Abstract
Objective
This systematic review aims to assess the efficacy chlorhexidine chip as an adjunctive therapy of scaling and root planning on periodontal disease treatment.
Material and methods
This study follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) and was registered in the PROSPERO database (CRD42019148221). The search was performed in PubMed/MEDLINE, Scopus, and Cochrane databases until April 2020. The PICO question was: “Is the chlorhexidine chip (CHX) effective as an adjunctive therapy of scaling and root planning on periodontal disease treatment?”. Inclusion criteria involved: randomized controlled clinical trials, with a minimum of 15 patients included on the sample and each patient has two sites of probing depth of ≥5 mm; The minimum follow up was at least 1 months of follow-up and the outcomes present in the studies probing depth (PD), plaque index (PI) and clinical attachment level (CAL) after scaling and root planning (SRP).
Results
After searching the databases, 13 articles were selected for qualitative and 8 for quantitative analysis. Were included 427 patients, with a mean age of 45.6 years. The results shown that the association of chlorhexidine chips to scaling and root planning reduce periodontal pocket depths (P < 0.00001; MD −0.77 [CI −1.0 to −0.55]; I2 = 23%, P = 0.24), gain on the clinical attachment level (P < 0.0001; MD −0.57 [CI −0.86 to −0.27]; I2 = 33%, P = 0.18P < 0.0001) and reduction on plaque index (P = 0.04; MD −0.23 [CI −0.45 to −0.01]; I2 = 91%, P < 0.00001).
Conclusions
Thus, we can conclude that chlorhexidine chip when used associated to scaling and root planning promoted a significant improvement the reduction of periodontal diseases.
Keywords: Chlorhexidine gluconate, Periodontal diseases, Dental scaling, Systematic review
1. Introduction
Scaling and root planning (SRP) are considered gold standard methods for dental plaque removal (Pai et al., 2013). Although mechanical elimination significantly reduces the level of microorganisms in the subgingival area, it does not eradicate all pathogens (Kasaj et al., 2007) because of the complex root anatomy (Kondreddy et al., 2012). To overcome the conventional treatment limitations, antibiotics and antiseptics have been used for periodontal therapy (Paolantonio et al., 2008a).
The association of systemic antibiotic therapy with SRP has been useful in the treatment of periodontal pockets (Kondreddy et al., 2012). One concern is that these drugs only reach low concentrations at the infection site (Paolantonio et al., 2008a), due to the fact that the crevicular fluid is constantly renewed (Kasaj et al., 2007); thus, a higher dosage is required, which can promote undesirable side effects, such as the development of bacterial resistance (Paolantonio et al., 2008a).
However, the use of local antimicrobials, inserted directly on the pocket, can reach 100 times the concentration of the same drug administered orally (Gottumukkala et al., 2014). Antimicrobials such as tetracycline, chlorhexidine (CHX), metronidazole (Bansal et al., 2019, Singh et al., 2018), 10% doxycycline, and 2% minocycline have been used as local drugs in periodontal disease treatment (Singh et al., 2018). CHX is considered the gold standard in periodontics (Jolkovsky & Ciancio, 2006) because it has a large antimicrobial spectrum, is biocompatible, and effective.
Regarding the CHX chips, its advantages are uncertain. Some studies have shown a low benefit in reducing microorganisms in comparison with SRP (Salvi et al., 2002, Daneshmand et al., 2002). However, other studies (Mızrak et al., 2006, Azmak et al., 2002) reported significant advantages in using CHX chips and SRP combined. Only one systematic review (Cosyn and Wyn, 2006) revealed inconclusive data, because the clinical and microbiological data available was limited and conflicting.
For these reasons, this systematic review aims to provide new evidence on the effectiveness of the use of CHX chips as adjunctive therapy for scaling and root planning in the treatment of periodontal disease. The null hypothesis is that there is no difference in clinical parameters with the use of CHX chips.
2. Materials and methods
2.1. Protocol registration
This systematic review follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria and was registered on the PROSPERO. (CRD42019148221).
2.2. Eligibility criteria
The PICO (patient, intervention, comparison, outcome) was: ““Is the chlorhexidine chip (CHX) effective as an adjunctive therapy of scaling and root planning on periodontal disease treatment?”. The “Population” included patients with periodontal disease; “Intervention” is the SRP associated to chlorhexidine chip; “Comparison” is only SRP as a treatment and the “Outcome” evaluated probing depths (PD) (primary outcome) and the clinical attachment level (CAL) and plaque index (PI) (secondary outcome).
Inclusion criteria involved: randomized controlled clinical trials, with a minimum of 15 patients included on the sample and each patient has two sites of probing depth of ≥5 mm; The minimum follow up was at least 1 months of follow-up and the outcomes present in the studies probing depth (PD), plaque index (PI) and clinical attachment level (CAL) after scaling and root planning (SRP).
