Abstract
Purpose
To describe the surgical technique and examine the feasibility and outcomes following robotic pelvic exenteration and extended pelvic resection for rectal and/or urological malignancy.
Materials and Methods
We present a case series of seven patients with locally advanced or synchronous urological and/or rectal malignancy who underwent robotic total or posterior pelvic exenteration between 2012–2016.
Results
In total, we included seven patients undergoing pelvic exenteration or extended pelvic resection. The mean operative time was 485±157 minutes and median length of stay was 9 days (6–34 days). There was only one Clavien–Dindo complication grade 3 which was a vesicourethral anastomotic leak requiring rigid cystoscopy and bilateral ureteric catheter insertion. Eighty-five percent of patients had clear colorectal margins with a median margin of 3.5 mm (0.7–8.0 mm) while all urological margins were clear. Six out of seven patients had complete (grade 3) total mesorectal excision. Three patients experienced recurrence at a median of 22 months (21–24 months) post-operatively. Of the three recurrences, one was systemic only whilst two were both local and systemic. One patient died from complications of dual rectal and prostate cancer 31 months after the surgery.
Conclusions
We report a large series examining robotic pelvic exenteration or extended pelvic resection and describe the surgical technique involved. The robotic approach to pelvic exenteration is highly feasible and demonstrates acceptable peri-operative and oncological outcomes. It has the potential to benefit patients undergoing this highly complex and morbid procedure.
Keywords: Colorectal cancer, Minimally invasive surgical procedures, Pelvic exenteration, Prostate cancer, Surgery
INTRODUCTION
First described by Brunschwig in 1948, pelvic exenteration is a highly complex surgical technique for advanced pelvic malignancy [1]. With advancements in surgical technology, particularly in the fields of laparoscopy and robotics, and trends towards decreasing invasiveness, a robotic minimally invasive approach to this highly morbid surgery could be advantageous. Rectal cancer afflicts more than 50 persons per 100,000 and accounts for more than 80,000 deaths per year in Europe [2,3]. Multiple clinical studies have examined outcomes following laparoscopic vs open resection of rectal cancer, the majority demonstrating similar oncological outcomes and improved perioperative outcomes [2,3,4,5,6]. Few studies have examined outcomes following robotic approach to rectal cancer excision. Prostate cancer is the most common form of cancer in males and the second leading cause of cancer related death. In 2012, it is estimated that 1 million men were diagnosed with prostate cancer world-wide. Robotic approaches for prostatectomy have been well-described [7], even in the setting of previous pelvic irradiation [8]. Despite its prevalence, invasion into surrounding pelvic viscera is far less common than rectal cancer and therefore pelvic exenteration or extended pelvic resections are often only required in end stage disease and rarely considered until recently as patient were mostly offered radiotherapy.
Pelvic exenteration and other similar extended pelvic resections are a highly invasive procedure that involves en bloc resection of involved pelvic organs. As such, there are multiple forms of pelvic resections that dependent on the organ involvement and degree of disease invasion. Total pelvic exenteration, as the name suggests involves the total surgical removal of the pelvic viscera including the bladder, rectum and reproductive organs. Anterior pelvic exenteration involves the en bloc excision of the bladder and reproductive organs and thus spares the rectum. This approach is used in advanced urogenital malignancy. Posterior pelvic exenteration involves the excision of the rectum and reproductive organs and preservation of the bladder and is utilized in advanced rectal malignancy. Pelvic exenteration and complex extended pelvic resections are conventionally performed with an open approach. The extreme invasiveness of pelvic exenteration meant an association with high morbidity and mortality. Indeed, in the past post-operative mortality had been reported as high as 23%, although peri-operative and medical advancements have seen improved outcomes in recent times [9]. Improved preoperative staging with medical imaging advancements and a multidisciplinary approach have likely improved patient selection for pelvic exenteration.
The theoretical advantages of robotic surgery are the enhanced three-dimensional and magnified views of enclosed spaces that can be achieved as well as increased dexterity with fine precision surgical instruments which make it ideal for operating in confined anatomical spaces such as the pelvis. With this in mind and considering the great complexity of pelvic exenterative surgery for pelvic malignancy, a robotic approach to this procedure may be advantageous. Despite this, very few case reports of this technique have been published in the literature, particularly pertaining to rectal and urological malignancy.
The aim of this study is to examine the intraoperative, post-operative and oncological outcomes following robotic pelvic exenteration and extended pelvic resections for rectal or urological malignancy, and to compare the findings to current literature on both open and robotic approach to pelvic exenteration for this indication. We also conducted a review of the current evidence regarding robotic exenteration and draw comparison to this case series.
