Highlights
-
•
Myrrh improved postoperative surgical-site edema, tenderness, and socket size.
-
•
There were no side effects, allergy, or signs of toxicity with Myrrh use.
-
•
Myrrh application significantly decreased inflammatory signs after one week.
-
•
There were no group differences in fever, fatigue, and limited mouth opening.
Keywords: Myrrh, Tooth extraction, Wound healing
Abbreviations: ISRCTN, International Standard Randomized Controlled Trial Number; IRB, Institutional Review Board
Abstract
Background
The early period after tooth extraction is a critical period for wound healing. Wound healing after tooth extraction is considered secondary intention healing. It passes through several stages in the following order: hemostasis, inflammatory phase, proliferative phase, and finally the remodeling phase.
Wounds usually heal normally unless there is interference by local or systemic factors. In certain circumstances, early wound healing can be enhanced by several interventions such as antibiotics, mouthwashes, or topical medications. Myrrh has been used as a topical medication for promoting wound healing after tooth extraction. The purpose of this study was to assess the wound healing effect of myrrh mouthwash during the early post-extraction period.
Methods
We enrolled 40 healthy adult patients in this study (20: study group and 20: control group). All the activities performed for each group were double-blinded. All the participants underwent dental extraction under local anesthesia using standard protocol. Next, the study group used Commiphora molmol (myrrh) extract as a mouthwash while the control group used normal saline mouthwash. The participants used the mouthwashes twice a day for 7 days starting from the first post-extraction day. Clinical examination data were recorded and analyzed using the Mann Whitney Wilcoxon test.
Results
There was a statistically significant between-group difference in postoperative surgical-site edema, tenderness, and socket size, with the test group showing greater improvements.
Conclusions
Myrrh mouthwash has an enhancement effect on wound healing during the early period after tooth extraction.
1. Introduction
The early period after tooth extraction is a critical period for wound healing. Wound healing after tooth extraction is considered secondary intention healing. It involves several stages as follows: hemostasis, inflammatory phase, proliferative phase, and finally the remodeling phase. Hemostasis involves primary platelet plug formation in a fibrin matrix, which normally occurs within minutes during the immediate post-extraction period. The inflammatory phase takes place during the first post-extraction week and involves formation of temporary fibrin. This phase is clinically characterized by redness, swelling, hotness, and pain. Neutrophils and monocytes are recruited to the extraction site to initiate phagocytosis and first-line defense. They also stimulate the release of cytokines and growth factors that promote the healing process. The proliferative phase usually starts from the 4th post-extraction day until the third week and involves proliferation of more specialized cells such as angiocytes and neurocytes. The blood vessels proliferate and woven bone formation begins. Granulation tissue is formed during the third week and re-epithelialization is almost completed. Bone remodeling continues until the 4th to 6th post-extraction month while the whole wound remodeling phase continues after about 22 days to 2 years with the fibroblasts playing a major role (Kane, 2007, Shetty and Schwartz, 2006, Werner and Grose, 2003).
Wounds usually heal normally unless there is interference by local or systemic factors. In certain circumstances, early wound healing can be enhanced by several interventions such as antibiotics, mouthwashes, or topical medications. Myrrh has been used as a topical medication for promoting wound healing after tooth extraction. It is an aromatic resin exudate obtained from Commiphora molmol and Commiphora myrrh trees, which are species of the Burseraceae family (Hanus et al., 2005). These species are found in southern Arabia, and from Northeast Africa to Northeast Kenya (Hanus et al., 2005). Myrrh is chemically composed of about 30–60% of water-soluble gum, 20–40% of alcohol-soluble resin, and 3–8% of volatile oil (Hanus et al., 2005, Zhu et al., 2003). The gum part consists of polysaccharides and proteins while the volatile oil part consists of steroids, sterols, and terpenes. The strong odor of myrrh comes from furanosesquiterpenes (Hanus et al., 2005).
Myrrh has been used as a medicine by Chinese people since the Tang Dynasty in A.D. 600 (Hanus et al., 2005). It has also been used for many years as a medicine in the Middle East (Al-Harbi et al., 1997, Huang, 1998, Tariq et al., 1985). Alternative medicine has been well accepted by the public with more than half of the worldwide population using it for treatment (Modak et al., 2007). Myrrh, which is used in alternative medicine, was traditionally used for wound care and to treat several diseases including joint pains, gastric problems, infections (Abdul-Ghani et al., 2009), sore throats, cough, and burns. It is also used as an oral mouthwash and as anticancer treatment (Al-Harbi et al., 1994, Dolara et al., 2000, Nomicos, E.Y.H., 2007).
Previous studies have demonstrated evidence of the potential anti-inflammatory, antihistaminic, hypolipidemic, hypocholesterolemic, and antiatherosclerotic effects of myrrh (Duwiejua et al., 1993, Lata et al., 1991, Malhotra et al., 1977, Tariq et al., 1985). Moreover, the efficacy of myrrh in infectious diseases has been reported by many studies. A previous study reported antimicrobial effects of various species of Commiphora against several Gram-positive and Gram-negative bacteria (El Ashry et al., 2003). Strains of Staphylococcus aureus, Salmonella enterica, and Klebsiella pneumoniae have been shown to be sensitive to Commiphora molmol (Rahman et al., 2008).
Moreover, a study reported minimum inhibitory concentrations of myrrh for a range of pathogens, including Pseudomonas aeruginosa, Escherichia coli, and Candida albicans (Dolara et al., 2000). Myrrh has been shown to have a therapeutic effect on fascioliasis (Massoud et al., 2004) and to be effective in Schistosoma-infected mice (El-Sherbmy et al., 2006). There are also reports of myrrh being used as a gingival anti-inflammatory toothpaste and mouthwash (Bradley, 2006, Saeedi et al., 2003). An animal model study reported that application of a diluted suspension of myrrh as a mouthwash stimulated wound healing in injured oral tissue (Al-Mobeeriek, 2011). However, the study suggested possible adverse reaction of the oral tissue to myrrh related to a higher dose, longer time of application, or as part of a reaction to myrrh constituents, regardless of the dose (Al-Mobeeriek, 2011).