Exclusion criteria were studies involving patients under age of 18; articles involving smokers, pregnant women, people allergic to chlorhexidine; individuals who went under systemic therapy with antimicrobials within 2 months before the study and patients that received periodontal treatment in less than 3 months of the preliminary consultation.
2.3. Search strategy
Two investigators (C.D.D.R.D.R and J.M.L.G) searched independently on the electronic databases of PubMed/MEDLINE, Scopus e Cochrane, studies published until April of 2020 according to eligibility criteria.
The selection strategy was based on the following combination: ‘‘(((“periodontitis”[MeSH Terms] OR “periodontitis”[All Fields]) OR (“periodontal diseases”[MeSH Terms] OR (“periodontal”[All Fields] AND “diseases”[All Fields]) OR “periodontal diseases”[All Fields])) AND ((“chlorhexidine gluconate”[Supplementary Concept] OR “chlorhexidine gluconate”[All Fields] OR “perio chip”[All Fields]) OR ((“chlorhexidine”[MeSH Terms] OR “chlorhexidine”[All Fields]) AND chip[All Fields]))) AND ((“dental scaling”[MeSH Terms] OR (“dental”[All Fields] AND “scaling”[All Fields]) OR “dental scaling”[All Fields]) OR (“root planing”[MeSH Terms] OR (“root”[All Fields] AND “planing”[All Fields]) OR “root planing”[All Fields]))”. Likewise, a manual search was completed on high impact periodontics journals such as Journal of Periodontology, Journal of Dental Research, Journal of Clinical Periodontology, Journal of Periodontal Research, Journal of Dentistry, Journal of the American Dental Association, Periodontology 2000, Clinical Oral Investigations.
2.4. Data analysis
One author (C.D.D.R.D.R.) was collect data from the included studies and a second author (C.A.A.L.) checked the information. When there was disagreement, a third reviewer (S.L.D.M.) was consulted. The qualitative data collected were author of study and year, number of patients, mean age of the study participants, range of follow-up in months, clinical evaluations, chlorhexidine chip application interval, outcomes, conclusion and effect of intervention in the studies. The quantitative data collected was mean and standard deviation (Mean ± SD) of the outcomes: PD, CAL, PI (Table 1).
Table 1.
Data from selected studies.
| Author | Patient, n | Mean age, years | Follow-up | Clinical Evaluations | CHX Chip ApplicationInterval | Outcomes Results | Conclusion | Effect |
|---|---|---|---|---|---|---|---|---|
| Heasman et al. (2001) | 24 | 47 (35–59) | 6 months | PD, CAL, PI | Periochip 2.5 mg, 1x at baseline | Only CAL at 6 months showed statistically significant differences for CHX chip group. | PerioChip is a safe and effective adjunctive to SRP in the management of previously non-responding sites in maintenance patients. | Positive only for CAL |
| Azmak et al. (2002) | 20 | 49 (36–62) | 6 months | PD, CAL, PI | Periochip 2.5 mg, 1x at baseline | PD, CAL and PI no showed statistically significant differences at 1, 3 or 6 months when compared control and treatment group. | CHX chip following SRP might be beneficial in improving periodontal parameters | None |
| Kasaj et al. (2007) | 20 | 40 (20–60) | 6 months | PD, CAL, PI | Periochip 2.5 mg, 1x at baseline and 1x at 3 months | CAL and PD at 1, 3 and 6 months showed statistically significant differences for CHX chip group. But PI scores were not significantly different. | Adjunctive application of the CHX chip to SRP is beneficial in improving clinical periodontal parameters | Positive for PD and CAL |
| Paolantonio et al. (2008a) | 116 | 49 (33–65) | 6 months | PD, PI | Periochip 2.5 mg, 1x at baseline | PD at 6 months showed statistically significant differences for CHX chip group. | CHX chip with SRP resulted in a clinically improvement in PD reduction and relative attachment level gain compared to SRP alone. | Positive only for PD |
| Paolantonio et al. (2008b) | 82 | 47 (31–63) | 6 months | PD, CAL | Periochip 2.5 mg, 1x at baseline | The PP and CAL were significantly lower at 6 months as compared to the baseline scores in both treatments (p < 0.01). | CHX chip with SRP resulted in a clinically improvement in PD reduction and CAL gain compared to SRP alone. | Positive for PD and CAL |
