Abstract
Robotic assistance in laparoscopic surgery was introduced at the turn of this millennium, marking a milestone in the history of surgery. Urologists were early adopters of robotic technology and the indications of robot-assisted surgery in urology are expanding. Over the last 20 years, the da Vinci surgical system was the dominant system in the robotic surgical market. However, the recent expiration of Intuitive patents has allowed new systems to enter the market more freely. We performed a nonsystematic literature review using the PubMed/MEDLINE search engines. The aim of this review was to briefly summarize the currently available robotic surgical systems for laparoscopic urologic surgery. New surgical devices have already been launched in the robotic market and the da Vinci systems have some competition. The innovation of robotic technology is continuing, and new features such as an open-console design, haptic feedback, smaller instruments, and separately mounted robotic arms have been introduced. A new robotic era is rising, and new systems and technologies enhancing patient care are welcomed.
Keywords: Robotic surgical procedures, Robotics, Urology
INTRODUCTION
Robot-assisted surgery revolutionized minimally invasive surgery (MIS) and overcame the technical limitations of laparoscopy. The robotic assistance expanded the indications of MIS in more delicate and complex procedures with the help of magnified three-dimensional (3D) and high-resolution (HD) visualization, improved dexterity, tremor filtration, and great precision of movement [1,2,3]. The da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was first introduced in 1999 and was approved for human use by the U.S. Food and Drug Administration (FDA) in 2000. Intuitive succeeded in making the da Vinci surgical system synonymous with robot-assisted laparoscopic surgery and dominated the surgical robot market for almost 20 years [4].
In recent years, many companies have developed robotic surgical systems, and more are currently under development. However, the protection of Intuitive's intellectual property with numerous patents worked as a minefield against potential competitors seeking to enter the market [5]. In 2019, some of the da Vinci patents expired and the market is now more accessible to new robotic surgical systems. This study aimed to review the current robotic surgical systems for laparoscopic surgery available in clinical practice.
METHODS
A thorough nonsystematic literature review was performed using the PubMed/MEDLINE search engines with the terms “new robotic surgical system” (n=2,130), “robotic surgical device” (n=8,423), “robotics AND urology” (n=7,465), and “da Vinci system” (n=2,505). Lists of references from the retrieved articles were also searched. Additional information was obtained by searching through google.com (Google Inc, Mountain View, CA, USA) and the official websites of the robot's companies. The review focused on surgical systems for laparoscopic surgery with regulatory approval for human use and urological applications.
1. Robotic surgical systems
The currently approved surgical robotic systems are summarized in Table 1.
Table 1. Summary of the currently approved robotic surgical systems and their features.
| Robotic system | Approval/year | Patient cart | Surgeon console | Controllers | Camera diameter (mm) | Instrument diameter/DOF | Instrument use | Additional feature |
|---|---|---|---|---|---|---|---|---|
| da Vinci Xi | FDA/2014 CE Mark/2014 |
Single | Closed | Finger loops | 8 | 8 mm/7° | 10 times | Multiquadrant surgery Port hopping camera Dual console |
| da Vinci SP | FDA/2018 | Single | Closed | Finger loops | 12×10 | 6 mm/7° | 10 times | Single-port 360° rotating boom Articulating camera |
| Senhance | FDA/2017 (not for urology) CE Mark/2016 |
Multiple | Open/3D glasses | Laparoscopic handles | 10 | 10 mm/7° 5 mm/6° 3 mm/6° |
Unlimited | Eye-tracking system Haptic feedback No port docking |
| Revo-I | KMFDS/2017 | Single | Closed | Finger loops | 10 | 7.4 mm/7° | 20 times | Extensive force use warning messages |
| Versius | CE Mark/2019 | Multiple | Open/3D glasses | Joystick handles | 10 | 5 mm/7° | NA | Haptic feedback Portable independent arms Surgeon in standing position No port docking |
| Avatera | CE Mark/2019 | Single | Semi-closed | Finger loops | 10 | 5 mm/7° | Single | Space-saving (2 units) |
| Hinotori | JMHLW/2020 | Single | Semi-closed | Finger loops | NA | NA | NA | No port docking |
DOF, degrees of freedom; FDA, U.S. Food and Drug Administration; SP, Single-Port; 3D, three-dimensional; KMFDS, Korean Ministry of Food and Drug Safety; NA, no available data; JMHLW, Japanese Ministry of Health, Labor and Welfare.
2. da Vinci surgical system
Since the first FDA clearance in 2000, the da Vinci system remained the main robotic surgical system for over 20 years. The system has been approved for urology, gynecology, cardiothoracic, colorectal, head & neck, and general surgery, both for adult and pediatric use [6]. During this period, four generations of multi-arm da Vinci systems have been launched: the da Vinci 2000, S, Si, and Xi. All generations followed the same concept: robotic arms mounted in a single patient cart, a closed surgeon console containing loop-like handles with Endowrist technology, and instruments of 8 mm in diameter with 7 degrees of freedom [7]. The latest system, the da Vinci Xi, which was released in 2014, is equipped with thinner robotic arms and newly designed joints, longer instrument shafts, and an 8-mm digital end-mounted camera that can be attached in any arm. These technical features are very helpful in multiquadrant procedures [8].
