Abstract
Purpose
Sexual performance is related to proprioception and pelvic floor muscle strength (PFMS). The aim of this study was to correlate sexual activity and orgasm with PFMS.
Materials and Methods
A total of 140 healthy continent female were prospectively distributed into 4 groups according to age: Group 1 (G1), 30–40; Group 2 (G2), 41–50; Group 3 (G3), 51–60; Group 4 (G4), over 60 years old. Evaluated parameters were: frequency of sexual activity and orgasm achievement; body mass index (BMI) and objective evaluation of PFMS using perineometer and surface electromyography.
Results
BMI was higher in G4 compared to G1 (p=0.042). Women who reported sexual activity was significantly higher in G1 compared to G3 and G4 (94.1% vs. 66.7% and 37.5%, respectively; p=0.001). Orgasm was more frequently in G1 compared to G3 and G4 (91.2% vs. 63.9% and 28.1%, respectively; p=0.001), demonstrating that sexual activity and orgasm decrease after age 51. The duration of PFM contraction was significantly higher in women who had sexual intercourse (p=0.033) and orgasm (p=0.018).
Conclusions
Although the frequency of sexual intercourse and orgasm may decrease with aging, a relationship between sexual activity and PFMS remains apparent, once both sexually active women and those who have orgasms showed better PFM endurance than non-sexually active ones.
Keywords: Aging, Muscle strength dynamometer, Muscular contraction, Sexual activities
Graphical Abstract
INTRODUCTION
Female sexual dysfunction is not a rare condition, with prevalence ranging from 38% to 85.2 % [1]. Different related risk factors have been described, such as post-menopausal status, long-term relationship with the partner, diabetes, pregnancy, alcohol and tobacco consumption, pelvic organ prolapses and urinary incontinence [2].
It is well known that aging increases the prevalence of sexual dysfunction, suggesting a possible anatomical correlation between pelvic floor muscle (PFM) function and female sexual performance [3]. Although the role of pelvic floor muscle strength (PFMS) is well established in urinary incontinence (UI), it is important to emphasize that there is a high correlation between UI and sexual disorders [4,5], where approximately 43% of incontinent women may complain of their sexual performance [6,7]. Additionally, different authors have demonstrated that successful non-surgical treatment of stress urinary incontinence is associated with improvements in incontinence-specific measures of sexual function [8,9].
Some authors suggested different mechanisms to explain how PFMS may influence female sexual function. According to some studies, the weakness of pelvic muscles could contribute to the inability of a woman to achieve orgasm [10] and women who had anorgasmia showed a significantly lower pubococcygeus muscle strength compared with those who had orgasms [11]. Likewise, an increase in the strength of muscles attached to the corpus cavernosum of the clitoris could lead to increased orgasm and arousal [3]. de Menezes Franco et al. [12], in a randomized and controlled study, reported that postmenopausal women with sexual dysfunction presented weakness of PFM compared to non-menopause women. However, despite these findings, others studies have found no association between sexual function and PFMS [13,14].
These apparently contradictory facts put in doubt which would be the role of PFMS on sexual dysfunction [15], demonstrating that there is a lack of information about this subject in the literature, especially in continent women. Thus, it would be very important to evaluate the role of PFMS in continent women during the physiological aging process and its impact on sexual function.
The aim of this study was to correlate sexual activity and orgasm with PFMS, assessed by perineometer and electromyography (EMG), in healthy continent women.
MATERIALS AND METHODS
From March, 2008 to January, 2009, 140 voluntees, female, were studied in the Maternidade Santa Isabel, Bauru, Brazil. All participants were healthy women, with no urological complaints and none of them had clinical comorbidities or reported any type of treatment in course, nor use of controlled medicine. They were informed about the procedures and study objectives and provided written consent, as approved by the Institutional Review Board of Universidade Sagrado Coração (approval number: 61/07), according to ethical standards of the Declaration of Helsinki (2013). Exclusion criteria were UI and/or lower urinary tract symptoms, neurological diseases, previous pelvic surgeries, diabetes mellitus, smoking, and cognitive deficit. All participants were prospectively distributed into four groups according to age: Group 1 (G1, n=34), 30–40 years old; Group 2 (G2, n=38), 41–50 years old; Group 3 (G3, n=35), 51–60 years old; Group 4 (G4, n=33), older than 60 years. The following parameters were evaluated: obstetric and gynecological history, frequency of sexual activity and orgasm achievement, and body mass index (BMI). Objective evaluation of PFMS was done using a perineometer and electromyography surface (EMGs) by vaginal electrode.
