Abstract
Objective
This study aimed to assess the clinical and radiographic findings obtained by using amniotic membrane (AM) to cover nano-hydroxyapatite (nHA) bone grafts coated with platelet-rich fibrin (PRF) and thereby evaluate the osseointegration of posterior mandibular implants inserted simultaneously during alveolar piezoelectric ridge splitting technique (RST).
Methods
A prospective cohort study was implemented with thirty patients who had a narrow posterior mandibular alveolar ridge and required implant restoration. Patients were distributed randomly into three groups (group I treated by piezoelectric RST and immediate implant insertion, augmented by the nHA bone graft only; group II treated by piezoelectric RST augmented by nHA bone graft and covered by AM; while group III was treated by piezoelectric RST augmented with PRF and nHA graft and covered by AM). Patients were evaluated clinically to assess the implant stability quotient (ISQ) and radiographically to assess horizontal ridge dimension, crestal bone level (CBL), and bone densitometric (BD) parameters.
Results
ISQ results showed a non-significant clinical difference between groups while CBL values showed a high statistically significant difference over the 12-month interval when comparing groups III and II with group I. BD outcomes showed statistically significant differences at all intervals in comparisons of group III with groups I and II.
Conclusions
The results of this study suggest that concomitant use of PRF with nHA graft covered with AM for augmentation around the dental implant in a narrow posterior mandible after piezoelectric alveolar ridge splitting accelerate osseointegration and significantly increase bone density around the osseointegrated implant and decrease bone resorption in comparison to that achieved with the graft alone.
Keywords: PRF, Ridge split, Piezoelectric, Implant
1. Introduction
Sufficient tissue around the dental implant is mandatory for successful osseointegration. However, this requirement creates major challenges in implant dentistry since alveolar atrophy occurs after tooth extraction, which complicates implant placement (Esposito et al., 2008).
Horizontal bone resorption after extraction may prevent proper implant placement and generate functional and esthetic problems that jeopardize the implant success rate (Hammerle et al., 2002).
In this regard, different techniques have been used to correct thin ridges, including onlay grafts, guided bone regeneration (GBR), and the ridge split technique (RST). In RST, a space is created that heals in a similar pattern as the extracted socket and thereby allows instantaneous implant installation. However, none of these approaches have completely met the requirements of successful width increase in the narrow ridge (Aghaloo and Moy, 2007, Castillo, 2010, Jensen and Terheyden, 2009).
The RST offers several advantages such as a reduced waiting time from surgery to the beginning of prosthetic treatment, minimal bone graft requirement, and prevention of the collapse of distended buccal and lingual/palatal walls (Demarosi et al., 2009, Donos et al., 2008). However, it shows limitations related to buccal plate resorption, gingival recession, and devitalization of out-fractured segments.
Several trials have attempted to solve these challenges using novel techniques such as piezoelectric surgery, which can facilitate osteotomy of the thin ridge. Moreover, the use of nano-hydroxyapatite (nHA), a bone substitute with a chemical composition corresponding to the bone mineral, along with the GBR membrane (Han et al., 2011, Sethi and Kaus, 2000) and platelet-rich fibrin (PRF), which contains various growth factors (Dohan et al., 2006, Dohan Ehrenfest et al., 2009), could help accelerate bone healing (Alt et al., 2016, Elsayed et al., 2014, Lewandrowski et al., 2003)
Thus, addition of PRF to nHA and covering it with amniotic membrane (AM) could help fill the area around the implant and promote the healing process. The current study aimed to assess the effect of the covering the combination of PRF and nHA with AM on osseointegration around the dental implant after ridge widening using piezoelectric RST.
2. Patients and methods
2.1. Subjects
Thirty patients, all of whom attended the Oral and Maxillofacial Surgery Clinics at Al Azhar University, were enrolled in this prospective cohort study. All patients signed an informed consent form before the surgery. The study was conducted in accordance with the declaration of Helsinki, and ethics committee approval was obtained from Al Azhar University Assuit branch.
Thorough clinical examinations and radiographic evaluations by cone-beam computed tomography (CBCT; Kodak 9500 cone beam 3D system) were performed for all patients to evaluate their medical condition and to assess the bone volume available for implantation.