MATERIALS AND METHODS
1. Study population
An institutional ethics review board of Royal Brisbane Hospital (Brisbane, QLD, Australia) approved retrospective review of a case series of seven patients who underwent robotic pelvic exenteration or similar extended pelvic resections at a single hospital in Australia over 4 years from 2012 to 2016 (approval number: HREC/17/QRBW/238). Patients were managed by two consultants (one colorectal surgeon and one urological surgeon).
2. Surgical approach
Within the current series, procedures were performed by the same two surgeons (both one urological and one colorectal surgeon). The mean operative time was 485±157 minutes.
The robotic approach was performed utilizing a routine 7 port trans-peritoneal technique for pelvic surgery using Da Vinci Si, Xi or S system (Intuitive Surgical, Sunnyvale, Ca, USA). The patient was positioned in steep Trendelenburg position (from 20° to 25°) and secured with a bean bag. Following safe peritoneal access, the and ascending and decending and bowel was mobilized medially along Toldts fascia and ureters was identified bilaterally. The ureters were then mobilized distally to the bladder.
In general, the pelvic dissection progressed from posterior to anterior with the posterior mesorectal dissection being performed first. For the colorectal resection, the mesorectal plane was identified and dissection was continued to the levator ani. The vascular pedicles were transected with endoGIA vascular staplers (Medtronic, Dublin, Ireland). Following colorectal mobilization, the urological resection was performed depending on primary disease. In cases of synchronous disease, the colorectal resection was completed prior to urologic resection. In cases of locally advanced disease, anterior rectal dissection was not performed as the mass was retrieved en bloc.
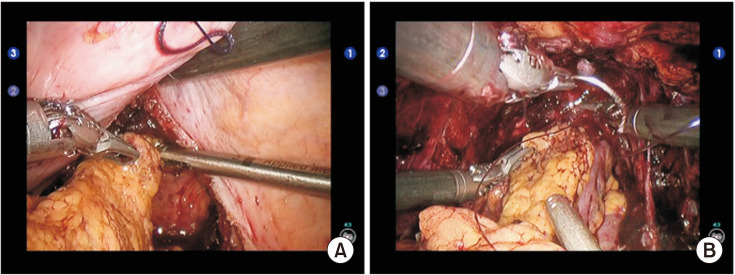
In cases of cystectomy, bladder dissection was performed by creating the space of Retzius to the prostatic apex. The dorsal venous complex was controlled with a 3-0 VLock suture (Medtronic) and the urethra was transected. The lateral pedicles of the bladder were controlled with endoGIA staplers. Where appropriate, the specimen was retrieved en bloc (Fig. 1). A mini laparotomy was required to retrieve the specimen. In such cases where cystectomy were performed, an ileal conduit was fashioned utilizing 15 cm of terminal ileum.
Fig. 1. En bloc resection combining abdominoperineal resection and cystoprostatectomy, right posterior pedicle dissection.
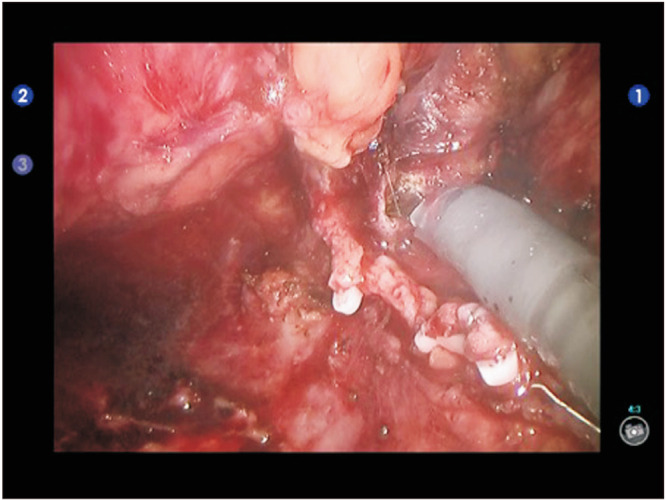
In cases of prostatectomy, the space of Retzius was developed to the prostatic apex. The dorsal venous complex was controlled with a 3-0 VLock suture. Bladder neck transection was performed and the seminal vesicles and vas deferens was dissected. Retrograde posterior dissection was performed where appropriate. For locally advanced disease, the specimen was retrieved en bloc following urethral transection was transected. For cases involving a bowel anastomosis with overlying vesico-urethral anastomosis an omental flap was mobilised at this point also. If complete abdominoperineal resection (APR) was not indicated the colo-anal anastomosis was then performed and covered with the previously mobilised omental flap prior to vesico-urethral anastomosis (Fig. 2).
Fig. 2. In case of ultra-low anterior resection with colo-anal anastomosis, an omental flap is inserted between the both digestive and urinary fistula. (A) First, the omental flap is tunneled posteriorly to the bladder toward the pelvis. (B) Then the omental flap is sutured anterior to the colo-anal anastomosis before performing the urethra-vesical anastomosis.