Sexual activity was assessed by a self-applicable anonymous questionnaire, composed of two simple questions: (1) Have you had sexual intercourse in the last 3 months? Yes/no; (2) Did you have orgasm during this intercourse? Yes/no.
BMI was calculated and classified according to the World Health Organization [16].
The objective measurements of PFMS were obtained using a Dynamed portable perineometer (Dynamed® model DM01; Dynamed, São Paulo, Brazil), in bent-knee lying position. The examiner introduced a balloon catheter covered with a non-lubricated condom into the vagina, sized 11×2.6 cm, and filled it with 60 mL of air to permit the contact with the vaginal wall. This value was standardized at 60 mL in all participants. The equipment was immediately set to zero, and three PFM contractions were requested and held as long as possible with nearly 30-seconds of rest interval between each one. Maximal peak of each contraction was registered in centimeters of water (cmH2O) and the length of each contraction was timed in seconds with a chronometer. The average of three measurements was used to avoid biased results.
EMGs evaluation was performed using electromyography equipment (MyoTrac® G3; Thought Technology, Montreal, QC, Canada) connected to a vaginal electrode. The contact electrodes were positioned under the upper-anterior iliac crests to assess the abdominal muscles activity. The reference surface electrode was positioned on subcostal left side (below the ribs), after cleaning with 70% alcohol. The used protocol consisted of three maximal and three sustained voluntary PFM contractions, recorded by the vaginal probe in microvolts (µV). The endurance was calculated from the measurement of the sustained contraction curve area (µV/s2).
To compare the four study groups, variance parametric analysis (ANOVA) for a model with one factor (one-way) was applied when the variable adhered to the normal distribution of probabilities, and nonparametric analysis when the variable did not. Parametric analyses were completed with Tukey's multiple comparison test, and non-parametric, with Dunn's test [17]. However, in comparing only two groups, the parametric procedure consisted of the Student's t-test for independent samples, and the nonparametric, Mann Whitney test [17]. The comparisons involving contrasts between binomial populations (sexual activity and orgasm) were performed using the Goodman Association Test [18]. All statistical procedures were performed using R-Gui (Foundation for Statistical Computing, Vienna, Austria) [19], a free software environment for statistical computing and graphics and discussed considering a 5% significance level.
RESULTS
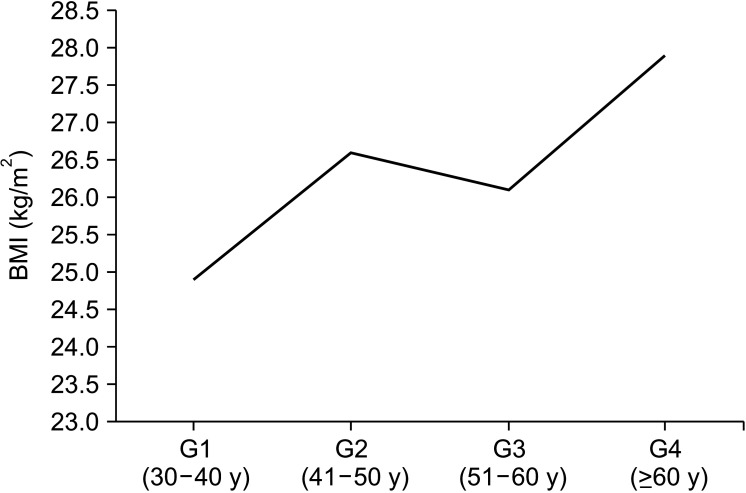
Average age in the G1, G2, G3 and G4 groups was respectively 34.7, 45.1, 54.5 and 66.6 years (p<0.001). BMI was significant higher in G4 compared to G1 group (27.93±3.6 kg/cm2 vs. 24.90±4.1 kg/cm2; p=0.042, respectively). There was no statistical difference among the other groups. It was observed a positive linear correlation between age and BMI (r=0.215, p=0.011) that showed a weight gain during physiologic aging (Fig. 1). BMI was significantly higher in women that had no sexual activity compared to those that reported sexual activity (28.1±4.8 vs. 25.5±3.9 kg/cm2; p<0.001, respectively).