There have been fewer studies on the toxicity of myrrh compared to those on its positive effects (Omer and Adam, 1999, Omer et al., 1999, Rao et al., 2001). A previous study investigated the effects of different concentrations of myrrh mouthwash on the tensile strength of 3 types of suture materials, i.e., silk, polyglactin, and polytetrafluoroethylene. They reported a significant decrease in the tensile strength of all the sutures when applying 100% myrrh solution, especially after use for 3 days (Alshehri et al., 2015).
An animal study reported that pre-treatment with myrrh had dose-dependent protection against the effects of ethanol treatment including adverse effects on the stomach wall leading to mucosal damage, necrosis, erosion, and hemorrhage. This protective effect of myrrh is due to its ability to induce mucous secretion in the gastric wall and increase the formation of nucleic acid and nonprotein sulfhydryl. These occur through prostaglandin and thyroid stimulation, as well as antioxidant effects (Al-Harbi et al., 1997, Bone, 2003).
Myrrh has been reported to exhibit an antitumor effect on Ehrlich ascites carcinoma similar to that of cyclophosphamide (Al-Harbi et al., 1994, Qureshi et al., 1993). In mice exposed to lead toxicity, myrrh emulsion has been reported to decrease oxidative injury and lipid peroxidation, and stimulate glutathione S-transferase antioxidant enzyme in liver tissue. It has also been shown to enhance immunity by stimulating lymphocytes and phagocytosis (Ashry et al., 2010, Assimopoulou et al., 2005, Auffray, 2007, Delaveau et al., 1980, El-Ashmawy et al., 2006).
Our study will have a strong implications in which its application has high prevalence and public health importance of complications that come with oral surgery procedures and the need to develop safe and effective treatments and management protocols. As addressed by literature review, this is the first study that discuss the application of myrrh to enhance wound healing.
The objective of this study was to prove the wound-healing potentiation effects of myrrh mouthwash during the early post-extraction period.
2. Materials and methods
Ethics approval was obtained from the Institutional Review Board (IRB) in King Saud University after a full board review and registered with the National Committee of Bio and Medical Ethics and assigned a reference number: E-17-2609. The study was registered in the International Standard Randomized Controlled Trial Number (ISRCTN) through BioMed Central given the ID: ISRCTN82716015 registered 19 April 2019, retrospectively registered. Written informed consent was obtained from each subject. The study was conducted according to the World Medical Association Declaration of Helsinki version 2008.
We enrolled 40 healthy nonsmoker adult patients in this study. To estimate the sample size for a randomized controlled trial (using EPI Info 7, StatCalc, CDC, USA), we assumed a 95% confidence level and statistical power of 90% and the sample size was estimated as 36 patients (18 cases + 18 controls). After allowing a 10% overestimation to accommodate for loss to follow-up, the final sample size was calculated as 40 patients (20 cases + 20 controls).
The inclusion criteria were as follows:
-
–
Young adults in the age range of 18–45 years and with American Society of Anesthesiologists physical status I.
-
–
Lack of infection at the tooth indicated for extraction.
-
–
Absence of any pathology at the area of the tooth indicated for extraction and neighboring teeth.
-
–
Teeth that require simple extraction.
-
–
Provision of written informed concent explaining all the study’s possible risks, aims, and methodology.
The exclusion criteria were as follows:
-
–
Medically compromised patients.
-
–
Pregnant females.
-
–
Teeth that require surgical extractions.
-
–
Teeth with abscess or pathology.
We conducted a randomized, double-blind, placebo-controlled study with both the participants and the examiner masked. The subjects were randomly assigned to the exposure or control group, and only the dental assistant was aware of the group allocation. All participants underwent dental extraction under local anesthesia at the Oral and Maxillofacial Surgery Clinic, College of Dentistry, King Saud University. The procedures adhered to the standard pre-operative and post-operative extraction protocols. All the patients received postoperative advice on good oral hygiene.
Patients were randomized into two groups. Group A (Test group) consisted of subjects who were prescribed to use Commiphora molmol (myrrh) extract as a mouthwash twice a day for 7 days, beginning from the 1st post-operative day. The myrrh was prepared by the researcher as a mouthwash in the following concentration: 0.5% w/w which indicates that 0.5% of the drug is present in the solution (El-Ashmawy et al., 2006, Pole, 2006). It means 0.5 gm of drug in 100 gm of water (0.5 gm myrrh/100gm of water), and 100gm water equals 100 ml water so we prepared the required mouthwash amount of 250 ml by providing 1.25 gm of grinded myrrh to be dissolved by the patient in a cup of 250 ml of warm water. The measurements were done using the laboratory analytical digital weighing balance (Mettler Toledo® MS205DU Semi-micro Analytical Balance). The researcher obtained the myrrh commercially after which it was ground and packed in unlabeled packs of white nontransparent filter paper, each contains 1.25 gm of myrrh (see Fig. 1). The patients were instructed to put each pack in a cup of 250 ml warm water for use as a mouthwash.
Fig. 1.

Commiphora myrrh powder weight measurement before packing.
Group B (Control group) consisted of patients who were prescribed to use saline mouthwash (0.90% w/v of NaCl) twice a day for 7 days beginning from the 1st post-operative day. The researcher prepared unlabeled packs of white nontransparent filter paper, each contains 2.25gm of sodium chloride. Similarly, the patients were instructed to put each pack in a cup of 250 ml warm water for use as a mouthwash.
