Abstract
In humans, inactivating mutations in MLL4, which encodes a histone H3-lysine 4-methyltransferase, lead to Kabuki syndrome (KS). While dwarfism is a cardinal feature of KS, the underlying etiology remains unclear. Here we report that Mll4 regulates the development of growth hormone-releasing hormone (GHRH)-producing neurons in the mouse hypothalamus. Our two Mll4 mutant mouse models exhibit dwarfism phenotype and impairment of the developmental programs for GHRH-neurons. Our ChIP-seq analysis reveals that, in the developing mouse hypothalamus, Mll4 interacts with the transcription factor Nrf1 to trigger the expression of GHRH-neuronal genes. Interestingly, the deficiency of Mll4 results in a marked reduction of histone marks of active transcription, while treatment with the histone deacetylase inhibitor AR-42 rescues the histone mark signature and restores GHRH-neuronal production in Mll4 mutant mice. Our results suggest that the developmental dysregulation of Mll4-directed epigenetic control of transcription plays a role in the development of GHRH-neurons and dwarfism phenotype in mice.
Subject terms: Disease model, Neurogenesis, Neuronal development
Mutations in the MLL4 gene can cause Kabuki syndrome, whose underlying molecular mechanisms are unclear. Here, the authors show that Mll4 epigenetically regulates the transcriptional program leading to the formation of GHRH-neurons in the developing mouse hypothalamus.
Introduction
Kabuki syndrome (KS), caused by haploinsufficiency of MLL4 or UTX, is a human developmental disorder that affects multiple tissues. One of the consistent cardinal features of KS is stunted growth and postnatal short stature1. For instance, a report on growth data in 39 KS patients revealed that postnatal growth retardation is a clinical feature in all cases2. To date, however, the molecular etiology underlying stunted growth and short stature in KS patients remains ambiguous. The postnatal growth is controlled by the hypothalamus–pituitary gland–liver axis3. First, growth hormone-releasing hormone (GHRH)-neurons in the hypothalamus release GHRH, which then stimulates secretion of the growth hormone (GH) from the pituitary gland. GH, in turn, induces the expression of insulin-like growth factor 1 (IGF1) in the liver, which controls bone epiphyses, muscle and adipose tissue development, growth plates development, and glucose homeostasis4. It is noteworthy that all 18 genetically confirmed prepubertal KS children successfully underwent catch-up growth after receiving a year-long treatment with recombinant human growth hormone (GH)5. These results suggest that the deficits of human KS patients in GH signaling should involve the production of GHRH (in the hypothalamus) and/or GH (in the pituitary) but not the events downstream of the pituitary in GH signaling pathway.
GHRH neurons are located in the hypothalamic arcuate nucleus (ARC, aka ARH), which centrally regulates diverse homeostatic processes critical for survival and reproduction3,6–8. The single-cell RNA-seq (scRNA-seq) analyses of the adult mouse ARC uncovered that the ARC contains as many as 24 distinct neuronal type9. Among these ARC neuronal types, GHRH-neurons constitute the hypothalamus–pituitary–liver regulatory axis, which directs linear growth. AgRP-neurons enhance food intake and reduce energy expenditure by releasing the neuropeptides NPY and AgRP, while POMC-neurons perform the opposite actions using the neuropeptides αMSH (cleaved from POMC) and CART6. Kiss1-neurons control reproduction via the Kiss1-encoded neuropeptide Kisspeptin, which triggers the secretion of gonadotrophin-releasing hormone (GnRH) from the hypothalamic GnRH-neurons8. To understand the developmental process for the ARC, we recently established gene expression profiles for each ARC neuronal type undergoing the cell fate specification and differentiation in the mouse embryonic hypothalamus and identified 83 genes that are most specifically enriched in developing GHRH-neurons relative to other developing ARC neurons10.
The genetic cause of KS has been relatively well understood. A majority of KS is caused by mutations in the MLL4 gene, but some cases of KS are due to mutations in the UTX gene1. The human genetics of KS corroborate the finding that MLL4 and UTX together form a histone-modifying enzyme complex named MLL4-complex, which controls gene expression, often in cell type-specific manners11,12. These data also strongly support the notion that the defect in MLL4/UTX-directed epigenetic gene regulation is a main contributing factor to human KS phenotypes. MLL4 is a histone H3-lysine 4 (H3K4)-methyltransferase. The methylation patterns in the histone H3K4 residue form versatile epigenetic marks that are intimately linked to the induction of associated genes. The promoters are decorated by H3K4 tri‐methylation (H3K4me3), while the enhancers are marked by H3K4 mono- and di‐methylations (H3K4me1 and H3K4me2)13. In lower eukaryotes, a singular H3K4‐methyltransferase Set1 performs H3K4 methylations through a large steady‐state Set1‐complex13. The H3K4‐methyltransferase action is divided among multiple complexes in higher eukaryotes. Drosophila has dSet1-, Trx-, and Trr-complexes that generate H3K4-methylation marks14. Mammals have six similar complexes, collectively named Set1-like complexes, and each complex contains one of the six H3K4‐methyltransferase, Set1α or its paralog Set1β, MLL1 or its paralog MLL2 (aka WBP7), or MLL3 (aka KMT2C) or its paralog MLL4 (aka KMT2D)11,12. We have identified the first two mammalian Set1‐like complexes, MLL3- and MLL4-complexes15,16. Interestingly, among the six mammalian Set1-like complexes, only the MLL3- and MLL4-complexes contain the H3K27‐demethylase UTX11, which removes the transcriptionally repressive chromatin mark H3K27 methylation13. Thus, MLL3/4-complexes have two distinct enzymatic activities that lead to the open chromatin formation for transactivation of the target genes. Consistently, the MLL3/4-complexes function as transcriptional coactivators that trigger target gene expression, and the subunits in the MLL3/4-complexes serve as adaptors that recruit MLL3/4 and UTX to enhancers of their target genes by partnering with transcription factors bound to those enhancers11,12.
With regards to MLL3/4-directed epigenetic gene regulation, the interplay between the two histone marks H3K4-methyation and H3K27-modification is noteworthy. MLL3/4 have been shown to be the main enzymes that generate the enhancer marks H3K4me1 and H3K4me2 (refs. 17–21). H3K4me1/2 marks concomitantly occur with another active enhancer mark H3K27ac, often preceding H3K27ac22,23. Several studies provide the molecular basis for the co-occurrences of H3K4me1/2 and H3K27ac marks23–26 and suggest that MLL3/4-directed H3K4me1/2 are intimately linked to H3K27ac.
In this paper, we report that Mll4 governs the development of GHRH-neurons in the mouse hypothalamus via its epigenetic regulatory activity. Further, we found that Mll4 triggers the expression of GHRH-neuronal genes during development, primarily by partnering with the transcription factor Nrf1 (for nuclear respiratory factor 1, aka α-Pal). Together, our study revealed that the deficiency of Mll4-directed epigenetic control of GHRH-neuronal genes results in a severe reduction of GHRH neurons and dwarfism in the mouse, providing crucial insights into the molecular etiology underlying dwarfism in human KS patients.