Fig. 1. Illustration of the correlation between body mass index (BMI) and aging in different studied groups: Group 1 (G1), n=34; Group 2 (G2), n=38; Group 3 (G3), n=35; Group 4 (G4), n=33.
The proportion of women who reported sexual activity was significantly higher in G1 compared to G3 and G4 groups (94.1% vs. 66.7% and 37.5%; p=0.001, respectively). There was no statistical difference among the other groups. Women reporting orgasm during sexual intercourse were significantly more frequent in G1 related to G3 and G4 (91.2% vs. 63.9% and 28.1%; p=0.001, respectively), demonstrating that sexual activity and orgasm decrease after 51 years old.
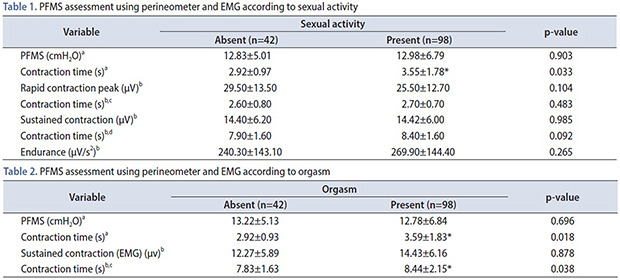
When the participants were analyzed according to sexual activity, we observed that the duration of PFM contraction assessed by the perineometer was significantly higher in women that had sexual intercourse when compared with those without (Table 1). Likewise, the duration of PFM contractions, both perineometer and EMG, were significantly higher in women with orgasm (Table 2). There was no statistical difference among all different parameters of PFM strength assessment either using perineometer or EMG.
Table 1. PFMS assessment using perineometer and EMG according to sexual activity.
| Variable | Sexual activity | p-value | |
|---|---|---|---|
| Absent (n=42) | Present (n=98) | ||
| PFMS (cmH2O)a | 12.83±5.01 | 12.98±6.79 | 0.903 |
| Contraction time (s)a | 2.92±0.97 | 3.55±1.78* | 0.033 |
| Rapid contraction peak (µV)b | 29.50±13.50 | 25.50±12.70 | 0.104 |
| Contraction time (s)b,c | 2.60±0.80 | 2.70±0.70 | 0.483 |
| Sustained contraction (µV)b | 14.40±6.20 | 14.42±6.00 | 0.985 |
| Contraction time (s)b,d | 7.90±1.60 | 8.40±1.60 | 0.092 |
| Endurance (µV/s2)b | 240.30±143.10 | 269.90±144.40 | 0.265 |
Values are presented as mean±standard deviation.
PFMS, pelvic floor muscle strength; EMG, electromyography; cmH2O, centimeters of water; µV, microvolts.
a:Perineometer. b:EMG. c:Time of rapid contraction in the EMG. d:Time of sustained contraction in the EMG.
*p<0.05.
Table 2. PFMS assessment using perineometer and EMG according to orgasm.
| Variable | Sexual activity | p-value | |
|---|---|---|---|
| Absent (n=42) | Present (n=98) | ||
| PFMS (cmH2O)a | 13.22±5.13 | 12.78±6.84 | 0.696 |
| Contraction time (s)a | 2.92±0.93 | 3.59±1.83* | 0.018 |
| Sustained contraction (EMG) (µv)b | 12.27±5.89 | 14.43±6.16 | 0.878 |
| Contraction time (s)b,c | 7.83±1.63 | 8.44±2.15* | 0.038 |
Values are presented as mean±standard deviation.
