Abstract
Purpose
Fifteen percent of patients undergoing elective sigmoidectomy will present a diverticulitis recurrence, which is associated with significant costs and morbidity. We aimed to systematically review the risk factors associated with recurrence after elective sigmoidectomy.
Methods
PubMed/MEDLINE, Embase, Cochrane, and Web of Science were searched for studies published until May 1, 2020. Original studies were included if (i) they included patients undergoing sigmoidectomy for diverticular disease, (ii) they reported postoperative recurrent diverticulitis, and (iii) they analyzed ≥ 1 variable associated with recurrence. The primary outcome was the risk factors for recurrence of diverticulitis after sigmoidectomy.
Results
From the 1463 studies initially screened, six studies were included. From the 1062 patients included, 62 patients recurred (5.8%), and six variables were associated with recurrence. Two were preoperative: age (HR = 0.96, p = 0.02) and irritable bowel syndrome (33.3% with recurrence versus 12.1% without recurrence, p = 0.02). Two were operative factors: uncomplicated recurrent diverticulitis as indication for surgery (73.3% with recurrence versus 49.9% without recurrence, p = 0.049) and anastomotic level (colorectal: HR = 11.4, p = 0.02, or colosigmoid: OR = 4, p = 0.033). Two were postoperative variables: the absence of active diverticulitis on pathology (39.6% with recurrence versus 26.6% without recurrence) and persistence of postoperative pain (HR = 4.8, p < 0.01).
Conclusion
Identification of preoperative variables that predict the occurrence of diverticulitis recurrence should help surgical decision-making for elective sigmoidectomy, while peri- and postoperative factors should be taken into account for optimal patient follow-up.
Electronic supplementary material
The online version of this article (10.1007/s00384-020-03762-0) contains supplementary material, which is available to authorized users.
Keywords: Diverticulitis, Sigmoidectomy, Postoperative recurrence, Risk factors
Introduction
Diverticulosis is defined by the presence of colonic diverticula which are protrusions of the mucosa and submucosa through the colonic wall. More than 90% of colonic diverticula are found in the left colon and sigmoid [1]. Based on an American population aged between 30 and 80 years undergoing outpatient colonoscopies, diverticulosis was present in 42% of patients [2]. This prevalence was increased in elderly, white population, overweight, smokers, and patients with decreased bowel movements [2]. Patients may remain asymptomatic, whereas others will develop diverticular disease, defined as symptomatic diverticulosis. Therefore 10–25% of patients with diverticulosis will manifest diverticular inflammation, and 12% of patients with diverticulitis will developed a complication such as abscess, perforation, fistula, stricture or obstruction [3].
The Hinchey classification modified by Wasvary et al. [4] is often used to classify severity of episode of diverticulitis. That classification includes four stages: stage Ia corresponds to a confined inflammation or phlegmon; stage Ib is characterized by a pericolic or mesenteric abscess; stage II is characterized by a distant abscess in the abdomen, pelvis, or retroperitoneum; and perforation leading to purulent or fecal peritonitis correspond to stages III or IV, respectively.
The European Association for Endoscopic Surgery (EAES) and other interventional techniques and Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) published guidelines in 2019 [5] recommending emergent sigmoid resection for Hinchey III and IV diverticulitis or after failure of conservative therapies for earlier stages. Elective sigmoidectomy was recommended in the case of decreased quality of life caused by diverticular disease. Moreover, chronic symptoms or smoldering disease, severity of prior episodes, comorbidities, and patient preferences should be taken into consideration [6].
Nevertheless, sigmoidectomy, although removing the segment of the colon the most affected by diverticula, as well as the recto-sigmoid junction, does not remove diverticula from the remaining colon. After a mean follow-up of 10 years, a recurrence rate of 15% after elective surgery for diverticulitis was reported [7]. Mechanism for these recurrences is not clear. However, several risk factors were identified. Prediction of these recurrences is important to prevent associated costs and morbidity [8]. Therefore, we aimed to systematically review the risk factors associated with recurrence of diverticulitis after elective sigmoidectomy.
Materials and methods
This systematic review was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement [9] (Supplementary Table 1).
Data source and search strategy
Two reviewers (GL, ZA) independently searched PubMed/MEDLINE, Embase, Cochrane, and Web of Science for studies published until May 1, 2020, without limitation based on the publication year. The following search terms were used: “diverticulitis” OR “diverticulum” AND “inflammation”, AND “surgery” OR “colectomy”, AND “recurrence” in MeSH terms; and “diverticula” OR “diverticulosis”, AND “resection” OR “sigmoidectomy” OR “Hartmann*”, AND “recurrent” OR “failure” in non-MeSH terms. Additionally, a manual cross-reference search of bibliographies of relevant articles was performed to identify additional studies.