The clinician and participants were double-blinded to randomization of the patients, which was documented only by a neutral dental assistant. To eliminate bias, each of the mouthwash packs was labeled with a code number based on a randomization sequence. Post-operative assessment, which included the recording of the patients’ symptoms and systemic and local findings, was performed on the 7th day. The patients' pain degree was measured using the Visual Analogue Scale. Local findings were obtained by assessing the presence/absence of swelling or facial asymmetry which is reported by visually comparing the extraction site with the contralateral side and measuring between facial points identified with permanent marker were made prior to surgery: angle of the mandible, nasal border, labial commissure, tragus, laterally to external corner of the eye, and on soft tissue pogonion. The distances were measured I: from angle of the mandible to tragus; II: from angle of the mandible to external corner of the eye; III: from angle of the mandible to nasal border; IV: from angle of the mandible to labial commissure; V: from angle of the mandible to soft tissue pogonion. Calculation done by measuring the distances differences between preoperative and postoperative assessment. Accordingly the average differences value < 10 mm, considered as mild swelling; 10–20 mm, considered as moderate swelling and value >20 mm, considered as severe swelling (Zhang et al, 2018). The oral condition including the color of the gingiva around the extracted socket by visually inspection of color changes other than the healthy pink color; presence of any infection signs such as pain, tenderness, abnormal or bad odor, and pus discharge; bleeding; socket size; loss of function such as limited mouth opening or numbness; and any other findings.
Bleeding is considered present if it is identified in any degree during patient examination in the determined postoperative period.
Socket size is recorded by measuring the socket size in the immediate postoperative time and compare it with its size in the 7th postoperative day and classified as shrunken if became narrower in any degree, same size if no change or increased in its size if became larger.
Limitation of mouth opening was measured using a calibrated digital caliper measuring the vertical distance between the maxillary and mandibular incisal edges normally ≥30 mm and if less means presence of limited mouth opening (Zhang et al, 2018).
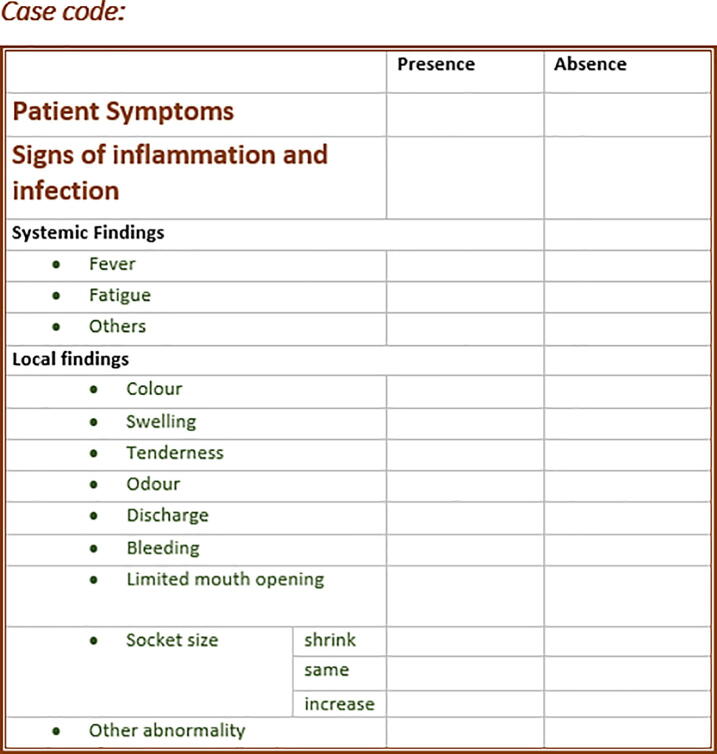
A single examiner performed all clinical measurements prior to surgery (baseline) and on the seventh postoperative day. Data obtained from the post-extraction assessment were tabulated, variables defined as categorical variables and statistically analyzed using the Mann Whitney Wilcoxon test. A data collection sheet was prepared to guide the examiner during the clinical examination (see Fig. 2). The data obtained were analyzed using the Statistical Package for Social Science version 25.0 program (SPSS Inc, Armonk, NY, USA). Descriptive statistical analysis using frequencies, percentages, means, and standard deviations, was used to describe the variables.
Fig. 2.
The clinical assessment sheet of the participants.
3. Results
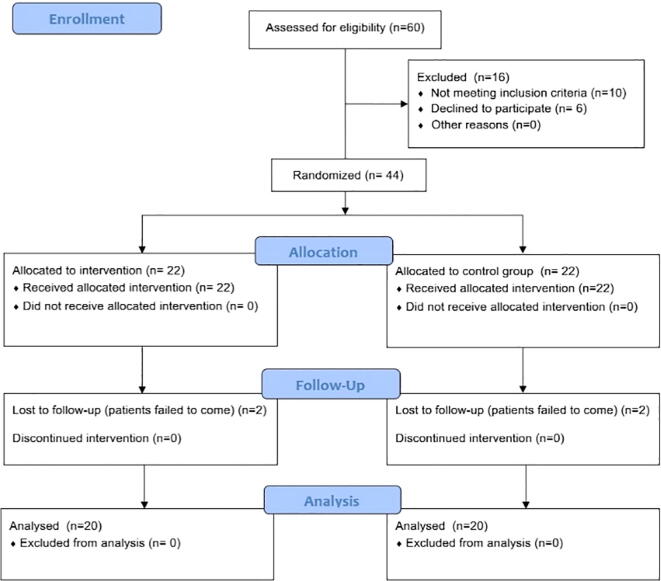
A total of 40 participants (20: study group and 20: control group) completed the study. The phases of the parallel randomized trial are shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (see Fig. 3).
Fig. 3.
Flow diagram of the randomized trial phases.
The frequencies and percentages of variables for experimental and control groups are shown in Table 1.
Table 1.