| Kondreddy et al. (2012) | 20 | 45 (35–55) | 6 months | PD, CAL, PI | Periocol CG 2.5 mg, 1x at baseline and 1x at 3 months | CAL and PI showed statistically significant differences for CHX chip group. | Use of PerioCol‑CG was safe and it is more favorable than SRP alone in the reduction of clinical parameters. | Positive for CAL and PI |
| Medaiah et al. (2014) | 15 | 45 (35–55) | 3 months | PD, CAL, PI | Periochip 2.5 mg, 1x at baseline | CAL and PD showed statistically significant differences for CHX chip group. But PI scores were not significantly different. | CHX chip by itself did provide clinical benefits | Positive for PD and CAL |
| John et al. (2015) | 20 | 45.5 (35–56) | 3 months | PD, CAL, PI | Periocol CG 2.5 mg, 1x at baseline | PD and CAL no showed statistically significant differences when compared control and treatment group. PI showed statistically significant differences for CHX chip group | CHX chip as an adjunct to SRP was safe and showed benefits in clinical and microbiological parameters | Positive for PI |
| Pattnaik et al. (2015) | 20 | 41.5 (29–54) | 3 months | PD, CAL | Periocol CG 2.5 mg, 1x at baseline | CAL and PD at 3 months showed statistically significant differences for CHX chip group. | SRP combined with CHX chip has a significantly better and prolonged effect compared to SRP alone on the PD, CAL and elimination of periodontopathogens, but not on gingival inflammation. | Positive for PD and CAL |
| Jose et al. (2016) | 15 | 45 (30–60) | 3 months | PD, CAL, PI | Periocol CG 2.5 mg, 1x at baseline | CAL and PD showed statistically significant differences for CHX chip group. But PI scores were not significantly different. | CHX chip is effective in improving oral hygiene, reducing gingival inflammation, reducing probing pocket depth and improving clinical attachment levels when used as adjuncts to SRP | Positive for PD and CAL |
| Lecic et al. (2016) | 15 | 36.5 (21–52) | 3 months | PD, CAL, PI | Periochip 2.5 mg, 1x at baseline | Only PD at 3 months showed statistically significant differences for CHX chip group. | CHX chip as an adjunct to SRP showed greater improvements in bleeding index and PPD compared to those obtained by SRP alone. | Positive only for PD |
| Singh et al. (2018) | 40 | 40 (30–50) | 3 months | PD, PI | Periocol CG 2.5 mg, 1x at baseline | PD at 3 months showed statistically significant differences for CHX chip group when compared SRP alone. | This study reveals the excellent clinical properties CHX | Positive for PD |
| Bansal et al. (2019) | 20 | 47.5 (30–65) | 1 month | PD, CAL, PI | Periocol CG 2.5 mg, 1x at baseline | PD, CAL and PI showed statistically significant differences for CHX chip group. | Adjunctive CHX chip therapy, appreciably improve the benefits of SRP | Positive for PD, CAL, PI |
PD = probing pocket depth; CAL = clinical attachment level; PI = plaque index; CHX = chlorhexidine; SRP = scaling and root planning.
2.5. Summary measurements
The meta-analysis was based on an inverse variance (IV) method. The primary outcome PD (primary) and the secondary outcomes: CAL and PI were considered continuous outcome and was evaluated using the mean difference (MD) evaluated by IV with 95% confidence interval (CI). The MD values were considered to be significant when P < 0.05. For statistically significant (P < 0.10) heterogeneity, a random-effects model was used to assess the significance of the treatment effects. When no statistically significant heterogeneity was found, an analysis was performed using a fixed-effects model. The software Reviewer Manager 5 (Cochrane Group) was used for the meta-analyses.
2.6. Risk of bias
Two authors (L.M., J.P.J.O.L.) performed risk of bias analysis on the included RCTs using the Cochrane risk of bias tool, the tool verifies selection, performance, attrition, reporting and other biases.
2.7. Additional analysis
As an additional analysis, the inter-rater test (Kappa), was used to measure the reliability of the database searches between the investigators (PubMed/MEDLINE, Scopus, Cochrane). Disagreements were analyzed and decided by a third author (E.P.P).
