Abstract
With cochlear implantation (CI) being the standard of care for profoundly deaf cases, more and more patients with low frequency residual hearing are currently being treated with CI. In view of preserving the residual hearing, the ultimate aim of both the surgeons and the CI companies is to achieve zero-degree of electrode insertion trauma. Variations in the size and shape of cochlea, cross-sectional dimensions of ST, electrode insertion techniques with and without metal stylet rod and the experience level of the operating surgeons, all play a role in the electrode array related insertion trauma. An effective electrode design must include flexible array to accommodate the cochlear shape variation, electrode with variety of array lengths to support the concept of cochlear size specific electrode array and finally smaller cross-sectional dimensions of electrode array in matching the cross-sectional dimensions of ST. As per published reports, FLEX electrode array design offers minimal degree of electrode insertion trauma along with the possibility of patient specific electrode array length matching their cochlear size. Looking at the cross-sectional dimensions of FLEX electrode array along with its volume, it appear to be highly safe to the cochlea by not taking too much volume inside the ST. To offer additional support, otological pre-planning software tool like OTOPLAN is now clinically available in measuring the cochlear size in finding the best electrode array match along with the possibilities of anatomy based post-operative speech processor fitting.
Keywords: Cochlear duct length, Cochlear anatomy, Cochlear volume, Scala tympani cross-sectional dimensions
Introduction
Cochlear implantation (CI) being the ultimate solution to restore hearing in deaf patients, the CI electrode array is considered the most important component of CI as it bridges the neural structures inside the scala tympani (ST) with the CI itself.1 While every surgeon aims at atraumatic (zero degree of trauma) electrode array placement inside the cochlea, 100% atraumatic electrode placement is still not guaranteed in every CI surgeries due to the varying degree of cochlear size, shape, anatomy and with the electrode designs from various CI brands differing in its insertion method resulting in some degree of intra-cochlear trauma.2, 3, 4 With recent reports having confirmed the importance of atraumatic electrode placement to the patient's post-operative hearing with CI,5, 6, 7 the pressure is now on CI surgeons and as well on implant designers.
The CI surgeons are professionally trained to implant any of the commercially available CI electrodes. Now it comes down to CI companies to ensure that the CI electrodearrays are cochlear friendly matching every variations in the cochlear geometries including the cochlear size, shape, anatomy and the cross-sectional dimensions of ST. While CI electrodes from every CI brand has its own design features, MED-EL's FLEX electrode array series gains more importance for its atraumatic features and this article addresses the rationale behind the FLEX electrode array design in matching every variation in the cochlear geometry.
Brief history behind FLEX electrode array design
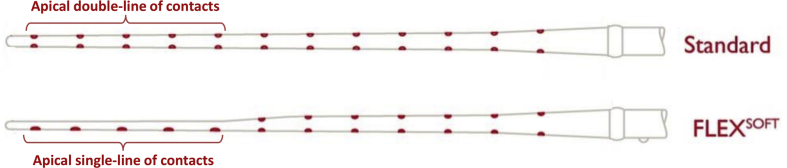
MED-EL started with the C40 implant system that had the 8 channel double line of contact electrode (in 1994) which was improved to 12 channel double-line of contact electrode (in 1996). Commercially this electrode is called as “STANDARD” electrode with an array length of 31.5 mm and is still commercially available. At that time (early 2000) with the growing interest of structure preservation, the STANDARD electrode design was further fine-tuned by reducing the mass at the apical end of the electrode, changing the apical 5 double-line of contacts into single-line of contacts. This was commercially named as FLEX SOFT electrode (in 2004) with the same array length of 31.5 mm (Fig. 1).
Figure 1.
Schematic drawing of STANDARD and FLEX SOFT electrode showing apical end double-line and single-line of contacts respectively.
FLEX electrode with varying array lengths of 28, 26, 24 and 20 mm were later introduced with the aim of matching every variation in the cochlear size, shape, anatomy and the hearing level.