Frequencies and percentages of variables.
| Control Group | Experimental Group | Statistically Significant Differences | |||
|---|---|---|---|---|---|
| Percentage | Percentage | ||||
| Patient Symptoms | No pain | 70.0 | 85.0 | – | |
| Some degree of pain | 20.0 | 5.0 | |||
| Sever pain | 10.0 | 0.0 | |||
| Color changes | Absence | 60.0 | 85.0 | – | |
| Presence | 40.0 | 15.0 | |||
| Swelling | Absence | 65.0 | 100.0 | ***p < 0.001 | |
| Mild | 30.0 | 0.0 | |||
| Moderate | 5.0 | 0.0 | |||
| Tenderness | Absence | 55.0 | 90.0 | **p < 0.01 | |
| Presence | 45.0 | 10.0 | |||
| Odor | Absence | 95.0 | 100.0 | – | |
| Presence | 5.0 | 0.0 | |||
| Discharge | Absence | 100.0 | 100.0 | – | |
| Bleeding | Absence | 100.0 | 100.0 | – | |
| Limited mouth opening | Absence | 100.0 | 100.0 | – | |
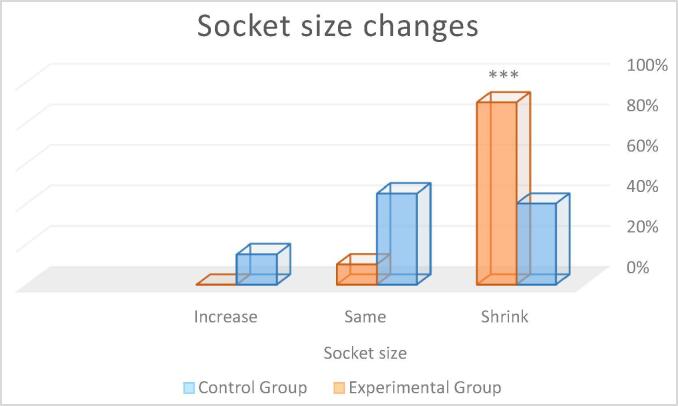
| Socket size | Shrink | 40.0 | 90.0 | ***p < 0.001 | |
| Same | 45.0 | 10.0 | |||
| Increase | 15.0 | 0.0 | |||
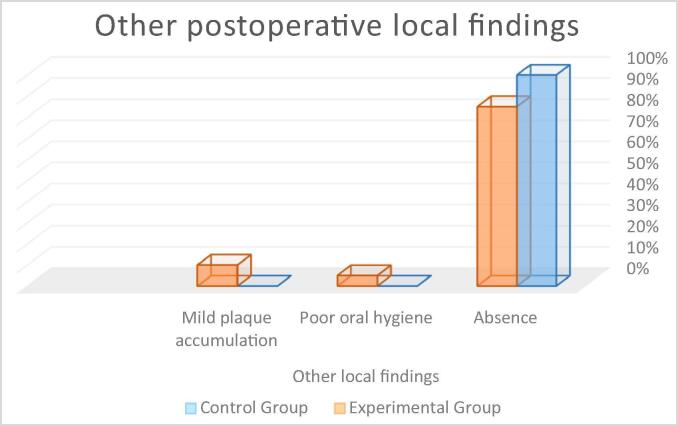
| Other local findings | Absence | 100.0 | 85.0 | – | |
| Presence | Poor oral hygiene | 0.0 | 5.0 | ||
| Mild plaque accumulation | 0.0 | 10.0 | |||
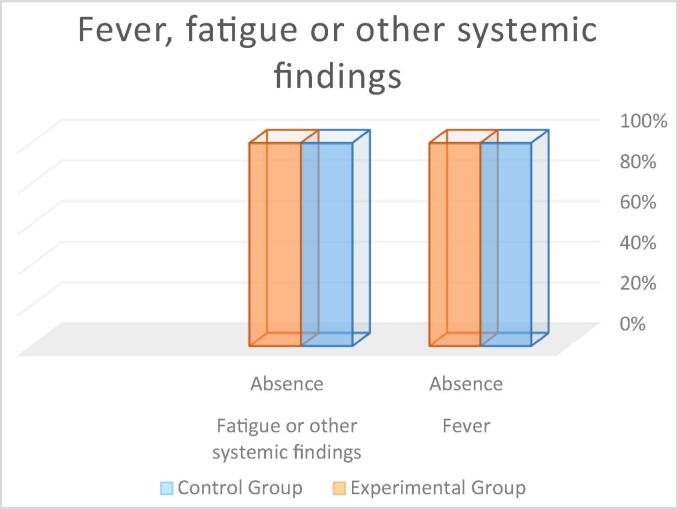
| Fever | Absence | 100.0 | 100.0 | – | |
| Fatigue or other systemic findings | Absence | 100.0 | 100.0 | – | |
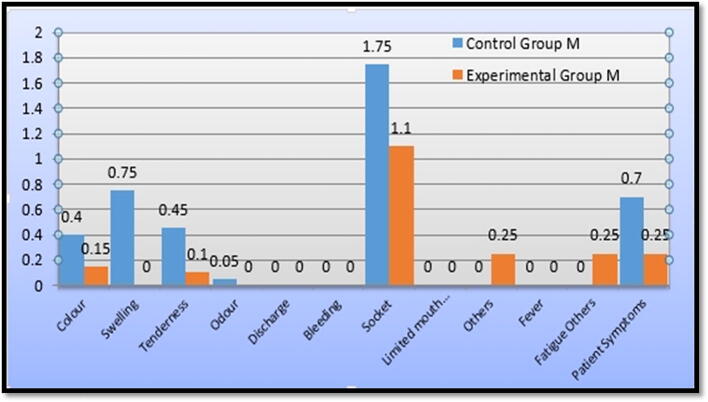
Regarding the patients’ symptoms in the control group, we found that 70% of the participants had no pain, 20% had some degree of pain, and 10% had severe pain. In the experimental group, 95% of the participants had no pain and 5% had some degree of pain (see Fig. 4). The Mann-Whitney Wilcoxon test revealed that there were no statistically significant between-group differences (p = 0.194): control group (M = 0.70) and experimental group (M = 0.25).
Fig. 4.
Percentage of patients with postoperative symptoms.