3. Results
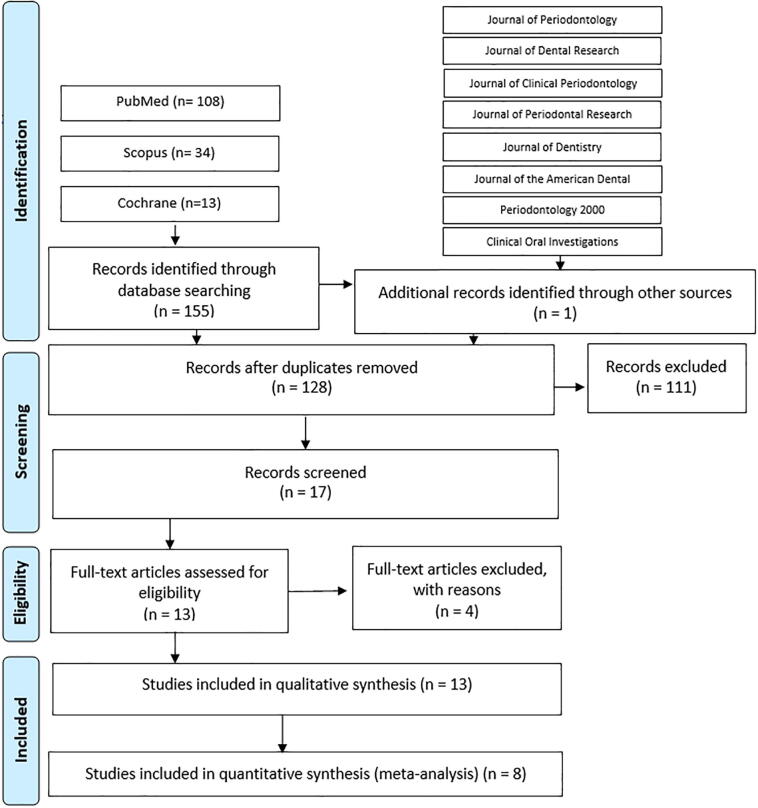
We found 156 studies from the previous selected bases: 108 from PubMed, 34 from Scopus, 13 from Cochrane, and one from the Journal of Periodontology. After removing duplicates, 128 articles were screened by title and abstract and 17 were screened by full text. Four references were excluded: two were not a split-mouth trial (Pai et al., 2013, Killoy, 1999), one included smokers (Carvalho et al., 2007), and the last one was not available in English (He et al., 2001). Therefore, 13 studies were included for the final qualitative analysis and eight were selected for the quantitative analysis. The details of the search strategy are illustrated in Fig. 1.
Fig. 1.
Search strategy.
The kappa test was applied to evaluate the agreement between examiners in the initial search, indicating high levels of agreement: 0.85 for PubMed/MEDLINE, 0.86 for Scopus, and 1.00 for the Cochrane Library.
3.1. Characteristics of selected studies
Detailed data from the seven selected studies are listed in Table 1. The total number of participants included was 427, with a mean age of 45.6 years. All participants had at least two sites of pockets, including 854 pockets divided into the treatment and control groups. The follow-up period ranged from 1 to 6 months.
All patients received SRP (manual and/or ultrasonic) and oral hygiene instructions. The authors used a manual periodontal probe (10 or 15 mm) to measure the pocket depth (PD) and CAL. Some studies (Kondreddy et al., 2012, John et al., 2015, Bansal et al., 2019, Singh et al., 2018, Jose et al., 2016, Medaiah et al., 2014) used occlusal guides to create a pattern on the clinical evaluations. All other studies used manual probing by only one examiner (Kasaj et al., 2007, Paolantonio et al., 2008b, Heasman et al., 2001, Lecic et al., 2016, Pattnaik et al., 2015, Azmak et al., 2002).
3.2. Risk of bias
For randomized clinical trials, the Cochrane scale was used (Table 2). On the domain “sequence generation”, three studies (Kasaj et al., 2007, Kondreddy et al., 2012, Heasman et al., 2001) were judged as having an uncertain risk of bias due to inconclusive information. On the domain “allocation concealment”, five studies (Kasaj et al., 2007, Kondreddy et al., 2012, Paolantonio et al., 2008a, Paolantonio et al., 2008a, Jose et al., 2016, Heasman et al., 2001) presented an uncertain risk of bias. In the domains of “blinding of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting and other sources of bias”, the studies had a low risk of bias.
Table 2.
Risk of bias of randomized controlled trials-cochrane scale.
| Heasman et al. (2001) | Azmak et al. (2002) | Kasaj et al. (2007) | Paolantonio et al. (2008a) | Paolantonio et al. (2008b) | Kondreddy et al. (2012) | Medaiah et al. (2014) | John et al. (2015) | Pattnaik et al. (2015) | Jose et al. (2016) | Lecic et al. (2016) | Singh et al. (2018) | Bansal et al. (2019) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence Generation | UNCLEAR | LOW | UNCLEAR | LOW | LOW | UNCLEAR | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Allocation Concealment | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW | LOW | UNCLEAR | LOW | LOW | LOW |
| Blinding of participants, personnel and outcome assessors | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Incomplete outcome data | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Selective outcome reporting | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Other sources of bias | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
3.3. Meta-analysis
The meta-analysis included eight studies that contained quantitative data related to the outcomes. Some studies could not be included in the analysis, considering that there was no mean or standard deviation to use when comparing the groups (Kasaj et al., 2007, Paolantonio et al., 2008a, Singh et al., 2018, Azmak et al., 2002, Heasman et al., 2001).