Variations in the size and shape of human cochlea
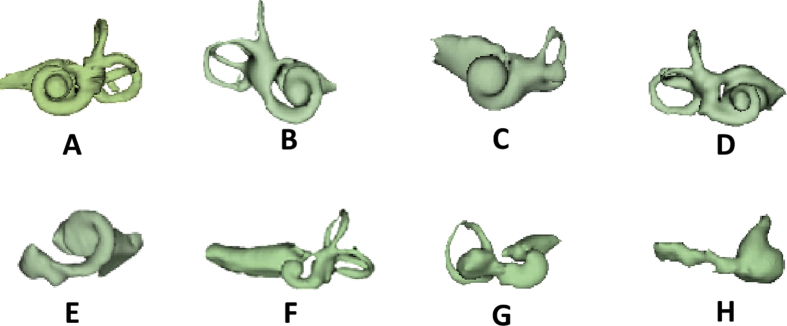
Escude et al8 in 2006 proposed a mathematical equation in estimating the cochlear duct length along the cochlear outer wall using an input value which is the basal turn diameter called as “A” value. Later Alexiades et al9 in 2014 fine-tuned the mathematical equations in estimating the cochlear duct length along the organ of corti which is more relevant in applying Greenwood frequency map and also in electrode array length selection. Erixon et al10 in 2009 reported on anatomical variations in overall size and shape of human cochlea from 73 corrosion cast models. Author himself has found such variations in size, shape and the anatomy of cochlea while 3D segmenting the inner-ear from 100s of pre-operative temporal bone CT scans for educational purposes as shown in Fig. 2.
Figure 2.
3D segmentation of inner ear from pre-operative CT images showing huge morphological variation. A & B: normal anatomy with differing cochlear size and shape; C: incomplete partition type II; D: incomplete partition type III; E–G: cochlear hypoplasia; H: common cavity. All images are in same scale and are in oblique coronal view (Images are from author's original work).
Meng et al2 in 2016 reported from 310 ears that some cochleae viewed at the basal turn were round in shape whereas some were elliptical in shape as seen from the coronal view conveying the message that the cochlear shape varies a lot. Liu et al11 in 2017 reported on the size and anatomical variations of cochlea from 102 patients in terms of cochlear height, cochlear basal turn diameter and as well on the anatomy. They further reported on variations in linear length of the cochlea measured along the outer wall. Thong et al12 in 2017 have raised the question, Will one size electrode fit all the cochlear duct length variations? In their study they have reported on cochlear size variation by estimating the basal turn length alone using Escude's mathematical equation among Chinese, Malays and Indian race. De Seta et al13 in 2016 reported on implanting STANDARD electrode in 19 adult patients and found deep insertion with smaller cochleae and shallower insertion with bigger cochleae conveying the message that insertion depth can be tailor made by choosing the electrode array length matching the cochlear size.
Timm et al14 in 2018 reported on patient specific selection of straight lateral wall cochlear implant electrode arrays based on anatomical indication ranges. In that study, they have confirmed the variations in cochlear duct length (CDL) by measuring the outer wall length and recommended patient specific electrode selection to minimize electrode insertion trauma and optimized stimulation. Ketterer et al6 in 2018 reported on heterogenous electrode-to-modiolus wall distance (wrapping factor) with one sized pre-curved electrode as a result of cochlear size and shape variation and they recommended CI companies to come up with custom-sized electrode arrays. This needs to be taken into serious consideration as the advantage of one-sized pre-curved electrode in bringing it close to the modiolus wall as commercially marketed is highly jeopardized with the variations in cochlear size and shape.