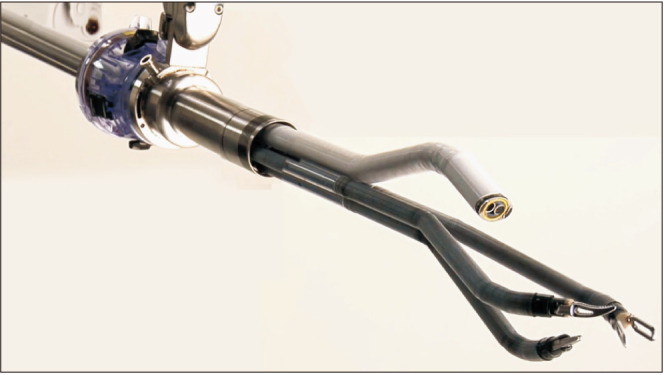
In May 2018 the FDA approved the da Vinci Single-Port (SP) system for use in urologic procedures, which is the first purpose-built single-site surgical system released to the market. The patient cart utilizes a single robotic arm with an oval 12x10-mm 3D-HD articulating camera and three 6-mm wristed instruments placed through a 25-mm multichannel cannula from a single point of entry (Fig. 1) [9]. The instruments are double-joined, with a distal (elbow) and a proximal (shoulder) site of articulation, and provide 7 degrees of freedom and intracorporeal triangulation to the target anatomy [10]. The surgeon console of the SP system is similar to its multiport counterpart, although several new features and technological advances have been added. A new “relocation” pedal moves the entire robotic arm while the instruments are maintained in the same position. The navigation interface, an image overlay, helps the surgeon track each instrument's relative position during surgery. Several high-volume surgeons and institutions have described their initial experience with the da Vinci SP in various procedures and have reported their series. These numerous clinical publications include descriptions for radical prostatectomy, radical cystectomy, radical and partial nephrectomy, pyeloplasty, and other reconstructive procedures [11,12,13,14,15,16,17].
Fig. 1. The da Vinci Single-Port (SP) surgical system. Original graph from Gosrisirikul C, et al. Asian J Endosc Surg 2018;11:291–9 [9], with permission of John Wiley and Sons.
3. Senhance
The Senhance surgical system (TransEnterix Surgical Inc., Morrisville, NC, USA), formerly known as Telelap ALF-X, was first developed by an Italian company (Sofar, Milan, Italy) and received the CE Mark certification in 2016 for all abdominal and noncardiac thoracic procedures. In October 2017, Senhance became the first new robotic system to receive FDA clearance since 2000; however, the FDA clearance does not include urology procedures and is limited to general surgery and gynecology procedures [18]. This multiport system comprises up to four independent robotic arms in separate carts (Fig. 2) [19]. The surgeon is ergonomically seated in an open console, called the “cockpit,” and a monitor provides 3D HD visualization with the use of polarized glasses. The camera manipulation is controlled by the surgeon's eye movements through an infrared eye-tracking system [20]. The handles are based on laparoscopic instruments and offer haptic feedback, which can help in a smooth transition for laparoscopy-experienced surgeons [21]. The available instruments have diameters ranging from 3 to 10 mm, and all can be resterilized and reused [22]. The first clinical applications mainly focused on general surgery and gynecologic surgeries [23,24]. Recently, the use of Senhance for radical prostatectomy and various urological procedures was described in Europe [21,25].
Fig. 2. Senhance robotic surgical system. Original graph from Rao PP. World J Urol 2018;36:537–41 [19], with permission of Springer Nature.
4. Revo-I
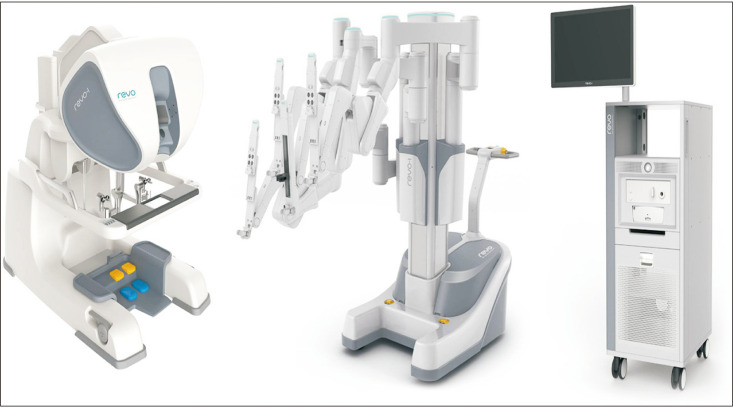
The Revo-I (Meere Company Inc., Yongin, Korea) surgical platform is a master-slave system, which received approval for human use from the Korean Ministry of Food and Drug Safety in August 2017. The system, which is quite similar to the da Vinci Si system, consists of a four-arm patient cart, a closed surgeon console, and a HD vision cart (Fig. 3) [26]. The 3D endoscope is 10 mm in diameter. The instruments are fully wristed, providing 7 degrees of freedom, with a diameter of 7.4 mm, and are reusable for up to 20 times [26]. The safety and feasibility of fallopian tube reconstruction, cholecystectomy, and partial nephrectomy were assessed in animal preclinical studies [27,28,29]. In 2018, the first human study using the Revo-I surgical system in Retzius-sparing robot-assisted radical prostatectomy was published [26].