Regarding local signs, the findings were divided into either inflammatory signs or other abnormalities as follows:
-
•
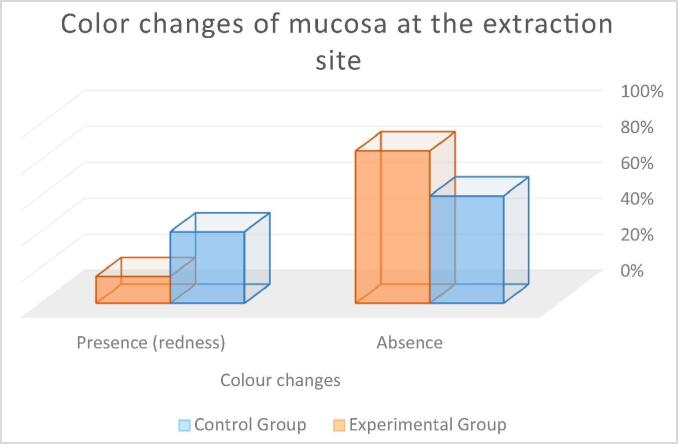
Color changes of mucosa at extraction area: In the control group, 60% of the participants showed no color changes of the gingiva and mucosa at the extraction site while 40% showed gingival redness. In the experimental group, 85% of the participants showed no gingival or mucosal color changes while 15% showed gingival redness (see Fig. 5). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 0.080): control group (M = 0.40) and experimental group (M = 0.15).
-
•
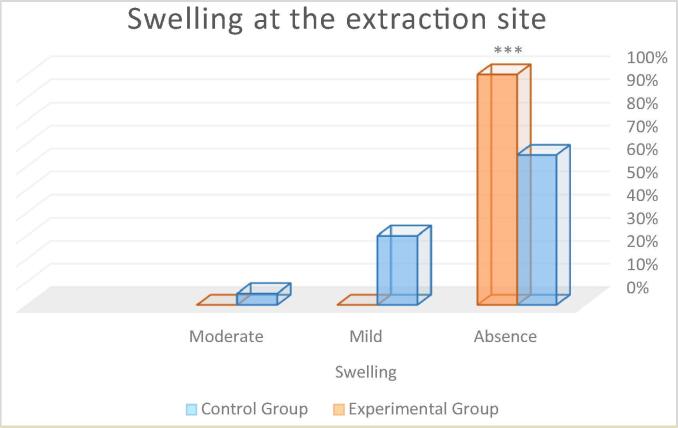
Swelling at the extraction site: In the control group, 65% of participants had no swelling, 30% had mild edema or swelling at the extraction site, 5% had moderate swelling, and none had severe swelling. In the experimental group, 100% of the participants showed absence of any swelling (see Fig. 6). The Mann-Whitney Wilcoxon test revealed statistically significant between-group differences ***p < 0.001 (control group: M = 0.75; experimental group: M = 0.00), with the control group having a higher frequency.
-
•
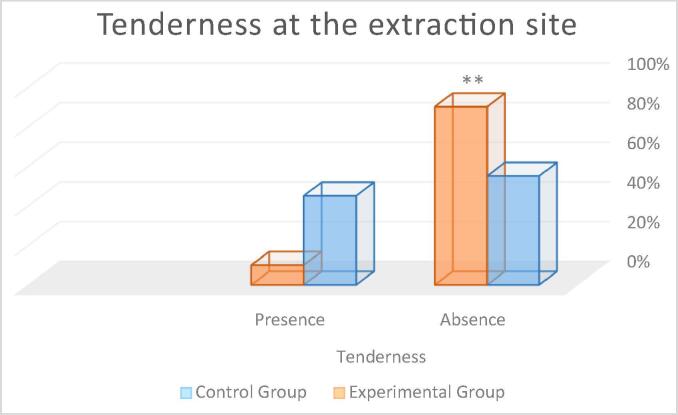
Tenderness at the extraction site: In the control group, 55% of participants showed absence of tenderness and 45% had tenderness. In the experimental group, 90% of the participants showed absence of tenderness and 10% had tenderness (see Fig. 7). The Mann-Whitney Wilcoxon test revealed statistically significant between-group differences **p < 0.01 (control group: M = 0.45; experimental group M = 0.10), with the control group having a higher frequency.
-
•
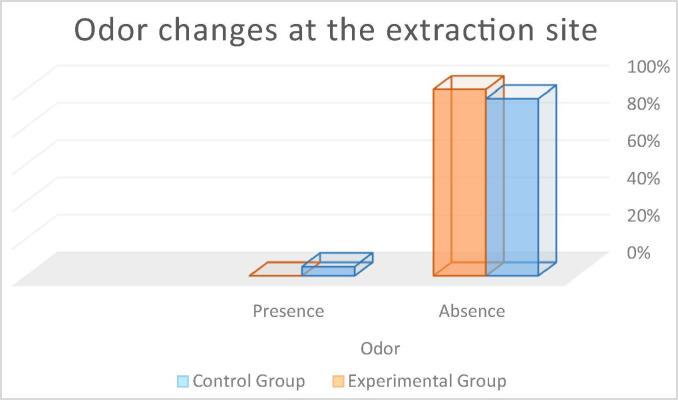
Odor at the extraction site: In the control group, 95% of the participants showed absence of odor changes and 5% showed changes in odor. In the experimental group, 100% of the participants were showed absence of odor changes (see Fig. 8). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 0.315) (control group M = 0.05; experimental group M = 0.00).
-
•
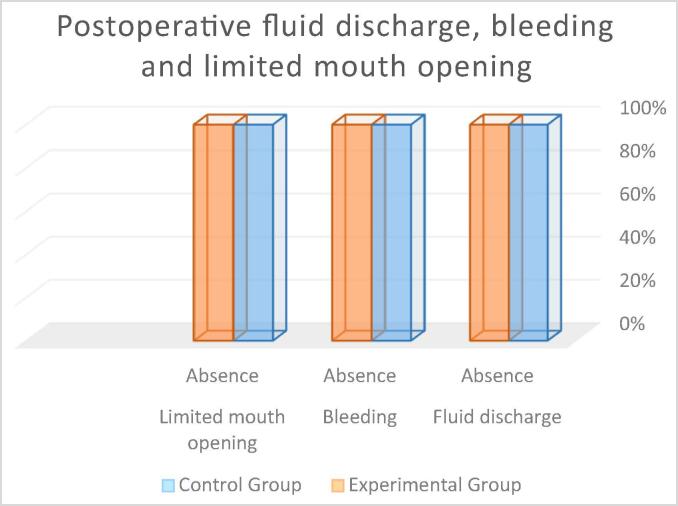
Discharge of pus or exudates from the extraction site: In both groups, 100% of the participants showed absence of any discharge from the extraction site (see Fig. 9). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 1.00) (control group M = 0.00; experimental group M = 0.00).