3. Data extraction
Patients were identified from an existing patient data base and data collected through online medical records and physical patient charts. Data was extracted for patient demographics (age, body mass index [BMI], American Society of Anesthesiologists [ASA] score, Charlson Comorbidity index), baseline oncological data (cancer type, grade, stage), intraoperative data (operation type, time, estimated blood loss, type of anastomosis and urinary diversion), post-operative outcomes (length of stay, intensive care unit length of stay, Clavien–Dindo complication grade, time to flatus/bowel motion) and oncological outcome data (histology, margins, positive nodes, grade of total mesorectal excision, recurrence, mortality).
RESULTS
1. Patient details
The median age was 60 years (range 46–70 years), all were male, median Charlson Comorbidity index was 3 (range 3–6), Median ASA was 1 (range 1–3) and median BMI was 25.1 kg/m2 (range 18.1–31.6 kg/m2) (Table 1). Two patients had rectal cancer with local extension, two patients had prostate cancer with local extension while three patients had dual rectal and prostate cancer. Three patients underwent APR plus cystoprostatectomy (CP) and ileoconduit (IC) formation, two patients underwent APR plus en bloc prostatectomy and vesicourethral anastomosis and two patients underwent ultra-low anterior resection (ULAR) plus en bloc prostatectomy (Table 2).
Table 1. Key demographic, operative, post-operative and oncological outcome data.
| Variable | Value |
|---|---|
| Age (y) | 60 (46–79) |
| Sex (male/female) | 7/0 |
| Body mass index (kg/m2) | 25.1 (18.1–31.6) |
| ASA score (1/2/3) | 3/3/1 |
| Charlson Comorbidity index | 3 (3–6) |
| Distance from anal verge of colorectal Ca (cm) | 4 (1–7) |
| Operative time (min) | 485±157 |
| Length of stay (d) | 9 (6–34) |
| Clavien-Dindo classification grade (I/II/III) | 4/3/0 |
| Colorectal oncological margins (mm) | 3.5 (0.7–8.0) |
| Grade of total meso-rectal excision (III/II/I) | 6/1/0 |
| Adjuvant therapy (yes/no) | 4/3 |
| Neoadjuvant therapy (yes/no) | 5/2 |
| Recurrence | 3 |
| Recurrence location (local/systemic) | 2/3 |
| Time to recurrence (mo) | 22 (21–24) |
| Death | 1 |
Values are presented as median (range), number only, or mean±standard deviation.
Ca, cancer; ASA, American Society of Anesthesiologists.
Table 2. Case series stratified by individual patients.
| Variable | Patient | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age (y) | 46 | 72 | 58 | 79 | 64 | 62 | 60 |
| Sex | Male | Male | Male | Male | Male | Male | Male |
| BMI (kg/m2) | 18.1 | 22.2 | 29.4 | 25.1 | 23.8 | 31.6 | * |
| ASA | 2 | 2 | 2 | 3 | 1 | 1 | 1 |
| Charlson Comorbidity | 3 | 6 | 3 | 6 | 4 | 4 | 3 |
| Colorectal malignancy | Primary rectal Ca | Primary rectal Ca | None | Rectal Ca recurrence | Primary rectal Ca | Primary rectal Ca | None |
| Urological malignancy | None | None | Primary prostate Ca | Primary prostate Ca | Primary prostate Ca | Primary prostate Ca | Primary prostate Ca |
| Pre-op PSA | None | None | * | 4.5 | 8.1 | 18 | 28 |
| T (CR/URO for dual) | 4 | 4a | 4 | 4b/2c | 1/3a | 2/3a | 4 |
| N | 2a | 0 | 0 | 1 | 0 | 0 | 1 |
| M | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Surgery | APR+CP+IC | APR+prostatectomy | APR+cystectomy+IC | APR+CP+IC | ULAR+prostatectomy | APR+prostatectomy | ULAR+prostatectomy |
| Urological anastomosis | Ileal-ileal | Vesicourethral | Ileal-ileal | Ileal-ileal | Vesicourethral | Vesicourethral | Vesicourethral |
| Colorectal anastomosis | None | None | None | None | Colorectal | None | Colorectal |
| Stoma | End-colostomy+urostomy | End-colostomy | End-colostomy+urostomy | End-colostomy+urostomy | Loop ileostomy | End colostomy | Loop ileostomy |
| Operation time (min) | 200 | 450 | 600 | 600 | 420 | 670 | 460 |
| EBL (mL) | * | * | * | 400 | 150 | 1,000 | 700 |
| LOS (d) | 9 | 13 | 12 | 34 | 8 | 6 | * |
| Clavien–Dindo complication | II | I | I | II | I | I | III |
| Morbidity | Recurrent UTI requiring IVABx. R) Ureteric stricture requiring stent | Ileus | Ileus | Ileus, perineal wound dehiscence/infection (VAC and IVABx) | UTI (POABx) | Ileus | Vesicourethral anastomotic leak and sepsis |
| Post-op transfusion | No | No | No | No | No | No | No |
| Colorectal histology | Adenocarcinoma | Adenocarcinoma (high grade) | None | Adenocarcinoma (high grade) | Adenocarcinoma | Adenocarcinoma | None |