Fig. 3. Revo-I surgical robot. Adapted from Chang KD, et al. BJU Int 2018;122: 441–8 [26].
5. Versius
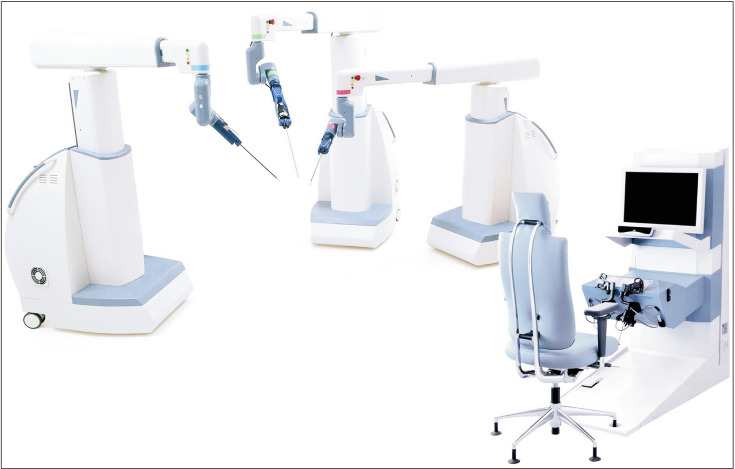
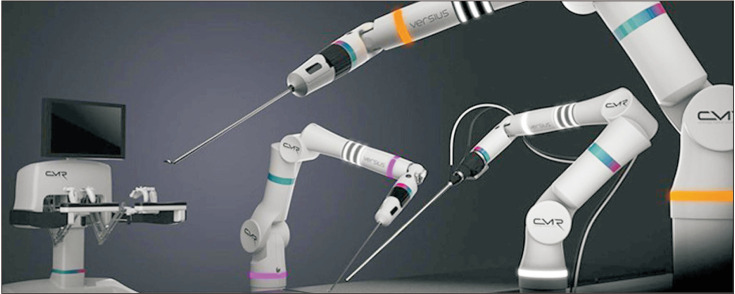
The Versius surgical system (Cambridge Medical Robotics Ltd., Cambridge, UK) received the European CE Mark in March 2019. The robotic arms of this surgical system are individually mounted in portable carts (Fig. 4) [30]. Each robotic arm has a shoulder, an elbow, and a wrist joint, mimicking the movements of the human arm. The console has an open design and requires polarized glasses for 3D HD vision [31]. The surgeon can choose to operate in a sitting or a standing position while controlling the system through joystick handles. The surgeon also receives haptic feedback from the handles. The instruments are 5 mm in diameter and are fully wristed with 7 degrees of freedom [32]. The system has been used in a preclinical setting, where multiple surgeons successfully performed prostate surgeries, renal surgeries, and pelvic lymph node dissection in cadavers and porcine models [33]. Furthermore, the completion of the first clinical series of gynecologic and upper gastrointestinal surgeries using the Versius surgical system in India was announced [34], and the first clinical report of 30 robotic radical hysterectomies was recently published [35].
Fig. 4. Versius surgical robotic system. Original graph from Peters BS, et al. Surg Endosc 2018;32:1636–55 [30], with permission of Springer Nature.
6. Avatera
The Avatera system (avateramedical GmbH, Jena, Germany) is another robotic system that was recently (November 2019) cleared in Europe for use in minimally invasive procedures focused in gynecology and urology surgeries. The company plans to launch the robot into the market later this year [36]. Avatera is composed of two main units, the patient cart or the surgical robot, and the console or the control unit. The patient cart has three robotic arms for the 5-mm fully articulating instruments and one for the 10-mm endoscope. Besides their diameter, the instruments offer 7 degrees of freedom. All the instruments are single-use and disposable. The surgeon sits at the console in an ergonomically designed position. A microscope-like eyepiece provides 3D full HD vision without enveloping the entire head of the surgeon for easier communication with the surgical team. Finally, the instruments are controlled by loop-like handles [37]. To date, there are no published data describing the use of the Avatera system, and the publication of clinical studies is awaited.
7. Hinotori
The Hinotori surgical robot system (Medicaroid Corporation, Kobe, Japan) is the first made-in-Japan robotic system, which most recently (August 2020) received regulatory approval from the Japanese Ministry of Health, Labor and Welfare [38]. Medicaroid will launch the robotic system to the Japanese market initially for use in urological surgeries, mainly for prostate cancer, and internationally in the next 2 to 3 years, after receiving approval for each country. Hinotori, a master-slave robot, has three components: the surgeon cockpit, the operative unit, and the vision unit. The four robotic arms, which are mounted in the operative unit, have multiple joints and can move in eight axes. The console is a semi-closed design, and a microscope-like eyepiece provides a 3D view of the surgical field. The wristed instruments are controlled by the surgeon with loop-like handles [39]. Publication of further information for this robotic system and the results of the first human trials are expected.