-
•
Bleeding from the extraction site: In both groups, 100% of the participants showed absence of bleeding from the extraction site (see Fig. 9). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 1.00) (control group M = 0.00; experimental group M = 0.00).
-
•
Limited mouth opening: In both groups, 100% of the participants showed absence of any limitation in mouth opening (see Fig. 9). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 1.00).
-
•
Socket size: In the control group, 40% of the participants had, as compared to the preoperative socket size, a shrunken socket size, 45% had a similar-sized socket, and 15% showed an increased socket size. In the experimental group, 90% of the participants had a shrunken socket size and 10% a similar-sized socket (see Fig. 10). The Mann-Whitney Wilcoxon test revealed statistically significant between-group differences ***p < 0.001 with the control group having a higher frequency of an increased or similar socket size.
-
•
Other inflammatory or abnormal findings: In the control group, 100% of participants did not show any other local findings while in the experimental group, 85% did not show any, 10% had mild plaque accumulation, and 5% had poor oral hygiene (see Fig. 11). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 0.076) (control group M = 0.00; experimental group M = 0.25).
Fig. 5.
Percentage of patients with postoperative color changes of mucosa at the extraction site.
Fig. 6.
Percentage of patients with postoperative swelling at the extraction site. Statistical differences ***p < 0.001 respect to control group.
Fig. 7.
Percentage of patients with tenderness at the extraction site. Statistical differences **p < 0.01 respect to control group.
Fig. 8.
Percentage of patients with odor changes at the extraction site.
Fig. 9.
Percentage of patients with postoperative fluid discharge, bleeding and limited mouth opening.
Fig. 10.
Percentage of patients with postoperative socket size changes. Statistical differences ***p < 0.001 respect to control group.
Fig. 11.
Percentage of patients with other postoperative local findings.
Regarding systemic signs of inflammation or abnormality, we observed the following findings:
-
•
Fever: In both groups, 100% of the participants showed absence of fever (see Fig. 12). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 1.00) (control group M = 0.00; experimental group M = 0.00).
-
•
Fatigue or other findings: In both groups, 100% of the participants showed absence of fatigue or any other systemic findings (see Fig. 12). The Mann-Whitney Wilcoxon test revealed no statistically significant between-group differences (p = 0.076) (control group M = 0.00; experimental group M = 0.25).
Fig. 12.
Percentage of patients with postoperative fever, fatigue or other systemic findings.
4. Discussion
Our findings indicate that most of our patients did not have significant complaints, symptoms, infection, or severe abnormality during the postoperative assessment, apart from a few patients in the control group who reported severe pain. There were no significant between-group differences in the local signs except for swelling, tenderness, and socket size, which had a higher frequency in the control group. Further, our findings indicated that the study group had a significant decrease in the inflammatory signs after one postoperative week. Since all the participants did not show preoperative signs of infection and were advised on good oral hygiene, the chance of error was lowered; therefore, the myrrh mouthwash can be considered to have a wound healing enhancement effect.
Our findings are consistent with previous reports that indicated the wound care efficacy (Al-Harbi et al., 1994, Dolara et al., 1996, Nomicos, E.Y.H., 2007) and anti-inflammatory activity (Duwiejua et al., 1993, Lata et al., 1991, Malhotra et al., 1977, Tariq et al., 1985) of Commiphora molmol. A similar animal model study by Al-Mobeeriek (2011) reported similar clinical results. They used several mouthwash types to compare with myrrh mouthwash and saline mouthwash, which was used by the control group. They irrigated incised wounds with 1 ml of 0.2% myrrh solution that was prepared using powdered myrrh obtained from a traditional market and dissolved in normal saline. The mouthwash was applied three times per week after the surgery for variable durations. The other mouthwashes used for comparison were 0.2% chlorhexidine gluconate and 0.25% tetracycline. They performed clinical and histological assessments for variable intervals for up to four weeks. They reported that the myrrh group started the remodeling stage earlier and showed good immunomodulation, as well as antibacterial and antifungal effects. Compared to the control group, they found decreased inflammation in all other groups after one week; however, the myrrh group had a higher number of mast cells. After four weeks, participants in all groups had normal mucosa, and a higher number of mast cells was maintained in the myrrh group. They concluded that the use of myrrh mouthwash provided antimicrobial activity, and shortened the time required for wound healing by avoiding a pronounced inflammatory response (Al-Mobeeriek, 2011). Their findings are consistent with ours regarding the effects short-term myrrh mouthwash use.
Regarding systemic signs, we did not find any abnormalities in either group, which might be explained by the simplicity of the surgical procedure and the absence of an infectious source. However, this could also be attributed to the antimicrobial activity of myrrh. As earlier discussed, myrrh has an inhibitory effect on the growth of many bacterial strains, fungi, and other microbial species; moreover, it has been reported to have a therapeutic effect on several severe infections such as fascioliasis and Schistosoma (El-Sherbmy et al., 2006, Massoud et al., 2004).
A double-blinded clinical study by Saeedi et al. (2003) assessed the effects of myrrh application to the oral mucosa. They prepared toothpastes containing either myrrh alone, or myrrh with chamomile that were applied on bleeding gingiva, and compared the results with those of a placebo control. They found a significant improvement in gingival bleeding in participants using myrrh-containing toothpaste compared to controls. This suggested that myrrh-containing toothpastes could be used in the management of patients with bleeding gingiva (Saeedi et al., 2003). This is consistent with our findings of inflammation reduction as shown by the less in inflammatory signs including tenderness and swelling at the extraction area, by the shrinkage of socket size and absence of postoperative bleeding in the myrrh-treated group.
Myrrh exerts its anti-inflammatory effects via inhibition of inflammatory mediators such as nitric oxide, prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) production. A previous animal study reported that myrrh inhibited lipopolysaccharide-induced peritoneal macrophages and had antimicrobial effects during sepsis in an animal model of cecal ligation and puncture. Cecal ligation and puncture is a method used to induce sepsis in animals via perforation of the cecum, which allows the release of fecal material into the peritoneal cavity (Kim et al., 2012). Myrrh has also been reported to have a stimulatory effect on leukocytes. It potentiates leukocyte migration to the injury site and maintains their function and proliferation during the healing process (Al-Said, 2010).