Results
Mll4+/− mice exhibit dwarfism and the reduction of GHRH neurons
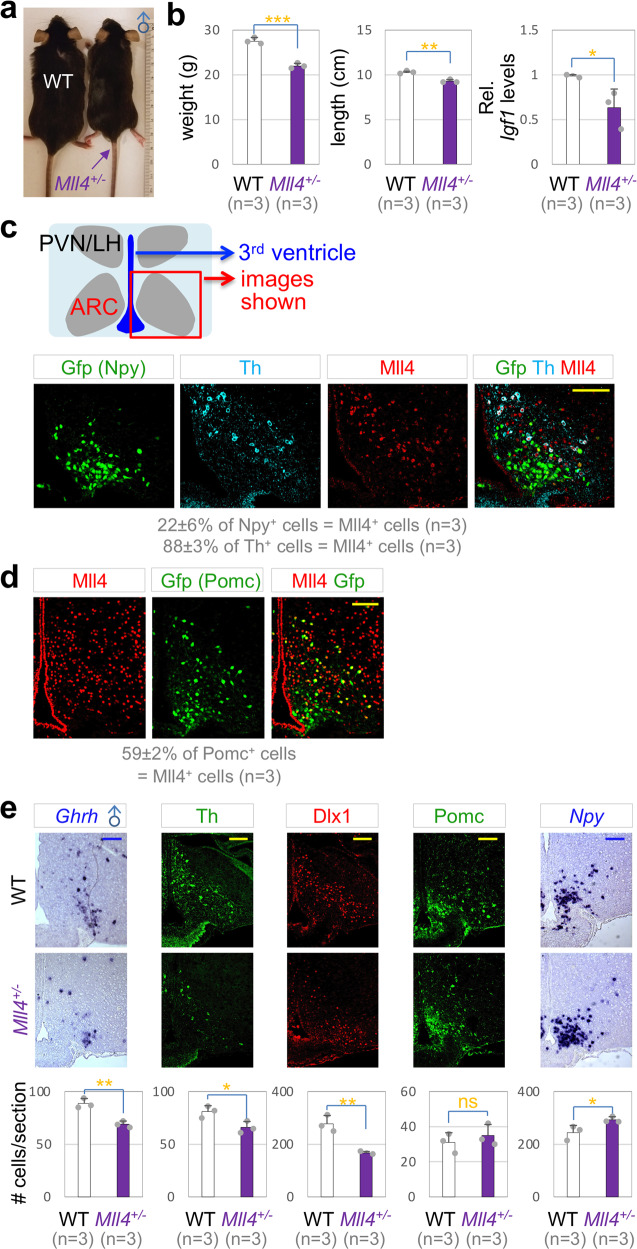
To test if Mll4 haploinsufficiency leads to dwarfism in mice as it does in human, we monitored the growth of Mll4+/− mice, in which a single Mll4 allele is inactivated27. Mll4+/− mice showed a significant reduction in body weight, linear length, and mRNA levels for Igf1 in the liver (Fig. 1a, b, Supplementary Fig. 1a), indicating that Mll4+/− mice have a deficit in GH signaling and faithfully recapitulate one of the cardinal features of KS, postnatal growth deficiency1.
Fig. 1. Dwarfism of Mll4+/− mice.
a Mll4+/− male mice are smaller than their littermate WT mice at P80. b A significant reduction in body weight as well as linear length and liver Igf1 levels in male Mll4+/− mice relative to their littermate WT mice at P80. c, e Coexpression of Mll4 (IHC) with either Gfp driven by Npy-Gfp or Th (IHC) at P33 (c), and with Gfp driven by Pomc-Gfp at P33 (d). e Expression of Ghrh, Th, Dlx1, Pomc, and Npy in the ARC of male WT mice and their littermate Mll4+/− mice at P80, assessed by ISH (Ghrh, Npy) and IHC (Th, Dlx1, POMC). Quantifications were done by counting the number of labeled cells in three rostral to caudal sections for each mouse, and the number of mice used is as indicated in parenthesis (c–e). The location of ISH/IHC images is schematically shown (c), which applies to all images in (c–e). Scale bars, 100 µm. Statistical differences were determined by two-sided Student’s t-test (b, e); *p < 0.05, **p < 0.01, ***p < 0.001, and not significant (ns). Column bars represent mean, error bars indicate the SD (b, e).
To test the role of Mll4 in hypothalamic GHRH neurons, we monitored the expression pattern of Mll4 in the ARC. GHRH neurons are one of tyrosine hydroxylase (Th)+ neuronal types in the ARC28. Interestingly, Mll4 was expressed in ~88% of Th+ neurons that include GHRH neurons, but only in ~22% of Npy+ AgRP-neurons at postnatal day 33 (P33) in Npy-Gfp reporter mice that express Gfp in AgRP-neurons29 (Fig. 1c). In contrast, Mll4 was expressed in ~59% of Pomc+ cells in P65 Pomc-eGfp reporter mice30 (Fig. 1d). These results indicate that most GHRH-neurons express Mll4, whereas only a subset of AgRP- and POMC-neurons express Mll4.
Next, we tested if the number of GHRH-, AgRP-, and POMC-neurons changes in Mll4+/− mice using a panel of markers. GHRH-neurons significantly reduced, as determined by GHRH-neuronal markers, Ghrh, Th, and Dlx1, in Mll4+/− mice relative to littermate WT control mice (Fig. 1e). In contrast, the number of POMC-neurons did not change and AgRP-neurons mildly increased in Mll4+/− mice (Fig. 1e). Our data indicate that the generation or survival of GHRH-neurons is impaired in Mll4+/− mice, and also suggest that the deficiency of GHRH-neurons is likely a strong contributing factor to the dwarfism in Mll4 haploinsufficiency.
The inactivation of Mll4 in the developing hypothalamus leads to a drastic reduction of GHRH neurons and dwarfism
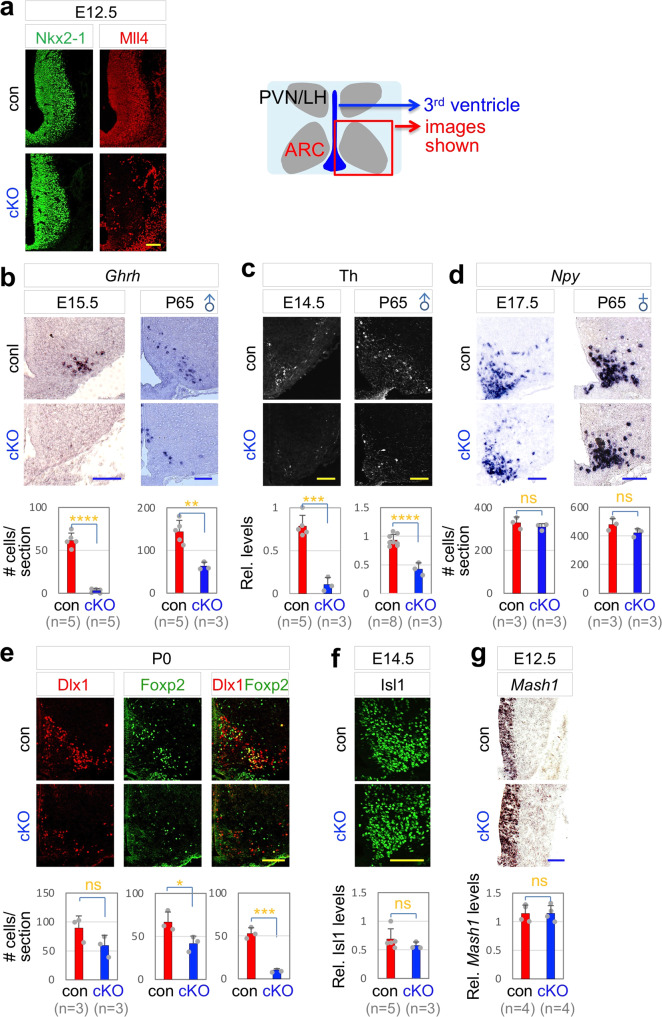
To test if Mll4 plays a cell-autonomous role in GHRH-neuronal development, we generated Mll4 conditional knockout (Mll4-cKO) mice, in which Mll4 was deleted in the embryonic ARC, by crossing Mll4f/f and Nkx2-1-Cre mice21,31. In Mll4-cKO mice, Mll4 expression was largely eliminated in the developing ARC, but not in surrounding tissues, by embryonic day (E) 12.5 (Fig. 2a). At E14.5~E15.5, GHRH-neurons expressing Ghrh and Th were markedly reduced in Mll4-cKO mice compared to littermate control mice (Fig. 2b, c), indicating that the development of GHRH-neurons was impaired in the absence of Mll4. Mll4-cKO mice also showed a striking reduction of GHRH-neurons at P65 (Fig. 2b, c), indicating that the developmental defects of GHRH-neurons in Mll4-cKO embryos were not recovered at adult stage. In contrast to GHRH-neurons, the number of AgRP-neurons did not significantly change in Mll4-cKO mice (Fig. 2d). We also tested the marker for other hypothalamic neurons; Trh, a marker for the dorsomedial hypothalamus that does not express Nkx2-132 and Sf1, a marker for a subregion of the ventromedial hypothalamus that expresses Nkx2-132,33. Neither Sf1+ nor Trh+ cells were significantly altered in their numbers in Mll4-cKO mice (Supplementary Fig. 2), highlighting a relatively selective loss of GHRH-neurons in the Mll4-deficient hypothalamus.