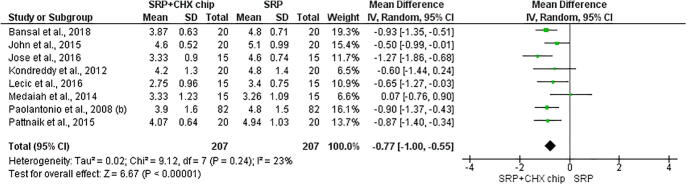
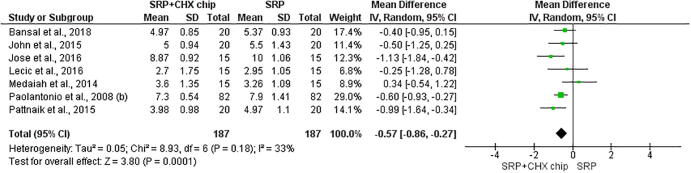
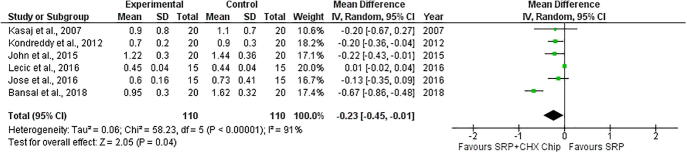
The eight studies were included for the first outcome on the probing depth (Kondreddy et al., 2012, John et al., 2015, Bansal et al., 2019, Jose et al., 2016, Medaiah et al., 2014, Paolantonio et al., 2008b, Lecic et al., 2016, Pattnaik et al., 2015). The results showed that associating CHX chips with scaling and root planning reduced periodontal pocket depths (P < 0.00001; MD −0.77 [CI −1.0 to −0.55]; I2 = 23%, P = 0.24) (Fig. 2), gain on the clinical attachment level (P < 0.0001; MD −0.57 [CI −0.86 to −0.27]; I2 = 33%, P = 0.18, P < 0.0001) (Fig. 3), and the plaque index (P = 0.04; MD −0.23 [CI −0.45 to −0.01]; I2 = 91%, P < 0.00001) (Fig. 4).
Fig. 2.
Forest plot evaluating Probing pocket depth. Statistically significant difference (p < 0.05) favorable to chlorhexidine chip.
Fig. 3.
Forest plot evaluating clinical attachment level. Statistically significant difference (p < 0.05) favorable to chlorhexidine chip.
Fig. 4.
Forest plot evaluating plaque index. Statistically significant difference (p < 0.05) favorable.
4. Discussion
The local administration of antimicrobials as an adjunctive therapy for the treatment of periodontal disease has been described in the literature for 40 years (Lindhe et al., 1979). The null hypothesis of this study was rejected, since CHX chips associated with SRP showed better results for PD, CAL, and PI. Access to root morphology is complex when the clinicians use SRP to remove the subgingival plaque (Fleischer et al., 1989). To achieve success in mechanical debridement, local antiseptics may be used to eradicate periodontal pathogens at different phases of the treatment (Jose et al., 2016), and CHX chips can be viable alternatives (John et al., 2015).
The commercial name of CHX chips is “Periochip” and each dose contains 2,5 mg of CHX gluconate that can inhibit more than 99% of subgingival microorganisms in the pocket, maintaining a concentration level higher than the minimum inhibitory concentration (MIC) (90) for more than a week (Stanley et al., 1989). This effect reduces the bacterial degradation of proteins and glycoproteins, and consequently, the availability of essential nutrients for bacterial development (Beighton et al., 1991). Additionally, CHX chips have been associated with a significant reduction of periodontal pathogens associated with chronic periodontitis and found in the deepest pockets, such as P. gingivalis and T. forsythia (Pattnaik et al., 2015).
Chlorhexidine molecules can connect to salivary bacteria and interfere with its tooth’s adsorption, reducing bacterial repopulation (Pattnaik et al., 2015). The reduction in PD in sites receiving treatment with SRP and CHP can be explained by the fact that these patients have a lower bacterial count to 1 month after therapy, when compared to patients with only SRP (Paolantonio et al., 2008a). Due to the antimicrobial effects of the CHX chip during the initial healing phase, the maturation of the bacterial biofilm was impaired (Pattnaik et al., 2015), promoting better healing of the periodontal tissues (Paolantonio et al., 2008a).
The results of this study show that the use of the CHX chip as a compliment to SRP demonstrates an advantage over treatment with SRP alone. During the three-month follow-up period, the average reduction in PD was 1.2 mm (Jose et al., 2016), 1.6 ± 0.5 mm (Kondreddy et al., 2012), 1.9 ± 0.32 mm, showing an additional 0.6 mm reduction in comparison with the SRP group (John et al., 2015). In the study by Lecic et al. (2016), the SRP + CHX group, with an average PD of 5.70 ± 0.97 mm, decreased to 2.75 ± 0.96 mm, while the SRP group, which had an average of 5.25 ± 1,01 mm, reduced to 3.40 ± 0.75 mm.