2.2. Inclusion and exclusion criteria
Patients were included in this study if they were systemically healthy and had edentulous space in the posterior mandible. The alveolar ridge width ranged from 3 to 5 mm with a height of at least 8 mm, and the edentulous site was free from any pathology.
Patients were excluded from the study if they showed infection at the surgical area, any medical disease that could complicate wound healing, a history of abnormal habits, or loss of stable posterior occlusion.
2.3. Patient grouping and randomization
In this study, all narrow ridges were augmented by piezoelectric RST. The graft material was placed after implant installation. On the basis of the grafting technique, equal numbers of patients were classified randomly into the following groups using online software (https://www.randomizer.org):
Group Ι: The nHA (19-nm size, Ostim, Heraeus Kulzer, Hanau, Germany) graft was placed in the gap. Group Π: The nHA graft was placed in the gap and covered by AM (25 cm2, sterile Biomembrane, The National Center for Radiation Research, Sensiti Xe, SAE). Group III: A mixture of PRF and nHA was placed in the gap and covered by AM.
2.4. PRF preparation
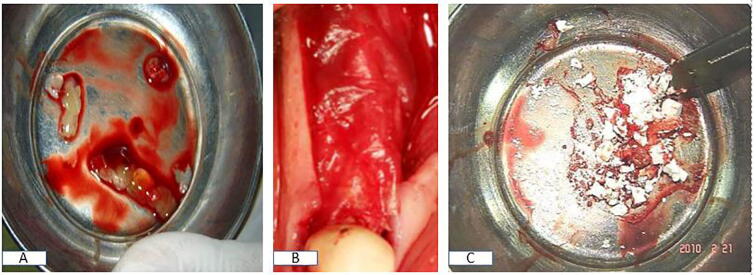
PRF was prepared according to the Dohan technique (Dohan et al., 2006). First, 10 mL of blood was collected in a glass-coated plastic vacutainer tube without anticoagulant and centrifuged at 3000 rpm for 10 min at room temperature. The PRF clot was isolated from the tube using forceps, the attached RBC base was separated from the PRF clot using scissors, and the clot was placed in a sterile metal cup to release serum slowly for 10 min into the cup. The clot was cut into small pieces and mixed with nHA III (Fig. 1A–C).
Fig. 1.
Intraoperative photograph showing (A) PRF prepared and cut down for group III, (B) AM placed above the split ridge in groups II and III, and (C) nHA used in all groups.
2.5. Surgical procedures
Local anesthesia was used in all procedures (4% articaine/adrenaline 1:100,000 1.8 mL (3 M™ ESPE™ Ubistesin™ Forte, 3 M Germany) using a mandibular nerve block and field block techniques for hemostasis.
Mid-crestal and sulcular incisions were completed at the edentulous site and adjacent teeth, respectively. A full-thickness flap was then raised with minimal retraction on both aspects of the cortical plates to preserve the periosteal attachment surrounding the bone. A single operator was responsible for all surgical procedures.
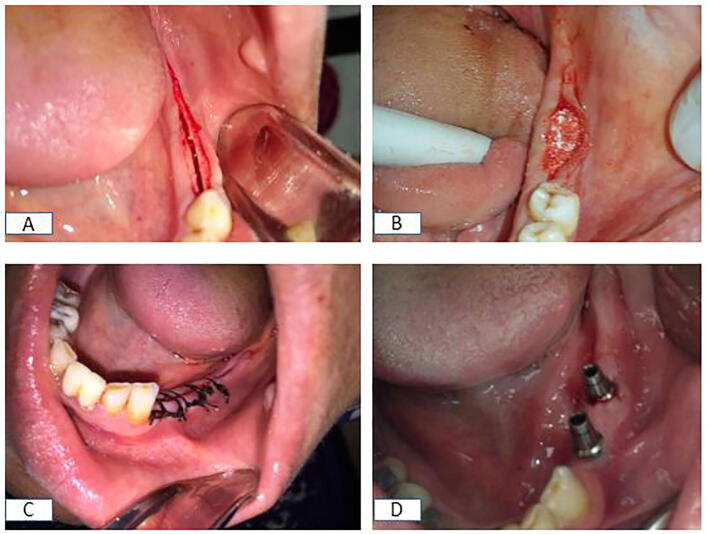
Using the piezosurgical device (Piezotome SOLO Satelec Acteon, Bordeaux-Merignac, France), a crestal cortical cut was made in the ridge. The crestal osteotomy was widened to a depth of 5 mm, and 2 mm away from the adjacent teeth, vertical cuts were made to a corresponding depth on the labial side. Once the buccal-relief osteotomies were completed, the buccal bone plate widened gradually (Fig. 2A).