PFMS, pelvic floor muscle strength; EMG, electromyography; cmH2O, centimeters of water; µV, microvolts.
a:Perineometer. b:EMG. c:Time of sustained contraction in the EMG.
*p<0.05.
DISCUSSION
There are few studies in the current literature demonstrating an association between PFMS and sexual function in women without self-reported sexual dysfunction, particularly considering the ageing process and its effects on these women. So, the cause–effect relation among these factors has not been well established yet.
In the present study we observed that women gained weight during physiological ageing, with a positive linear correlation between age and BMI. At the same time, BMI was significantly higher in women that had no sexual activity suggesting that obesity could influence in the sexual function. Other authors also reported, using specific female sexual dysfunction questionnaires, that BMI is associated with pain, poor lubrication and hampered orgasm [20]. Probably both women weight gain and sexual dysfunction are correlated and are influenced, among other factors, by the hormonal alterations throughout the ageing process [21,22]. These same reasons could be used to explain the decrease in sexual activity and orgasm observed in these women, particularly after age 51. Also, this finding is in agreement with other authors that have reported sexual dysfunction due to ageing or in association with metabolic diseases [22,23].
It seems intuitive to imagine that, since PFM performance in women is positively related to sexual satisfaction and arousal [24], women with stronger pelvic floor muscle should benefit. EMG is among the different methods of measuring the activity of PFMS [25] but, although the voluntary and involuntary contractions of PFM have been studied during sexual arousal [26], few studies evaluated the EMG responses in this process. Mohktar et al. [27] using the EMG measurement as a quantitative way to observe the improvement of female sexual function after performing Kegel exercises conclude that female sexual function can be enhanced with Kegel exercise if the strength of 2 or more of her abdominal or PFM muscles (measured using the EMG technique) display improvement. In our series, considering the objective evaluation of PFMS, the duration of contraction was higher in women that reported sexual activity and orgasm. Some authors have already identified an anatomical correlation between PFMS and sexual function [3]. According to these studies the increase in vaginal tonus, especially in the anterior vaginal area (Grafenberg spot), would improve the contact with the penis during sexual intercourse, increasing the female orgasm [28]. Likewise, other authors observed better sexual performance in women with higher PFMS [12,29].
Lowenstein et al. [30] reported similar results to those found by us, observing a positive correlation between duration of PFM contraction and sexual function, suggesting that the PFM could play an important role in the female orgasmic response. Despite this, Baytur et al. [13] did not found a clear influence of PFMS in the sexual function of women.
Although several anatomical studies pointed to the importance of PFMS in the female sexual function, we need to keep in mind that this is a complex process, influenced by several different mechanisms, such as physical, psychological, social and cultural factors. Some of the limitations of our study may have been to fail to assess other conditions that could interfere with the sexual function of these patients, such as menopause status, inadequate lubrification and vaginal atrophy; the lack of a more detailed description about alcohol consumption (not abuse) or the prevalence of conditions that may affect pelvic blood flow, for example. Another point that could be questioned was the use of a nonspecific questionnaire, which resulted in the lack of quantitative data such as the number of sexual activities for 3 months and the rate of orgasm per sexual activity. Thus, additional controlled trials are necessary to further elucidate the participation of PFMS in this function, helping to better define different ways to approach this problem.
Despite the limitations of our study, as it did not use a specific questionnaire for the evaluation of female sexual dysfunction, we conclude that PFMS is greater in sexually active women achieving orgasm. As sex and orgasm frequency decreases with age, there probably is a relationship between sexual activity and PFM other than age itself.
CONCLUSIONS
Although the frequency of sexual intercourse and orgasm may decrease with aging, a relationship between sexual activity and PFMS remains apparent, once both sexually active women and those who have orgasms showed better PFM endurance than non-sexually active ones.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Paulo Roberto Kawano and João Luiz Amaro.