DISCUSSION
Urologists have always been keen on adopting new technological advances to benefit their patients and were also pioneers in the field of robotic surgery. Initially, the da Vinci system was approved for cardiac surgery and was applied in coronary artery bypass surgery [40]. In 2001, the first cases of robot-assisted radical prostatectomy were performed in Europe [41,42,43], and the first urologic robotic program for radical prostatectomy was established at the Vattikuti Urology Institute in the USA [44]. Since then, the application of the da Vinci system in urology has exponentially grown to include kidney, bladder, reconstructive, and functional urologic surgery [45]. Even nowadays, urology leads the way in robotic innovation; the da Vinci SP, the first specialty-purpose engineered system for single-site surgery, was initially cleared for use in urological surgical procedures.
The da Vinci SP can be characterized as a game-changing technology, and Intuitive Surgical is credited for the development and clearance of this innovative system. The application of robotics in laparoendoscopic single-site surgery (R-LESS) in urology was started in 2008 when four cases of R-LESS radical prostatectomy were successfully performed [46]. Since then, multiple small series of R-LESS have been published in the literature, including mainly prostate and kidney surgery [47,48]. However, this approach was not widely adopted by the urological community, as the robotic system used was not specially designed for R-LESS, and issues related to loss of triangulation, instrument clashing, and limited assistant workspace were noted [49]. The da Vinci SP was specially designed to overcome these challenges and has already been used in several demanding urologic procedures [11,12,13,14,15,16,17].
The personal experience of the authors with the da Vinci SP system includes mainly radical prostatectomies through the transperineal and Retzius-sparing approaches. Both procedures could be safely performed without conversion and in acceptable operative time. The instruments of the SP robot provide adequate traction and counter-traction for the dissections, in addition to their smaller diameter and two sites of articulation. The handling of the robotic instruments through the console is similar to the previous da Vinci systems. The learning curve for an expert robotic surgeon is minimal and could be readily familiar with the different mechanics of the instruments' movements, as the articulation is provided by an elbow and a shoulder. True single-port surgery is feasible with this device and the assistant's ports can be placed from a single incision through a GelPOINT advanced access platform. However, the working space of the assistant's instruments is limited owing to collisions with the bulky multichannel port and extracorporeal robotic arm, and an additional port is helpful in the initial cases. Better cosmesis is the incontrovertible advantage of this system, albeit further comparative studies are needed to assess the impact of the adoption of this system on surgical outcomes and patient morbidity.
For over 20 years, Intuitive built a practically insurmountable competitive moat blocking the entrance of other companies into the market by developing a superior product, protecting its intellectual portfolio, clearing multiple regulatory hurdles, establishing worldwide training centers, amassing a worldwide installation base, building a huge network of distributors and technical support, and gaining surgeons' trust [50]. According to Intuitive, over 5,500 da Vinci systems have been installed in 67 countries around the world and more than 7.2 million robot-assisted surgical procedures have been performed using their systems. The development of new robotic systems from different companies and the recent expiration of Intuitive's patents will change the robotic surgical market [4]. Currently, other than the da Vinci systems, five other robotic surgical systems are commercially available: Senhance has regulatory approval for human use in the USA, Europe, and Japan; Versius and Avatera hold a CE Mark certification for use in Europe; whereas Revo-I and Hinotori are available in the Korean and Japanese markets, respectively.
Competition is considered as an essential element of all markets. Competition in robotic surgical systems might promote faster innovation and development of high-quality products. Surgeons and hospitals will be able to choose the robotic system which best fits their needs and setting. Furthermore, the acquisition and maintenance of a robotic system is highly costly for hospitals; the equation of the cost also includes instruments and accessories. In 2017, the cost of the da Vinci system was estimated to be $3,568 per robotic procedure and questions were raised about the cost-effectiveness [51]. Expectantly, an open, competitive market could decrease the expense and increase the availability of robotic surgery around the world. New robotic companies should study well their marketing and pricing policy in order to be competitive.
Even though the da Vinci system brought many robots with significant developments for each generation to the market, there has been no significant improvement in the console. The da Vinci closed console design envelops the face of the surgeon and decreases his or her awareness of the surrounding operative theatre. The communication is mainly verbal through the microphone of the console and the speakers of the system, and an experienced surgical team with excellent communication skills is needed. Senhance and Versius offer an open-console design, which promotes verbal and nonverbal communication between the surgeon and the surgical team [20,31]. However, the use of polarized glasses decreases the brightness of the operating field and the adoption of a glasses-free 3D display technology could be a more innovative step [52]. The novel eye-tracking control of the camera is an interesting feature of the Senhance system. The image is centered at the point at which the surgeon is focusing; as with all indirect camera control systems, the risk of unintentional movements and malfunctions should be clinically assessed [53].