Fig. 2.
Intraoperative photograph showing (A) a mid-crestal incision and piezoelectric RST in the posterior mandible, (B) an n-HA graft inserted in the created gaps, (C) suturing of the flap, and (D) the abutment fixed in place.
The implant site was prepared according to the standard technique, and two implants (Zimmer implants®; Zimmer dental, 1900 Aston Avenue Carlsbad, CA, USA) were inserted in each surgical site.
After implant placement, the created gaps were treated as mentioned previously and the surgical sites in groups II & III were covered by AM after soaking in saline to allow easy adaptation (Fig. 2B and C). The wound was closed by interrupted non-resorbable sutures.
Each patient received an antibiotic (Augmentin 1 g tablet every 12 h for seven days; GlaxoSmithKline, Fifth district, Cairo, Egypt), and was asked to use mouth wash for 15 days (DG-wash; Al Esraa Pharmaceuticals, Fourth Industrial Zone, Badr Industrial City, Egypt.) and analgesic if needed (Cataflam 75 mg amp; Novartis Pharma, Heliopolis, Egypt).
The patients were instructed to avoid incising food in the operated sites for 6 weeks. Stitches were removed after 7 days, and every 4 weeks, the patients underwent examinations to identify any complications. Abutments were tightened into the implant after 6 months, with the torque adjusted to 35 N·cm and the final cemented restoration delivered (Fig. 2D).
2.6. Postoperative measurements
2.6.1. Clinical evaluation
Implant Stability Quotient (ISQ): The primary stability was recorded using Osstell TM. ISQ values were obtained immediately after implant placement. These measurements were obtained in triplicate and averaged to yield the mean baseline ISQ value for each implant. Additional resonance frequency analysis (RFA) measurements were taken at a 6-month follow-up examination performed during reentry in the clinic for prosthetic procedures.
2.6.2. Radiographic evaluation
2.6.2.1. Post-operative ridge width
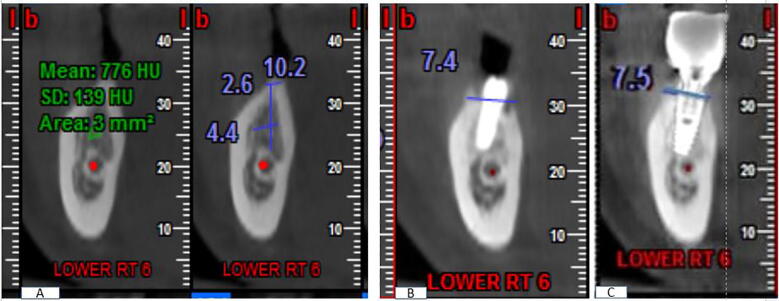
The bone width measurements were taken before implant placement and at 6 months after placement. We ensured that each cut showed an undistorted view of the featured implant in its entirety plus at least 5 mm of bone apical to the apex of the implant (Fig. 3A).
Fig. 3.
CBCT image showing (A) measurements of ridge width and bone density on a preoperative cross-sectional cut of the lower right first molar and (B) 6 months postoperative CBCT and (C) 12 months post-operative CBCT showing the bone level on both buccal and lingual cortices.
2.6.2.2. Marginal bone loss evaluation
The crestal bone level was assessed on CBCT sagittal cuts on computerized software. CBCT imaging exposure was performed by the same operator and the same machine under a standardized protocol.
On the multiplanar screen, navigation was performed until an accurate same-view place of the implant was determined on the reformatted panorama and cross-sectional cut. Marginal bone loss around the implant was evaluated on the day of implant placement (baseline) and at follow-up visits at 3, 6, and 12 months. The distance from the implant apex to the reference point on the implant surface where the marginal bone contacts the implant was measured.