Fig. 2. Ghrh expression impaired in Mll4-cKO mice.
a E12.5 Mll4-cKO embryos show a drastic reduction in Mll4 expression in the developing hypothalamus. Similar results were obtained from at least three independent experiments. b–d Expression of Ghrh (b) and Th (c), but not Npy (d), is significantly reduced in Mll4-cKO at both embryonic and adult stages. e Cells expressing Dlx1 or Foxp2 only as well as Dlx1/Foxp2 double-positive cells are reduced in P0 Mll4-cKO pups relative to their littermate P0 control pups. f, g Expression of Isl1 (f) and Mash1 (g) was not significantly different between control embryos and their littermate Mll4-cKO embryos. The location of ISH/IHC images is schematically shown (a), which applies to all images in (a–g). Scale bars, 100 µm. Quantifications were done by counting the number of labeled cells or the relative intensity of signals in three rostral to caudal sections for each mouse. The number of mice used is as indicated below each genotype in parenthesis (b–g). Statistical differences were determined by two-sided Student’s t-test (b–g); *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and not significant (ns). Column bars represent mean, error bars indicate the SD (b–g).
To determine if the mere expression of Ghrh gene alone or the developmental program for GHRH-neurons was disrupted in Mll4-cKO mice, we examined the expression pattern of transcription factors that are crucial to drive GHRH-neuronal development. We have previously reported that Dlx1 and its paralog Dlx2 are required for the development of mouse Th+ neurons, which include GHRH-neurons34 and that Foxp2 is essential for the generation of mouse GHRH-neurons10. Mash1, expressed in neural progenitors in the ARC, and Isl1, expressed in ARC neurons, are involved in the development of multiple ARC neuronal types35–37. Interestingly, in Mll4-cKO mice, the number of cells co-expressing Dlx1 and Foxp2 was drastically reduced (Fig. 2e), but the levels of Isl1 and Mash1 did not significantly change (Fig. 2f, g). These results strongly suggest that the development of GHRH-neurons, rather than just the expression of Ghrh, is impaired in Mll4-cKO mice.
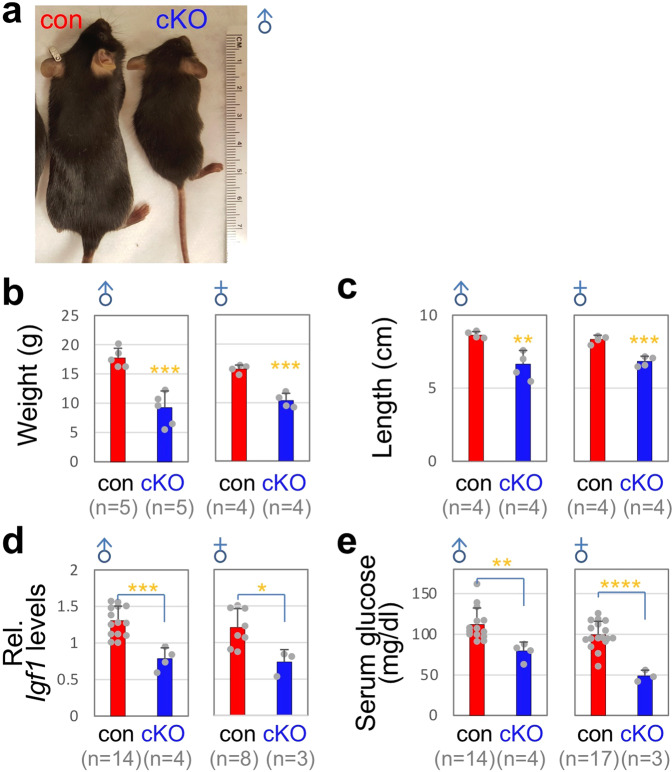
Next, we examined if Mll4-cKO mice exhibit any growth phenotypes, reflecting the developmental deficiency of GHRH-neurons. Mll4-cKO mice showed a marked reduction of both body weight and linear length (Fig. 3a–c). Furthermore, serum glucose levels and hepatic Igf1 mRNA levels drastically decreased in Mll4-cKO mice (Fig. 3d, e), indicating deficits in the signaling of GH, downstream of GHRH. Milder but similar results were also obtained with Mll4-cHET (for conditional heterozygous knockout) mice (Supplementary Fig. 1b).
Fig. 3. Dwarfism of Mll4-cKO mice.
a Mll4-cKO mice are smaller than their littermate control mice (a representative image for males). b–e A significant reduction in body weight (b), linear length (c), liver Igf1 mRNA levels (d), and serum glucose levels (e) in male and female Mll4-cKO mice relative to their littermate control mice at P40. The number of mice used is as indicated below each genotype in parenthesis (b–e). Statistical differences were determined by two-sided Student’s t-test; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Column bars represent mean, error bars indicate the SD (b–e).
Together, our results establish that Mll4 is required for the proper generation of GHRH-neurons in the developing hypothalamus. Our data also clearly link the stunted growth of Mll4 mutant mice to the impaired development of GHRH-neurons.
Nrf1 is a major partner transcription factor of Mll4 in developing hypothalamus
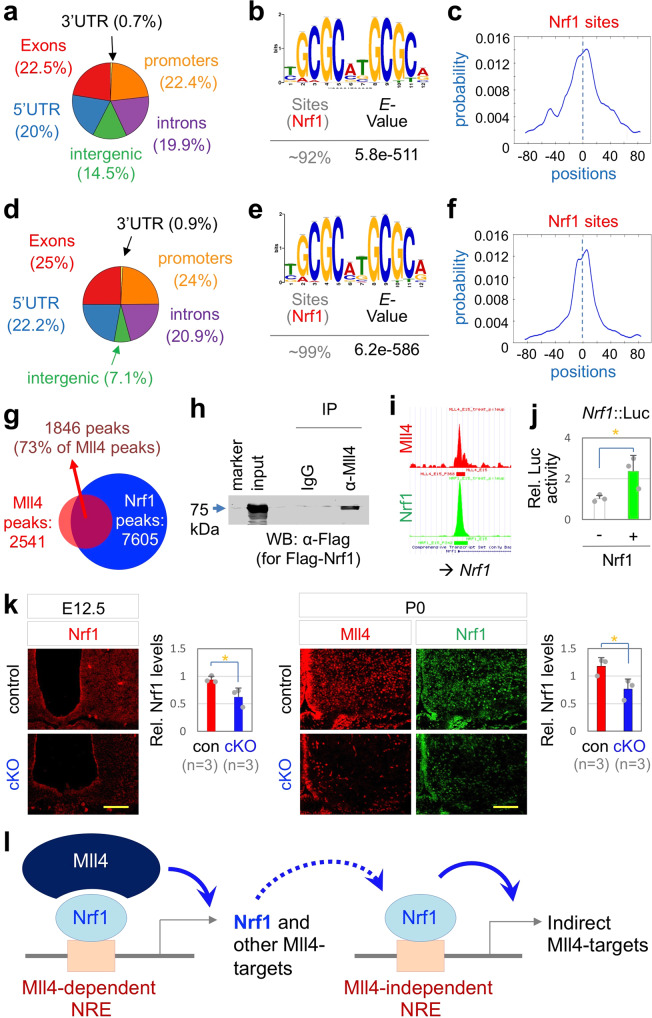
To determine the molecular mechanism by which Mll4 directs the development of GHRH-neurons, we defined genome-wide Mll4-binding loci in the developing hypothalamus by performing ChIP-seq in the mouse hypothalamus at E15, a time point when GHRH-neurons are being actively specified, with the ChIP-seq quality Mll4 antibody that we developed27. Our ChIP-seq analyses identified 2541 Mll4-bound ChIP-seq peaks (p < 0.001, FDR < 10%). Interestingly, ~85.5% of Mll4 ChIP-seq peaks were located in the promoters and gene bodies and only ~14.5% of Mll4-bound peaks were found in the intragenic regions in the developing hypothalamus (Fig. 4a). This Mll4-occupany pattern differs from the previous finding that Mll4 is mainly enriched in the intergenic enhancer regions in the adipocytes21.