| Urological histology | None | None | Prostate adenocarcinoma (high grade) | Prostate adenocarcinoma | Prostate adenocarcinoma | Prostate adenocarcinoma | Prostate adenocarcinoma |
| Lymph nodes positive | 4/12 | 0/13 | 0/0 | 1/8 | 0/15 | 0/13 | 4/12 |
| Grade of TME | 3 | 3 | 3 | 3 | 2 | 3 | 3 |
| Colorectal margins CRM (mm) | 4 | 0.7 | None | 3.5 | 3 | 8 | None |
| Colorectal margins DRM (mm) | 100 | 35 | Clear, mm not reported | Clear, mm not reported | 10 | 15 | Clear, mm not reported |
| Urological margins | None | None | Clear | Clear | Clear | Clear | Clear |
| Neo-adjuvant therapy/response | Yes/Grade 3 | Yes/Grade 1 | No | Yes/Grade 3 | No | Yes/Grade 1 | Yes/Grade 1 |
| Type of therapy | Chemoradiotherapy | Chemoradiotherapy | None | Chemoradiotherapy+5-FU | Chemoradiotherapy (45 Gy XRT and 5-FU) | Chemoradiation 5-FU+45 Gy XRT | ADT |
| Adjuvant therapy | Yes | Yes | No | Yes | No | Yes | No |
| Type of therapy | Modified FOLFOX-6 | 5-FU | None | 5-FU | None | Capecitabine incomplete course | None |
| Recurrence (months post-op) | 21 | None | 22 | 24 | None | None | None |
| Recurrence (local) | None | None | Right internal iliac node | Pelvic | None | None | None |
| Recurrence (systemic) | Retrocrural/supraclavicular nodes, lung | None | Thoracic vertebrae | Lung, femoral neck | None | None | None |
| Survival | Yes | Yes | Yes | No | Yes | Yes | Yes |
Grade 3 is considered complete. Grade 2 is considered near complete. Response to neoadjuvant therapy adjudged by grades where Grade 1 is moderate response and Grade 3 is poor response. BMI, body mass index; ASA, American Society of Anesthesiologists; Ca, cancer; op, operative; PSA, prostate specific antigen; CR/URO, colorectal or urological; ARP, abdominoperineal resection; ULAR, ultra-low anterior resection; CP, cystoprostatectomy; IC, ileal conduit; EBL, estimated blood loss; LOS, length of stay; UTI, urinary tract infections; IVABx, intravenous antibiotics; VAC, vacuum dressing; POABx, per oral antibiotics; TME, total mesorectal excision; CRM, circumferential resection margin; DRM, distal resection margin; XRT, external beam radiotherapy; FU, Fluorouracil; ADT, androgen deprivation therapy; FOLFOX, folinic acid, fluorouracil, oxaliplatin.
*Denotes missing data.
2. Post-operative outcomes
Post-operatively, all patients were monitored in intensivecare. No cases suffered cognitive compromise as a result of prolonged Trendelenburg positioning. There was one vesicourethral anastomotic leak occurred in a patient who underwent ULAR and prostatectomy and bilateral ureteric re-implantation requiring rigid cystoscopy and bilateral ureteric catheter insertion. Two patients developed urinary tract infections (UTIs) while 3 patients were managed conservatively for ileus. Median time to bowel motion was 4 days (range 1–8 days), no patients required post-operative transfusions or prolonged intensive care unit (ICU) stay. Median length of stay was 9 days (range 6–34 days), median ICU length of stay was 1 day (range 1 day).
3. Oncological outcomes
All patients had clear urological margins. With respect to colorectal margins, one patient was considered R1 as the circumferential margin was 0.7 mm (total mesorectal excision grade 3), with a median colorectal margin of 3.5 mm (range 0.7–8.0 mm). Six out of seven patients had complete (grade 3) total meso-rectal excision, one had grade 2. Three out of six patients who underwent lymph node dissection had positive pelvic nodes. Three patients experienced recurrence at a median of 22 months (range 21–24 months) post-operatively with recurrence experienced in one advanced rectal adenocarcinoma, one advanced prostate cancer, and one dual rectal and prostate cancer. Of the three recurrences, one was systemic whilst two were both local and systemic. One local recurrence represented a right internal iliac node in a patient that did not undergo concurrent lymph node dissection. The other patient with local recurrence had disease characterized by a large left lateral pelvic soft tissue mass that was avid on flurodeoxyglucose positron emission tomography. These findings, along with location of systemic disease are characterized in Table 2. One patient died from locally advanced dual rectal and prostate cancer leading to small bowel obstruction and aspiration pneumonitis 31 months after the surgery.