2.6.2.3. Bone density measurement
Average density at the implant marginal bone was determined using Bioquant® (G Power, Ver. 3.192 copyright 1992–2014). A region of interest (ROI) was selected on the cross-sectional view and traced (color density selection) by counting the threshold pixels in each ROI (Yang, 2017). The bone density values were registered at a distance of 1.2 mm parallel from the implant fixture in a spot diameter of 1 mm. Bone density was measured immediately after implant placement (baseline) and on follow-up visits at 3, 6, and 12 months. All measurements were assessed by two different assessors who were blinded to the patient grouping, after which the average was calculated and tabulated (Fig. 3B and C).
2.7. Data analysis
Data were coded and entered into a Microsoft Excel sheet and transferred to IBM SPSS statistical software version 21 to display the descriptive distribution of each variable, including frequency and percentage in addition to the mean and standard deviation. This was followed by comparative paired and unpaired t-tests that were used to compare continuous variables, and the significance level was set at ≤0.05.
3. Results
Thirty patients (20 males and 10 females) participated in this study. The mean age of the patients was 37.5 ± 2.3 years. Group I contained 7 males & 3 females, group II contained 6 males and 4 females, while group III contained 7 males and 3 females. The mean age of the patients was 35.8 ± 4.28 years in group I, 36.9 ± 2.4 years in group II, and 39.8 ± 3.2 years in group III.
In this study, implant diameter ranged from 4.2 to 4.8 mm while implant length ranged from 8 to 12 mm. One patient in group I showed implant mobility, while another patient in group II exhibited partial membrane exposure at 14 days postoperatively, which was allowed to heal by secondary measures through application of good oral hygiene & irrigation.
3.1. Implant stability quotient (ISQ)
All groups showed statistically significant differences in a comparison of the mean changes in ISQ at the 6-month observation interval. The mean stability difference in Group I was higher than that in groups II and III (3.72, 1.54, and 2.45, respectively) (Table 1). However, intergroup comparisons of ISQ showed no statistically significant differences at both baseline and 6 months postoperatively.
Table 1.
Illustrating range, mean, minimum, maximum and standard deviation of implant stability quotient (ISQ) and alveolar bone width in all groups and comparison between the two different intervals.
| Intervals | Range | Min | Max | Mean | ±SD | Baseline Vs. 6 Month | ||
|---|---|---|---|---|---|---|---|---|
| ISQ | Group I | baseline | 20 | 55 | 75 | 68.45 | ±5.22 | 0.0001** |
| 6 month | 21 | 50 | 71 | 64.73 | ±5.83 | |||
| Group II | baseline | 9 | 65 | 74 | 69.09 | ±2.84 | 0.015* | |
| 6 month | 9 | 63 | 72 | 67.55 | ±2.46 | |||
| Group III | baseline | 10 | 65 | 75 | 69.00 | ±3.03 | 0.004** | |
| 6 month | 9 | 60 | 69 | 66.55 | ±2.66 | |||
| Alveolar bone width | Group I | Pre-operative | 1.7 | 3.3 | 5 | 3.99 | ±0.4 | 0.0001** |
| Post-operative | 3.1 | 4.9 | 8 | 6.84 | ±1.1 | |||
| Group II | Pre-operative | 0.9 | 3.2 | 4.1 | 3.64 | ±0.3 | 0.0001** | |
| Post-operative | 1.9 | 6.1 | 8 | 6.90 | ±0.6 | |||
| Group III | Pre-operative | 1.9 | 3.1 | 5 | 4.17 | ±0.7 | 0.0001** | |
| Post-operative | 1.3 | 6.2 | 7.5 | 6.65 | ±0.4 | |||
Statistically significant: (P < 0.05).
High statistically significant: (P < 0.01).
3.2. Ridge width evaluation
The ridge width increased significantly in all groups. In group I, the mean ridge width was 3.99 ± 0.4 mm preoperatively, which increased to 6.84 ± 1.1 mm postoperatively (P = 0.001). In group II, the mean ridge width was 3.64 ± 0.3 mm preoperatively, which increased to 6.90 ± 0.6 mm postoperatively (P = 0.001). In group III, the mean ridge width was 4.17 ± 0.7 mm preoperatively, which increased to 6.65 ± 0.4 mm postoperatively (P = 0.001). The difference within group II was higher than those in groups I and III, but there were no significant postoperative differences between groups (Table 1).
