 × 10−6 cm/sec in A2B direction without P-gp inhibitor (Tables 3 and 4). In the presence of elacridar (tested only for active compounds), A2B peremability, i.e. passive permeability, becomes moderate for compounds 5c–e and 5 g. For two remaining compounds, 5 h and 6a, permeability remains low (<2 × 10−6 cm/sec) even in the presence of P-gp inhibitor. In addition, all active compounds could be classified as P-gp substrates with efflux ratio >2 in the absence of P-gp inhibitor and its significant decrease (at least 50%) in the presence of elacridar. Non-active compounds could also be classified as P-gp substrates based on efflux ratio (>2) in the absence of inhibitor. The only exception is 6 b, low permeable compound whose transport through membrane seems not to be influenced by P-gp (efflux ratio <2).
4. Conclusion
Novel amidino substituted benzimidazole/benzothiazole derivatives 5a–5i and 6a–6g were synthesised and tested for their antiproliferative activity using 2 D and 3 D assays. The compounds were prepared by using conventional synthetic methods. Obtained results revealed that in generaly, the benzothiazole derivatives were more active in comparison to their benzimidazole analogues with the exception of 2-phenyl substituted benzimidazole 6a which showed enhanced activity, especially for HCC827 cell lines. All active compounds showed strong activity in BrdU assay, in comparison with MTS assay especially for the NCI-H358 cell line.
Additionally, ADME properties of the most active compounds were determined in various in vitro assays including solubility, lipophilicity, permeability, metabolic stability and binding to plasma proteins. Five compunds from benzothiazole series (5c–5e, 5 g, 5 h) showed moderate to good kinetic solubility, in general good metabolic stability and high binding to plasma proteins. They are also characterised by low permeability and could be classified as P-gp substrates.
Similar ADME properties were obtained for one benzimidazole derivative (6a), with the exception of lower lipophilicity and consequently low plasma protein bidning and more pronounced metabolism in liver microsomes. In addition, no significant difference in ADME profile was observed for seven selected non-active compounds.
Tested compouds showed simillar mode of action to the doxorubicin and confirmed obtained low IC50s levels on BrdU assay. Due to promissing antiproliferative effect and lower cell cytotoxicity, described compounds have potential to be part of further developing process for new drugs with antitumor activity and likely with less toxic side effects.
Funding Statement
The authors greatly appreciate the financial support of the Croatian Science Foundation under the projects 4379 entitled Exploring the antioxidative potential of benzazole scaffold in the design of novel antitumor agents.
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Barta JA, Powell CA, Wisnivesky JP.. Global epidemiology of lung cancer. Ann Glob Health 2019;85:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal Y, Silakari O.. The therapeutic journey of benzimidazoles: a review. Bioorg Med Chem 2012;20:6208–36. [DOI] [PubMed] [Google Scholar]
- 3.Yadav G, Ganguly S.. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: a mini-review. Eur J Med Chem 2015;97:419–43. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar J, Khan AA, Ali Z, et al. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem 2017;125:143–89. [DOI] [PubMed] [Google Scholar]
- 5.Irfan A, Batool F, Naqvi SAZ, et al. Benzothiazole derivatives as anticancer agents. J Enzyme Inhib Med Chem 2020;35:265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma PC, Sinhmar A, Sharma A, et al. Medicinal significance of benzothiazole scaffold: an insight view. J Enzyme Inhib Med Chem 2013;28:240–66. [DOI] [PubMed] [Google Scholar]
- 7.Gill RK, Rawal RK, Bariwal J.. Recent advances in the chemistry and biology of benzothiazoles. Arch Pharm Chem Life Sci 2015;348:155–24. [DOI] [PubMed] [Google Scholar]
- 8.Silverman RB. The organic chemistry of drug design and drug action. 2nd ed. Burlington (MA): Elsevier Academic Press; 2004. [Google Scholar]
- 9.Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM.. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem Biol Drug Des 2015;86:19–65. [DOI] [PubMed] [Google Scholar]
- 10.More GS, Thomas AB, Chitlange SS, et al. Nitrogen mustards as alkylating agents: a review on chemistry, mechanism of action and current USFDA status of drugs. Anticancer Agents Med Chem 2019;19:1080–102. [DOI] [PubMed] [Google Scholar]
- 11.Tariq S, Kamboj P, Amir M.. Therapeutic advancement of benzothiazole derivatives in thelast decennial period. Arch Pharm Chem Life Sci 2019;352:e1800170. [DOI] [PubMed] [Google Scholar]
- 12.Perin N, Nhili R, Cindrić M, et al. Amino substituted benzimidazo[1,2-a]quinolines: Antiproliferative potency, 3D QSAR study and DNA binding properties. Eur J Med Chem 2016;122:530–45. [DOI] [PubMed] [Google Scholar]