Study selection
Original studies written in English were eligible for inclusion if they fulfilled all the following criteria: (i) they included patients undergoing elective sigmoidectomy for diverticular disease, (ii) they reported postoperative recurrent diverticulitis, and (iii) they reported ≥ 1 variable associated with recurrence. Studies were excluded if postoperative recurrence was not confirmed by imaging or if the definition of recurrence was not specified. Studies including surgical procedure without resection (i.e., peritoneal lavage, surgical drainage) were excluded. Other exclusion criteria were case reports, conference abstracts, editorials, and protocols. There was no restriction based on the design or sample size of the study.
Data extraction
Two authors (GL, ZA) independently extracted the data, including general and methodological information of the study and baseline characteristics of the study population: sample size, age, gender, classification of diverticulitis, the number of previous episodes of diverticulitis, and indication for surgery. Intraoperative data were also extracted, including elective/emergency intervention, type of resection (sigmoidectomy/left-sided hemicolectomy/anterior resection), splenic flexure mobilization, laparoscopic/open resection, conversion, creation of ostomy, and type of anastomosis (stapled/handsewn, colorectal/colosigmoid). Postoperative extracted data were follow-up duration, pathology report (specimen length, inflammation state [active, chronic, none]), persistent complaints, complications, recurrence, and treatment for recurrence. Variables associated with recurrence on quantitative analysis and variables significant on uni- or multivariate regression analysis were also extracted.
Outcome measures
The primary outcome of the systematic review was to identify risk factors for postoperative recurrence of diverticulitis. The secondary outcomes were the incidence of postoperative recurrence of diverticulitis, treatment for postoperative recurrence of diverticulitis (medical versus surgical), postoperative complications, and mortality.
Recurrence was defined as left lower quadrant pain, inflammation (fever, elevated white blood cell, or C-reactive protein), and imaging consistent with the diagnosis of diverticulitis. Complication was defined as any deviation from the normal postoperative course and did not include recurrence.
Results
Studies selection and characteristics
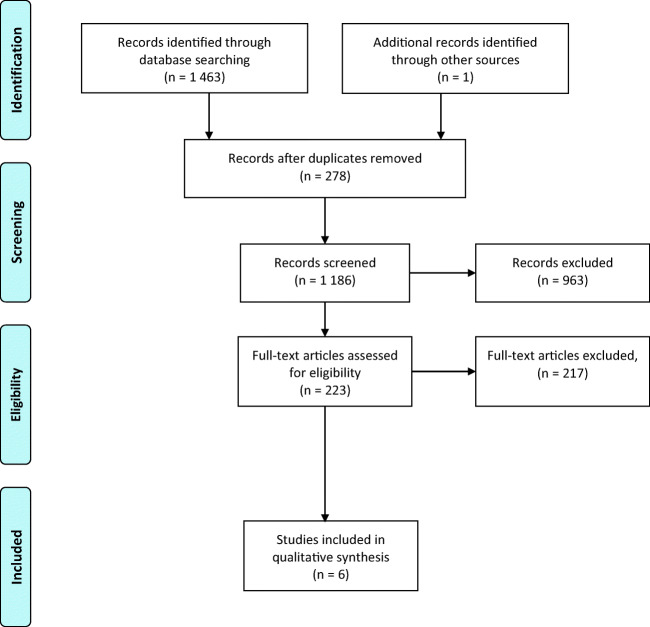
The initial search identified 1463 studies (Fig. 1). After duplicates removal, 1186 records were screened. Based on the title and abstract, 963 studies were removed. From the 223 full text articles assessed for eligibility, 208 were excluded because they did not fulfill all inclusion criteria. Furthermore, nine other studies were removed: one study [10] contained duplicated data from another included study; two studies [11, 12] included peritoneal lavage, surgical drainage, or diverticulectomy in the resection group; in five studies [13–17] recurrences were not defined or confirmed by imaging; and one study [18] contained insufficient data. One study [19] was identified by cross-referencing. Finally, six articles [19–24] were included in the present review.
Fig. 1.
Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) flowchart showing selection of publications for review
From the included studies, five studies were monocentric [19–23] and retrospective observational cohort [19–22, 24] (Table 1). Overall, 1062 patients were included (518 males and 544 females) with a mean age ranging from 56 to 63 years. Data regarding the mean follow-up duration was reported by 4 studies [19–21, 24], which ranged from 55 to 86 months. Before the surgical procedure, 765 (72%) patients experienced recurrent attacks of diverticulitis, and 128 (12%) presented their first episode (data unavailable for 169 (16%) of cases). Only two studies [22, 23] reported the severity of the diverticulitis episode, classified either with the Hinchey classification [25] or with the Hansen and Stock classification [26].
Table 1.