Fig. 4. Nrf1 is a major partner transcription factor of Mll4 in developing hypothalamus.
a–f Location of ChIP-seq peaks in E15 hypothalamus shown for Mll4 ChIP-seq (a) and Nrf1 Chip-seq (d), Nrf1-binding motif found in de novo motif analysis of the top 300 Mll4 peaks (b) and Nrf1 peaks (e), and the enrichment of the Nrf1-binding motif in the center of the peaks for Mll4 (c) and Nrf1 (f). g 73% of Mll4 ChIP-seq peaks overlap with Nrf1 ChIP-seq peaks, suggesting recruitment of Mll4 to a majority of its targets via Nrf1. h CoIP of Mll4 and Flag-Nrf1 in HEK293 cells. Similar results were obtained from at least three independent experiments. i An identical locus of the promoter region of Nrf1 gene is associated with Mll4 and Nrf1 ChIP-seq peaks. j A luciferase reporter construct directed by the genomic region containing the Nrf1-associated Nrf1/Mll4-binding locus (i) is activated by ectopic expression of Nrf1 in HEK293 cells. Three independent experiments (each experiment done in duplicate) were analyzed together. k Nrf1 expression is reduced in Mll4-cKO at both E12.5 and P0 relative to their littermate controls (n = 3, each genotype). Scale bars, 100 µm. Statistical differences were determined by two-sided Student’s t-test (j, k); *p < 0.05. Column bars represent mean, error bars indicate the SEM (j) and SD (k). l A model for direct and indirect target genes of Mll4 in the developing hypothalamus. The Nrf1 gene is a direct target of Mll4:Nrf1, and therefore genes regulated by Nrf1 alone can be classified as indirect target genes of Mll4.
To identify partner transcription factors that mediate the recruitment of Mll4 to its target genomic loci in the developing hypothalamus, we performed de novo motif analyses on the top 300 Mll4 ChIP-seq peaks using the algorithms MEME, DREME, and TOMTOM38–40. Intriguingly, a single motif, which resembles the consensus binding site for the transcription factor Nrf1 (ref. 41), was found in ~92% of the top 300 Mll4 ChIP-seq peaks (Fig. 4b). The Nrf1 motif was enriched in the summit of Mll4 ChIP-seq peaks (Fig. 4c). This genome-wide analyses indicate that Nrf1 is primarily responsible for recruiting Mll4 to most Mll4-target loci in E15 developing hypothalamus.
To further test the role of Nrf1 in Mll4-directed gene regulation in the developing hypothalamus, we performed ChIP-seq in E15 hypothalamus using ChIP-seq quality Nrf1 antibody that we generated, and identified 7605 Nrf1-bound genomic regions in the developing hypothalamus (p < 0.001, FDR < 10%). Similar to Mll4, Nrf1 primarily occupied the promoters and gene bodies (Fig. 4d). De novo motif analyses uncovered that ~99% of Nrf1-bound genomic regions contain the Nrf1 motif (Fig. 4e). Validating that the Nrf1 motif is the binding site for Nrf1, the motif was enriched in the summit of Nrf1 ChIP-seq peaks (Fig. 4f). The direct comparison of genome-wide binding patterns of Mll4 and Nrf1 revealed that 73% of Mll4 ChIP-seq peaks are also the binding sites for Nrf1 (Fig. 4g). These data suggest that Nrf1 mediates the recruitment of Mll4 to a majority of Mll4-target genes in the developing hypothalamus (Fig. 4g). Further supporting this notion, Mll4 associated with Nrf1 in HEK293 cells, as determined by co-immunoprecipitation (coIP) assays (Fig. 4h).
Notably, Mll4 and Nrf1 co-occupied the promoter of the Nrf1 gene itself (Fig. 4i), raising the possibility that Nrf1 auto-regulates its own transcription by recruiting Mll4 as a transcriptional coactivator. Indeed, the ectopic expression of Nrf1 enhanced the transcriptional activity of the Nrf1/Mll4-binding region in the Nrf1 gene in HEK293 cells, as monitored using luciferase reporter assays (Fig. 4j). The expression of Nrf1 was severely reduced in the ARC region of Mll4-cKO mice relative to their littermate controls at E12.5 and P0 (Fig. 4k), supporting the notion that Mll4 induces the expression of Nrf1 by binding the Nrf1 gene in the developing hypothalamus (Fig. 4i).
Together, these results suggest that, in the developing hypothalamus, Mll4 employs Nrf1 as the major partner transcription factor to bind and induce its ‘direct’ target genes. Given that Mll4 activates the expression of Nrf1, Mll4 is also expected to positively regulate the genes bound by Nrf1 alone without Mll4, which represent ‘indirect’ target genes of Mll4 (Fig. 4l).
Mll4 positively regulates GHRH-neuronal genes in the developing hypothalamus
To identify Mll4/Nrf1-target genes that are important in GHRH-neuronal development, we integrated Mll4 and Nrf1 ChIP-seq datasets with our scRNA-seq analyses of E15 ARC neurons, which revealed 83 genes that are most specifically enriched in developing GHRH-neurons relative to other ARC neuronal types10. Among the 83 genes, 7 genes recruit both Mll4 and Nrf1 to the same loci (Fig. 5a, b, Supplementary Fig. 3), suggesting that Nrf1 mobilizes Mll4 to these 7 genes thereby activating their expression (Fig. 4l). These Nrf1-Mll4-co-occupancy genes include two transcription factor genes Prox1 and Pbx3 and five non-transcription factor genes, Vat1, Flywch2, Plekhg1, Tmem200a, and Them6 (Fig. 5a, b, Supplementary Fig. 3). Among the 83 genes, 24 genes including the transcription factor gene Egr1, are associated with the genomic regions that recruit only Nrf1, not Mll4, thus representing potential ‘indirect’ Mll4-target genes (Fig. 4l). Interestingly, Mll4 binds to the promoter/5′UTR of the Dlx1 gene, but Nrf1 does not (Fig. 5a, b), suggesting that Mll4 induces Dlx1 expression in the developing hypothalamus by collaborating with other transcription factor. Overall, our systematic bioinformatic analyses revealed several ‘direct’ and ‘indirect’ target genes of Mll4 in the developing GHRH-neurons. Notably, these Mll4-target genes include Dlx1, Prox1, Egr1, and Pbx3, which have been shown or proposed to play a role in the development of GHRH-neurons10,34.
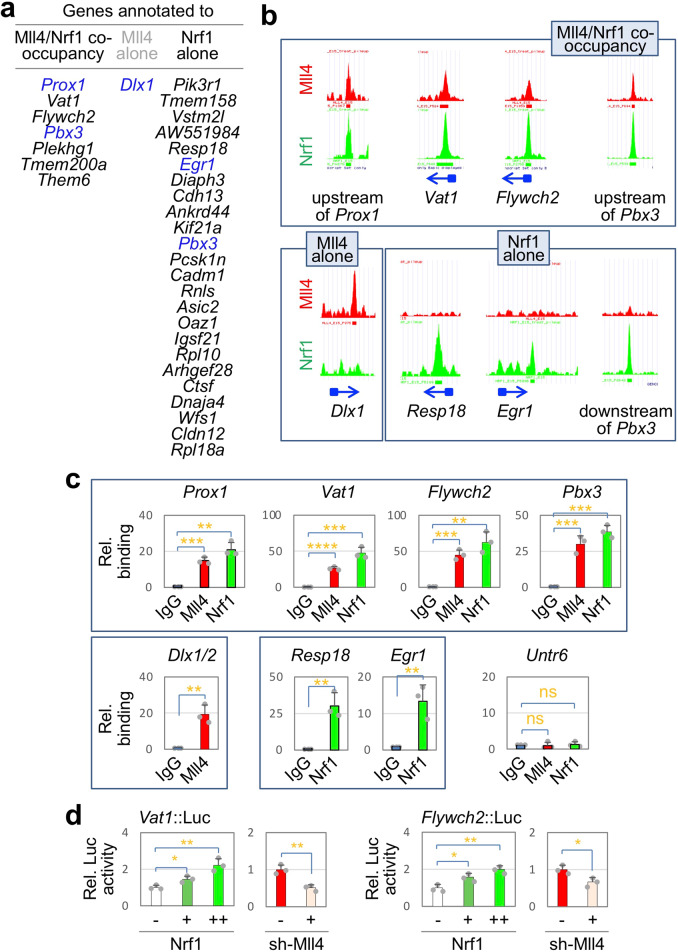
Fig. 5. Target genes of Mll4 in developing mouse GHRH neurons.