We did not find any side effects, allergy, and signs of toxicity, or unexplained abnormality with the use of the myrrh mouthwash. This could be attributed to the low dose, short application duration, and high tissue tolerance toward myrrh. This is consistent with previous findings of the ability of myrrh to reduce tissue toxicity via several mechanisms. As discussed earlier, myrrh has been reported to have a protective effect on tissue from the adverse effects of ethanol and lead toxicity. This is through its stimulatory effect on mucous secretion, antioxidant, antitumor, and immune stimulatory potentials (Al-Harbi et al., 1994, Al-Harbi et al., 1997, Al-Said, 2010, Ashry et al., 2010, Assimopoulou et al., 2005, Auffray, 2007, Bone, 2003, Delaveau et al., 1980, El-Ashmawy et al., 2006, Qureshi et al., 1993). This indicates the tolerance of myrrh and the safety of its use at a relatively low dose for wound healing enhancement.
5. Conclusions
Myrrh mouthwash has an enhancement effect on wound healing during the early period after tooth extraction. The conclusion from our findings can be generalized to the population. As has been well highlighted by discussing the findings in light of those of previous studies, the study makes a significant novel contribution to the existing research, as it is the first study to assess the wound-healing potentiation effects of myrrh mouthwash during the early post-extraction period.
Suggestions: Developing a policy of implication of myrrh mouthwash with standards in its prescription, indications, availability in the market, clear instruction and follow up assessments of the patients.
We recommend further research on the application of myrrh after a more complicated oral surgical procedure, which could reveal more findings on the myrrh potentiation ability to enhance wound healing.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgements
Praises be to Allah who grant us in accomplishing this work. Then I would like to express my special thanks to Dr. Sundar Ramalingam (a faculty member in the Oral and Maxillofacial Surgery department at King Saud University) for providing priceless guide and insights. Thanks to the Elsevier editors for the constructive comments, language edit and high quality of service.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Study registration
The study was registered in the International Standard Randomized Controlled Trial Number (ISRCTN) through BioMed Central given the ID: ISRCTN82716015 registered 19 April 2019, retrospectively registered.
This study was ethically approved by the Institutional Review Board (IRB) in King Saud University after a full board review and registered with the National Committee of Bio and Medical Ethics and assigned a reference number: E-17-2609.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdul-Ghani R.A., Loutfy N., Hassan A. Myrrh and trematodoses in Egypt: an overview of safety, efficacy and effectiveness profiles. Parasitol. Int. 2009;58:210–214. doi: 10.1016/j.parint.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Al-Harbi M.M., Qureshi S., Raza M., Ahmed M.M., Afzal M., Shah A.H. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J. Ethnopharmacol. 1997;55:141–150. doi: 10.1016/s0378-8741(96)01488-2. [DOI] [PubMed] [Google Scholar]
- Al-Harbi M.M., Qureshi S., Raza M., Ahmed M.M., Giangreco B., Shah A.H. Anticarcinogenic effect of Commiphora molmol on solid tumors induced by Ehrlich carcinoma cells in mice. Chemotherapy. 1994;40:337–347. doi: 10.1159/000239216. [DOI] [PubMed] [Google Scholar]
- Al-Mobeeriek A. Effects of myrrh on intra-oral mucosal wounds compared with tetracycline-and chlorhexidine-based mouthwashes. Clin. Cosmet. Investig. Dent. 2011;3:53. doi: 10.2147/CCIDEN.S24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Said A.H. Effect of Commiphora molmol on leukocytes proliferation in relation to histological alterations before and during healing from injury. Saudi. J. Biol. Sci. 2010;17:139–146. doi: 10.1016/j.sjbs.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri M.A., Baskaradoss J.K., Geevarghese A., Ramakrishnaiah R., Tatakis D.N. Effects of myrrh on the strength of suture materials: an in vitro study. Dent. Mater. J. 2015;34:148–153. doi: 10.4012/dmj.2013-317. [DOI] [PubMed] [Google Scholar]
- Ashry K.M., El-Sayed Y.S., Khamiss R.M., El-Ashmawy I.M. Oxidative stress and immunotoxic effects of lead and their amelioration with myrrh (Commiphora molmol) emulsion. Food Chem. Toxicol. 2010;48:236–241. doi: 10.1016/j.fct.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Assimopoulou A.N., Zlatanos S.N., Papageorgiou V.P. Antioxidant activity of natural resins and bioactive triterpenes in oil substrates. Food Chem. 2005;92:721–727. [Google Scholar]
- Auffray B. Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil. Int. J. Cosmet. Sci. 2007;29:23–29. doi: 10.1111/j.1467-2494.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Bone K. Elsevier Health Sciences; 2003. A Clinical Guide to Blending Liquid Herbs e-book: Herbal Formulations for the Individual Patient. [Google Scholar]
- Bradley P. vol. 2. British Herbal Medicine Association; Bournemouth: 2006. (A Handbook of Scientific Information of Widely used Plant Drugs). [Google Scholar]
- Delaveau P., Lalloutte P., Tessier A.M. A clinical guide to blending liquid herbs, herbal formulations for the individual patient. Planta Med. 1980;40:49–54. [Google Scholar]