3.3. Bone density measurements
Bone density measurements showed significant differences in all groups during the observation periods, with the bone density changes being higher in group III than those in groups II and I (Table 2). Group III showed high statistically significant differences at both the 6th and 12th month evaluations when compared with groups II and I.
Table 2.
Illustrating range, minimum, maximum, means and standard deviations of bone density (BD) in all groups and measurements comparison during different intervals within groups.
| Intervals | Range | Min | Max | Mean | ±SD | P | |
|---|---|---|---|---|---|---|---|
| Group I | baseline | 17 | 71 | 88 | 77.91 | ±5.54 |
0.032* 0.009** |
| 3 month | 13 | 77 | 90 | 80.55 | ±4.97 | ||
| 6 month | 11 | 78 | 99 | 84.82 | ±6.05 | ||
| 12 month | 30 | 69 | 101 | 90.09 | ±8.92 | ||
| Group II | baseline | 19 | 70 | 89 | 80.55 | ±5.75 |
0.008** 0.0001** |
| 3 month | 26 | 73 | 99 | 87.82 | ±7.17 | ||
| 6 month | 26 | 74 | 100 | 90.91 | ±7.98 | ||
| 12 month | 34 | 75 | 109 | 98.91 | ±9.32 | ||
| Group III | baseline | 11 | 79 | 90 | 83.91 | ±4.01 |
0.041* 0.001** |
| 3 month | 19 | 85 | 99 | 90.55 | ±4.97 | ||
| 6 month | 15 | 90 | 105 | 97.45 | ±4.46 | ||
| 12 month | 10 | 105 | 115 | 111.09 | ±3.45 | ||
Statistically significant: (P < 0.05).
High statistically significant: (P < 0.01).
3.4. Marginal bone level
The marginal bone loss changes between the baseline measurements and the 12-month measurements were higher in group I than in groups II and III; the marginal bone loss increased from 0.00 ± 0.00 mm at baseline to 0.64 ± 1.28 mm at 12 months in group I, from 0.00 ± 0.00 mm to 0.17 ± 0.04 mm in group II, and from 0.00 ± 0.00 mm to 0.11 ± 0.04 mm in group III. The results showed a high statistically significant difference over the 12-month interval when comparing groups III and II with group I.
4. Discussion
This study investigated the effect of PRF and AM on osseointegration around dental implants in a piezoelectrically created gap. The clinical and radiological results obtained after one year revealed stable osseointegration with no soft tissue recessions or peri-implant bone loss in groups II & III, with superior results for group III.
The effect of PRF has been studied in vitro, and it showed promising effects on the proliferation and differentiation of osteoblasts (Dohan et al., 2006, Lewandrowski et al., 2003). Moreover, PRF stimulated osteoblast adhesion and upregulated collagen production (Baiomy et al., 2016, Yang, 2017). The fibrin architecture of PRF serves as an excellent scaffold for cell migration and angiogenesis, as well as a reservoir for growth factors, providing slow release over 7 days (Zandi et al., 2010). In addition, the high leukocyte content in PRF might play a role in the regulation of inflammation and prevention of infection (Abdallah Edrees et al., 2017, Knapen et al., 2015).
AM was used in this study to cover the surgical site before flap closure. It acts as a scaffold for cell proliferation and differentiation due to the existence of fibronectin, elastin, nidogen, collagen, elastin, and hyaluronic acid. The ability of AM to promote epithelialization; its anti-inflammatory, antifibrotic, antibacterial, and antiangiogenic properties; and its non-immunogenicity makes it an ideal regenerative tool for wound healing. AM promotes epithelial cell migration and adhesion and enhances the growth of epithelial cells by increasing their lifespan (Knapen et al., 2015, Zandi et al., 2010).
In this study, a full-thickness flap was raised with minimal tissue reflection on either aspect of the cortical plates to preserve the periosteal attachment surrounding the bone. This was performed to prevent bone plate cracks.