a List of genes in developing mouse GHRH-neurons, which are associated with either Mll4 or Nrf1 ChIP-seq peaks or both Mll4 and Nrf1 ChIP-seq peaks. Genes encoding transcription factors are highlighted in blue. b Mll4 and Nrf1 ChIP-seq peaks for representative target genes of Mll4 in developing GHRH-neurons. The location of each peak with regard to its associated gene is marked by an arrow for the direction of the gene and a square for the 5′ UTR (both in blue). c Independent validation ChIP experiments with E15 hypothalamus and IgG (controls) and antibodies against Mll4 and Nrf1 reveal recruitment of Mll4 and Nrf1 to representative target genes of Mll4. Column bars represent mean of experiments with three independent embryos, error bars indicate the SD. d Luciferase reporter assays reveal that the common ChIP-seq peak areas for Mll4 and Nrf1 in Vat1 and Flywch2 are responsive to ectopic expression of Nrf1 and shRNA against Mll4. Column bars represent mean of three independent experiments, error bars indicate the SEM. Statistical differences were determined by two-sided Student’s t-test; not significant (ns), *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
To validate the ChIP-seq data, we performed independent ChIP assays in E15 hypothalamus using antibodies against Mll4 and Nrf1. Consistent with ChIP-seq data, both Mll4 and Nrf1 were recruited to the common ChIP-seq peak regions for Mll4 and Nrf1 regions annotated to Prox1, Vat1, Flywch2, and Pbx3, but not to the negative control genomic region Untr6 (for Untranslated region on chromosome 6)42 (Fig. 5c). Also, Nrf1 bound to ChIP-seq peak regions in Resp18 and Egr1, and Mll4 bound to Dlx1 (Fig. 5c).
To test if Nrf1/Mll4-bound genomic genes act as enhancers responding to Nrf1 and Mll4, we generated the luciferase reporters linked to Nrf1-Mll4-co-occupancy regions annotated to Vat1 and Flywch2, named Vat1::Luc and Flywch2::Luc. Both luciferase reporters were activated by Nrf1 in a dose-dependent manner and suppressed by Mll4 knockdown with shRNA against Mll4 in HEK293 cells (Fig. 5d), suggesting that Nrf1 and Mll4 enhance the transcription activity of their binding regions in cells.
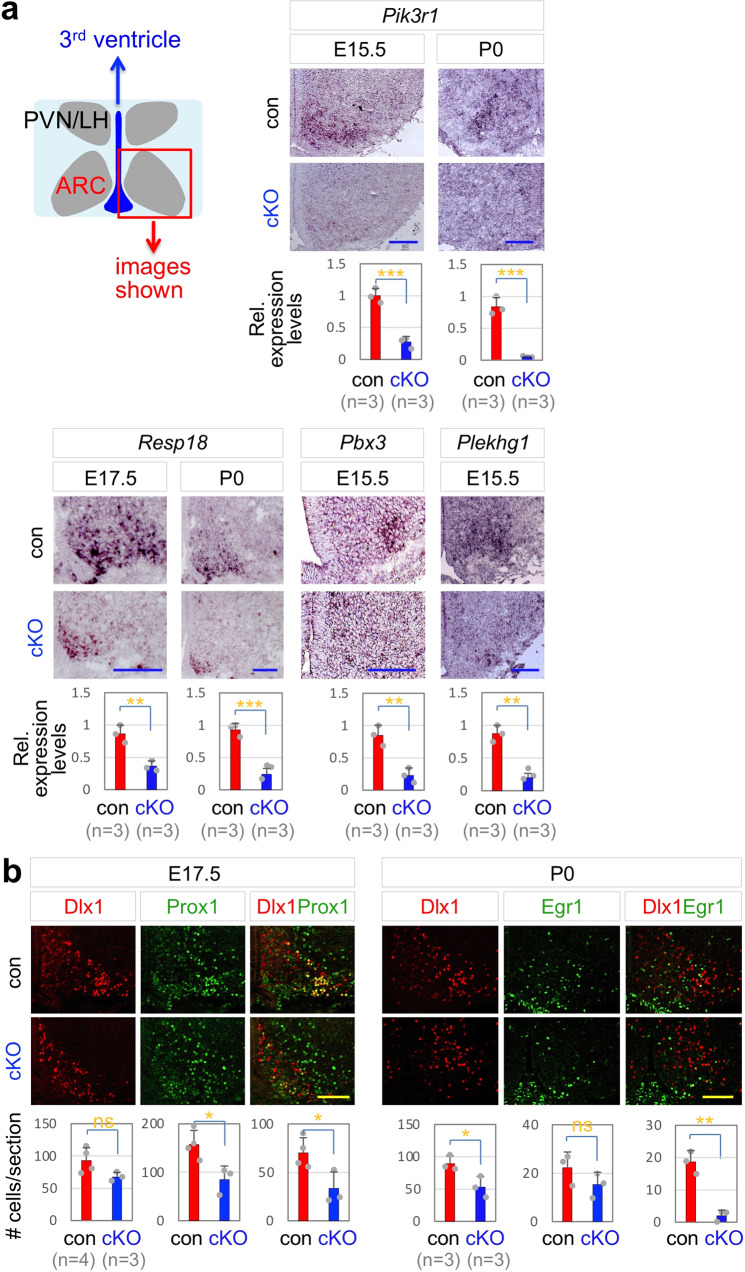
We predicted that the expression of the direct and indirect Mll4-target genes in mouse developing GHRH-neurons would be downregulated in the absence of Mll4. To test this idea, we examined the expression levels of potential Mll4-target genes in the ARC of Mll4-cKO mice using in situ hybridization (ISH) and immunohistochemistry (IHC) analyses. Mll4-cKO mice showed significant downregulation of all tested target genes, such as Pbx3 and Plekhg1, which are annotated to Nrf1-Mll4-co-occupancy regions (i.e., direct Mll4-target genes), and Pik3r1 and Resp18, which are associated with Nrf1 peak but not Mll4 peak (i.e., indirect Mll4-target genes) (Fig. 6a). While Dlx1, Prox1, and Egr1 are highly and significantly enriched in developing GHRH-neurons, they are also expressed in other ARC neuronal types, such as the expression of Dlx1 in developing n8/n9 TH- neurons10. Thus, to monitor the expression of Dlx1, Prox1, and Egr1 in developing GHRH-neurons, we performed the double immunofluorescence staining with a combination of antibodies against Dlx1 and Prox1 or Egr1. Dlx1/Prox1- and Dlx1/Egr1 double-positive cells in the ARC were markedly reduced in the ARC of Mll4-cKO mice relative to their littermate controls (Fig. 6b), indicating the downregulation of these transcription factors in GHRH-neuronal lineage and the impaired transcription program directing the development of GHRH-neurons.
Fig. 6. Mll4-cKO show reduced expression of Mll4-target genes in developing GHRH-neurons.
a ISH analyses of expression of Mll4-target genes in Mll4-cKO and their littermate controls at indicated stages. b IHC analyses of coexpression of transcription factors whose genes are targeted by Mll4; the number of Dlx1 only, Prox1 only, and Dlx1/Prox1 double-positive cells at E17.5, and the number of Dlx1 only, Egr1 only, and Dlx1/Egr1 double-positive cells at P0 in Mll4-cKO and controls. The number of mice used is as indicated below each genotype in parenthesis (a, b). The location of ISH/IHC images is schematically shown (a), which applies to all images in (a, b). Statistical differences were determined by two-sided Student’s t-test; *p < 0.05, **p < 0.01, and ***p < 0.001. Column bars represent mean, error bars indicate the SD. Scale bars, 100 µm.
Together, these studies strongly suggest that Mll4 governs GHRH-neuronal development by inducing transcription program crucial for GHRH-neuronal differentiation and also by upregulating non-transcription factor genes that are relatively specifically enriched in developing GHRH-neurons.