- Dolara P., Corte B., Ghelardini C., Pugliese A.M., Cerbai E., Menichetti S., Nostro A.L. Local anaesthetic, antibacterial and antifungal properties of sesquiterpenes from myrrh. Planta Med. 2000;66:356–358. doi: 10.1055/s-2000-8532. [DOI] [PubMed] [Google Scholar]
- Dolara P., Luceri C., Ghelardini M.C., Monserrat C., Aiolli S., Luceri F., Lodovici M., Menichetti S., Romanelli M.N. Analgesic effects of myrrh. Nature. 1996;379:29. doi: 10.1038/379029a0. [DOI] [PubMed] [Google Scholar]
- Duwiejua M., Zeitlin I.J., Waterman P.G., Chapman J., Mhango G.J., Provan G.J. Anti-inflammatory activity of resins from some species of the plant family Burseraceae. Planta Med. 1993;59:12–16. doi: 10.1055/s-2006-959594. [DOI] [PubMed] [Google Scholar]
- El-Ashmawy I.M., Ashry K.M., El-Nahas A.F., Salama O.M. Protection by turmeric and myrrh against liver oxidative damage and genotoxicity induced by lead acetate in mice. Basic Clin. Pharmacol. Toxicol. 2006;98:32–37. doi: 10.1111/j.1742-7843.2006.pto_228.x. [DOI] [PubMed] [Google Scholar]
- El Ashry E.S., Rashed N., Salama O.M., Saleh A. Components, therapeutic value and uses of myrrh. Pharmazie. 2003;58:163–168. [PubMed] [Google Scholar]
- El-Sherbmy M., Abdel-Aziz M.M., Abbas A.T. Immune response on mice infected with Schistosoma monsoni and treated with myrrh. J. Med. Sci. 2006;6:858–861. [Google Scholar]
- Hanus L.O., Rezanka T., Dembitsky V.M., Moussaieff A. Myrrh-Commiphora chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2005;149:3–28. doi: 10.5507/bp.2005.001. [DOI] [PubMed] [Google Scholar]
- Huang K.C. second ed. Routledge; 1998. The Pharmacology of Chinese Herbs. [Google Scholar]
- Kane D.P. HMP Communications; Pennsylvania: 2007. Chronic Wound Healing and Chronic Wound Management. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals. [Google Scholar]
- Kim M.S., Bae G.S., Park K.C., Koo B.S., Kim B.J., Lee H.J., Seo S.W., Shin Y.K., Jung W.S., Cho J.H., Kim Y.C., Kim T.H., Song H.J., Park S.J. Myrrh inhibits LPS-induced inflammatory response and protects from cecal ligation and puncture-induced sepsis. Evid. Based Complement. Alternat. Med. 2012;2012:278718. doi: 10.1155/2012/278718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata S., Saxena K.K., Bhasin V., Saxena R.S., Kumar A., Srivastava V.K. Beneficial effects of Allium sativum, Allium cepa and Commiphora mukul on experimental hyperlipidemia and atherosclerosis–a comparative evaluation. J. Postgrad. Med. 1991;37:132–135. [PubMed] [Google Scholar]
- Malhotra S.C., Ahuja M.M., Sundaram K.R. Long-term clinical studies on the hypolipidaemic effect of Commiphora mukul (Guggulu) and clofibrate. Indian J. Med. Res. 1977;65:390–395. [PubMed] [Google Scholar]
- Massoud A.M., El-Kholy N.M., El-Shennawy F.A., Farag R.E. Study of some immune aspects in patients with fascioliasis before and after Commiphora molmol (mirazid) treatment. J. Egypt. Soc. Parasitol. 2004;34:315–332. [PubMed] [Google Scholar]
- Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T.P.A. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomicos E.Y.H. Myrrh: medical marvel or myth of the magi? Holist. Nurs. Pract. 2007;21:308–323. doi: 10.1097/01.HNP.0000298616.32846.34. [DOI] [PubMed] [Google Scholar]
- Omer S.A., Adam S.E. Toxicity of Commiphora myrrha to goats. Vet. Hum. Toxicol. 1999;41:299–301. [PubMed] [Google Scholar]
- Omer S.A., El Adam S., Khalid H.E. Effects on rats of Commiphora myrrha extract given by different routes of administration. Vet. Hum. Toxicol. 1999;41:193–196. [PubMed] [Google Scholar]
- Pole S. Elsevier Health Sciences; 2006. Ayurvedic Medicine: The Principles of Traditional Practice. [Google Scholar]
- Qureshi S., al-Harbi M.M., Ahmed M.M., Raza M., Giangreco A.B., Shah A.H. Evaluation of the genotoxic, cytotoxic, and antitumor properties of Commiphora molmol using normal and Ehrlich ascites carcinoma cell-bearing Swiss albino mice. Cancer Chemother. Pharmacol. 1993;33:130–138. doi: 10.1007/BF00685330. [DOI] [PubMed] [Google Scholar]
- Rahman M.M., Garvey M., Piddock L.J., Gibbons S. Antibacterial terpenes from the oleo-resin of Commiphora molmol (Engl.) Phytother. Res. 2008;22:1356–1360. doi: 10.1002/ptr.2501. [DOI] [PubMed] [Google Scholar]
- Rao R.M., Khan Z.A., Shah A.H. Toxicity studies in mice of Commiphora molmol oleo–gum–resin. J. Ethnopharmacol. 2001;76:151–154. doi: 10.1016/s0378-8741(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Saeedi M., Azadbakht M., Semnani K., Khandan M. Formulation of herbal toothpaste from chamomile and myrrh, a preliminary clinical evaluation of bleeding gum. J. Mazandaran Univ. Med. Sci. 2003;13:61–69. [Google Scholar]
- Shetty V., Schwartz H.C. Wound healing and perioperative care. Oral Maxillofac. Surg. Clin. North. Am. 2006;18:107–113. doi: 10.1016/j.coms.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Tariq M., Ageel A.M., Al-Yahya M.A., Mossa J.S., Al-Said M.S., Parmar N.S. Anti-inflammatory activity of Commiphora molmol. Agents Actions. 1985;17:381–382. doi: 10.1007/BF01982655. [DOI] [PubMed] [Google Scholar]
- Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Li, J., Li, Z.-B., Li, Z., 2018. Predicting postoperative facial swelling following impacted mandibular third molars extraction by using artificial neural networks evaluation Scientific reports, Nature Publishing Group, vol. 8, pp. 12281. [DOI] [PMC free article] [PubMed]
- Zhu N., Sheng S., Sang S., Rosen R.T., Chi-Tang H. Isolation and characterization of several aromatic sesquiterpenes from Commiphora myrrha. Flavour Fragr. J. 2003;18:282–285. [Google Scholar]