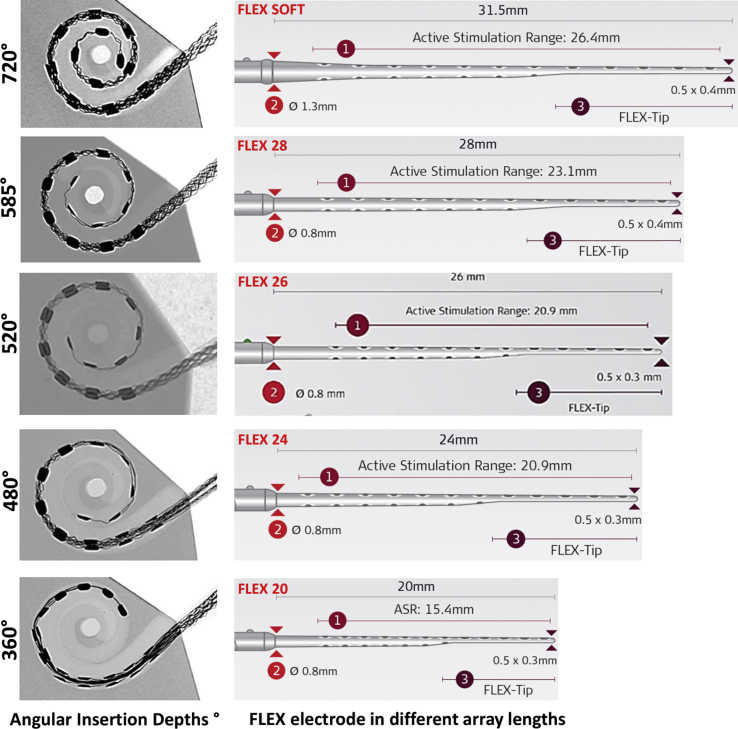
Similar findings were reported by Wang et al15 in 2017 that encouraged them to conclude “Our results show that perimodiolar EAs, more often than not, do not sit adjacent to the modiolus where they are likely most effective”. Cochlear duct length variations were reported by enough number of centers from across the world that motivated the CI companies to provide straight lateral wall electrodes in variety of array lengths. Fig. 3 shows FLEX electrode in different array lengths providing various angular insertion depths (AID) in an average sized cochlear model. Considering the huge variation in the cochlear size, shape and anatomy, having flexible straight electrode arrays in varying array lengths makes absolute sense for the surgeons to choose the best fitting electrode to their patient's cochlear needs.
Figure 3.
Showing FLEX electrode arrays in variety of array length with wave shaped wires in the electrode central core. Angular insertion depth (AID) provided by FLEX electrode array variants in an average sized cochlear model (AID image is from author's own work and the electrode array pictures were taken from MED-EL's homepage upon their approval).
Variation in cross-sectional dimensions of scala tympani
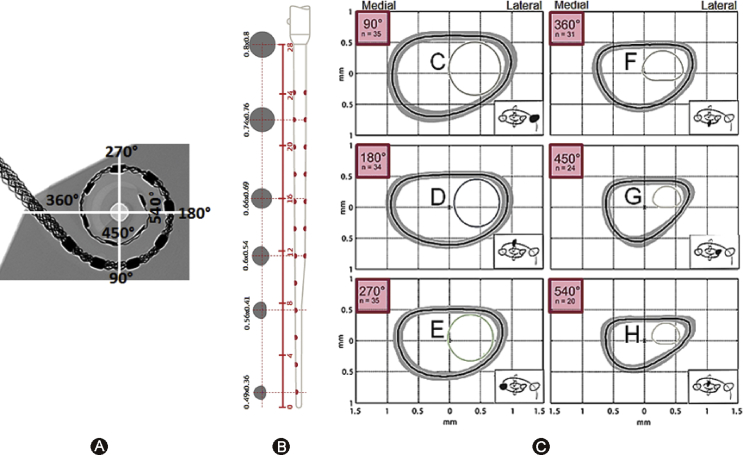
Variations are not just seen in the cochlear size, shape and anatomy but also in the intra-cochlear level of ST. Rebscher et al16 in 2008 reported on variations in cross-sectional dimensions of human ST from 79 cadaveric temporal bones at various insertion depths with 90° increments. Cross-sectional shape of ST is in the form of distorted ellipse as shown in Fig. 4. The height of ST towards the lateral position is getting tighter as the angular insertion depth goes beyond 450°. At this depth, the height of ST along the lateral position is around 0.6 mm and creeps down to 0.5 mm at 540°. Avci et al17 in 2014 reported similar results measured from 16 cadaveric temporal bones.
Figure 4.
A: AID of FLEX 28 with full insertion in an average sized cochlea. B: Cross sectional dimensions of FLEX28 electrode array. C: Cross-sectional dimensions of scala tympani (ST) for various insertion depths taken from Rebscher et al16 (the journal does not exist anymore to request copyright permission) and modified with the addition of FLEX 28 electrode array cross-section in it.