- Data acquisition: Dulcegleika Vilas Boas Sartori and Pedro Rochetti Pajolli.
- Statistical analysis: Hamilto Akihissa Yamamoto and Rodrigo Guerra.
- Data analysis and interpretation: Dulcegleika Vilas Boas Sartori, Paulo Roberto Kawano, Hamilto Akihissa Yamamoto, Rodrigo Guerra, Pedro Rochetti Pajolli, and João Luiz Amaro.
- Drafting of the manuscript: Dulcegleika Vilas Boas Sartori, Hamilto Akihissa Yamamoto, Rodrigo Guerra, and Pedro Rochetti Pajolli.
- Critical revision of the manuscript: Paulo Roberto Kawano and João Luiz Amaro.
- Administrative, technical, or material support: João Luiz Amaro.
- Supervision: Paulo Roberto Kawano and João Luiz Amaro.
- Approval of the final manuscript: João Luiz Amaro.
References
- 1.Jaafarpour M, Khani A, Khajavikhan J, Suhrabi Z. Female sexual dysfunction: prevalence and risk factors. J Clin Diagn Res. 2013;7:2877–2880. doi: 10.7860/JCDR/2013/6813.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diehl A, Silva RL, Laranjeira R. Female sexual dysfunction in patients with substance-related disorders. Clinics (Sao Paulo) 2013;68:205–212. doi: 10.6061/clinics/2013(02)OA14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:361–376. doi: 10.1007/pl00004028. [DOI] [PubMed] [Google Scholar]
- 4.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 5.Zincir H, Demir G, Günaydin Y, Ozen B. Sexual dysfunction in married women with urinary incontinence. Urol J. 2018;15:193–198. doi: 10.22037/uj.v0i0.4006. [DOI] [PubMed] [Google Scholar]
- 6.Sutherst J, Brown M. Sexual dysfunction associated with urinary incontinence. Urol Int. 1980;35:414–416. doi: 10.1159/000280359. [DOI] [PubMed] [Google Scholar]
- 7.Clark A, Romm J. Effect of urinary incontinence on sexual activity in women. J Reprod Med. 1993;38:679–683. [PubMed] [Google Scholar]
- 8.Handa VL, Whitcomb E, Weidner AC, Nygaard I, Brubaker L, Bradley CS, et al. Sexual function before and after non-surgical treatment for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2011;17:30–35. doi: 10.1097/SPV.0b013e318205e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacomori C, Cardoso FL. Predictors of improvement in sexual function of women with urinary incontinence after treatment with pelvic floor exercises: a secondary analysis. J Sex Med. 2015;12:746–755. doi: 10.1111/jsm.12814. [DOI] [PubMed] [Google Scholar]
- 10.Kegel AH. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521–524. [PubMed] [Google Scholar]
- 11.Graber B, Kline-Graber G. Female orgasm: role of pubococcygeus muscle. J Clin Psychiatry. 1979;40:348–351. [PubMed] [Google Scholar]
- 12.de Menezes Franco M, Driusso P, Bø K, Carvalho de Abreu DC, da Silva Lara LA, de Sá Rosa E Silva ACJ, et al. Relationship between pelvic floor muscle strength and sexual dysfunction in postmenopausal women: a cross-sectional study. Int Urogynecol J. 2017;28:931–936. doi: 10.1007/s00192-016-3211-5. [DOI] [PubMed] [Google Scholar]
- 13.Baytur YB, Deveci A, Uyar Y, Ozcakir HT, Kizilkaya S, Caglar H. Mode of delivery and pelvic floor muscle strength and sexual function after childbirth. Int J Gynaecol Obstet. 2005;88:276–280. doi: 10.1016/j.ijgo.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Handa VL, Harvey L, Cundiff GW, Siddique SA, Kjerulff KH. Sexual function among women with urinary incontinence and pelvic organ prolapse. Am J Obstet Gynecol. 2004;191:751–756. doi: 10.1016/j.ajog.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Li-Yun-Fong RJ, Larouche M, Hyakutake M, Koenig N, Lovatt C, Geoffrion R, et al. Is pelvic floor dysfunction an independent threat to sexual function? A cross-sectional study in women with pelvic floor dysfunction. J Sex Med. 2017;14:226–237. doi: 10.1016/j.jsxm.2016.11.323. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. BMI classification [Internet] Geneva: World Health Organization; 2006. [cited 2018 Nov 18]. Available from: www.who.int/bmi. [Google Scholar]
