Abstract
Purpose
The aim of this study was to compare the prognostic value of pretreatment inflammation-based scoring systems in terms of overall survival (OS) and progression-free survival (PFS) in patients with germ cell tumors (GCTs) receiving bleomycin, etoposide, and cisplatin (BEP) chemotherapy.
Materials and Methods
We evaluated 63 patients with GCTs retrospectively. The Glasgow prognostic score (GPS), neutrophil-to-lymphocyte ratio, prognostic index, platelet-to-lymphocyte ratio (PLR), prognostic nutritional index (PNI), systemic immune-inflammation index, and albumin-to-globulin ratio (AGR) were measured in all patients before chemotherapy. To assess the predictive ability of each scoring system, areas under the receiver operating characteristic curve were calculated, and multivariate analysis was performed to identify associations between the predictive scores and OS.
Results
Of all the inflammation-based scoring systems, the GPS had the greatest area under the curve (0.847) for predicting OS, followed by the PNI (0.829) and AGR (0.810). Kaplan–Meier analyses revealed that the GPS, PNI, and AGR were significantly associated with OS, whereas the GPS, PLR, and PNI were significantly associated with PFS. In the multivariate analysis, the GPS was an independent predictor of OS and PFS.
Conclusions
We demonstrated that the GPS was the most valuable biomarker of OS and PFS in patients with GCTs.
Keywords: Germ cell tumor, Glasgow prognostic score, Prognostic nutritional index
Graphical Abstract
INTRODUCTION
A germ cell tumor (GCT) is a common malignancy in men [1]. Several studies have reported a favorable prognosis of GCTs because chemotherapy is often effective, even for advanced cases [2,3]. The bleomycin, etoposide, and cisplatin (BEP) regimen has been used as the standard chemotherapy regimen for GCTs [4]. However, some cases are resistant to this treatment, resulting in relapse or death.
Alpha fetoprotein, human chorionic gonadotropin, and lactate dehydrogenase are serum tumor markers of GCTs that have been used for several decades [5]. According to the International Germ Cell Consensus Classification (IGCCC), the combination of these biomarkers is essential for determining the prognosis and treatment of advanced GCTs [6]. However, the IGCCC is based on cases enrolled between 1975 and 1990 and thus may not reflect the current situation of patients with GCTs. In particular, some reports have shown that the survival rate of recently treated patients with a poor prognosis has improved [7,8]. Therefore, a modified prognostic classification that meets current needs is needed.
Recently, inflammation-based scoring systems for several types of malignant tumors have attracted attention [9,10,11]. Because of the advantage of inflammation-based markers, which can be easily measured by use of routine blood tests, these scoring systems are increasingly being reported as predictors of outcomes in numerous cancers [12]. In GCTs, only a few studies have assessed the prognostic value of inflammation-based biomarkers, and the only comparative study of inflammation-based scoring systems was conducted to predict the pulmonary toxicity of bleomycin [12]. GCTs have a good survival outcome on the whole compared with other cancers when they are treated appropriately. However, in a specific cohort of patients, poor prognosis and unfavorable therapy response profiles are observed. Thus, we were interested in investigating several inflammatory markers within retrospectively followed patients with GCTs who initiated systemic chemotherapy. The purpose of this study was to compare the prognostic value of inflammation-based scoring systems in terms of the overall survival (OS) and progression-free survival (PFS) of patients with GCTs treated with BEP chemotherapy.
MATERIALS AND METHODS
1. Patients
In this study, we recruited patients who were newly diagnosed with a GCT and received first-line chemotherapy in our institution between February 2007 and December 2018. Patients who underwent radical orchiectomy only were excluded. GCT diagnosis was confirmed pathologically, and both seminoma and nonseminoma cases arising in the testis or extragonadal regions were included. All patients received three or four cycles of BEP chemotherapy according to the National Comprehensive Cancer Network guidelines, version 2. The BEP regimen consisted of bleomycin 30 mg once weekly on days 2, 9, and 16; etoposide 100 mg/m2 on days 1–5 every 3 weeks; and cisplatin 20 mg/m2 on days 1–5 every 3 weeks, according to the IGCCC [6]. Patients who were treated at other institutions or had clinical evidence of an infection were excluded.