Gnansia et al18 in 2016 reported from a sample size of 9 on the height of ST to be around 0.6 mm at an insertion depth of 720°. Hatsushika et al19 in 1990 reported on the height of ST as 1 mm at an insertion depth of 25 mm from the round window entrance from a sample size of 8. These data make the tip dimensions of electrode arrays to be considered very critical. With FLEX 28 array reaching an insertion depth of 540° in an average sized cochlea, there is a good match between the cross-sectional dimensions of ST and electrode itself as shown in Fig. 4.
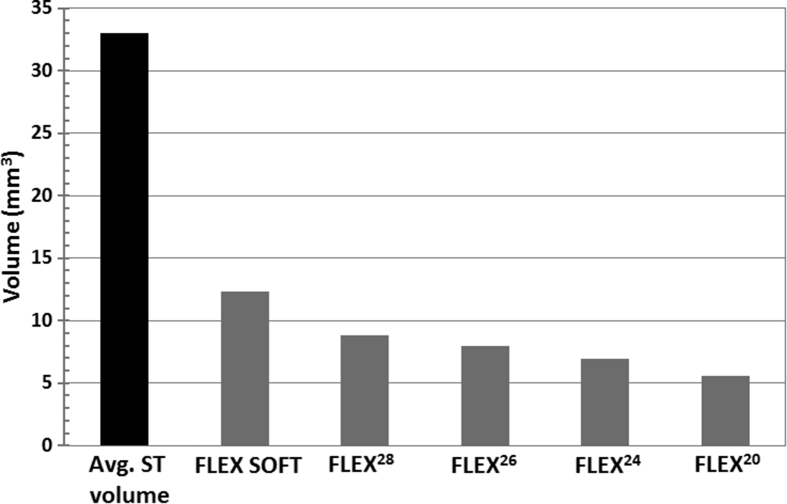
The average volume of ST alone accounts to 33 mm3 and it can go up to 41 mm3 as reported by Al-Dhamari et al.20 The volume of all FLEX electrode array variants as measured from its corresponding 3D computer model shows that the FLEX electrode array volume does not even occupy half the volume of ST itself. The FLEX SOFT electrode which is the longest of all, has a volume of 12.3 mm3 followed by FLEX 28 array with 8.8 mm3, FLEX 26 array with 7.96 mm3, FLEX 24 array with 6.96 mm3 and FLEX 20 array with 5.59 mm3 as given in Fig. 5.
Figure 5.
Volume of average scala tympani (ST) and all FLEX electrode array variants (Electrode array volume is author's own work).
Takahashi et al21 in 2017 reported on higher cochlear volume associated with bigger cochleae from 65 patient cases. They measured the cochlear length using Alexiades equation and calculated the cochlear volume from magnetic resonance (MR) images. Further interesting report from this work was higher hearing preservation rates were associated with higher cochlear volume. In that aspect, CI electrode array occupying lesser volume in the ST is highly preferable and FLEX electrode volumes reported here are well in favor for hearing preservation surgeries.
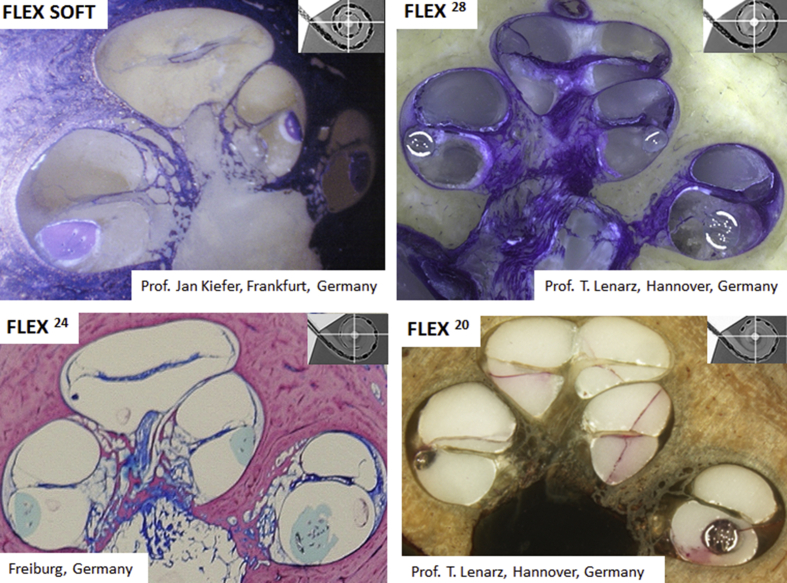
Though there is enough number of scientific reports available on the cochlear dimensions, with which the new electrode arrays can be designed reliably, still it is the histology section of human cochlea with electrode array inside is considered the gold standard in proving the safety of the electrode arrays. In that aspect, Fig. 6 shows histology section of human cochlea with FLEX electrode array variants placed completely inside the ST along with the space available around the electrode confirms the fact that FLEX electrode array variants do not occupy too much volume inside the ST.