- 13.Perin N, Nhili R, Ester K, et al. Synthesis, antiproliferative activity and DNA binding properties of novel 5-aminobenzimidazo[1,2-a]quinoline-6-carbonitriles. Eur J Med Chem 2014;80:218–27. [DOI] [PubMed] [Google Scholar]
- 14.Hranjec M, Kralj M, Piantanida I, et al. Novel cyano- and amidino-substituted derivatives of styryl-2-benzimidazoles and benzimidazo[1,2-a]quinolines. Synthesis, photochemical synthesis, DNA binding, and antitumor evaluation, part 3. J Med Chem 2007;50:5696–711. [DOI] [PubMed] [Google Scholar]
- 15.Racane L, Tralić-Kulenović V, Kraljević Pavelić S, et al. Novel diamidino-substituted derivatives of phenyl benzothiazolyl and dibenzothiazolyl furans and thiophenes: synthesis, antiproliferative and dna binding properties. J Med Chem 2010;53:2418–32. [DOI] [PubMed] [Google Scholar]
- 16.Sović I, Cindrić M, Perin N, et al. Biological potential of novel methoxy and hydroxy substituted heteroaromatic amides designed as promising antioxidative agents: synthesis, 3D-QSAR analysis, and biological activity. Chem Res Toxicol 2019;32:1880–92. [DOI] [PubMed] [Google Scholar]
- 17.Hranjec M, Piantanida I, Kralj M, et al. Novel amidino-substituted thienyl- and furylvinylbenzimidazole: derivatives and their photochemical conversion into corresponding diazacyclopenta[c]fluorenes. Synthesis, interactions with DNA and RNA, and antitumor evaluation. 4. J Med Chem 2008;51:4899–910. [DOI] [PubMed] [Google Scholar]
- 18.Cindrić M, Jambon S, Harej A, et al. Novel amidino substituted benzimidazole and benzothiazole benzo[b]thieno-2-carboxamides exert strong antiproliferative and DNA binding properties. Eur J Med Chem 2017;136:468–79. [DOI] [PubMed] [Google Scholar]
- 19.Ćaleta I, Kralj M, Marjanović M, et al. Novel cyano- and amidinobenzothiazole derivatives: synthesis, antitumor evaluation, and X-ray and quantitative structure-activity relationship (QSAR) analysis. J Med Chem 2009;52:1744–56. [DOI] [PubMed] [Google Scholar]
- 20.Starčević K, Kralj M, Ester K, et al. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem 2007;15:4419–26. [DOI] [PubMed] [Google Scholar]
- 21.Racané L, Butković K, Martin-Kleiner I, et al. Synthesis and antiproliferative activity in vitro of amidino substituted 2-phenylbenzazoles. Croat Chem Acta 2019;92:181–9. [Google Scholar]
- 22.Racané L, Kraljević Pavelić S, Ratkaj I, et al. Synthesis and antiproliferative evaluation of some new amidino-substituted bis-benzothiazolyl-pyridines and pyrazine. Eur J Med Chem 2012;55:108–16. [DOI] [PubMed] [Google Scholar]
- 23.Racané L, Kraljević Pavelić S, Nhili R, DOI:, et al. New anticancer active and selective phenylene-bisbenzothiazoles: Synthesis, antiproliferative evaluation and DNA binding. Eur J Med Chem 2013;63:882–91. [DOI] [PubMed] [Google Scholar]
- 24.Racané L, Kralj M, Šuman L, et al. Novel amidino substituted 2-phenylbenzothiazoles: synthesis, antitumor evaluation in vitro and acute toxicity testing in vivo. Bioorg Med Chem 2010;18:1038–44. [DOI] [PubMed] [Google Scholar]
- 25.Racané L, Stojković R, Tralić-Kulenović V, et al. Interactions with polynucleotides and antitumor activity of amidino and imidazolinyl substituted 2-phenylbenzothiazole mesylates. Eur J Med Chem 2014;86:406–19. [DOI] [PubMed] [Google Scholar]
- 26.Perin N, Bobanović K, Zlatar I, et al. Antiproliferative activity of amino substituted benzo[b]thieno[2,3-b]pyrido[1,2-a]benzimidazoles explored by 2D and 3D cell culture system. Eur J Med Chem 2017;125:722–35. [DOI] [PubMed] [Google Scholar]
- 27.Estman A. Improving anticancer drug development begins with cell culture: misinformation perpetrated by the misuse of cytotoxicity assays. Oncotarget 2017;8:8854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:5–63. [DOI] [PubMed] [Google Scholar]
- 29.Zlatar I, Jelić D, Kelava V, et al. Comparison of antitumor activity of some benzothiophene and thienothiophene carboxanilides and quinolones in 2D and 3D cell culture system. Croatica Chemica Acta 2017;90:413–24. [Google Scholar]
- 30.Di L, Kerns EH, Carter G.. Drug-like property concepts in pharmaceutical design. Curr Pharm Des 2009;15:2184–94. [DOI] [PubMed] [Google Scholar]
- 31.Di L, Kerns EH.. Pharmaceutical profiling in drug discovery. Drug Discov Today 2003;8:316–23. [DOI] [PubMed] [Google Scholar]
- 32.Boggust WA, Cocker W.. Experiments in the chemistry of benzthiazole. J Chem Soc 1949:355–62. [Google Scholar]
- 33.Wlodkowic D, Skommer J, Darzynkiewicz Z.. Flow cytometry-based apoptosis detection. Methods Mol Biol 2009;559:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racanè L, Tralić-Kulenović V, Mihalić Z, et al. Synthesis of new amidino-substituted 2-aminothiophenoles: mild basic ring opening of benzothiazole. Tetrahedron 2008;64:11594–602. [Google Scholar]
- 35.Brajša K, Trzun M, Zlatar I, Jelić D.. Three-dimensional cell cultures as a new tool in drug discovery. Period. Biol 2016;118:59–65. [Google Scholar]