2. Methods
Blood samples were collected before the initial treatment to measure C-reactive protein (CRP), albumin, and globulin levels and neutrophil, lymphocyte, white blood cell, and platelet counts. In order to avoid the influence of surgical inflammation resulting from the radical orchiectomy, we analyzed the preoperative blood sample. In patients with extragonadal GCTs, we analyzed the blood sample before initial chemotherapy. These parameters were used to calculate the Glasgow prognostic score (GPS), neutrophil-to-lymphocyte ratio (NLR), prognostic index (PI), platelet-to-lymphocyte ratio (PLR), prognostic nutritional index (PNI), systemic immune-inflammation index (SII), and albumin-to-globulin ratio (AGR) (Table 1). The cutoff values for each inflammation-based scoring system were defined as shown in Table 1. The GPS and PI cutoff values were determined on the basis of previous studies, whereas the cutoff values of the other markers were determined by receiver operating characteristic (ROC) curve analyses [9].
Table 1. Scoring systems for inflammation-based markers.
| Scoring system | Score |
|---|---|
| GPS | |
| CRP <1.0 mg/dL and albumin ≥3.5 g/dL | 0 |
| CRP ≥1.0 mg/dL or albumin <3.5 g/dL | 1 |
| CRP ≥1.0 mg/dL and albumin <3.5 g/dL | 2 |
| NLR | |
| Neutrophil count/lymphocyte count <4.1 | 0 |
| Neutrophil count/lymphocyte count ≥4.1 | 1 |
| PI | |
| CRP <1.0 mg/dL and white blood cell count <11×103/µL | 0 |
| CRP ≥1.0 mg/dL or white blood cell count ≥11×103/µL | 1 |
| CRP ≥1.0 mg/dL and white blood cell count ≥11×103/µL | 2 |
| PLR | |
| Platelet count/lymphocyte count <320 | 0 |
| Platelet count/lymphocyte count ≥320 | 1 |
| PNI | |
| 10×albumin (g/dL)+0.005×total lymphocyte count ≥32 | 0 |
| 10×albumin (g/dL)+0.005×total lymphocyte count <32 | 1 |
| SII | |
| Platelet count×neutrophil count/lymphocyte count <120×104/m3 | 0 |
| Platelet count×neutrophil count/lymphocyte count ≥120×104/m3 | 1 |
| AGR | |
| Albumin level/globulin level ≥1.1 | 0 |
| Albumin level/globulin level <1.1 | 1 |
GPS, Glasgow prognostic score; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio.
3. Statistical analysis
All statistical analyses were performed by using EZR, version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R [13]. The patients' characteristics and laboratory data were analyzed by using Fisher's exact test for categorical variables and the Mann–Whitney U-test for continuous variables. The ability of each inflammation-based scoring system to predict survival (OS and PFS) was assessed by calculating the area under the ROC curve (AUC). The Kaplan–Meier method was used to estimate survival outcomes by use of each scoring system, and differences between survival curves were determined by using a two-tailed log-rank test. Univariate logistic regression analyses were conducted to identify potential predictive factors for OS and PFS. Variables determined to be significant in the univariate analyses were entered into the multivariate logistic regression analysis. We considered a p-value <0.05 to be significant.
4. Ethics
This retrospective study complied with the standards of the Declaration of Helsinki and current ethical guidelines and was approved by the Institutional Review Board of Okayama University (approval number: 1803013).
RESULTS
1. Patient characteristics
During the study period (February 2007 to December 2018), 63 patients were newly diagnosed with a GCT and underwent BEP chemotherapy. The median follow-up period was 63.4 months (range: 1.6–150.5). The baseline characteristics of the patients are shown in Table 2. Seven patients died from their GCT, all of whom had a histology of nonseminoma. No patient died of any other cause. Six of the seven patients who died had high-risk disease, and the remaining patient had intermediate-risk disease, according to the IGCCC. In this cohort, seven patients had tumors involving the extragonadal regions (of whom three patients died from the GCT). One patient with T0 disease was diagnosed with a burned-out GCT based on histological examination of tissue removed during inguinal orchiectomy. The laboratory data of the patients before the start of chemotherapy are shown in Table 3.