Figure 6.
Histology sections of human cochleae with FLEX electrode variants showing it full placement inside the scala tympani (ST). Images were provided by MED-EL GmbH, Austria.
Clinical reports on ST placement with FLEX CI electrode variants
Boyer et al22 in 2015 reported on ST placement in 29 CI patients out of 30 (96.6%, 29/30) who were implanted with MED-EL's FLEX electrode array variants. O'Connell et al23 in 2016 reported on 100% ST placement in 48 CI patients implanted with various FLEX electrode array variants. As per the report of Fischer et al24 in 2016, out of 63 patients implanted with FLEX 28 electrodes, 58 patients had complete ST placement (92%, 58/63) while the electrode deviated from ST to scala vestibuli (SV) in 5 patients in whom cochleostomy approach of electrode insertion was applied. An et al's25 report in 2017 showed that out of 21 patients implanted with FLEX 28 electrode array, 20 patients (95.2%, 20/21) retained the electrode in dfST while electrode deviated from ST to SV in 1 patient. In all these reports, post-operative computed tomography (CT) imaging method was used to confirm the scalar position of the electrode.
Implications of choosing electrode array matching the cochlear size
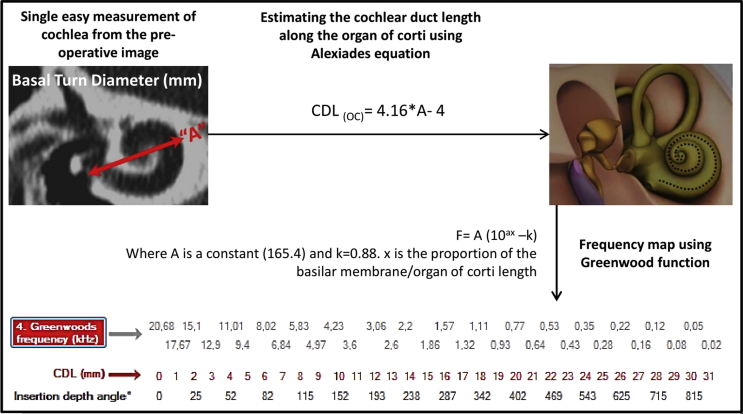
Cochlear frequency map provides the exact intra-cochlear location at which the neural elements are responsible for processing sound signal of various frequencies. Depending on the cochlear size, the frequency map will vary individually. Greenwood et al26 in 1990 proposed a mathematical function to create the individual frequency map just with the help of CDL estimated/measured along the organ of Corti. Fig. 7 schematically explains the “A” value measurement from the pre-operative image of the cochlea and applying Alexiades and Greenwood functions in the estimation of CDL and mapping the frequencies respectively.
Figure 7.
Schematic representation of “A” value measurement from the pre-operative image of the cochlea and the application of Alexiades9 and Greenwood26 function in estimating the cochlear duct length (CDL) and mapping the frequencies respectively.
Combining the CDL estimated along the organ of Corti, patient's pre-operative audiogram and Greenwood frequency map, it is possible to locate the exact position/insertion depth inside the cochlea at which it is responsible for various characteristic frequencies.