Mll4 directs GHRH-neuronal development via its epigenetic regulatory activity
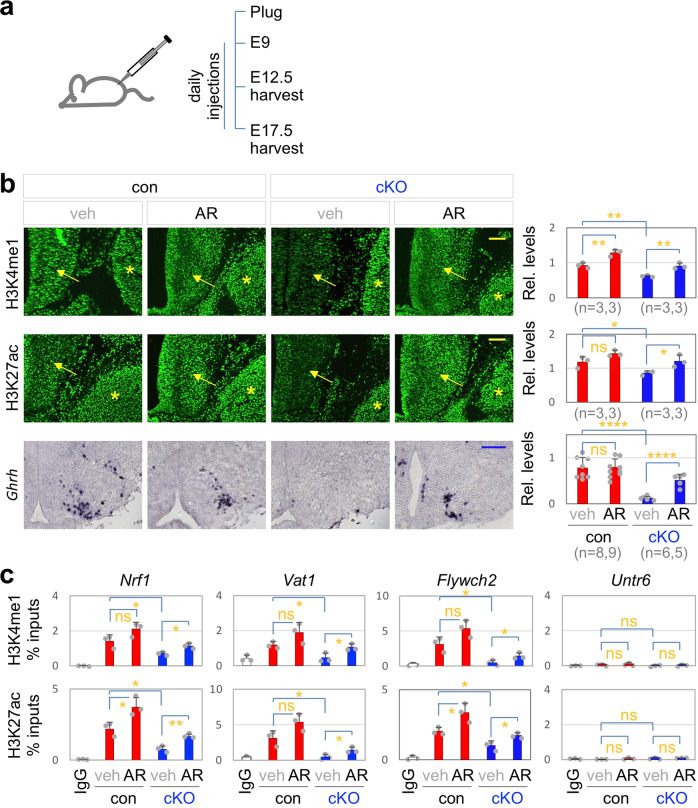
Next, to ask if Mll4 triggers the transcriptional activation of its target genes in the GHRH-neuronal lineage via its epigenetic regulatory activity that induces transcriptionally active chromatin landscape, we sought for the method to trigger transcriptionally active chromatin, marked by H3K27ac, in Mll4-deficient mice. Given that the HDAC inhibitor AR-42 restored the active chromatin in the hippocampus and also rescued the hippocampal memory defects in Mll4+/− mice43, we employed AR-42. We performed daily intraperitoneal injection of 50 mg/kg of AR-42 into pregnant dams from 9 days after a vaginal plug detection until harvesting the embryos (Fig. 7a). We first monitored the active chromatin marks at E12.5. H3K4me1 and H3K4me2 levels substantially reduced in the developing ARC, but not in the surrounding tissues, in Mll4-cKO embryos, whereas H3K4me3 levels did not show any change (Fig. 7b, Supplementary Fig. 4a). These data suggest that Mll4 is the main enzyme generating H3K4me1 and H3K4me2 marks in the developing ARC. Interestingly, H3K27ac levels also significantly deceased in Mll4-deficient ARC (Fig. 7b), consistent with the notion that enhancer marks H3K4me1/2 and H3K27ac occur concomitantly22,23. AR-42 treatment restored both H3K4me1/2 and H3K27ac levels in E12.5 Mll4-cKO embryos (Fig. 7b). To monitor the epigenetic modification in individual Mll4-target genomic regions in the developing hypothalamus, we performed ChIP with antibodies against H3K4me1/2 and H3K27ac in E15 hypothalamus isolated from Mll4-cKO and littermate control mice following the treatment with AR-42 or vehicle. H3K4me1/2 and H3K27ac levels in Mll4-target loci reduced in Mll4-cKO mice, which was restored by AR-42 treatment (Fig. 7c). Such chromatin mark change was not observed in the negative control genomic region Untr6 (Fig. 7c). These results suggest that AR-42 is capable of rescuing the defective chromatin landscape caused by Mll4 inactivation. Remarkably, AR-42 treatment also significantly increased the number of cells expressing key GHRH-neuronal fate markers Ghrh, Dlx1, and Th in E17.5 Mll4-cKO embryos (Fig. 7b, Supplementary Fig. 4b), indicating that deficiency of GHRH-neuronal developmental program in Mll4-null hypothalamus was at least partially repaired by AR-42 treatment. Collectively, these data suggest that the restored active chromatin landscapes by AR-42 led to the partial rescue of GHRH-neuronal development in Mll4-cKO mice.
Fig. 7. AR-42 restores Ghrh expression in Mll4-cKO mice.
a Schematics for AR-42 experiments. From 9 days after a plug was observed, vehicle or AR-42 was injected to pregnant dams daily, followed by embryo harvests at E12.5 or E17.5. b IHC analyses of relative levels of H3K4me1 and H3K27ac in embryos harvested at E12.5 as well as ISH analyses of Ghrh expression in embryos harvested at E17.5. Quantification of H3K4me1/H3K27ac levels in the developing ARC (arrows) done relative to the signals in the adjacent tissues (the trigeminal ganglion, indicated by asterisks). The number of mice used is as indicated below each genotype in parenthesis. Scale bars, 100 µm. c Embryos harvested at E15 (n = 3, each genotype and condition) were subject to ChIP analyses with IgG (controls) or antibodies against H3K4me1 or H3K27ac, followed by qPCR quantification of H3K4me1/H3K27ac levels on genomic region containing the Mll4/Nrf1 ChIP-seq peaks associated with Nrf1, Vat1, Flywch2, and Untr6 (negative control locus). Statistical differences were determined by two-sided Student’s t-test (b, c); *p < 0.05, **p < 0.01, ****p < 0.0001, and not significant (ns). Column bars represent mean, error bars indicate the SD (b, c).
Discussion
Mutations in MLL4 result in human developmental disorder KS, whose hallmarks include dwarfism1. Providing important insights into the etiology for the dwarfism in KS, our studies uncovered that Mll4 governs the development of mouse GHRH-neurons during hypothalamic development by establishing transcriptionally active chromatin landscapes in collaboration with Nrf1 and other partner transcription factors (Fig. 8). First, the two distinct mouse models, Mll4+/− mice, which mimic the haploinsufficiency for MLL4 in KS, and Mll4-cKO mice, in which Mll4 was inactivated in the developing hypothalamus, showed impaired GHRH-neuronal development, reduced hepatic Igf1 levels, and stunted growth. Second, our comprehensive genome-wide ChIP-seq studies for Mll4 and Nrf1 uncovered that Mll4 partners mainly with Nrf1 to activate the expression of GHRH-neuronal genes in the developing hypothalamus. Notably, Mll4, and Nrf1 collaborate to trigger the expression of the Nrf1 gene itself, suggesting that Mll4 indirectly controls Nrf1 alone-occupied genes via increasing Nrf1 levels (Fig. 8). Our studies also suggest that Mll4 may cooperate with other transcription factors. For instance, Mll4 may be recruited to the promoter/5′UTR region of Dlx1 by an unknown partner transcription factor. Last, the HDAC inhibitor AR-42 restored the active chromatin marks, followed by a partially rescued GHRH-neuronal developmental program, in the ARC of Mll4-cKO mice.
Fig. 8. Model for Mll4 action in developing GHRH-neurons.
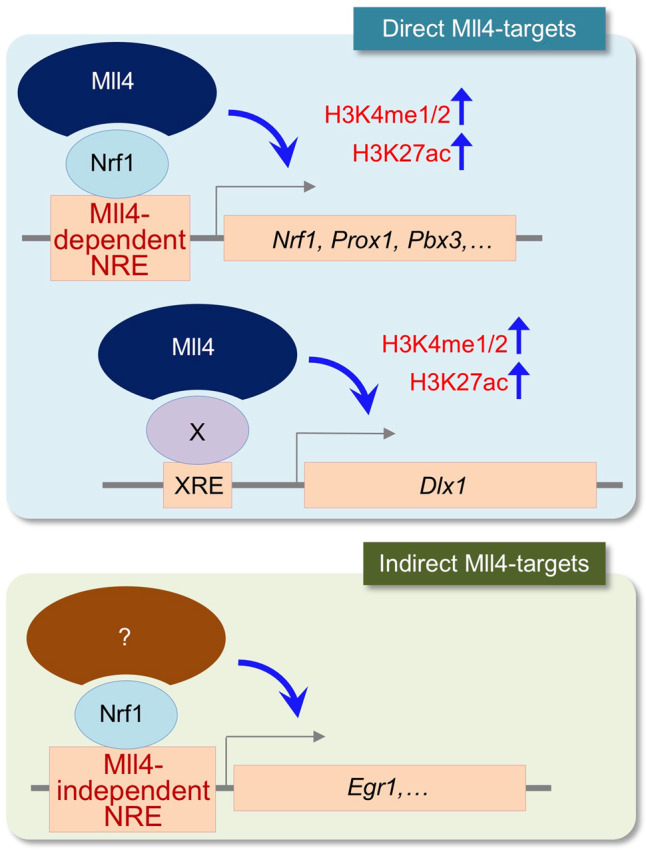
Mll4 partners mainly with Nrf1 epigenetically activating the common target genes of Mll4 and Nrf1 by enhancing the levels of H3K4me1/2 and H3K27ac. These common targets also include the Nrf1 gene itself, resulting in indirect activation of Mll4-independent target genes of Nrf1 by Mll4 (i.e., indirect target genes of Mll4). Mll4 also partners with unknown transcription factors, for instance to activate Dlx1.