In the six-month follow-up period, the mean PD reduction ≥ 2 mm in the SRP + CHX chip group was significantly higher than that in the SRP group (Kasaj et al., 2007, Paolantonio et al., 2008b, Heasman et al., 2001). Azmak et al. (2002) also showed a significant reduction in PD (≥2 mm) in 94.4% of the SRP + CHX group versus 77.8% of the SRP group. For Kondreddy et al., 2012, the mean reduction was 3.2 ± 0.6 mm for the SRP + CHX group. Regarding very deep pockets, similar results were found in the article by Paolantonio et al., 2008a, when the PD subgroup (≥7 mm) demonstrated a significant reduction in the SRP + CHX chip group in the sixth month.
In 2007, Kasaj et al. found that the mean probing depth on the sixth month was 2.2 mm in the SRP + CHX chip group, while in the SRP group the reduction was 0.7 mm. This difference was the highest seen in studies, which can be explained by the depth of the pockets at the beginning of the trial because the reduction in PD and the gain in CAL is higher in the deepest pockets after SRP (Ramfjord et al., 1987). In addition, in the third month of this study, all sites underwent SRP and the test sites received a second CHX chip (Kasaj et al., 2007), maintaining pockets reduction after this period (Mızrak et al., 2006).
Probing depth and CAL are important indicators in diagnosing and evaluating the success of periodontal disease therapy (Lecic et al., 2016). The met analysis showed a significant reduction in CAL using the SRP + CHX chip in the group. When evaluating the 3-month follow-up period, Konkredy et al. in 2012 observed an average gain in CAL of 1.3 ± 0.5 mm in the SRP group and 1.8 ± 0.6 mm in the SRP + CHX group. Similar results can also be observed in the study by John et al., 2015, which demonstrated that the average gain of CAL in the SRP group was 1.0 ± 0.47 mm, in contrast with the results of the SRP + CHX chip of 1.9 ± 0.32 mm.
When evaluating the 180-day period, Konkredy et al. (2012) found an average CAL gain of 2.7 ± 1.0 mm in the SRP group and 3.2 ± 0.9 mm in the SRP + CHX group. The SRP + CHX chip group demonstrated a higher average gain in CAL (average: 1.4 mm) compared to the SRP group (average: 0.9 mm; P < 0.05) (Paolantonio et al., 2008b). The highest gain in CAL was reported by Kasaj et al. (2007), explained by the methods used by the authors. The pockets that received SRP and CHX chips had a significant gain in CAL on the 1st, third and sixth months when compared with those on SRP alone (P < 0,05). On the third month, all pockets treated with SRP had an average gain of 0.5 mm in comparison with SRP + CHX chip sites that reached a gain of 1.6 mm. After 6 months, the average gain in CAL was 0.6 mm on the SRP sites and 1.9 mm on the SRP + CHX chip sites.
The plaque index was determined to evaluate the general oral hygiene status. The results indicate a significant reduction in the scores on baseline and follow-up visits (Kasaj et al., 2007, Paolantonio et al., 2008a, Singh et al., 2018, Azmak et al., 2002, Jose et al., 2016, Lecic et al., 2016). This score reduction can be assigned to the SRP and patient adhesion to the oral hygiene instructions (Medaiah et al., 2014). Adherence to oral hygiene habits is important in obtaining and maintaining good results in periodontal therapy (Sarsilmazer and Atilla, 2020). A significant reduction in plaque scores was observed in the SRP + CHX group (Kondreddy et al., 2012, John et al., 2015, Bansal et al., 2019). This can be explained by the interference of chlorhexidine in its adsorption of bacteria to the teeth, and thus interfering with the bacterial aggregation that leads to the formation of dental plaque (Pattnaik et al., 2015), an effect that is enhanced by the property of chlorhexidine, known as substantivity (James et al., 2010).
The split-mouth design, involving periodontal pockets in different quadrants, was chosen because this type of study can compare patients with themselves, allowing ease of trial interpretation, minimizing the variability effects between patients (Jose et al., 2016). Regarding heterogeneity in the PD and CAL meta-analysis, the I2 value demonstrated that the studies variability was low (Higgins and Green, 2011).
5. Conclusions
The CHX chips, when used as adjunctive therapy for scaling and root planning, had a significant improvement in reducing probing depth, gaining clinical attachment level, and reducing the plaque index. Therefore, chlorhexidine chip therapy can be considered effective, mainly to the pockets with probing depth over 5 mm.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This work had in part the support of the scholarship provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Cleber Davi Del Rei Daltro Rosa, Email: cleberdavi2@hotmail.com.