- 17.Zar JH. Biostatistical analysis. 5th ed. Upper Saddle River: Prentice-Hall; 2009. p. 994. [Google Scholar]
- 18.Goodman LA. Simultaneous confidence intervals for contrasts among multinomial populations. Ann Math Stat. 1964;35:716–725. [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing [Internet] Vienna: R Foundation for Statistical Computing; 2020. [cited 2020 May 25]. Available from: https://cran.r-project.org/ [Google Scholar]
- 20.Polland AR, Davis M, Zeymo A, Iglesia CB. Association between comorbidities and female sexual dysfunction: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Int Urogynecol J. 2019;30:377–383. doi: 10.1007/s00192-018-3739-7. [DOI] [PubMed] [Google Scholar]
- 21.Jamali S, Rahmanian A, Javadpour S. Examining the sexual function and related attitudes among aged women: a cross-sectional study. Int J Reprod Biomed. 2016;14:29–38. [PMC free article] [PubMed] [Google Scholar]
- 22.Ozdemir FC, Pehlivan E, Melekoglu R. Pelvic floor muscle strength of women consulting at the gynecology outpatient clinics and its correlation with sexual dysfunction: a cross-sectional study. Pak J Med Sci. 2017;33:854–859. doi: 10.12669/pjms.334.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appa AA, Creasman J, Brown JS, Van Den Eeden SK, Thom DH, Subak LL, et al. The impact of multimorbidity on sexual function in middle-aged and older women: beyond the single disease perspective. J Sex Med. 2014;11:2744–2755. doi: 10.1111/jsm.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara LA, Montenegro ML, Franco MM, Abreu DC, Rosa e, Ferreira CH. Is the sexual satisfaction of postmenopausal women enhanced by physical exercise and pelvic floor muscle training? J Sex Med. 2012;9:218–223. doi: 10.1111/j.1743-6109.2011.02516.x. [DOI] [PubMed] [Google Scholar]
- 25.Enck P, Vodusek DB. Electromyography of pelvic floor muscles. J Electromyogr Kinesiol. 2006;16:568–577. doi: 10.1016/j.jelekin.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Devreese A, Staes F, Janssens L, Penninckx F, Vereecken R, De Weerdt W. Incontinent women have altered pelvic floor muscle contraction patterns. J Urol. 2007;178:558–562. doi: 10.1016/j.juro.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 27.Mohktar MS, Ibrahim F, Mohd Rozi NF, Mohd Yusof J, Ahmad SA, Su Yen K, et al. A quantitative approach to measure women's sexual function using electromyography: a preliminary study of the Kegel exercise. Med Sci Monit. 2013;19:1159–1166. doi: 10.12659/MSM.889628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambless DL, Stern T, Sultan FE, Williams AJ, Goldstein AJ, Lineberger MH, et al. The pubococcygens and female orgasm: a correlational study with normal subjects. Arch Sex Behav. 1982;11:479–490. doi: 10.1007/BF01542473. [DOI] [PubMed] [Google Scholar]
- 29.Martinez CS, Ferreira FV, Castro AA, Gomide LB. Women with greater pelvic floor muscle strength have better sexual function. Acta Obstet Gynecol Scand. 2014;93:497–502. doi: 10.1111/aogs.12379. [DOI] [PubMed] [Google Scholar]
- 30.Lowenstein L, Gruenwald I, Gartman I, Vardi Y. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553–556. doi: 10.1007/s00192-009-1077-5. [DOI] [PubMed] [Google Scholar]