Haptic feedback refers to the ability to sense the degree of force a surgeon is applying to the tissue. Currently, haptic feedback is lacking in da Vinci surgical systems. Robotic surgeons have compensated for this limitation by developing a “pseudo-haptic” ability, which relies on optical cues to assess the tension on tissues [54]. Senhance and Versius feature haptic feedback; the force and its direction applied from the tips of the instruments on tissues are translated in counter-movements in console handles [21,32]. The haptic feedback could provide additional advantages of robot-assisted surgery, namely, improved tissue manipulation, structure characterization, and less suture breakage and could further reduce the learning curve of robotics [55].
All new companies tried to reduce the instruments' diameter of their robots to promote less tissue trauma and invasiveness and move toward better cosmesis. Revo-I utilizes 7.4-mm instruments, but the port size remains at 8 mm [26]. Versius and Avatera use instruments of 5 mm in diameter [32,37]. Senhance, except for the 5-mm instruments, offers the option of microlaparoscopy with 3-mm instruments, although the lack of articulation is a significant drawback [56].
The global market for robotic surgery was valued at $2.4 billion in 2019, and it is continuously expanding and is anticipated to reach $8.2 billion by 2025 [57]. Many companies are interested in entering this global market and are developing a surgical robotic system. Titan Medical Inc. (Toronto, Canada) has been developing the Single Port Orifice Robotic Technology (SPORT) surgical system for many years, which is a purpose-built system for single-site surgery with a flexible camera and two multi-articulated instruments [58]. File submission for FDA clearance is still pending and as the da Vinci SP has already received clearance, the SPORT robot will face high competition. Multiport robotic systems under development which have already been unveiled without receiving approval for any market are the Hugo RAS system (Medtronic, Dublin, Ireland), a modular, open-console surgical robot [59]; the BITRACK system (Rob Surgical, Barcelona, Spain), which is a three-arm, open-console design with a sensory feedback system [60]; and the Tumai surgical robot (MicroPort, Shanghai, China) [61]. The question is, even if most of these new robotic systems receive FDA/CE mark approval, how many of them will commercially succeed.
The da Vinci surgical system is considered the gold standard in robot-assisted laparoscopic surgery. Unavoidably, each emerging system has to compete with and be compared to the existing gold standard and generate data evaluating potential effectiveness and safety. New companies seem to have carefully studied the features lacking in the established system, and developers have implemented new technologies to try to improve the capabilities of the previous system. Whether these new features will attract surgeons to adopt and utilize the new robotic systems in everyday clinical practice is an issue that will be answered in the near future.
CONCLUSIONS
We are at the verge of a new era of robot-assisted surgery. The hegemony of the da Vinci surgical systems is subject to change as a handful of platforms for robot-assisted laparoscopic surgical procedures are available in the market worldwide. The innovation of robotic surgery is still growing; new robotic systems are in the process of development and hopefully will approach the market in the next few years. As surgeons, we are looking forward to welcoming new systems and technologies that will enable us to improve patient care in a cost-effective manner.
ACKNOWLEDGMENTS
Dr. Periklis Koukourikis acknowledges the Hellenic Urological Association for the scholarship for training in an International Fellowship program.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Periklis Koukourikis and Koon Ho Rha.
- Data acquisition: Periklis Koukourikis.
- Data analysis and interpretation: Periklis Koukourikis.
- Drafting of the manuscript: Periklis Koukourikis.
- Critical revision of the manuscript: Koon Ho Rha.
- Supervision: Koon Ho Rha.
- Approval of the final manuscript: Periklis Koukourikis and Koon Ho Rha.
References
- 1.Luciani LG, Chiodini S, Mattevi D, Cai T, Puglisi M, Mantovani W, et al. Robotic-assisted partial nephrectomy provides better operative outcomes as compared to the laparoscopic and open approaches: results from a prospective cohort study. J Robot Surg. 2017;11:333–339. doi: 10.1007/s11701-016-0660-2. [DOI] [PubMed] [Google Scholar]