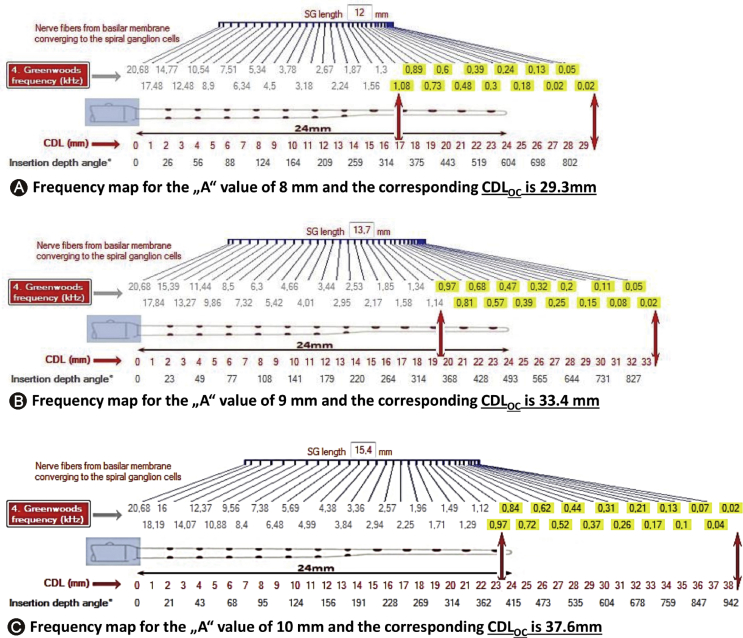
Fig. 8 demonstrates how the frequency position changes as the CDL changes. As CI is becoming standard of care even in cases of partial deafness, it is believed by many in the CI field that physical placement of electrode in functional residual hearing region is detrimental to cochlear health. In contrast to this belief, Manjaly et al27 in 2018 reported on complete hearing preservation with full insertion of FLEX 28 electrode array in patients with pre-operative hearing ≤85 dB HL at 250 Hz. Sierra et al28 in 2019 reported on complete/partial hearing preservation using both FLEX SOFT (array length 31.5 mm), STANDARD (array length 31.5 mm) and FLEX 28 (array length 28 mm) electrode in majority of the cases. All these electrodes are capable of going beyond the basal turn of the cochlea is an indirect proof that the electrode array did not cause any intra-cochlear structural damage. In both of these studies, they calculated hearing preservation percentages using the formula described in HEARRING group that involves pure tone average (PTA) before and after the CI surgery. Complete hearing preservation is considered with the hearing preservation percentage of >75% and anything between 25% and 75% is considered as partial hearing preservation.
Figure 8.
Three different cochlear duct lengths (CDLs) with one electrode array length of 24 mm. With the help of Greenwood frequency map on top of electrode and CDL on the bottom of electrode, the place pitch position of each electrode channel can be identified. In all three cases, with an assumption of the residual hearing starting at 1000 Hz towards the low frequency which is highlighted in yellow [Image taken from author's own work (CDL-frequency map software)].
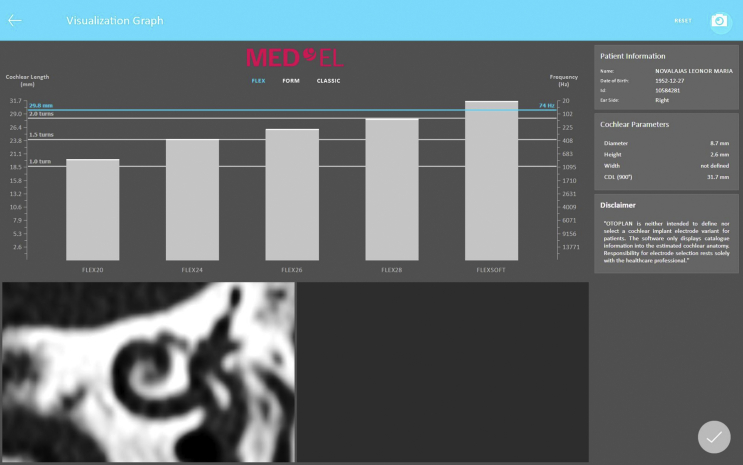
OTOPLAN (www.otoplan.ch), the tablet based otological pre-planning software tool is now commercially available that simplifies the overall process of measuring the cochlear size (Fig. 9).
Figure 9.
Screenshot of OTOPLAN pre-planning software tool showing the cochlear parameters along with various electrode arrays placed parallel to the patient specific Greenwood frequency map.