Table 2. Demographic and clinical characteristics of the patients.
| Variable | Value |
|---|---|
| Age (y) | 35 (16–67) |
| Smoking history | 26 (41) |
| Underlying lung disease | 6 (10) |
| BMI (kg/m2) | 23 (13–34) |
| Primary site | |
| Testis | 56 (89) |
| Extragonadal | 7 (11) |
| Histology | |
| Seminoma | 17 (27) |
| Non-seminoma | 46 (73) |
| IGCCC risk group | |
| Good | 26 (41) |
| Intermediate | 23 (37) |
| Poor | 14 (22) |
| T stagea | |
| 0 | 1 (2) |
| 1 | 27 (43) |
| 2 | 19 (30) |
| 3 | 7 (11) |
| 4 | 2 (3) |
| Lymph node metastasis | 49 (78) |
| Lung metastasis | 22 (35) |
| Non-pulmonary solid metastasis | 9 (14) |
Values are presented as median (range) or number (%).
BMI, body mass index; IGCCC, International Germ Cell Consensus Classification.
aExtragonadal cases not included.
Table 3. Patient laboratory data prior to initiation of chemotherapy.
| Variable | Value |
|---|---|
| CRP (mg/dL) | 0.84 (0.01–18.2) |
| ALB (g/dL) | 4.3 (2.2–5.1) |
| White blood cell count (×109/L) | 7.5 (3.7–18.0) |
| Neutrophil count (×109/L) | 5.5 (2.0–14.1) |
| Lymphocyte count (×109/L) | 1.4 (0.2–2.9) |
| Platelet count (×104/m3) | 22.8 (6.1–77.6) |
| GPS | |
| 0 | 33 (54) |
| 1 | 20 (33) |
| 2 | 8 (13) |
| NLR | 4.4 (0.9–18.2) |
| PI score | |
| 0 | 27 (44) |
| 1 | 23 (37) |
| 2 | 12 (19) |
| PLR | 208 (73–840) |
| PNI | 43 (22–51) |
| SII (×104/m3) | 127 (19–557) |
| AGR | 1.3 (0.6–2.2) |
Values are presented as median (range) or number (%).
CRP, C-reactive protein; ALB, albumin; GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio.
2. Predictive factors
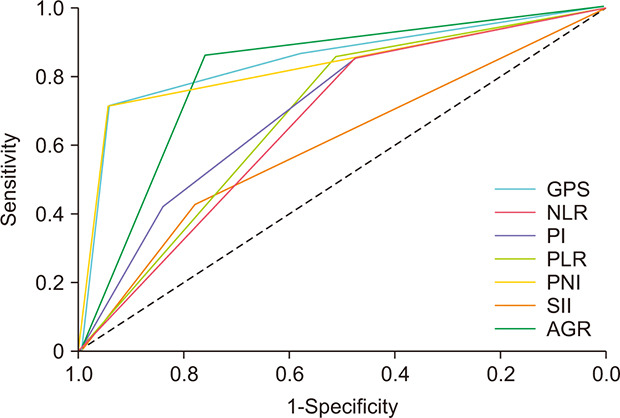
The ROC curves for each inflammation-based score for predicting OS are summarized in Fig. 1. We compared the AUCs to evaluate the discriminatory ability of each score (Table 4). All scores except the PLR were associated with OS. The GPS had the highest AUC (0.847; 95% confidence interval [CI], 0.66–1.00; p<0.01) of all the inflammation-based scoring systems examined, followed by the PNI (0.829; 95% CI, 0.65–1.00; p<0.01) and the AGR (0.810; 95% CI, 0.66–0.96; p<0.01). When comparing the statistical significance between the AUC of each score, we found that only the comparison of GPS with PLR (p=0.02) and that of PLR with PNI (p=0.02) showed statistical significance.