The mean changes in ISQ at the 6-month observation interval when compared to the baseline in all groups might be explained by the fact that all groups underwent the same technique, wherein soft tissue and blood supply at the gap site are preserved when the piezosurgical device is used for preparation of osteotomy. This result matched with another study (kumar Post Graduate Student et al., 2015), which pointed that the piezoelectric device ensures micrometric accurate cutting, safety, rapid healing and better hemostasis during surgery.
The mean change from the primary stability in group I was higher than those in groups II and III (3.72, 1.54, and 2.45, respectively). However, in a comparison of the ISQ between groups, there was no statistically significant difference at both baseline and 6-month postoperative intervals. This can be explained by the fact that the use of nHA and AM to cover the surgical area in both groups would benefit from the properties of nHA and AM in enhancing bone induction, wound healing, and reepithelialization as well as the antimicrobial and anti-viral properties of AM that were demonstrated by previous studies (Elsayed et al., 2014, Kjaergaard et al., 2001).
Regarding marginal bone level, there was a statistically significant difference during the 12-month interval in favor of group III over group II. The marginal bone loss change between the baseline measurements and the 12-month measurements was higher in group 1 than in groups II and III. This result was expected since in group I, only the nHA graft was applied, which showed a higher resorption rate during the initial post-implant insertion period. However bone loss readings in the present study were in accordance with the reported bone loss levels during the first year of implant insertion in other studies (Alt et al., 2016, Elsayed et al., 2014).
There was a significant increase in bone density measurements in all groups during the observation periods, where the difference in bone density changes was higher in group III than in groups II and I, respectively. These results are in accordance with the results reported in a previous study (Zhang et al., 2012), which showed no difference in density after 6 months of using bovine hydroxyapatite alone or in combination with PRF during sinus lift. These findings may be attributable to the use of another form of hydroxyapatite and the adjunctive use of AM as cover in our study. Moreover, our results agreed with those reported in a study that pointed out the role of PRF in regeneration of bone defects (Saluja and Dehane, 2011).
5. Conclusion
Concomitant use of PRF with an nHA graft covered with AM for augmentation around the dental implant in narrow posterior mandibles after piezoelectric alveolar ridge splitting accelerated osseointegration and significantly increased bone density around the osseointegrated implant while decreasing bone resorption in comparison to that observed with the graft alone
Ethical considerations
The present study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The study was approved by Research Ethical Committee of Al Azhar University. All patients’ personal information was not identified and only the researchers had access to the records.
Declaration of Competing Interest
The authors declare no conflict of interest
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Alaa Abdelqader Altaweel, Email: alaarezk77@azhar.edu.eg.
Abdel Aziz Baiomy Abdullah Baiomy, Email: abdelazizbaiomy@azhar.edu.eg.
Shadia Abdel-Hameed Elsayed, Email: shadiaelsayed@azhar.edu.eg.
References
- Abdallah Edrees M.F., Abdullah Baiomy A.A.B., Fahmy Gobran H.G., Dameer H.A. Leukocyte platelet-rich fibrin versus platelet-rich fibrin block in ridge splitting technique with simultaneous implant placement, Egypt. J. Oral Maxillofac. Surg. 2017;8:32–37. doi: 10.1097/01.omx.0000515465.79426.e0. [DOI] [Google Scholar]
- Aghaloo T.L., Moy P.K. Are the most successful in furnishing bony support for implant placement? Int. J. oral Maxillofac. Implant. 2007;22:49–73. [PubMed] [Google Scholar]
- Alt V., Cheung W.H., Chow S.K.H., Thormann U., Cheung E.N.M., Lips K.S., Schnettler R., Leung K.S. Bone formation and degradation behavior of nanocrystalline hydroxyapatite with or without collagen-type 1 in osteoporotic bone defects - An experimental study in osteoporotic goats. Injury. 2016;47:S58–S65. doi: 10.1016/S0020-1383(16)47010-5. [DOI] [PubMed] [Google Scholar]