With few button clicks the greenwood frequency map corresponding to that particular cochlear size is also visualized. With the CDL known along with Greenwood frequency map, MED-EL's FLEX electrode array length selection becomes a simple process. The software screenshot as shown in Fig. 9 corresponds to a cochlear size measured by the “A” value of 8.7 mm. If the suggested electrode array is implanted to its full length, then the OTOPLAN offers the possibility of anatomy based post-operative speech processor fitting with patient's individual frequency map.
Conclusions
Knowing the cochlear size before the CI surgery helps in many possible ways like choosing the best matching electrode array which might reduce the electrode insertion trauma and as well in preserving the residual hearing. Advanced otological pre-planning software tool like OTOPLAN is now clinically available offering the possibility in measuring the cochlear size, choosing the best fitting electrode array and also in post-operative speech processor fitting. Flexible and atraumatic electrode arrays available in different array length is absolutely making sense and would assist CI surgeons in choose the best matching electrode array for their patient's cochlear needs.
Declaration of Competing Interest
Author is employed at MED-EL GmbH Austria as the Head of Translational Science Communication, which is purely a scientific role with no sales or marketing activities.
Acknowledgement
Dr. Claude Jolly, Dr. Andreas Jäger and Mr. Stefan Bryde Nielsen (all from MED-EL) are highly acknowledged for their master minds behind the FLEX CI electrode array design. Dipl. Ing. Alexander Mayr (MED-EL) is specially acknowledged for his early work together with Dr. Techn. DDr. Med. Hc. Ingeborg Hochmair (CEO- MED-EL) that resulted in the CI electrode design with “wavy” wires which is unique in the MED-EL's electrode array design. Author personally thanks all those CI surgeons across the world who shared their positive views on FLEX electrodes from their clinical experience that motivated to write this article.
Edited by Qiuyi Qu and Xin Jin
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Roland P.S., Tobey E. A tribute to a remarkably sound solution. Cell. 2013;154:1175–1177. doi: 10.1016/j.cell.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Meng J., Li S., Zhang F., Li Q., Qin Z. Cochlear size and shape variability and implications in cochlear implantation surgery. Otol Neurotol. 2016;37:1307–1313. doi: 10.1097/MAO.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 3.Dhanasingh A. Variations in the size and shape of human cochlear malformation types. Anat Rec (Hoboken) 2019;302:1792–1799. doi: 10.1002/ar.24136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhanasingh A., Dietz A., Jolly C., Roland P. Human inner-ear malformation types captured in 3D. J Int Adv Otol. 2019;15:77–82. doi: 10.5152/iao.2019.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaul C., Dragovic A.S., Stringer A.K., O'Leary S.J., Briggs R.J. Scalar localisation of peri-modiolar electrodes and speech perception outcomes. J Laryngol Otol. 2018;132:1000–1006. doi: 10.1017/S0022215118001871. [DOI] [PubMed] [Google Scholar]
- 6.Ketterer M.C., Aschendorff A., Arndt S. The influence of cochlear morphology on the final electrode array position. Eur Arch Otorhinolaryngol. 2018;275:385–394. doi: 10.1007/s00405-017-4842-y. [DOI] [PubMed] [Google Scholar]
- 7.O'Connell B.P., Hunter J.B., Gifford R.H. Electrode location and audiologic performance after cochlear implantation: a comparative study between nucleus CI422 and CI512 electrode arrays. Otol Neurotol. 2016;37:1032–1035. doi: 10.1097/MAO.0000000000001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escudé B., James C., Deguine O., Cochard N., Eter E., Fraysse B. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurootol. 2006;11(Suppl 1):27–33. doi: 10.1159/000095611. [DOI] [PubMed] [Google Scholar]
- 9.Alexiades G., Dhanasingh A., Jolly C. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015;36:904–907. doi: 10.1097/MAO.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 10.Erixon E., Högstorp H., Wadin K., Rask-Andersen H. Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol. 2009;30:14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y.K., Qi C.L., Tang J. The diagnostic value of measurement of cochlear length and height in temporal bone CT multiplanar reconstruction of inner ear malformation. Acta Otolaryngol. 2017;137:119–126. doi: 10.1080/00016489.2016.1221132. [DOI] [PubMed] [Google Scholar]