Fig. 1. Comparison of the area under the receiver operating characteristic curve for predicting overall survival of patients with germ cell tumors among the inflammation-based scoring systems evaluated. GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio.

Table 4. Comparison of the AUC for predicting overall survival according to inflammation-based factors.
| Factor | AUC | 95% confidence interval | p-value |
|---|---|---|---|
| GPS | 0.847 | 0.66–1.00 | <0.01 |
| NLR | 0.665 | 0.51–0.82 | 0.04 |
| PI | 0.708 | 0.51–0.90 | 0.04 |
| PLR | 0.605 | 0.40–0.81 | 0.32 |
| PNI | 0.829 | 0.65–1.00 | <0.01 |
| SII | 0.683 | 0.53–0.84 | 0.02 |
| AGR | 0.810 | 0.66–0.96 | <0.01 |
AUC, area under the receiver operating characteristic curve; GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio.
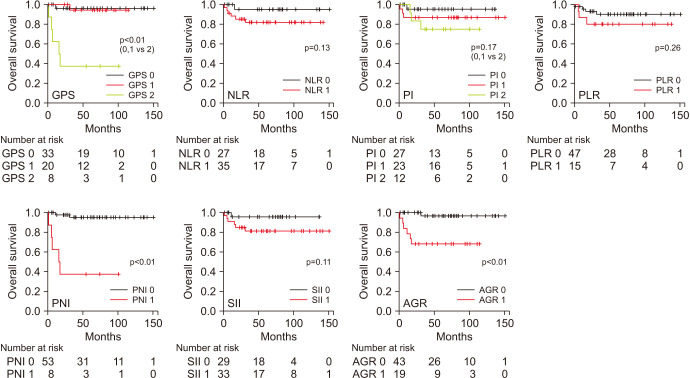
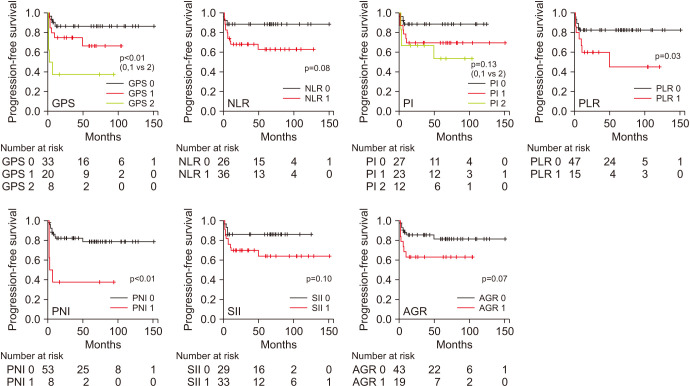
Kaplan–Meier analyses revealed that the GPS (p<0.01), PNI (p<0.01), and AGR (p<0.01) were significant predictors of OS, and the GPS (p<0.01), PLR (p=0.03), and PNI (p<0.01) were significant predictors of PFS (Figs. 2, 3). Patients with a GPS of 0 or 1 had a significantly better OS (p<0.001, Fig. 2) and PFS (p<0.001, Fig. 3) than did those with a GPS of 2.
Fig. 2. Overall survival predictions, according to the Kaplan–Meier method, in patients with germ cell tumors using the GPS (p<0.01), NLR (p=0.13), PI (p=0.17), PLR (p=0.26), PNI (p<0.01), SII (p=0.11), and AGR (p<0.01). GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio.
Fig. 3. Progression-free survival of patients as predicted by the GPS (p<0.01), NLR (p=0.08), PI (p=0.13), PLR (p=0.03), PNI (p<0.01), SII (p=0.10), and AGR (p=0.07). GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio.