Characteristics of included studies and baseline characteristics of participants
| Study | Methodological characteristics | Population characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Journal of publication | Study design | Monocentric or multicentric | Study period | Sample size | Age (mean ± SD or range) | Gender (male/female) | Follow-up (mean ± SD or range) | Diverticulitis severity | Previous episode of diverticulitis | |||||
| 0 | 1 | 2 | 3 | ≥ 4 | ||||||||||
|
Bergamaschi et al. 1998, France |
Surgical endoscopy | ORC | Monocentric | 1990–1994 | 75 | 60.9 | 48/27 | 55 months | -ª | - | ||||
|
Thaler et al. 2003, USA |
Disease of the colon & rectum | ORC | Multicentric | 1992–2000 | 236 | 60.4 ± 10 | 131/105 | 67 ± 30 months | -° | 1* | 62* | 113* | 10* | 0 |
|
Regenet et al. 2005, France |
Hepatogastroenterology | OPC + ORC | Monocentric | 1996–2001 | 94 | 56.4 | 47/47 | NA |
Hinchey classification: 72 patients stage 0 22 patients stage 1 |
- | ||||
|
Andeweg et al. 2008, Netherlands |
World journal of surgery | ORC | Monocentric | 1985–2003 | 183 | 63, 26–93 | 84/99 | 86, 0–216 months | - | 63 | 16 | 88 | 11 | 5 |
|
Holmer et al. 2011, Germany |
Langenbeck’s archives of surgery | OPC | Monocentric | 2004–2008 | 113 | 62.4, 38–92∏ | 46/67 | NA |
Hansen and Stock classification: 12 patients stage I 59 patients stage 2a 42 patients stage 2b |
45 | 68: ≥ 1 episode | |||
|
Choi et al. 2019, USA |
Journal of gastrointestinal surgery | ORC | Monocentric | 2002–2016 | 361 | 56 ± 10.7 | 162/199 | 86, 6–190 months | - | 19 | 214: 1–3 episodes | 128 | ||
SD standard deviation, ORC observational retrospective cohort, - not available, OPC observational prospective cohort
*Based on the previous admission, data not available for all patients but 235 cases with more than one episode of diverticulitis
∏expressed as median, with range
ªComplicated diverticulitis were excluded
°Perforated diverticulitis were excluded
Perioperative and postoperative outcomes
Only one study [20] included emergency surgical resections, representing 73 (7%) of patients included in the review (Table 2). The remaining patients represented 953 (93%) cases, which underwent elective intervention for uncomplicated diverticulitis [19–21, 24] or complicated diverticulitis [20–22]. Other reported indications for surgery were symptomatic diverticulosis [23], persistent abdominal pain/smoldering disease [21, 22], recurrent bleeding [22], failure of conservative treatment for diverticulitis [22], and first attack of diverticulitis in immunosuppressed patients [22]. Sigmoidectomy was the procedure of choice in 928 (90%) patients, while anterior resection or left-sided hemicolectomy were not routinely performed (11 patients and 38 patients, respectively; type of intervention performed not reported for 85 patients). Details of the operative techniques are detailed in Table 2.
Table 2.
Operative characteristics
| Study | Indication for surgery | Elective/emergency | Type of operation | Laparoscopic/open// conversion |
Splenic flexure mobilization | Ostomy creation | Anastomosis | ||
|---|---|---|---|---|---|---|---|---|---|
| Sigmoidectomy | Other | Stapler/Handswen | Colorectal/Colosigmoid | ||||||
| Bergamaschi et al. | Uncomplicated diverticulitis | 75/0 | - | 40/35 // 1 | 46 | 0 | 51/24 | 50/25 | |
| Thaler et al. | Uncomplicated diverticulitis | 236/0 | 236 | 0 | 96/140 // 18 | 109 | 0 | 171 / 65 | 143 / 93 |
| Regenet et al. | Symptomatic diverticulosis | 94/0 | 94 | 0 | 72/22 // 0 | 94 | 0 | 94/0 | 94/0 |
| Andeweg et al. | Uncomplicated and complicated diverticulitis | 110/73 | 150 |
5 LH 18 AR* |
- | - | 68 | - | 21/90¥ |
| Holmer et al. |
Persistent abdominal pain Covered perforation ± abscess Recurrent bleeding Failure of conservative treatment First attack in immunosuppressed |
113/0 | 113 | 0 | - | 113 | 0 | 113/0 | - |
| Choi et al. |
Uncomplicated recurrent diverticulitis Localized perforation ± abscess Fistula Stricture Smoldering disease |
361/0 | 335 |
6 LH 20 AR |
359 / 2 // 41 | 0 | 5 | 361/0 | 361/0 |
- not available, LH left-sided hemicolectomy, AR anterior resection
*10 classified as miscellaneous
¥4 anastomosis classified as other
Pathology showed a mean specimen length from 14 to 26 cm [19–24] and the presence of diverticulitis in 536 cases, but no inflammation in 177 cases [19–21, 23] (Table 3). Moreover, inflammation at the proximal margin was reported by two studies in 13 [19] and 30 [24] patients. Data on the presence of postoperative persistent complaints was reported by one study [20] in 36 patients. Five studies [19–22, 24] reported postoperative complications, corresponding to a total of 136 events and an incidence of 14.0% (136/968); and four studies [19, 20, 22, 24] reported postoperative death, corresponding to 8 patients and a mortality of 1.3% (8/607).