DISCUSSION
We present here a large single case-series of robotic pelvic exenteration and extended pelvic resections for synchronous or locally advanced urological and/or rectal malignancies. Pelvic exenteration and similar resections are complex surgeries used for locally advanced pelvic malignancies that involve multi-visceral resection with the goal of achieving negative resection margins [10,11]. It is traditionally performed as open surgery and has been associated with high morbidity and mortality [11]. Indeed, in the past, pelvic exenteration was initially intended as a palliative procedure and had high operative mortality [11]. With medical advancement, there has been a shift to curative intent and operative mortality has been reported as less than 5% with overall 5-year survival of more than 50% in many recent studies [11]. The invasiveness of the procedure however remains problematic, with high rates of complication including anastomotic fistulas reported in the post-operative period.
Recent times have seen a trend towards minimally invasive surgical techniques such as laparoscopic and robotic surgery [11]. There is good evidence supporting a laparoscopic approach to localized rectal cancer with multiple multicentre trials having demonstrated similar oncological outcomes [10]. A smaller body of evidence supports a robotic approach to these cases [11,12]. In the field of Uro-Oncology, the use of robotic surgery for localized prostate and bladder cancer has evidence for equivalent or non-inferior operative, perioperative and oncological outcomes [7,13]. For advanced pelvic malignancies requiring exenterative surgery, there exists a number of studies regarding the use of laparoscopic pelvic exenteration for primary gynaecological and urological malignancy, and isolated case reports for rectal cancer. Robotic approach to pelvic exenteration has not been well described in the literature, but could theoretically be beneficial given the improved access and visualization of robotic surgery in the pelvis.
The current case series is among the largest to date that evaluates outcomes following robotic pelvic exenteration. We have found the robotic approach to pelvic exenteration and extended pelvic resections to be feasible (Table 1). While laparoscopic surgery has been limited in exenterative surgery by limited visualization, mobility and dexterity deep in the pelvis, robotic surgery has an enhanced three-dimensional view and superior ergonomics which allow for enhanced dissections. Intra-operative outcomes were acceptable, while only one grade III Clavien–Dindo complication was encountered. No patients required post-operative transfusions and the median length of stay 9 days (range 6–34 days). Of our series, 85.7% had clear colorectal and all had clear urological margins though there were three cases of recurrence (two of which were local) and one death which was related to primary malignancy.
Review of the literature pertaining to robotic pelvic exenteration identified three articles and seven patients (Table 3) [10,14,15]. These cases did not demonstrate any significant differences in patient demographic data or peri-operative outcomes to the reported case series. Oncological outcome data was generally lacking and could not be included in this literature review, although 85.7% of cases did achieve negative surgical margins. Similarly, long term follow-up data, perhaps the most meaningful, was not included in any case included in the meta-analysis and therefore cannot be commented on. Recurrence and survival data are particularly significant for evaluation of the success of pelvic exenteration surgery and the absence of this data is a significant limitation that makes it very difficult to assess overall outcomes following robotic approach to pelvic exenteration. Similarly, functional outcomes following pelvic exenteration have not been assessed by the cases included in the literature review, but form an important parameter used to gauge the success of this procedure.
Table 3. Outcomes summary for cases of robotic pelvic exenteration included in literature review.