Tables 5 and 6 summarize the results of the univariate and multivariate analyses of predictive factors for OS and PFS. The univariate analysis showed that the GPS (p<0.01), PNI (p<0.01), and AGR (p=0.01) were associated with OS. These significant variables were then included in the multivariate analysis, which showed that only the GPS remained independently associated with OS (hazard ratio 9.97, p=0.04, Table 5). The factors associated with poor PFS in the univariate analyses included GPS (p<0.01), PLR (p=0.04), and PNI (p<0.01). In the multivariate analysis, only the GPS was significantly associated with PFS (hazard ratio 4.56, p<0.01) and OS (hazard ratio 9.97, p=0.04) (Table 6).
Table 5. Univariate and multivariate analyses of predictive factors for overall survival.
| Risk factor | Univariate analysis | p-value | Multivariate analysis | p-value | ||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | Hazard ratio | 95% confidence interval | |||
| GPS (0, 1 vs. 2) | 22.98 | 4.42–119.30 | <0.01 | 9.97 | 1.16–85.88 | 0.04 |
| NLR, ≥4.1 | 4.42 | 0.53–36.75 | 0.17 | |||
| PI (0, 1 vs. 2) | 2.72 | 0.61–12.17 | 0.19 | |||
| PLR, ≥320 | 2.29 | 0.51–10.25 | 0.28 | |||
| PNI, <32 | 22.98 | 4.42–119.30 | <0.01 | NA | NA | NA |
| SII, ≥120×104/m3 | 4.87 | 0.59–40.47 | 0.14 | |||
| AGR, <1.1 | 14.73 | 1.78–122.50 | 0.01 | 3.41 | 0.21–54.53 | 0.39 |
GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio; NA, not available.
Table 6. Univariate and multivariate analyses of predictive factors for progression-free survival.
| Risk factor | Univariate analysis | p-value | Multivariate analysis | p-value | ||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | Hazard ratio | 95% confidence interval | |||
| GPS (0, 1 vs. 2) | 4.99 | 1.69–14.75 | <0.01 | 4.56 | 1.52–13.73 | <0.01 |
| NLR, ≥4.1 | 3.17 | 0.89–11.26 | 0.07 | |||
| PI (0, 1 vs. 2) | 2.25 | 0.77–6.58 | 0.14 | |||
| PLR, ≥320 | 2.97 | 1.07–8.21 | 0.04 | 2.59 | 0.92–7.25 | 0.07 |
| PNI, <32 | 4.99 | 1.69–14.75 | <0.01 | NA | NA | NA |
| SII, ≥120×104/m3 | 2.49 | 0.79–7.85 | 0.12 | |||
| AGR, <1.1 | 2.54 | 0.89–7.24 | 0.08 | |||
GPS, Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; PI, prognostic index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AGR, albumin-to-globulin ratio; NA, not available.
DISCUSSION
This retrospective study demonstrated that, among the inflammation-based scoring systems examined, the GPS is the most suitable predictor of OS and PFS. This is the first report to assess the association between the GPS and the survival of patients with GCTs.
Patients with cancer generally have chronic activation of systemic inflammatory responses and tend to have poor nutrition. To assess such conditions, inflammation-based scoring systems have been proposed, which can easily be calculated by simple blood sampling. It is assumed that combining a blood parameter with either inflammatory response or nutritional status factors can improve the sensitivity of prognostic predictions. We previously reported that preoperative inflammatory markers are associated with pulmonary toxicity due to BEP chemotherapy [14]. This paper suggested that systemic inflammation could affect the response to chemotherapy in patients with GCTs. In addition, there are several lines of research being reported in which preoperative or prechemotherapy systemic inflammation is related to poor prognosis and unfavorable therapy response profiles in patients with carcinoma. On the basis of these findings, we guess that preoperative systemic inflammatory status may affect survival outcomes in patients with GCTs. We examine several inflammatory markers before administering first-line BEP chemotherapy. The reason for collecting blood samples at this time is that it is difficult to evaluate postchemotherapy inflammatory markers because bleomycin itself causes inflammation. In this context, we included the patients who received BEP chemotherapy, so we excluded the patients who underwent radical orchiectomy only.