Table 3.
Postoperative outcomes
| Study | Pathology | Complications (timepoint) | Persistent complains | Mortality (timepoint) | |
|---|---|---|---|---|---|
| Specimen length (mean ± SD or range) | Pathologist assessment | ||||
| Bergamaschi et al. | 14.3 cm | 75 diverticulitis∏ | 8 | - | 0 |
| Thaler et al. | 17.9 ± 5.9 cm | -∏ | 54 (30 days) | - | 1 (30 days) |
| Regenet et al. | 26.3 cm | 94 diverticulitis | - | - | - |
| Andeweg et al. | 17.3, 7-35 cm |
166 acute diverticulitis 17 no inflammation |
9* | 36 | 7 |
| Holmer et al. | - | - | 16 | - | 0 |
| Choi et al. | 17.7 cm |
141 acute diverticulitis 60 chronic diverticulitis 158 diverticular disease 2 no disease |
49 (perioperative) | - | - |
SD standard deviation, cm centimeter, - not available
∏inflammation at the proximal margin was reported in 13 cases by Bergamaschi et al. and in 30 cases by Thaler et al.
*only severe complications were reported
Postoperative recurrence of diverticulitis
A total of 62 diverticulitis recurred after surgical resection for diverticular disease, representing 5.8% of the population included in the review. Mean time to recurrence ranged between 38 [20] and 78 months [24], and the cumulative time-related incidence at 15 years ranged between 6.3 [21] and16% [20] (Table 4). The treatment for postoperative recurrence was conservative in 43 patients, but 14 patients required another surgical intervention (treatment not reported by one study [23]).
Table 4.
Reported recurrences of included studies
| Study | Number of recurrence (%) |
Time until recurrence (mean ± SD or range) | Cumulative time-related incidence of recurrence | Treatment for recurrent diverticulitis | |
|---|---|---|---|---|---|
| Conservative | Surgical | ||||
| Bergamaschi et al. | 4 (5.3%) | - | - | 4 | 0 |
| Thaler et al. | 12 (5.1%) | 78 ± 25 months | - | 11 | 1 |
| Regenet et al. | 5 (5.3%) | - | - | - | |
| Andeweg et al. | 16 (8.7%) | 38, 6–144 months |
1 year: 3% (SE 1.3) 5 years: 8.2% (SE 2.3) 10 years: 12% (SE 3.0) 15 years: 16% (SE 3.7) |
8 | 8∏ |
| Holmer et al. | 4 (3.5%) | - | - | 4 | 0 |
| Choi et al. | 21 (5.8%) | 55, 6–109 months |
1 year: 0.3% 5 years: 3.0% 10 years: 6.3% 15 years: 6.3% |
16 | 5* |
| Overall | 62 (5.8%) | - | - | 43 | 14 |
SD standard deviation, - not available, SE standard error
∏3 left hemicolectomy, 3 partial resection of transverse colon, 2 subtotal colectomy
*5 left colectomies
Preoperative variables associated with postoperative recurrence of diverticulitis
Eight preoperative variables were considered for their association with recurrence of postoperative diverticulitis (Table 5). Age was not associated with postoperative recurrence in the retrospective study by Choi et al. [21] (p = 0.12). However, Andeweg et al. [20] reported a lower age to be associated with recurrence (mean 54, range 33–75, versus without recurrence: mean 64, range 27–93, p < 0.02). On regression analysis (Cox-model), younger age was still an independent predictor of recurrence (univariate: hazard ratio (HR) = 0.96, 95% CI 0.93–0.99, p = 0.02; multivariate: stated as significant but no value reported). Irritable bowel syndrome was the other preoperative variable associated with recurrence on univariate analysis (33.3% with recurrence versus 12.1% without recurrence, p = 0.02) [21]. Nevertheless, the latter was not significant on regression analysis (p = 0.053). Six preoperative variables showed no significant association with recurrence: the number of preoperative episodes of diverticulitis (reported by four studies [20–22, 24]), gender (reported by three studies [20, 21, 24]), American society of anesthesiologists (ASA) class and previous abdominal surgery (both reported by one study [24]), comorbidity, and previous treatment modality (both reported by one study [21]).