| Variable | Study | ||||||
|---|---|---|---|---|---|---|---|
| 1 (Shin et al. [14]) | 2 (Shin et al. [14]) | 3 (Shin et al. [14]) | 4 (Castillo et al. [10]) | 5 (Winters et al. [15]) | 6 (Winters et al. [15]) | 7 (Winters et al. [15]) | |
| Age (y) | 47 | 42 | 41 | 71 | 57 | 78 | 61 |
| Sex | Male | Male | Male | Male | Male | Male | Male |
| BMI (kg/m2) | 19 | 27 | 18 | - | 26.8 | 21.8 | 24 |
| ASA | 1 | 1 | - | - | - | - | - |
| Charlson Comorbidity | - | - | - | - | 2 | 2 | 3 |
| Colorectal malignancy | Rectal Ca and colon Ca | Rectal Ca | Rectal Ca | None | None | None | Rectal Ca |
| Urological malignancy | None | None | None | Prostate Ca | Prostate Ca | Urothelial cell Ca of bladder | None |
| Surgery | Robotic ISR and en bloc prostatectomy with a simultaneous laparoscopic right hemicolectomy | Robotic abdominopelvic resection with en bloc prostatectomy | Robotic abdominopelvic resection with en bloc prostatectomy | Robotic abdominopelvic resection with en bloc cystoprostatectomy | Robotic abdominopelvic resection with en bloc prostatectomy | Robotic abdominopelvic resection with en bloc cystoprostatectomy | Robotic abdominopelvic resection with en bloc prostatectomy+rectus flap |
| Urological anastomosis | Vesicourethral | Vesicourethral | None | None | None | None | None |
| Colorectal anastomosis | Colo-anal | None | None | None | None | None | None |
| Stoma | None | Colostomy | Ileal conduit and end colostomy | Double barrelled wet colostomy and loop colostomy | Ileal conduit and end colostomy | Ileal conduit and end colostomy | Ileal conduit and end colostomy |
| Operation time (min) | 585 | 550 | 480 | 249 | 660 | 600 | 530 |
| EBL (mL) | 700 | 600 | 300 | 600 | 800 | 500 | 350 |
| LOS (day) | 28 | - | 8 | 7 | 7 | 8 | 7 |
| Clavien–Dindo complication (I to IV) | 0 | III | 0 | 0 | 0 | II | 0 |
| Colorectal histology | Adenocarcinoma | Adenocarcinoma | Poorly differentiated adenocarcinoma | None | None | None | Adenocarcinoma |
| Urological histology | None | None | None | Acinar adenocarcinoma | Adenocarcinoma | Urothelial cell Ca of bladder | None |
| Lymph nodes positive | 0 | 7/52 | 4/12 | 1–17 | 0 | 0 | 0 |
| Colorectal margins | Positive | Negative | Negative | - | - | - | Negative |
| Urological margins | - | - | - | Negative | Negative | Negative | - |
| Neo-adjuvant therapy | None | Yes | Yes | None | None | None | Yes |
| Adjuvant therapy | Yes | Yes | Yes | Yes | None | None | None |
| Recurrence (months post-op) | None | None | None | None | None | None | None |
| Recurrence (local) | None | None | None | None | None | None | None |
| Recurrence (systemic) | None | None | None | None | None | None | None |
| Survival | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
BMI, body mass index; ASA, American Society of Anesthesiologists; Ca, cancer; ISR, intersphincteric resection; EBL, estimated blood loss; LOS, length of stay; op, operation; -, not available.
On comparison with historical open exenteration metaanalysis data by Platt et al. [9], and additionally multicenter cohort data from the PelvEx collaborative and single centre cohort data from Quyn et al. [16], the robotic exenteration case series compares favourably in peri-operative outcome measures including estimated blood loss, length of stay and return to theatre (Table 4) [9,16]. Platt et al. [9] analyzed 1,016 patients from across 16 studies and the PelvEx collaborative included 1,184 patients from 27 centres. Both studies included complex resections including sacrectomy which should be kept in mind when drawing comparison [9,16]. Quyn et al. [16] reports the outcomes of 104 exenterations for locally advanced primary rectal cancer and is generally comparable across clinico-histopathological parameters [16]. Drawing meaningful oncologic comparisons from these series is not suitable given the variation in disease processes between the published data. Specifically, the previous pelvic exenteration data pertains to a single locally advanced disease process, while our series pertains to synchronous primary tumours. The current series reports one case of recurrence with the remainder locally advanced primary or synchronous primary rectal or prostate malignancy.
Table 4. Comparison of outcomes between case series, review of literature of robotic pelvic exenteration and open pelvic exenteration.
| Variable | Case series | Literature review of robotic exenteration | Platt et al. [9] | Quyn et al. [16] | PelvEx Collaborative |
|---|---|---|---|---|---|
| Sample size | 7 | 7 | 1,016 | 104 | 1,184 |
| Age (y) | 60 (46–79) | 57 (41–78) | 59 (50–65) | 62 (27–86) | 63 (IQR, 56–69) |
| Sex (male/female) | 7/0 | 7/0 | 640/376 | 54/50 | 752/432 |
| Body mass index (kg/m2) | 25.1 (18.1–31.6) | 22.9 (18–27) | - | - | 25 (22–28) |
| ASA score (1/2/3/4) | 3/3/1/0 | 2/0/0/0 | - | 17/54/17/0 | - |
| Charlson Comorbidity index | 3 (3–6) | 2 (2–3) | - | - | - |
| Operative time (min) | 485±157 | 522±133 | 444 (266–726) | 438 (132–930) | 509±201 |
| Blood loss (mL) | 375 (0–1,000) | 600 (300–800) | 2,114 (540–7,550) | 1,500 (100–13,000) | - |
| Length of stay | 9 (6–34) | 8 (7–28) | 18 (9–49) | 19 (7–97) | 15 (IQR, 10–46) |
| Clavien-Dindo Classification Grade (I–II/III–IV) | 7/0 | 1/1 | 380/280 | -/8 | -/380 |
| Return to theatre (%) | 0.0 | 0.0 | 14.6 | 5.0 | 7.2 |
| 30 day mortality (%) | 0.0 | 0.0 | 0.0 (0.0–8.7) | 1.0 | 1.8 |
| R0 resection (%) | 85.7 | 85 | 74 | 86 | 55.4 |
| Adjuvant therapy (yes/no) | 4/3 | 3/7 | - | - | - |
| Neoadjuvant therapy (yes/no) | 5/2 | 4/7 | - | - | 614/515 |
| 5 year survival (%) | 85.7 | 100.0 | 32.0 | * | 22.5 |
Values are presented as number only, median (range), mean±standard deviation, percent only, or median only.