The GPS is an inflammation-based prognostic score based on serum levels of CRP and albumin. Hypoalbuminemia is known to be a negative prognostic marker in cancer patients and is verified in various types. Hypoalbuminemia is also a systemic inflammation parameter. The GPS was constructed on the basis of the cross-linkage between CRP and albumin [15], and its prognostic value is increasingly being reported in various cancers [16,17,18,19,20,21]. The relationship between the GPS and a poorer prognosis of cancer patients is unclear and likely involves a complex mechanism. However, it is believed that CRP plays a pivotal role in the tumor–host relationship, with an elevation in CRP reflecting compromised cell-mediated immunity. In, addition, an elevated CRP level and hypoalbuminemia are also associated with upregulation of components of the innate immune system. Thus, a high GPS score (i.e., elevated CRP and reduced albumin levels) indicates an immune system imbalance, thus compromising effective host–tumor immune responses [20,21].
In this study, the GPS had the highest prognostic ability, with 54% of the patients having a score of 0, 33% a score of 1, and 13% a score of 2. The proportion of patients with a high GPS score seems lower for GCTs than for colorectal and metastatic renal cancers [16,17,18]. We believe that this is because of the more favorable prognosis of GCTs than of other cancers. Using the Kaplan–Meier method, we showed that both OS and PFS were significantly lower in patients with a GPS of 2 than in patients with a GPS of 0 or 1. Furthermore, in the multivariate logistic regression analysis, the GPS was an independent prognostic factor for both OS and PFS. These results suggest that the GPS has a strong predictive ability, and we can assess tumor aggressiveness more accurately by evaluating the GPS in combination with the existing IGCCC.
The PNI was the second most useful predictor after the GPS in this study. The PNI is calculated on the basis of the serum albumin level and total lymphocyte count, which reflect both pretreatment nutrition and inflammation status [22]. Malnutrition causes immune dysfunction and an altered inflammatory response [23]. Thus, pretreatment nutritional status predicts not only the risk for complications but also the long-term outcomes of cancer patients [19,22,23]. In this study, we set the cutoff PNI to 32 based on the ROC analysis. This cutoff value is lower than those used for other diseases, which often range from 40 to 50 [22,23]. This is because GCTs are is more common in younger men whose blood albumin levels are higher than in older men. As a result, the PNI value decreases with age [24].
Even though no study has investigated the predictive value of the GPS or PNI for GCT outcomes, one study revealed that low albumin and high CRP levels are associated with a shorter OS in patients with metastatic GCTs undergoing first-line chemotherapy [25]. This was similar to our findings in that albumin and CRP levels are components of the GPS and PNI, albeit with differences before and after chemotherapy. Several studies have demonstrated that the NLR or SII (a combination of the NLR and PLR) is an independent predictor of OS in addition to the IGCCC risk group [25,26]. However, it is unknown which of the inflammation-based scoring systems most accurately predicts the survival outcomes of patients with GCTs, as comparative assessments have not been performed previously. Herein, we compared the GPS, NLR, PI, PLR, PNI, SII, and AGR to determine which scoring system is most suitable for predicting the survival outcomes of patients with GCTs. The GPS and PNI followed by the AGR showed superiority in terms of the AUC. The AGR, which is obtained by dividing the serum albumin level by the globulin level, also reflects both the nutritional status and inflammatory response [27]. As in a previous study, the PNI was more predictive of malnutrition than was the AGR, because the PNI is based on two independent factors, the albumin level and lymphocyte count, whereas the AGR is based on two related factors [12]. The PLR was the only inflammatory marker not associated with OS in the ROC analysis, yet was a valuable predictor of PFS. It has been reported that changes in the PLR are affected by other systemic inflammatory markers, especially the NLR [28]. The NLR, reflecting neutrophil and lymphocyte numbers, has been reported to be an independent predictor of bleomycin pulmonary toxicity and prognosis in patients with GCTs [12]. However, the predictive value of the NLR was not superior to that of the other inflammatory markers assessed in this study. The NLR cutoff tends to increase with disease aggressiveness [12].