Mll4-cKO mice showed not only a reduced number of Ghrh-expressing cells but also a downregulation of many genes highly enriched in developing GHRH neurons10, indicating that Mll4 inactivation led to impaired GHRH-neuronal development, rather than just downregulation of the Ghrh gene. These GHRH-neuronal genes include transcription factor genes Dlx1, Egr1, Pbx3, and Prox1, which are directly bound and controlled by Mll4 according to our ChIP-seq and subsequent analyses. Given that Dlx1 is crucial for GHRH-neuron generation and other transcription factors are candidates for key regulators of GHRH-neuronal development10,34, we propose that Mll4 activates the central gene regulatory network for GHRH-neuronal development, thus serving as a primary epigenetic regulator for GHRH-neuronal production (Fig. 8).
The findings that KS patients respond well to recombinant human GH in catch-up growth5 indicate that KS patients have an intact GH signaling pathway. These studies imply that the hypothalamus–pituitary axis producing GH is defective in KS. While our studies demonstrate an essential role of Mll4 for the generation of GHRH neurons, it is possible that Mll4 plays an additional role in GH production in the pituitary. In this regard, it is noteworthy that Nkx2-1 regulates GH and prolactin transcription in the rat pituitary44. Notably, Nkx2-1 is also expressed in other cell types of the hypothalamus as well as forebrain and lung31,45,46. Therefore, we do not exclude the possibility that the dwarfism observed with our Mll4-cKO mice may also involve deletion of Mll4 in Nkx2-1+ non-GHRH-neuronal populations in the hypothalamus or Nkx2-1+ cell types in the pituitary, forebrain or lung, either directly or indirectly. In particular, further studies are needed to investigate the role of Mll4 in the development and function of the pituitary, particularly regarding the pituitary production of GH.
The hippocampal memory defects of Mll4+/− mice can be rescued by the HDAC inhibitor AR-42 or a ketogenic diet that increases the endogenous HDAC inhibitor β-hydroxybutyrate43,47. Both methods robustly increased H3K27ac levels43,47. Here, we discovered that AR-42 also rescued the expression of Ghrh and other GHRH-neuronal genes impaired in the ARC of Mll4-cKO embryos. Notably, we wished to further test if the restored expression of Ghrh by AR-42 also reverses the postnatal stunted growth of Mll4-cKO mice, but AR-42-treated pups showed perinatal lethality to severe developmental deficits, consistent with the reported embryonic lethality of knockout mouse models for several Hdac genes48. Interestingly, we found that both H3K27ac and H3K4me1 increased upon AR-42 treatment in Mll4-cKO mice, raising the question of which enzyme contributed to the induction of H3K4me1 in the absence of Mll4. The compensatory increase in Mll3, the paralog of Mll4, in the ARC region of Mll4-cKO mice (Supplementary Fig. 4c) may have been responsible for the increased H3K4me1 levels following AR-42 treatment in Mll4-cKO mice, which warrants future studies. Given that MLL3 mutation has not been linked to KS or dwarfism in human, MLL3 is unlikely to function redundantly with MLL4 in the hypothalamus at least under normal condition.
Our results also highlight the intimate link between the two enhancer marks H3K27ac and H3K4me1/2. MLL4 has been shown to play a role to recruit H3K27-acetyltransferases p300 and CBP to its target genes24–26. Combined with these previous findings, our study suggests that MLL4 orchestrates the establishment of transcriptionally active chromatin landscape. It remains to be addressed whether MLL4-directed H3K4me1/2 modification plays an active role in gene induction or simply serves as a co-occurring mark with H3K27ac. In this regard, it will be interesting to test whether the rescue of GHRH-neuronal gene expression requires a restoration of levels of H3K27ac alone or both H3K27ac and H3K4me1/2. To this end, the chemical inhibitors of the H3K4‐methyltransferase activity of Mll4 and the H3K27-acetyltransferase activity of p300 can be explored. Such inhibitors exist for p300 (ref. 49), but not for Mll4 yet. Overall, our results suggest that the missing epigenetic regulatory activity of Mll4 is the main driver for the loss of GHRH-neurons in Mll4-cKO mice, and further provide the proof-of-concept that MLL4 can be a feasible target to develop epigenetic therapeutics for KS.
In summary, by combining mouse genetics, genome-wide studies, and pharmacological approaches, we demonstrated that Mll4 directs the development of mouse GHRH-neurons via its ability to modulate H3K4me1/2 and H3K27ac levels on its target genes during hypothalamus development. We also found Nrf1 as the major partner transcription factor of Mll4 in directing the development of mouse GHRH-neurons. These results strongly suggest that the dysregulation of MLL4-directed epigenetic regulation of GHRH-neuronal genes is likely a molecular etiology underlying the dwarfism in human KS patients.
Methods
Mouse work
All mice were maintained on a normal 12 h light, 12 h dark cycle with ad libitum access to chow and water, unless otherwise noted. Mll4+/−, Mll4f/f, Pomc-Gfp, Npy-Gfp, and Nkx2-1-Cre mice have been described previously21,27,29–31. Mll4f/f mice were crossed with Mll4f/+;Nkx2-1-Cre mice to generate Mll4f/f;Nkx2-1Cre mice (Mll4-cKO mice). Pregnant mice were intraperitoneally injected with AR-42 (50 mg/kg, purchased from Selleck Chemicals) or vehicle (0.5% methylcellulose, 0.1% Tween-80, water) from 9 days after detecting a vaginal plug till harvest at E12.5 or E17 for analyses. All studies were approved by the Institutional Animal Care & Use Committee of University at Buffalo and Oregon Health & Science University.
Cell culture and luciferase assay
HEK293 cells were obtained from ATCC and cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Thermo Scientific), penicillin/streptomycin, and l-glutamine (both Lonza). For luciferase assays, cells were seeded into 48-well plates and transfected with Nrf1-, Vat1-, or Flywch2-luciferase reporters and expression vectors for Nrf1 or Dlx1 or control shRNA or sh-Mll4 (ref. 27) using SuperFect (Qiagen) according to the manufacturer’s instruction. Two days after transfection, luciferase activity was measured. The actin-β-galactosidase plasmid was cotransfected for normalization of the luciferase activities to transfection efficiency. Data were shown in relative luciferase units (mean ± SEM).
CoIP
For coIP, HEK293 cells were transfected with expression vector for Flag-Nrf1 using calcium phosphate transfection method. Transfected HEK293 cells were subsequently dissolved in RIPA buffer and rotated for 2 h at 4 °C. After centrifugation, the lysate was subjected to immunoprecipitation using 1 mg of Mll4 antibody o/n, followed by 2 h incubation with Protein A/G agarose beads (Thermo Fisher Scientific). Beads were than dissolved in SDS loading buffer and separated on SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in Odyssey blocking buffer, and incubated with primary antibodies against Flag-tag and incubated o/n. Next day, the blots were incubated with secondary antibodies, and fluorescence was detected with the Odyssey System (LI-COR), as shown in Fig. 4h (the uncropped original image in Supplementary Fig. 5).
RNA isolation and quantitative RT-PCR
Total RNA was isolated with Trizol, and converted to cDNA with a RevertAid kit (Thermo Scientific). Twenty nanograms of cDNA was used for qPCR with the ViiA7 platform and primers for amplification of Igf1 and Gapdh. Igf1 mRNA expression values were normalized to Gapdh using the ΔΔCt method. Relative or fold expression levels were calculated from three individual experimental replicates.