Sandra Lúcia Dantas de Moraes, Email: sandra.moraes@upe.br.
Eduardo Piza Pellizzer, Email: ed.pl@uol.com.br.
References
- Azmak N., Atilla G., Luoto H., Sorsa T. The effect of subgingival controlled-release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J. Periodontol. 2002;73(6):608–615. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- Bansal V., Gupta R., Dahiya P., Kumar M., Samlok J.K. A clinico-microbiologic study comparing the efficacy of locally delivered chlorhexidine chip and diode LASER as an adjunct to non-surgical periodontal therapy. J. Oral Biol. Craniofacial Res. 2019;9(1):67–72. doi: 10.1016/j.jobcr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D., Decker J., Homer K.A. Effects of chlorhexidine on proteolytic and glycosidic enzyme activities of dental plaque bacteria. J. Clin. Periodontol. 1991;18(2):85–89. doi: 10.1111/j.1600-051x.1991.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Carvalho J., Novak M.J., Mota L.F. Evaluation of the effect of subgingival placement of chlorhexidine chips as an adjunct to scaling and root planing. J. Periodontol. 2007;78(6):997–1001. doi: 10.1902/jop.2007.060122. [DOI] [PubMed] [Google Scholar]
- Cosyn J., Wyn I. A systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2006;77(2):257–264. doi: 10.1902/jop.2006.050216. [DOI] [PubMed] [Google Scholar]
- Daneshmand N., Jorgensen M.G., Nowzari H., Morrison J.L., Slots J. Initial effect of controlled release chlorhexidine on subgingival microorganisms. J. Periodontal Res. 2002;37(5):375–379. doi: 10.1034/j.1600-0765.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- Fleischer H.C., Mellonig J.T., Brayer W.K., Gray J.L., Barnett J.D. Scaling and root planing efficacy in multirooted teeth. J. Periodontol. 1989;60(7):402–409. doi: 10.1902/jop.1989.60.7.402. [DOI] [PubMed] [Google Scholar]
- Gottumukkala S.N., Sudarshan S., Mantena S.R. Comparative evaluation of the efficacy of two controlled release devices: chlorhexidine chips and indigenous curcumin based collagen as local drug delivery systems. Contemp. Clin. Dentistry. 2014;5(2):175. doi: 10.4103/0976-237X.132310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Geng S., Cao C. The efficacy of the chlorhexidine chip following scaling and root planing (SRP) and compared to SRP alone. Zhonghua kou qiang yi xue za zhi= Zhonghua kouqiang yixue zazhi= Chinese J. Stomatol. 2001;36(6):443–445. [PubMed] [Google Scholar]
- Heasman P.A., Heasman L., Stacey F., McCracken G.I. Local delivery of chlorhexidine gluconate (PerioChipTM) in periodontal maintenance patients. J. Clin. Periodontol. 2001;28(1):90–95. doi: 10.1034/j.1600-051x.2001.280114.x. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P.T., Green, S. (eds.). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- James P., Parnell C., Harding M., Whelton H., Worthington H.V., Beirne P.V. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2010 doi: 10.1002/14651858.cd0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose K.A., Ambooken M., Mathew J.J., Issac A.V., Kunju A.P., Parameshwaran R.A. Management of chronic periodontitis using chlorhexidine chip and diode laser-a clinical study. J. Clin. Diagn. Res.: JCDR. 2016;10(4):ZC76. doi: 10.7860/JCDR/2016/13241.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P., Lazarus F., George J.P., Selvam A., Prabhuji M.L.V. Adjunctive effects of a piscean collagen-based controlled-release chlorhexidine chip in the treatment of chronic periodontitis: a clinical and microbiological study. J. Clin. Diagn. Res.: JCDR. 2015;9(5):ZC70. doi: 10.7860/JCDR/2015/11534.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkovsky D.L., Ciancio S. Chemotherapeutic agents. In: Newman M.G., Takei H.H., Klokkevold P.R., Carranza K.A., editors. Carranza’s Clinical Periodontology. 10th edition. Elsevier; Missouri Saunders: 2006. pp. 798–812. [Google Scholar]
- Kasaj A., Chiriachide A., Willershausen B. The adjunctive use of a controlled-release chlorhexidine chip following treatment with a new ultrasonic device in supportive periodontal therapy: a prospective, controlled clinical study. Int. J. Dental Hygiene. 2007;5(4):225–231. doi: 10.1111/j.1601-5037.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- Killoy W.J. Assessing the effectiveness of locally delivered chlorhexidine in the treatment of periodontitis. J. Am. Dental Assoc. 1999;130(4):567–570. doi: 10.14219/jada.archive.1999.0253. [DOI] [PubMed] [Google Scholar]