Recently, the incidence of GCTs has been increasing, especially in developed countries [29,30]. A GCT is a malignancy that can be cured by multidisciplinary treatments such as chemotherapy and surgical resection. However, despite IGCCC risk prediction and potent chemotherapy regimens, a subset of patients still show a failure of response to treatment after chemotherapy, and some of those patients eventually die of their GCT. Thus, it is important to identify the high-risk patients and intensify the treatment algorithm or follow-up schedule. The inflammation-based scoring systems used in this study are useful for predicting prognosis because we can easily determine inflammation and nutrition statuses before treatment via blood tests.
Our study has several limitations. First, this was a retrospective, small-scale, single-center study, subjecting it to potential selection bias. Second, we included patients who were admitted to our institution for chemotherapy after inguinal orchiectomy at other institutions or patients who experienced recurrence after surgery. Third, different times of day of correcting of blood sampling may have influenced our analysis. Finally, most of the cutoff values in this study were determined by ROC analysis in our institution. There was no established cutoff definition, which was also discussed in previous report. However, if there had been a difference between each of the inflammation-based scoring systems, it is likely that a trend would have been observed. To determine the most suitable cutoff values for GCT, further research is required.
CONCLUSIONS
This study demonstrated that the GPS was the most suitable marker of the seven examined for predicting the OS and PFS of patients with GCTs. Both components of the GPS are easily measured and highly reproducible. The GPS appears to be an optimal tool for predicting prognosis.
ACKNOWLEDGMENTS
The authors thank the clinical laboratory technicians of Okayama University Hospital for their technical support.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Takuya Sadahira and Yuki Maruyama.
- Data acquisition: Kasumi Yoshinaga and Takuya Sadahira.
- Statistical analysis: Kasumi Yoshinaga and Yuki Maruyama.
- Data analysis and interpretation: Kasumi Yoshinaga, Takuya Sadahira, Yosuke Mitsui, and Takehiro Iwata.
- Drafting of the manuscript: Kasumi Yoshinaga, Takuya Sadahira, Yuki Maruyama, and Motoo Araki.
- Critical revision of the manuscript: Yosuke Mitsui, Takehiro Iwata, Koichiro Wada, and Toyohiko Watanabe.
- Obtaining funding: none.
- Administrative, technical, or material support: Yosuke Mitsui.
- Supervision: Takuya Sadahira and Yasutomo Nasu.
- Approval of the final manuscript: Motoo Araki, Toyohiko Watanabe, and Yasutomo Nasu.
References
- 1.McGlynn KA, Cook MB. Etiologic factors in testicular germcell tumors. Future Oncol. 2009;5:1389–1402. doi: 10.2217/fon.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RH, Vasey PA. Part II: testicular cancer--management of advanced disease. Lancet Oncol. 2003;4:738–747. doi: 10.1016/s1470-2045(03)01279-8. [DOI] [PubMed] [Google Scholar]
- 3.Hanna NH, Einhorn LH. Testicular cancer--discoveries and updates. N Engl J Med. 2014;371:2005–2016. doi: 10.1056/NEJMra1407550. [DOI] [PubMed] [Google Scholar]
- 4.de Wit R, Skoneczna I, Daugaard G, De Santis M, Garin A, Aass N, et al. Randomized phase III study comparing paclitaxel-bleomycin, etoposide, and cisplatin (BEP) to standard BEP in intermediate-prognosis germ-cell cancer: intergroup study EORTC 30983. J Clin Oncol. 2012;30:792–799. doi: 10.1200/JCO.2011.37.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. 2016;13:715–725. doi: 10.1038/nrurol.2016.170. [DOI] [PubMed] [Google Scholar]