- 2.Hyams ES, Mufarrij PW, Stifelman MD. Robotic renal and upper tract reconstruction. Curr Opin Urol. 2008;18:557–563. doi: 10.1097/MOU.0b013e32830fe43d. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AJ, Pariser JJ, Anderson BB, Pearce SM, Gundeti MS. The robotic appendicovesicostomy and bladder augmentation: the next frontier in robotics, are we there? Urol Clin North Am. 2015;42:121–130. doi: 10.1016/j.ucl.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Tindera M. Robot wars: $60B Intuitive Surgical dominated its market for 20 years. Now rivals like Alphabet are moving in [Internet] Jersey City: Forbes; 2019. Feb 14, [cited 2020 Jun 21]. Available from: https://www.forbes.com/sites/michelatindera/2019/02/14/intuitive-surgical-stock-robot-surgery-da-vinci-alphabet-jnj-ceo-gary-guthart/#565d4979a37b. [Google Scholar]
- 5.Intuitive. da Vinci robotic assisted, Patent notices [Internet] Sunnyvale: Intuitive Surgical; 2020. [cited 2020 Jun 20]. Available from: https://www.intuitive.com/en-us/about-us/company/legal/patent-notice. [Google Scholar]
- 6.Oleynikov D. Robotic surgery. Surg Clin North Am. 2008;88:1121–1130. viii. doi: 10.1016/j.suc.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Freschi C, Ferrari V, Melfi F, Ferrari M, Mosca F, Cuschieri A. Technical review of the da Vinci surgical telemanipulator. Int J Med Robot. 2013;9:396–406. doi: 10.1002/rcs.1468. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Kim H, Kwak S, Baek K, Na G, Kim JH, et al. The settings, pros and cons of the new surgical robot da Vinci Xi system for transoral robotic surgery (TORS): a comparison with the popular da Vinci Si system. Surg Laparosc Endosc Percutan Tech. 2016;26:391–396. doi: 10.1097/SLE.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 9.Gosrisirikul C, Don Chang K, Raheem AA, Rha KH. New era of robotic surgical systems. Asian J Endosc Surg. 2018;11:291–299. doi: 10.1111/ases.12660. [DOI] [PubMed] [Google Scholar]
- 10.Dobbs RW, Halgrimson WR, Talamini S, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robotic surgery: the next generation of minimally invasive urology. World J Urol. 2020;38:897–905. doi: 10.1007/s00345-019-02898-1. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal DK, Sharma V, Toussi A, Viers BR, Tollefson MK, Gettman MT, et al. Initial experience with da Vinci single-port robot-assisted radical prostatectomies. Eur Urol. 2020;77:373–379. doi: 10.1016/j.eururo.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Covas Moschovas M, Bhat S, Rogers T, Onol F, Roof S, Mazzone E, et al. Technical modifications necessary to implement the da Vinci single-port robotic system. Eur Urol. 2020;78:415–423. doi: 10.1016/j.eururo.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Kaouk J, Garisto J, Eltemamy M, Bertolo R. Step-by-step technique for single-port robot-assisted radical cystectomy and pelvic lymph nodes dissection using the da Vinci®SP™ surgical system. BJU Int. 2019;124:707–712. doi: 10.1111/bju.14744. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Thomas D, Salama G, Ahmed M. Single port robotic radical cystectomy with intracorporeal urinary diversion: a case series and review. Transl Androl Urol. 2020;9:925–930. doi: 10.21037/tau.2020.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na JC, Lee HH, Yoon YE, Jang WS, Choi YD, Rha KH, et al. True single-site partial nephrectomy using the SP surgical system: feasibility, comparison with the Xi single-site platform, and step-by-step procedure guide. J Endourol. 2020;34:169–174. doi: 10.1089/end.2019.0528. [DOI] [PubMed] [Google Scholar]
- 16.Heo JE, Kang SK, Koh DH, Na JC, Lee YS, Han WK, et al. Pure single-site robot-assisted pyeloplasty with the da Vinci SP surgical system: initial experience. Investig Clin Urol. 2019;60:326–330. doi: 10.4111/icu.2019.60.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billah MS, Stifelman M, Munver R, Tsui J, Lovallo G, Ahmed M. Single port robotic assisted reconstructive urologic surgerywith the da Vinci SP surgical system. Transl Androl Urol. 2020;9:870–878. doi: 10.21037/tau.2020.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. 510(k) premarket notification [Internet] Silver Spring: U.S. Food and Drug Administration; 2018. May 25, [cited 2020 Jun 21]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?start_search=1&productcode=GCJ&applicant=TRANSENTERIX%2C INC. [Google Scholar]
- 19.Rao PP. Robotic surgery: new robots and finally some real competition. World J Urol. 2018;36:537–541. doi: 10.1007/s00345-018-2213-y. [DOI] [PubMed] [Google Scholar]
- 20.Bozzini G, Gidaro S, Taverna G. Robot-assisted laparoscopic partial nephrectomy with the ALF-X robot on pig models. Eur Urol. 2016;69:376–377. doi: 10.1016/j.eururo.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Kaštelan Ž, Knežević N, Hudolin T, Kuliš T, Penezić L, Goluža E, et al. Extraperitoneal radical prostatectomy with the Senhance Surgical System robotic platform. Croat Med J. 2019;60:556–559. doi: 10.3325/cmj.2019.60.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TransEnterix. The first in digital laparoscopy. Senhance Surgical System [Internet] Morrisville: TransEnterix; 2020. [cited 2020 Jun 13]. Available from: https://www.senhance.com/us/digital-laparoscopy. [Google Scholar]