- Baiomy A.A.B.A., Habib Mohamed, Gobran Hany G. Versatility of nano-hydroxyapatite versus nano-β-tricalcium phosphate in grafting of mandibular bone defects: experimental study. Egypt. Dent. J. 2016;62:4689–4700. [Google Scholar]
- Castillo R. Horizontal ridge augmentation before placing implants using a double-bone,double resorbable membrane technique: two clinical cases. Eur. J. Esthet. Dent. 2010 https://doi.org/19834 [pii] [PubMed] [Google Scholar]
- Demarosi F., Leghissa G.C., Sardella A., Lodi G., Carrassi A. Localised maxillary ridge expansion with simultaneous implant placement: a case series. Br. J. Oral Maxillofac. Surg. 2009;47:535–540. doi: 10.1016/J.BJOMS.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg., Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006;101:e45–e50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest D.M., de Peppo G.M., Doglioli P., Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63–69. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- Donos N., Mardas N., Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy) J. Clin. Periodontol. 2008;35:173–202. doi: 10.1111/j.1600-051X.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- Elsayed S.A., Attia M.S., Gawish A.S. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite bone graft in treatment of peri-implant defects in diabetic patients, Egypt. J. Oral Maxillofac. Surg. 2014;5:33–38. doi: 10.1097/01.omx.0000444061.56326.e8. [DOI] [Google Scholar]
- Esposito M., Grusovin M.G., Willings M., Coulthard P., Worthington H.V. The effectiveness of immediate, early, and conventional loading of dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int. J. Oral Maxillofac. Implants. 2008;22:893–904. doi: 10.1016/S0022-3913(08)60070-0. [DOI] [PubMed] [Google Scholar]
- Hammerle, C.H.R, Jung, R.E., F.A., 2002. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J. Clin. Periodontol. 29, 226–31. [DOI] [PubMed]
- Han J.Y., Shin S. Il, Herr Y., Kwon Y.H., Chung J.H. The effects of bone grafting material and a collagen membrane in the ridge splitting technique: an experimental study in dogs. Clin. Oral Implants Res. 2011;22:1391–1398. doi: 10.1111/j.1600-0501.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- Jensen S.S., Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implants. 2009;24(Suppl):218–236. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- Kjaergaard N., Hein M., Hyttel L., Helmig R.B., Schønheyder H.C., Uldbjerg N., Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;94:224–229. doi: 10.1016/S0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Knapen M., Gheldof D., Drion P., Layrolle P., Rompen E., Lambert F. Effect of leukocyte- and platelet-rich fibrin (L-PRF) on bone regeneration: a study in rabbits. Clin. Implant Dent. Relat. Res. 2015;17:e143–e152. doi: 10.1111/cid.12146. [DOI] [PubMed] [Google Scholar]
- Kumar Post Graduate Student, P.K., Tukaram Kshirsagar, J., Kumar, P.K., Maria, N.T., 2015. Piezosurgery: ultrasonic bone surgery in periodontics and oral implantology-review. Int. J. Appl. Dent. Sci. 1, 19–22.
- Lewandrowski Kai-Uwe Bondre, Wise Shrikar P., Trantolo Donald L. D.J. Enhanced bioactivity of a poly(propylene fumarate) bone graft substitute by augmentation with nano-hydroxyapatite. Biomed. Mater. Eng. 2003;13:115–124. [PubMed] [Google Scholar]
- Saluja H., Dehane V. M.U. Platelet –rich fibrin: a second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann. Maxillofac. Surg. 2011;1:53–57. doi: 10.4103/2231-0746.83158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A., Kaus T. Maxillary ridge expansion with simultaneous implant placement: 5-year results of an ongoing clinical study. Int. J. Oral Maxillofac. Implants. 2000;15:491–499. [PubMed] [Google Scholar]
- Yang C. Feasibility of CBCT in evaluating bone density of dental implant placement sites. Res. Rev. J. Dent. Sci. 2017;5:87–91. [Google Scholar]
- Zandi M., Mirzadeh H., Mayer C., Urch H., Eslaminejad M.B., Bagheri F., Mivehchi H. Biocompatibility evaluation of nano-rod hydroxyapatite/gelatin coated with nano-HAp as a novel scaffold using mesenchymal stem cells. J. Biomed. Mater. Res. - Part A. 2010;92:1244–1255. doi: 10.1002/jbm.a.32452. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tangl S., Huber C.D., Lin Y., Qiu L., Rausch-Fan X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J. Cranio-Maxillofacial Surg. 2012;40:321–328. doi: 10.1016/J.JCMS.2011.04.020. [DOI] [PubMed] [Google Scholar]