ASA, American Society of Anesthesiologists; IQR, interquartile range; -, not available.
*Denotes missing data.
The current series reports a higher proportion of Clavien–Dindo grade I–II complications compared to Platt et al. [9], however this may have been secondary to enhanced reporting of minor complications in the case series, with the majority of the complications accounted for by ileus and UTI, and many larger studies (including the cohort studies used for comparison) only reported on the major complications (Table 5). Indeed, Quyn et al. [16] have not reported any cases of ileus or UTI, which may suggest these weren't considered minor complications. There was a higher proportion of Clavien–Dindo grade III–IV complications, returns to theatre and ICU in the open exenteration studies. Furthermore, there were improved rates of R0 resections and 5 years survivals, although comparison are not definitive given the limitation of sample size in the robotic exenteration case series, and it is difficult to attribute any statistical significance to these findings at this stage.
Table 5. Comparison of complications between case series, review of literature of robotic pelvic exenteration and open pelvic exenteration.
| Variable | Complication | ||||
|---|---|---|---|---|---|
| Current series (n=7) | Robotic exenterations in the literature (n=7) | Platt et al. [9] (n=1,016) | Quyn et al. [16] (n=104) | PelvEx collaborative (n=1,184) | |
| Ileus | 4 | 0 | - | - | - |
| UTI | 2 | 1 | - | - | - |
| Pneumonia | 0 | 0 | - | 5 | - |
| Wound infection/dehiscence | 1 | 1 | - | 3 | - |
| Anastomotic leak | 1 | 1 | - | 4 | - |
| VTE | 0 | 0 | - | - | - |
| Abdominal sepsis /abscess | 0 | 1 | - | 4 | - |
| Line sepsis | 0 | 0 | - | 1 | - |
| AKI | 0 | 0 | - | 1 | - |
| Cardiac event | 0 | 0 | - | 15 | - |
| CVA | 0 | 0 | - | 1 | - |
| Organ failure | 0 | 0 | - | - | - |
| Return to theatre | 0 | 0 | - | 5 | - |
| Return to ICU | 0 | 0 | - | 2 | - |
| Total Clavien–Dindo I/II | 7 | 2 | 381 | 41 | - |
| Total Clavien–Dindo III/IV | 0 | 1 | 348 | 8 | 380 |
| Death within 30 days | 0 | 0 | - | 1 | 21 |
Values are presented as number only.
UTI, urinary tract infections; VTE, venous thromboembolism; AKI, acute kidney injury; CVA, cerebrovascular accident; ICU, intensive care unit; -, not available.
Despite the promise of this approach, there are inherent limitations of a case series including small sample size, retrospective design and selection bias, mean that meaningful outcomes are difficult to draw from this data set. All cases where discussed at multidisciplinary meeting, however due to the lack of uniformity in the primary disease, variations in neoadjuvant and adjuvant therapy protocols occurred. Long term functional outcomes and perineal morbidity were not recorded and thus cannot be commented on in the current manuscript. The lack of data pertaining to recurrence, survival and function is a significant limitation for a procedure of this nature. Further, data from our institution pertaining to open exenteration and extended resections were not available for comparison with the current series. Subsequently, definitive comments on the overall safety and success of a robotic approach have to be cautious until a greater number of cases are made available for critical appraisal. Based upon this small case series however we feel that robotic approach to pelvic exenteration has the potential to produce acceptable outcomes and warrants further investigation.
CONCLUSIONS
The results from our cohort of robotic pelvic exenteration and extended pelvic resections suggest it may be a feasible approach for selected patients to treat locally advanced urological or colorectal malignancies. In terms of surgical technique, it is possible and it demonstrates acceptable perioperative and morbidity outcomes and compares favorably to the published data from open exenteration cohorts. While it is difficult to draw meaningful conclusions from the currently available evidence, the advantages of robotic surgery with its minimally invasive approach has the potential to benefit patients undergoing this highly complex and morbid procedure, and therefore warrants further investigation.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Michael Williams and François-Xavier Nouhaud.