Table 5.
Preoperative variables associated with postoperative recurrence of diverticulitis
| Study | Univariate analysis | Univariate regression analysis | Multivariate regression analysis |
|---|---|---|---|
| Number of preoperative episodes of diverticulitis | |||
| Thaler et al. | - | NS | - |
| Andeweg et al. | NS | NS | - |
| Choi et al. | NS | - | - |
| Holmer et al. | NS | - | - |
| Gender | |||
| Thaler et al. | - | NS | - |
| Andeweg et al. | NS | - | - |
| Choi et al. | NS | - | - |
| Age: mean, range with recurrence versus without recurrence | |||
| Andeweg et al. | 54, 33–75 versus 64, 27–93, p < 0.02 | HR 0.96, 95% CI 0.93–0.99, p = 0.02 | Significant (value NA) |
| Choi et al. | NS | - | - |
| ASA class | |||
| Thaler et al. | - | NS | - |
| Comorbidity | |||
| Choi et al. | NS | - | - |
| Irritable bowel syndrome: n (%) with recurrence versus without recurrence | |||
| Choi et al. | 5 (33.3%) versus 42 (12.1%), p = 0.02 | NS | - |
| Previous treatment modality: antibiotic IV versus antibiotic PO versus drainage | |||
| Choi et al. | NS | - | - |
| Previous abdominal surgery | |||
| Thaler et al. | - | NS | - |
NS not significant, ASA American Society of Anesthesiologists, IV intravenous, PO per os, - not available
Operative variables associated with postoperative recurrence of diverticulitis
From the eight operative variables, only two [20, 21, 24] were associated with recurrence (Table 6). The first variable was uncomplicated recurrent diverticulitis as indication for surgery (73.3% with recurrence versus 49.9% without recurrence, p = 0.049), but the association was not significant on univariate regression analysis [21]. Anastomotic level was considered by three studies [20, 23, 24], but significant in two studies [20, 24] on univariate regression analysis. Andeweg et al. [20] reported increased recurrences associated with colorectal anastomosis compared with colostomy (univariate regression analysis: HR = 11.4, 95% CI 1.2-109.5, p = 0.02; multivariate regression analysis: stated as significant but no value reported). In the other hand, Thaler et al. [24] reported colosigmoid anastomosis as a risk factor for postoperative recurrent diverticulitis (univariate regression analysis: odds ratio (OR) = 4, 95%CI 1.1-15.0, p = 0.033; no multivariate regression analysis). Six other factors were not associated with postoperative recurrence of diverticulitis: emergency/elective surgery [20], laparoscopic/open approach [19, 23, 24], length of resected bowel [20, 21, 23], type of resection [20, 21], splenic flexure mobilization [24], stapled/handsewn anastomosis [24].
Table 6.
Operative variables associated with recurrence associated with postoperative recurrence of diverticulitis
| Study | Univariate analysis | Univariate regression analysis | Multivariate regression analysis |
|---|---|---|---|
|
Unomplicated recurrent diverticulitis as indication for surgery: n (%) with recurrence versus without recurrence | |||
| Choi et al. | 11 (73.3) versus 171 (49.9%), p = 0.049 | NS | - |
| Emergency versus elective surgery | |||
| Andeweg et al. | NS | - | - |
| Laparoscopic versus open | |||
| Thaler et al. | - | NS | - |
| Regenet et al. | NS | - | - |
| Bergamaschi et al. | NS | - | - |
| Anastomotic level: n (%) of recurrence | |||
| Andeweg et al. | Colorectal: 3 (14.3%), colosigmoid: 12 (13.3%) versus colostomy 1 (1.5%), p = 0.04 | Colorectal: HR 11.4, 95% CI 1.2-109.5, p = 0.02, colosigmoid: NS | Colorectal: Significant (value NA) |
| Thaler et al. | - | Colosigmoid: 7 (12.5%) versus colorectal: 4 (2.8%); OR = 4, 95% CI 1.1–15.0, p = 0.033 | - |
| Regenet et al. | NS | - | - |
| Length of resected bowel | |||
| Choi et al. | NS | - | - |
| Andeweg et al. | NS | - | - |
| Regenet et al. | NS | - | - |
| Type of resection: sigmoidectomy versus anterior resection versus left-sided hemicolectomy | |||
| Choi et al. | NS | - | - |
| Andeweg et al. | NS | - | - |
| Splenic flexure mobilization | |||
| Thaler et al. | NS | - | - |
| Anastomotic technique: stapled versus handsewn | |||
| Thaler et al. | NS | - | - |
NS not significant
- not available
Postoperative variables associated with postoperative recurrence of diverticulitis
Four postoperative variables were included in the analysis for their association with diverticulitis (Table 7). The absence of active diverticulitis on pathology was significant on univariate analysis only in the study by Choi et al. [21] (39.6% with recurrence versus 26.6% without recurrence, p = 0.01). However, two studies [20, 24] reported no association between the pathology and the recurrence of postoperative diverticulitis. Persistence of postoperative pain was associated with recurrence on univariate analysis but also on uni- and multivariate regression analysis (22% with recurrence versus 5.4% without recurrence, p < 0.01; HR = 4.8, 95% CI 1.8–12.5, p < 0.01; stated as significant but no value reported; respectively) [20]. Two postoperative factors were not associated with recurrence, as reported by one study [24]: postoperative complications and reoperation.