- 6.International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk MR, Steyerberg EW, Habbema JD. Survival of non-seminomatous germ cell cancer patients according to the IGCC classification: an update based on meta-analysis. Eur J Cancer. 2006;42:820–826. doi: 10.1016/j.ejca.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Shintaku I, Satoh M, Okajima E, Fujimoto H, Kamoto T, Ogawa O, et al. Survival of metastatic germ cell cancer patients assessed by international germ cell consensus classification in Japan. Jpn J Clin Oncol. 2008;38:281–287. doi: 10.1093/jjco/hyn009. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988–993. doi: 10.1038/bjc.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirahara N, Matsubara T, Hayashi H, Takai K, Fujii Y, Tajima Y. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol. 2015;41:1308–1315. doi: 10.1016/j.ejso.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama Y, Sadahira T, Araki M, Mitsui Y, Wada K, Edamura K, et al. Comparison of the predictive value among inflammation-based scoring systems for bleomycin pulmonary toxicity in patients with germ cell tumors. Int J Urol. 2019;26:813–819. doi: 10.1111/iju.14017. [DOI] [PubMed] [Google Scholar]
- 13.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruyama Y, Sadahira T, Mitsui Y, Araki M, Wada K, Tanimoto R, et al. Prognostic impact of bleomycin pulmonary toxicity on the outcomes of patients with germ cell tumors. Med Oncol. 2018;35:80. doi: 10.1007/s12032-018-1140-5. [DOI] [PubMed] [Google Scholar]
- 15.Melling N, Grüning A, Tachezy M, Nentwich M, Reeh M, Uzunoglu FG, et al. Glasgow prognostic score may be a prognostic index for overall and perioperative survival in gastric cancer without perioperative treatment. Surgery. 2016;159:1548–1556. doi: 10.1016/j.surg.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Eren T, Burcu B, Tombalak E, Ozdemir T, Leblebici M, Ozemir IA, et al. Clinical significance of the Glasgow prognostic score for survival after colorectal cancer surgery. J Gastrointest Surg. 2016;20:1231–1238. doi: 10.1007/s11605-016-3114-2. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–212. doi: 10.1002/cncr.22400. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Wang H, Liu CC, Lu Y, Tang H. The Glasgow prognostic score (GPS) is a novel prognostic indicator in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer Res Clin Oncol. 2016;142:2339–2345. doi: 10.1007/s00432-016-2228-y. [DOI] [PubMed] [Google Scholar]
- 19.Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Pretreatment Glasgow prognostic score and prognostic nutritional index predict overall survival of patients with advanced small cell lung cancer. Lung Cancer (Auckl) 2017;8:249–257. doi: 10.2147/LCTT.S142880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am J Surg. 2012;203:101–106. doi: 10.1016/j.amjsurg.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249:788–793. doi: 10.1097/SLA.0b013e3181a3e738. [DOI] [PubMed] [Google Scholar]
- 22.Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients] Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. Japanese. [PubMed] [Google Scholar]
- 23.Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20:10537–10544. doi: 10.3748/wjg.v20.i30.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakabayashi H, Otani T, Kondo A, Mori S. Re-evaluation of the usefulness of the prognostic nutritional index: reported by Onodera, especially for the elderly patients undergoing gastrointestinal surgery for the gastric and colon cancer. Jpn J Gastroenterol Surg. 2004;37:472–478. [Google Scholar]
- 25.Fankhauser CD, Sander S, Roth L, Gross O, Eberli D, Sulser T, et al. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br J Cancer. 2018;118:825–830. doi: 10.1038/bjc.2017.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan YG, Sia J, Huang HH, Lau WKO. Neutrophil-to-lymphocyte ratio independently predicts advanced pathological staging and poorer survival outcomes in testicular cancer. Investig Clin Urol. 2019;60:176–183. doi: 10.4111/icu.2019.60.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koparal MY, Polat F, Çetin S, Bulut EC, Sözen TS. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int Braz J Urol. 2018;44:933–946. doi: 10.1590/S1677-5538.IBJU.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39:345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 30.Nigam M, Aschebrook-Kilfoy B, Shikanov S, Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J Urol. 2015;33:623–631. doi: 10.1007/s00345-014-1361-y. [DOI] [PubMed] [Google Scholar]