- 23.Fanfani F, Restaino S, Rossitto C, Gueli Alletti S, Costantini B, Monterossi G, et al. Total laparoscopic (S-LPS) versus TELELAP ALF-X robotic-assisted hysterectomy: a case-control study. J Minim Invasive Gynecol. 2016;23:933–938. doi: 10.1016/j.jmig.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Spinelli A, David G, Gidaro S, Carvello M, Sacchi M, Montorsi M, et al. First experience in colorectal surgery with a new robotic platform with haptic feedback. Colorectal Dis. 2018;20:228–235. doi: 10.1111/codi.13882. [DOI] [PubMed] [Google Scholar]
- 25.Samalavicius NE, Janusonis V, Siaulys R, Jasėnas M, Deduchovas O, Venckus R, et al. Robotic surgery using Senhance®robotic platform: single center experience with first 100 cases. J Robot Surg. 2020;14:371–376. doi: 10.1007/s11701-019-01000-6. [DOI] [PubMed] [Google Scholar]
- 26.Chang KD, Abdel Raheem A, Choi YD, Chung BH, Rha KH. Retzius-sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: surgical technique and results of the first human trial. BJU Int. 2018;122:441–448. doi: 10.1111/bju.14245. [DOI] [PubMed] [Google Scholar]
- 27.Abdel Raheem A, Troya IS, Kim DK, Kim SH, Won PD, Joon PS, et al. Robot-assisted Fallopian tube transection and anastomosis using the new REVO-I robotic surgical system: feasibility in a chronic porcine model. BJU Int. 2016;118:604–609. doi: 10.1111/bju.13517. [DOI] [PubMed] [Google Scholar]
- 28.Lim JH, Lee WJ, Park DW, Yea HJ, Kim SH, Kang CM. Robotic cholecystectomy using Revo-i Model MSR-5000, the newly developed Korean robotic surgical system: a preclinical study. Surg Endosc. 2017;31:3391–3397. doi: 10.1007/s00464-016-5357-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim DK, Park DW, Rha KH. Robot-assisted partial nephrectomy with the REVO-I robot platform in porcine models. Eur Urol. 2016;69:541–542. doi: 10.1016/j.eururo.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Peters BS, Armijo PR, Krause C, Choudhury SA, Oleynikov D. Review of emerging surgical robotic technology. Surg Endosc. 2018;32:1636–1655. doi: 10.1007/s00464-018-6079-2. [DOI] [PubMed] [Google Scholar]
- 31.Robotics Research. Next-generation robot revealed to the world for the first time [Internet] Swaffham Bulbeck: Robotics Research; 2018. Sep 07, [cited 2020 Jun 21]. Available from: https://www.roboticsresear.ch/articles/15309/next-generation-robot-revealed-to-the-world-for-the-first-time. [Google Scholar]
- 32.Morton J, Hardwick RH, Tilney HS, Gudgeon AM, Jah A, Stevens L, et al. Preclinical evaluation of the versius surgical system, a new robot-assisted surgical device for use in minimal access general and colorectal procedures. Surg Endosc. 2020 May 13; doi: 10.1007/s00464-020-07622-4. [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas BC, Slack M, Hussain M, Barber N, Pradhan A, Dinneen E, et al. Preclinical evaluation of the Versius Surgical System, a new robot-assisted surgical device for use in minimal access renal and prostate surgery. Eur Urol Focus. 2020 Mar 10; doi: 10.1016/j.euf.2020.01.011. [Epub]. [DOI] [PubMed] [Google Scholar]
- 34.CMR Surgical. CMR. Surgical successfully completes first set of robotically assisted surgical procedures in humans [Internet] Cambridge: CMR Surgical; 2019. May 13, [cited 2020 Jun 14]. Available from: https://cmrsurgical.com/cmr-surgical-successfully-completes-first-set-of-robotically-assisted-surgical-procedures-in-humans/ [Google Scholar]
- 35.Puntambekar SP, Goel A, Chandak S, Chitale M, Hivre M, Chahal H, et al. Feasibility of robotic radical hysterectomy (RRH) with a new robotic system. Experience at Galaxy Care Laparoscopy Institute. J Robot Surg. 2020 Jul 24; doi: 10.1007/s11701-020-01127-x. [Epub]. [DOI] [PubMed] [Google Scholar]
- 36.avateramedical GmbH. CE mark for avatera®, the first German system for robot-assisted, minimally invasive surgery, setting the foundation for strategic growth plans [Internet] Jena: avateramedical GmbH; 2019. Nov 14, [cited 2020 Aug 7]. Available from: https://www.avatera.eu/en/company/news/detail?tx_news_pi1%5Bnews%5D=19&cHash=0b499a1adf30ef40b4d441aa562e0a7b. [Google Scholar]
- 37.avateramedical GmbH. Avatera system [Internet] Jena: avateramedical GmbH; 2020. [cited 2020 Jun 13]. Available from: https://www.avatera.eu/en/avatera-system. [Google Scholar]
- 38.SurgRob. Medicaroid's hinotori Surgical Robot System approved in Japan [Internet] SurgRob; 2020. Aug 13, [cited 2020 Oct 17]. Available from: http://surgrob.blogspot.com/2020/08/medicaroids-hinotori-surgical-robot.html. [Google Scholar]
- 39.Medicaroid. Hinotori robotic assisted surgery system [Internet] Kobe: Medicaroid; 2020. [cited 2020 Oct 17]. Available from: http://www.medicaroid.com/en/product/hinotori/ [Google Scholar]