Table 7.
Postoperative variables associated with recurrence associated with postoperative recurrence of diverticulitis
| Study | Univariate analysis | Univariate regression analysis | Multivariate regression analysis |
|---|---|---|---|
| Acute diverticulitis on pathology: n (%) with recurrence versus without recurrence | |||
| Choi et al. | 4 (26.6%) versus 137 (39.6%), p = 0.01 | NS | - |
| Andeweg et al. | NS | - | - |
| Thaler et al. | - | NS∏ | - |
| Persistent postoperative pain: n (%) of recurrences in patients with persistent pain versus n (%) of recurrences without persistent pain | |||
| Andeweg et al. | 8 (22.2%) versus 8 (5.4%), p < 0.01 | HR 4.8, 95% CI 1.8–12.5, p < 0.01 | Significant (value NA) |
| Postoperative complication | |||
| Thaler et al. | - | NS | - |
| Reoperation | |||
| Thaler et al. | - | NS | - |
NS not significant
- not available
∏described as inflammation at the proximal margin
Discussion
The present systematic review included six observational cohorts [19–24], totalizing 1062 patients with diverticular disease. Recurrence occurred in 62 cases and needed conservative (43 cases) or surgical (14 cases) treatment. Three variables were significantly associated with postoperative recurrence of diverticulitis, one for each pre-, peri- or postoperative category. From the eight preoperative variables, a lower age [20] was associated with recurrence. From the eight perioperative factors, the anastomotic level was significant on regression analysis. Three studies [20, 21, 24] integrated four postoperative variables, of which persistent postoperative pain [20] was associated with recurrence on Cox regression model.
Our review had several limitations. Firstly, regression analysis was not undertaken by all the included studies. Secondly, risk of bias was high due to the design of the included studies (one prospective [22], four retrospectives [19–21, 24], and one mixed [23] observational cohorts). Thirdly, the study populations were small, and only one study [24] was multicentric. Fourthly, data were heterogeneous across studies (i.e. severity staging of the diverticulitis, indication fur surgery, operative technique, and definition of complications). Fifthly, the search strategy may have not retrieved all relevant studies.
Importantly, diverticulosis in limited to the descending colon and sigmoid in > 90% of cases [1] and sigmoidectomy seemed a good option for the treatment of diverticulitis [5]. However, it might not be a definitive cure for all patients, as showed by a cumulative time-related incidence of postoperative recurrence at 15 years ranging between 6.3 and 16% [20, 21]. Risk factors for recurrence should be identified, to avoid increased costs and morbidity. A systematic review by Hupfeld et al. [27] identified three factors with high likelihood to increase the risk of diverticulitis recurrence after non-surgical management: young age, diverticulitis complicated by an abscess formation, and recurrent diverticulitis. Compared with the latter review [27], we presently included two studies [20, 21] which assessed the relationship between age and postoperative recurrence. While one study [21] failed to find an association, another study [20] showed decreased recurrence in older patients (HR = 0.96, 95 % CI 0.93–0.99, p = 0.02). This might be explained by the decreased life expectancy while reappearance of diverticulitis could occur.
Herein, we presented the first systematic review of variables associated with postoperative recurrence. Identification of these factors could help optimization of the treatment strategy. From six identified variables, only the anastomotic level is modifiable. Based on a low level of evidence, the EAES and SAGES recommended colorectal anastomosis to decrease the risk of postoperative recurrence. This statement is supported by the study by Thaler et al. [24] reporting increased recurrences with colosigmoid anastomosis versus colorectal anastomosis. However, Andeweg et al. [20] showed increased recurrences with colorectal anastomosis versus colostomy, and Regenet et al. [23] found no association between the anastomotic level and postoperative recurrence. Because the results are conflictual, we could not favor an anastomotic level over another. Moreover, five additional non-modifiable risk factors were identified. Because elective sigmoidectomy is associated with postoperative complication rate of 22.5% and 30-day mortality rate of 0.5% [28], benefices should be weight against the risks. This balance should consider postoperative recurrence and associated risk factors, together with the patient preferences and global condition.