- 12.Thong J.F., Low D., Tham A., Liew C., Tan T.Y., Yuen H.W. Cochlear duct length- one size fits all? Am J Otolaryngol. 2017;38:218–221. doi: 10.1016/j.amjoto.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 13.De Seta D., Nguyen Y., Bonnard D. The role of electrode placement in bilateral simultaneously cochlear-implanted adult patients. Otolaryngol Head Neck Surg. 2016;155:485–493. doi: 10.1177/0194599816645774. [DOI] [PubMed] [Google Scholar]
- 14.Timm M.E., Majdani O., Weller T. Patient specific selection of lateral wall cochlear implant electrodes based on anatomical indication ranges. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Dawant B.M., Labadie R.F., Noble J.H. Retrospective evaluation of a technique for patient-customized placement of precurved cochlear implant electrode arrays. Otolaryngol Head Neck Surg. 2017;157:107–112. doi: 10.1177/0194599817697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebscher S.J., Hetherignton A., Bonham B., Wardrop P., Whinney D., Leake P.A. Considerations for design of future cochlear implant electrode arrays: electrode array stiffness, size and depth of insertion. J Rehabil Res Dev. 2008;45:731–747. doi: 10.1682/jrrd.2007.08.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avci E., Nauwelaers T., Lenarz T., Hamacher V., Kral A. Variations in microanatomy of the human cochlea. J Comp Neurol. 2014;522:3245–3261. doi: 10.1002/cne.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnansia D., Demarcy T., Vandersteen C. Optimal electrode diameter in relation to volume of the cochlea. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(Suppl 1):S66–S67. doi: 10.1016/j.anorl.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Hatsushika S., Shepherd R.K., Tong Y.C., Clark G.M., Funasaka S. Dimensions of the scala tympani in the human and cat with reference to cochlear implants. Ann Otol Rhinol Laryngol. 1990;99:871–876. doi: 10.1177/000348949009901104. [DOI] [PubMed] [Google Scholar]
- 20.Al-Dhamari I., Bauer S., Paulus D., Helal Rm, Lisseck F., Jacob R. 2018. Automatic Cochlear Length and Volume Size Estimation.https://or20.univ-rennes1.fr/sites/or20.univ-rennes1.fr/files/asset/document/aldhamarietal2018_0.pdf [Google Scholar]
- 21.Takahashi M., Arai Y., Sakuma N. Cochlear volume as a predictive factor for residual-hearing preservation after conventional cochlear implantation. Acta Otolaryngol. 2018;138:345–350. doi: 10.1080/00016489.2017.1393840. [DOI] [PubMed] [Google Scholar]
- 22.Boyer E., Karkas A., Attye A., Lefournier V., Escude B., Schmerber S. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol. 2015;36:422–429. doi: 10.1097/MAO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell B.P., Hunter J.B., Haynes D.S. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017;127:2352–2357. doi: 10.1002/lary.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer N., Pinggera L., Weichbold V., Dejaco D., Schmutzhard J., Widmann G. Radiologic and functional evaluation of electrode dislocation from the scala tympani to the scala vestibuli in patients with cochlear implants. AJNR Am J Neuroradiol. 2015;36:372–377. doi: 10.3174/ajnr.A4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An S.Y., An C.H., Lee K.Y., Jang J.H., Choung Y.H., Lee S.H. Diagnostic role of cone beam computed tomography for the position of straight array. Acta Otolaryngol. 2018;138:375–381. doi: 10.1080/00016489.2017.1404639. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood D.D. A cochlear frequency-position function for several species- 29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 27.Manjaly J.G., Nash R., Ellis W. Hearing preservation with standard length electrode in pediatric cochlear implantation. Otol Neurotol. 2018;39:1109–1114. doi: 10.1097/MAO.0000000000001917. [DOI] [PubMed] [Google Scholar]
- 28.Sierra C., Calderón M., Bárcena E., Tisaire A., Raboso E. Preservation of residual hearing after cochlear implant surgery with deep insertion electrode arrays. Otol Neurotol. 2019;40:e373–e380. doi: 10.1097/MAO.0000000000002170. [DOI] [PubMed] [Google Scholar]