- 40.Mohr FW, Falk V, Diegeler A, Autschback R. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg. 1999;117:1212–1214. doi: 10.1016/s0022-5223(99)70261-8. [DOI] [PubMed] [Google Scholar]
- 41.Pasticier G, Rietbergen JB, Guillonneau B, Fromont G, Menon M, Vallancien G. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. 2001;40:70–74. doi: 10.1159/000049751. [DOI] [PubMed] [Google Scholar]
- 42.Abbou CC, Hoznek A, Salomon L, Olsson LE, Lobontiu A, Saint F, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165(6 Pt 1):1964–1966. doi: 10.1097/00005392-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 43.Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–410. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 44.Jeong W, Kumar R, Menon M. Past, present and future of urological robotic surgery. Investig Clin Urol. 2016;57:75–83. doi: 10.4111/icu.2016.57.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikhail D, Sarcona J, Mekhail M, Richstone L. Urologic robotic surgery. Surg Clin North Am. 2020;100:361–378. doi: 10.1016/j.suc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Kaouk JH, Goel RK, Haber GP, Crouzet S, Desai MM, Gill IS. Single-port laparoscopic radical prostatectomy. Urology. 2008;72:1190–1193. doi: 10.1016/j.urology.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Won Lee J, Arkoncel FR, Rha KH, Choi KH, Yu HS, Chae Y, et al. Urologic robot-assisted laparoendoscopic single-site surgery using a homemade single-port device: a single-center experience of 68 cases. J Endourol. 2011;25:1481–1485. doi: 10.1089/end.2010.0656. [DOI] [PubMed] [Google Scholar]
- 48.White MA, Autorino R, Spana G, Hillyer S, Stein RJ, Kaouk JH. Robotic laparoendoscopic single site urological surgery: analysis of 50 consecutive cases. J Urol. 2012;187:1696–1701. doi: 10.1016/j.juro.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 49.Autorino R, Kaouk JH, Stolzenburg JU, Gill IS, Mottrie A, Tewari A, et al. Current status and future directions of robotic single-site surgery: a systematic review. Eur Urol. 2013;63:266–280. doi: 10.1016/j.eururo.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 50.Rassweiler JJ, Autorino R, Klein J, Mottrie A, Goezen AS, Stolzenburg JU, et al. Future of robotic surgery in urology. BJU Int. 2017;120:822–841. doi: 10.1111/bju.13851. [DOI] [PubMed] [Google Scholar]
- 51.Childers CP, Maggard-Gibbons M. Estimation of the acquisition and operating costs for robotic surgery. JAMA. 2018;320:835–836. doi: 10.1001/jama.2018.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Cui F, Li J, Shao W, Wang W, Li J, et al. Development and clinical applications of glasses-free three-dimensional (3D) display technology for thoracoscopic surgery. Ann Transl Med. 2018;6:214. doi: 10.21037/atm.2018.05.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rassweiler JJ, Teber D. Advances in laparoscopic surgery in urology. Nat Rev Urol. 2016;13:387–399. doi: 10.1038/nrurol.2016.70. [DOI] [PubMed] [Google Scholar]
- 54.Hagen ME, Meehan JJ, Inan I, Morel P. Visual clues act as a substitute for haptic feedback in robotic surgery. Surg Endosc. 2008;22:1505–1508. doi: 10.1007/s00464-007-9683-0. [DOI] [PubMed] [Google Scholar]
- 55.Saracino A, Deguet A, Staderini F, Boushaki MN, Cianchi F, Menciassi A, et al. Haptic feedback in the da Vinci Research Kit (dVRK): a user study based on grasping, palpation, and incision tasks. Int J Med Robot. 2019;15:e1999. doi: 10.1002/rcs.1999. [DOI] [PubMed] [Google Scholar]
- 56.Montlouis-Calixte J, Ripamonti B, Barabino G, Corsini T, Chauleur C. Senhance 3-mm robot-assisted surgery: experience on first 14 patients in France. J Robot Surg. 2019;13:643–647. doi: 10.1007/s11701-019-00955-w. [DOI] [PubMed] [Google Scholar]
- 57.Grand View Research. Surgical robots market size [Internet] San Francisco: Grand View Research; 2019. Dec, [cited 2020 Oct 17]. Available from: https://www.grandviewresearch.com/industry-analysis/surgical-robot-market. [Google Scholar]
- 58.Titan Medical Inc. Sport surgical system [Internet] Toronto: Titan Medical Inc; 2020. [cited 2020 Oct 17]. Available from: https://titanmedicalinc.com/technology/ [Google Scholar]
- 59.Newmarker C. Medtronic finally unveils its new robot-assisted surgery system [Internet] Cleveland: MassDevice; 2019. Sep 24, [cited 2020 Oct 17]. Available from: https://www.massdevice.com/medtronic-finally-unveils-its-new-robot-assisted-surgery-system/ [Google Scholar]
- 60.Rob Surgical. Bitrack system for minimally invasive surgery [Internet] Barcelona: Rob Surgical; 2020. [cited 2020 Oct 18]. Available from: https://www.robsurgical.com/bitrack/ [Google Scholar]
- 61.Robot performs first prostatectomy in Shanghai [Internet] Beijing: People's Daily Online; 2019. Nov 19, [cited 2020 Oct 18]. Available from: http://en.people.cn/n3/2019/1115/c90000-9632516.html. [Google Scholar]