- Data acquisition: Michael Williams.
- Statistical analysis: Michael Williams.
- Data analysis and interpretation: Marlon Perera, François-Xavier Nouhaud, and Geoffrey Coughlin.
- Drafting of the manuscript: Michael Williams, Marlon Perera, and François-Xavier Nouhaud.
- Critical revision of the manuscript: Marlon Perera and Geoffrey Coughlin.
- Supervision: Geoffrey Coughlin.
- Approval of the final manuscript: Michael Williams, Marlon Perera, François-Xavier Nouhaud, and Geoffrey Coughlin.
References
- 1.Shin US, Nancy You Y, Nguyen AT, Bednarski BK, Messick C, Maru DM, et al. Oncologic outcomes of extended robotic resection for rectal cancer. Ann Surg Oncol. 2016;23:2249–2257. doi: 10.1245/s10434-016-5117-3. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Li Q, Qiu P, Jiang L, Fu Z, Fan Y, et al. Comparison of perioperative outcomes between laparoscopic and open surgery for mid-low rectal cancer with total mesorectal excision following neoadjuvant chemoradiotherapy. J Cancer Res Ther. 2016;12(Supplement):C199–C204. doi: 10.4103/0973-1482.200600. [DOI] [PubMed] [Google Scholar]
- 3.COLOR Study Group. COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Dig Surg. 2000;17:617–622. doi: 10.1159/000051971. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Cao G, Chen B, Wang M, Xu X, Cai W, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis of classic randomized controlled trials and high-quality nonrandomized studies in the last 5 years. Int J Surg. 2017;39:1–10. doi: 10.1016/j.ijsu.2016.12.123. [DOI] [PubMed] [Google Scholar]
- 5.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 6.Toshniwal S, Perera M, Lloyd D, Nguyen H. A 12-year experience of the Trendelenburg perineal approach for abdominoperineal resection. ANZ J Surg. 2013;83:853–858. doi: 10.1111/ans.12137. [DOI] [PubMed] [Google Scholar]
- 7.Yaxley JW, Coughlin GD, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 8.Wetherell D, Bolton D, Kavanagh L, Perera M. Current role of salvage robotic-assisted laparoscopic prostatectomy. World J Urol. 2013;31:463–469. doi: 10.1007/s00345-013-1025-3. [DOI] [PubMed] [Google Scholar]
- 9.Platt E, Dovell G, Smolarek S. Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Tech Coloproctol. 2018;22:835–845. doi: 10.1007/s10151-018-1883-1. [DOI] [PubMed] [Google Scholar]
- 10.Castillo OA, Vidal-Mora I, Rodriguez-Carlin A, Silva A, Schatloff O. First report: robot-assisted total pelvic exenteration for locally advanced prostate cancer. J Laparoendosc Adv Surg Tech A. 2015;25:592–594. doi: 10.1089/lap.2015.0213. [DOI] [PubMed] [Google Scholar]
- 11.Harji DP, Griffiths B, Velikova G, Sagar PM, Brown J. Systematic review of health-related quality of life in patients undergoing pelvic exenteration. Eur J Surg Oncol. 2016;42:1132–1145. doi: 10.1016/j.ejso.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 13.Barnajian M, Pettet D, 3rd, Kazi E, Foppa C, Bergamaschi R. Quality of total mesorectal excision and depth of circumferential resection margin in rectal cancer: a matched comparison of the first 20 robotic cases. Colorectal Dis. 2014;16:603–609. doi: 10.1111/codi.12634. [DOI] [PubMed] [Google Scholar]
- 14.Shin JW, Kim J, Kwak JM, Hara M, Cheon J, Kang SH, et al. First report: robotic pelvic exenteration for locally advanced rectal cancer. Colorectal Dis. 2014;16:O9–O14. doi: 10.1111/codi.12446. [DOI] [PubMed] [Google Scholar]
- 15.Winters BR, Mann GN, Louie O, Wright JL. Robotic total pelvic exenteration with laparoscopic rectus flap: initial experience. Case Rep Surg. 2015;2015:835425. doi: 10.1155/2015/835425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quyn AJ, Austin KKS, Young JM, Badgery-Parker T, Masya LM, Roberts R, et al. Outcomes of pelvic exenteration for locally advanced primary rectal cancer: overall survival and quality of life. Eur J Surg Oncol. 2016;42:823–828. doi: 10.1016/j.ejso.2016.02.016. [DOI] [PubMed] [Google Scholar]