Future researches are needed to identify risk factors for postoperative recurrence. Our review reported conflicting results, and significant association between variable and recurrence were reported by isolated study. Moreover, future trials should include larger prospective cohorts.
Conclusions
To conclude, surgeons should be aware of the risk of postoperative diverticulitis recurrence, and patients should be informed. Preoperative variables associated with postoperative recurrence should be considered by clinicians for adequate patient selection and aid surgical decision-making for elective sigmoidectomy. Moreover, peri- and postoperative variables should be emphasized for optimal patient follow-up and early recognition of recurrence to avoid complication and reoperation.
Electronic supplementary material
(DOC 70 kb)
Authors’ contribution
GL and ZA conceived and designed the study. GL and ZA acquired the data. GL, ZA, JM, CT, NCB, and FR interpreted the data. GL, ZA, JM, CT, NCB, and FR contributed to the writing of the manuscript and to its critical revision. GL, ZA, JM, CT, NCB, and FR approved the final version of the manuscript.
Funding
Open access funding provided by University of Geneva.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Compliance with ethical standards
Conflicts of interests
The authors have no conflicts of interest to disclose.
Ethics approval
Not applicable
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med. 2007;357(20):2057–2066. doi: 10.1056/NEJMcp073228. [DOI] [PubMed] [Google Scholar]
- 2.Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, Sandler RS. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266–272. doi: 10.1053/j.gastro.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strate LL, Morris AM. Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology. 2019;156(5):1282–1298. doi: 10.1053/j.gastro.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasvary H, Turfah F, Kadro O, Beauregard W. Same hospitalization resection for acute diverticulitis. Am Surg. 1999;65(7):632–635. [PubMed] [Google Scholar]
- 5.Francis NK, Sylla P, Abou-Khalil M, Arolfo S, Berler D, Curtis NJ, Dolejs SC, Garfinkle R, Gorter-Stam M, Hashimoto DA, Hassinger TE, Molenaar CJL, Pucher PH, Schuermans V, Arezzo A, Agresta F, Antoniou SA, Arulampalam T, Boutros M, Bouvy N, Campbell K, Francone T, Haggerty SP, Hedrick TL, Stefanidis D, Truitt MS, Kelly J, Ket H, Dunkin BJ, Pietrabissa A. EAES and SAGES 2018 consensus conference on acute diverticulitis management: evidence-based recommendations for clinical practice. Surg Endosc. 2019;33(9):2726–2741. doi: 10.1007/s00464-019-06882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regenbogen SE, Hardiman KM, Hendren S, Morris AM. Surgery for diverticulitis in the 21st century: a systematic review. JAMA Surg. 2014;149(3):292–303. doi: 10.1001/jamasurg.2013.5477. [DOI] [PubMed] [Google Scholar]
- 7.Mizrahi I, Al-Kurd A, Chapchay K, Ag-Rejuan Y, Simanovsky N, Eid A, et al. Long-term outcomes of sigmoid diverticulitis: a single-center experience. J Surg Res. 2018;221:8–14. doi: 10.1016/j.jss.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Frattini J, Longo WE. Diagnosis and treatment of chronic and recurrent diverticulitis. J Clin Gastroenterol. 2006;40(Suppl 3):S145–S149. doi: 10.1097/01.mcg.0000225507.52300.b9. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;21:6(7). [PMC free article] [PubMed] [Google Scholar]
- 10.Thaler K, Weiss E, Nogueras J, Arnaud JP, Wexner S, Bergamaschi R. Recurrence rates at minimum 5-year follow-up: laparoscopic versus open sigmoid resection for uncomplicated diverticulitis. Surg Laparosc Endosc Percutan Tech. 2003;13(5):325–327. doi: 10.1097/00129689-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lee IK, Lee YS, Kim SJ, Gorden DL, Won DY, Kim HJ, Cho HM, Jeon HM, Kim JG, Oh ST. Laparoscopic and open surgery for right colonic diverticulitis. Am Surg. 2010;76(5):486–491. [PubMed] [Google Scholar]
- 12.Gregersen R, Andresen K, Burcharth J, Pommergaard H-C, Rosenberg J. Long-term mortality and recurrence in patients treated for colonic diverticulitis with abscess formation: a nationwide register-based cohort study. Int J Color Dis. 2018;33(4):431–440. doi: 10.1007/s00384-018-2990-1. [DOI] [PubMed] [Google Scholar]