- Kondreddy K., Ambalavanan N., Ramakrishna T., Kumar R.S. Effectiveness of a controlled release chlorhexidine chip (PerioCol™-CG) as an adjunctive to scaling and root planing when compared to scaling and root planing alone in the treatment of chronic periodontitis: a comparative study. J. Indian Soc. Periodontol. 2012;16(4):553. doi: 10.4103/0972-124X.106909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecic J., Cakic S., Janjic Pavlovic O., Cicmil A., Vukotic O., Petrovic V., Cicmil S. Different methods for subgingival application of chlorhexidine in the treatment of patients with chronic periodontitis. Acta Odontol. Scand. 2016;74(6):502–507. doi: 10.1080/00016357.2016.1206964. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Heijl L., Goodson J.M., Socransky S.S. Local tetracycline delivery using hollow fiber devices in periodontal therapy. J. Clin. Periodontol. 1979;6:141–149. doi: 10.1111/j.1600-051x.1979.tb02193.x. [DOI] [PubMed] [Google Scholar]
- Medaiah S., Srinivas M., Melath A., Girish S., Polepalle T., Dasari A.B. Chlorhexidine chip in the treatment of chronic periodontitis–a clinical study. J. Clin. Diagn. Res.: JCDR. 2014;8(6):ZC22. doi: 10.7860/JCDR/2014/8808.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mızrak T., Güncü G.N., Çağlayan F., Balcı T.A., Aktar G.S., İpek F. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J. Periodontol. 2006;77(3):437–443. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- Pai B.J., Rajan S.A., Srinivas M., Padma R., Suragimath G., Walvekar A., Kamath V. Comparison of the efficacy of chlorhexidine varnish and chip in the treatment of chronic periodontitis. Contemp. Clin. Dentistry. 2013;4(2):156. doi: 10.4103/0976-237X.114848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolantonio M., D'Angelo M., Grassi R.F., Perinetti G., Piccolomini R., Pizzo G.…Guida L. Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: a multicenter study. J. Periodontol. 2008;79(2):271–282. doi: 10.1902/jop.2008.070308. [DOI] [PubMed] [Google Scholar]
- Paolantonio M., Dolci M., Perfetti G., Sammartino G., Spoto G., Ciampoli C., Tete S. Effect of a subgingival chlorhexidine chip on the clinical parameters and the levels of alkaline phosphatase activity in gingival crevicular fluid during the non-surgical treatment of periodontitis. J. Biol. Regul. Homeost. Agents. 2008;22(1):63–72. [PubMed] [Google Scholar]
- Pattnaik S., Anand N., Chandrasekaran S.C., Chandrashekar L., Mahalakshmi K., Satpathy A. Clinical and antimicrobial efficacy of a controlled-release device containing chlorhexidine in the treatment of chronic periodontitis. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34(10):2103–2110. doi: 10.1007/s10096-015-2459-x. [DOI] [PubMed] [Google Scholar]
- Ramfjord S.P., Caffesse R.G., Morrison E.C., Hill R.W., Kerry G.J., Appleberry E.A., Stults D.L. 4 modalities of periodontal treatment compared over 5 years. J. Clin. Periodontol. 1987;14(8):445–452. doi: 10.1111/j.1600-051x.1987.tb02249.x. [DOI] [PubMed] [Google Scholar]
- Salvi G.E., Mombelli A., Mayfield L., Rutar A., Suvan J., Garrett S., Lang N.P. Local antimicrobial therapy after initial periodontal treatment: a randomized clinical trial comparing three biodegradable sustained release polymers. J. Clin. Periodontol. 2002;29(6):540–550. doi: 10.1034/j.1600-051x.2002.290611.x. [DOI] [PubMed] [Google Scholar]
- Sarsilmazer G., Atilla G. The relationship between oral hygiene related self-efficacy, general self-efficacy and daily plaque control. Int. J. Dental Hygiene. 2020 doi: 10.1111/idh.12429. [DOI] [PubMed] [Google Scholar]
- Singh A., Sridhar R., Shrihatti R., Mandloy A. Evaluation of turmeric chip compared with chlorhexidine chip as a local drug delivery agent in the treatment of chronic periodontitis: a split mouth randomized controlled clinical trial. J. Alternative Complement. Med. 2018;24(1):76–84. doi: 10.1089/acm.2017.0059. [DOI] [PubMed] [Google Scholar]
- Stanley A., Wilson M., Newman H.N. The in vitro effects of chlorhexidine on subgingival plaque bacteria. J. Clin. Periodontol. 1989;16(4):259–264. doi: 10.1111/j.1600-051x.1989.tb01651.x. [DOI] [PubMed] [Google Scholar]