- 13.Benn PL, Wolff BG, Ilstrup DM. Level of anastomosis and recurrent colonic diverticulitis. Am J Surg. 1986;151(2):269–271. doi: 10.1016/0002-9610(86)90085-1. [DOI] [PubMed] [Google Scholar]
- 14.Gervaz P, Mugnier-Konrad B, Morel P, Huber O, Inan I. Laparoscopic versus open sigmoid resection for diverticulitis: long-term results of a prospective, randomized trial. Surg Endosc. 2011;25(10):3373–3378. doi: 10.1007/s00464-011-1728-8. [DOI] [PubMed] [Google Scholar]
- 15.Klarenbeek BR, Bergamaschi R, Veenhof AAFA, van der Peet DL, van den Broek WT, de Lange ESM, Bemelman WA, Heres P, Lacy AM, Cuesta MA. Laparoscopic versus open sigmoid resection for diverticular disease: follow-up assessment of the randomized control Sigma trial. Surg Endosc. 2011;25(4):1121–1126. doi: 10.1007/s00464-010-1327-0. [DOI] [PubMed] [Google Scholar]
- 16.Letarte F, Hallet J, Drolet S, Charles Grégoire R, Bouchard A, Gagné J-P, Thibault C, Bouchard P. Laparoscopic emergency surgery for diverticular disease that failed medical treatment: a valuable option? Results of a retrospective comparative cohort study. Dis Colon Rectum. 2013;56(12):1395–1402. doi: 10.1097/DCR.0b013e3182a760b6. [DOI] [PubMed] [Google Scholar]
- 17.Turunen P, Wikström H, Carpelan-Holmström M, Kairaluoma P, Kruuna O, Scheinin T. Smoking increases the incidence of complicated diverticular disease of the sigmoid colon. Scand J Surg. 2010;99(1):14–17. doi: 10.1177/145749691009900104. [DOI] [PubMed] [Google Scholar]
- 18.Thörn M, Graf W, Stefànsson T, Påhlman L. Clinical and functional results after elective colonic resection in 75 consecutive patients with diverticular disease. Am J Surg. 2002;183(1):7–11. doi: 10.1016/s0002-9610(01)00847-9. [DOI] [PubMed] [Google Scholar]
- 19.Bergamaschi R, Arnaud JP. Anastomosis level and specimen length in surgery for uncomplicated diverticulitis of the sigmoid. Surg Endosc. 1998;12(9):1149–1151. doi: 10.1007/s004649900803. [DOI] [PubMed] [Google Scholar]
- 20.Andeweg C, Peters J, Bleichrodt R, van Goor H. Incidence and risk factors of recurrence after surgery for pathology-proven diverticular disease. World J Surg. 2008;32(7):1501–1506. doi: 10.1007/s00268-008-9530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KK, Martinolich J, Canete JJ, Valerian BT, Chismark DA, Ata A, Lee EC. Elective Laparoscopic Sigmoid Colectomy for Diverticulitis-an Updated Look at Recurrence After Surgery. J Gastrointest Surg. 2020;24(2):388–395. doi: 10.1007/s11605-018-04083-y. [DOI] [PubMed] [Google Scholar]
- 22.Holmer C, Lehmann KS, Engelmann S, Gröne J, Buhr HJ, Ritz J-P. Long-term outcome after conservative and surgical treatment of acute sigmoid diverticulitis. Langenbeck's Arch Surg. 2011;396(6):825–832. doi: 10.1007/s00423-011-0815-6. [DOI] [PubMed] [Google Scholar]
- 23.Regenet N, Pessaux P, Tuech J-J, Hennekinne S, Lermite E, Ridereau-Zins C, Aube C, Bergamaschi R, Jean-Pierre A. Prospective evaluation of the quality of laparoscopic sigmoid resection for diverticular disease. Hepatogastroenterology. 2005;52(65):1427–1431. [PubMed] [Google Scholar]
- 24.Thaler K, Baig MK, Berho M, Weiss EG, Nogueras JJ, Arnaud JP, Wexner SD, Bergamaschi R. Determinants of recurrence after sigmoid resection for uncomplicated diverticulitis. Dis Colon Rectum. 2003;46(3):385–388. doi: 10.1007/s10350-004-6560-y. [DOI] [PubMed] [Google Scholar]
- 25.Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85–109. [PubMed] [Google Scholar]
- 26.Hansen O, Graupe F, Stock W. Prognostic factors in perforating diverticulitis of the large intestine. Chir Z Alle Geb Oper Med. 1998;69(4):443–449. doi: 10.1007/s001040050436. [DOI] [PubMed] [Google Scholar]
- 27.Hupfeld L, Burcharth J, Pommergaard H-C, Rosenberg J. Risk factors for recurrence after acute colonic diverticulitis: a systematic review. Int J Color Dis. 2017;32(5):611–622. doi: 10.1007/s00384-017-2766-z. [DOI] [PubMed] [Google Scholar]
- 28.Haas JM, Singh M, Vakil N. Mortality and complications following surgery for diverticulitis: Systematic review and meta-analysis. United European Gastroenterol J. 2016;4(5):706–713. doi: 10.1177/2050640615617357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 70 kb)
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.