ChIP and ChIP-seq
ChIP on E15.5 hypothalamus was performed using our homemade Mll4 and Nrf1 antibodies (ref. 27 and Supplementary Fig. 6). Hypothalami were dissected out, crosslinked for 10 min with PFA, and then quenched by 125 mM glycine. After washing in PBS, cells were lysed with lysis buffer, followed by lysis of nuclei with nuclei lysis buffer and sonification with the Pico Biorupter to generate 200–400 bp fragments. Chromatin was then 10× diluted in ChIP dilution buffer and, for immunoclearing, incubated with IgG and protein A agarose beads for 1 h. ChIP antibodies were added for o/n incubation followed by 1 h of incubation with agarose beads. Then, the beads were washed with RIPA buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, 150 mM NaCl), high salt buffer (same components as in TSE, except 500 mM NaCl), Buffer III (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.0), LICI buffer and TE, dissolved into TE supplemented with 10% SDS and proteinse K, incubated for 4 h at 37 °C, and decrosslinked at 65 °C o/n. DNA was subsequently isolated and subject to ChIP-seq50,51.
ISH and IHC
Embryonic and P0 brains were removed and fixed in 4% PFA o/n, cryoprotected with sucrose gradients, and frozen in OCT blocks, followed by sectioning using a microtome with a thickness of 12 µm per section. P33 and P65 mice were intraperitoneally injected with Avertin before performing standard perfusion with PBS and 4% PFA, followed by fixation in 4% PFA o/n. ISH was performed at 68 °C overnight with indicated RNA probes. After hybridization, slices were incubated in washing buffer (50% formamide, 1× SSC solution, and 0.1% Tween20) for 1 h, blocked in MABT buffer + 4% BSA for 1 h, and incubated with an anti-digoxigenin-AP antibody (11093274910 Roche, 1:5000) in MABT buffer + 2% BSA. Next day, the color reaction was performed with NBP/BCIP after washing with MABT buffer. For subsequent co-staining of visualized RNA probes, hybridized sections were incubated with our homemade antibodies against Mll4 (ref. 27, 1:1000) or Dlx134 (1:1500). Next day, Vectastain ABC Elite kit (PK-6101, Vector labs) was used for the color reaction. In addition to our previously described ISH probes against Ghrh, Npy, Trh, Sf1, and Mash1, we also generated new RNA probes for Pik3r1, Resp18, Pbx3, and Plekhg1 by converting hypothalamic RNA of P0 mice to cDNA using the primers listed in Supplementary Table 1. PCR products were then digested with the indicated enzymes and ligated into pBluescript. Digoxigenin-labeled riboprobes were generated using T7 RNA polymerase followed by purification over a column.
IHC was performed by incubating brain sections with homemade antibodies against Mll3, Mll4, Isl1, Dlx1, or Gfp (Supplementary Fig. 7) and commercial antibodies against Foxp2 (Abcam 16046, 1:2000), Prox1 (AngioBio, 1:500), Th (AB152, Millipore, 1:500), H3K4me1 (Abcam, 1:2000), H3K4me2 (Abcam, 1:1500), H3K4me3 (Abcam, 1:1000), H3K27Ac (Abcam, 1:750), Nkx2-1 (Abcam, 1:1000) in blocking buffer o/n at 4 °C. Next day, slices were washed with PBST and incubated with secondary fluorescence antibodies followed by washing and counter staining with DAPI.
Quantification and statistical analyses
For quantification of ISH/IHC images, serial sectioning was performed on embryo/mouse brain with the distance between sections 84–216 μm. One slide from each mouse that contains matched sections was used to compare controls and mutants. Zeiss Axio imager 2 with apotome was used to image ISH and IHC results. Integrated density measurement in Image J software was used to analyze densitometry. For cell counting, specifically immuno-stained cells in the arcuate nucleus were counted. Quantifications were done by analyzing three rostral to caudal sections for each embryo/mouse and at least three embryos/mice per each experimental group. Statistical differences were determined by two-sided Student’s t-test. Statistical significance is displayed as follows: ns for not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Supplementary information
Acknowledgements
We are thankful to Dr. Kai Ge for kindly sharing Mll4f/f mice with us. This work was supported by grants from NIH/NINDS (R01 NS111760 and R01 NS100471 to S.-K.L.) and NIH/NIDDK (R01 DK103661 to S.-K.L. and J.W.L.), by the Soongsil University Research Fund of 2018 (to S.K.), and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2019R1A2C2087068), Medical Research Center (MRC) (NRF-2018R1A5A2024425) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C0447) (S.L.)
Source data
Author contributions
C.H., M.K.C., Y.A.K., S.J., B.S., J.C., and Y.P. designed and performed experiments and analyzed the data. S.J.L., S.M.Y., and S.K. analyzed the ChIP-seq data. S.L, S.-K.L., and J.L. directed the project and, together with C.H., wrote the paper. All authors discussed the results and commented on the manuscript.
Data availability
All data supporting the findings of this study are provided within the paper and its supplementary information. A source data file is provided with this paper. The ChIP-seq dataset have been deposited in GEO (GSE149439). All additional information will be made available upon reasonable request to the authors. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Alexandre Fisette, Lori Zeltser and Paolo Giacobini for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seunghee Lee, Email: leeseung@snu.ac.kr.
Jae W. Lee, Email: jlee269@buffalo.edu
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-20511-7.
References
- 1.Adam, M. P., Hudgins, L. & Hannibal, M. In Kabuki Syndrome in Gene Reviews(R) (eds. Pagon, R. A. et al.) (University of Washington, Seattle, 1993). [PubMed]
- 2.Schott DA, et al. Growth pattern in Kabuki syndrome with a KMT2D mutation. Am. J. Med. Genet. Part A. 2016;170:3172–3179. doi: 10.1002/ajmg.a.37930. [DOI] [PubMed] [Google Scholar]
- 3.Bluet-Pajot MT, et al. Growth hormone secretagogues and hypothalamic networks. Endocrine. 2001;14:1–8. doi: 10.1385/ENDO:14:1:001. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P, Rosenfeld RG. Physiologic and clinical relevance of the insulin-like growth factor binding proteins. Curr. Opin. Pediatr. 1994;6:462–467. doi: 10.1097/00008480-199408000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Schott DA, Gerver WJM, Stumpel C. Growth hormone therapy in children with kabuki syndrome: 1-year treatment results. Horm. Res Paediatr. 2017;88:258–264. doi: 10.1159/000479368. [DOI] [PubMed] [Google Scholar]
- 6.Biebermann, H., Kuhnen, P., Kleinau, G. & Krude, H. The neuroendocrine circuitry controlled by POMC, MSH, and AGRP. Handb. Exp. Pharmacol. 47–75 10.1007/978-3-642-24716-3_3 (2012). [DOI] [PubMed]
- 7.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am. J. Physiol. Endocrinol. Metab. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hrabovszky E. Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology. 2014;99:33–48. doi: 10.1159/000356903. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci.10.1038/nn.4495 (2017). [DOI] [PMC free article] [PubMed]
- 10.Huisman C, et al. Single cell transcriptome analysis of developing arcuate nucleus neurons uncovers their key developmental regulators. Nat. Commun. 2019;10:3696. doi: 10.1038/s41467-019-11667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froimchuk E, Jang Y, Ge K. Histone H3 lysine 4 methyltransferase KMT2D. Gene. 2017;627:337–342. doi: 10.1016/j.gene.2017.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Roeder RG, Lee JW. Roles of histone H3-lysine 4 methyltransferase complexes in NR-mediated gene transcription. Prog. Mol. Biol. Transl. Sci. 2009;87:343–382. doi: 10.1016/S1877-1173(09)87010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heintzman ND, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 14.Mohan M, et al. The COMPASS family of H3K4 methylases in Drosophila. Mol. Cell. Biol. 2011;31:4310–4318. doi: 10.1128/MCB.06092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, et al. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc. Natl. Acad. Sci. USA. 2006;103:15392–15397. doi: 10.1073/pnas.0607313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goo YH, et al. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol. Cell. Biol. 2003;23:140–149. doi: 10.1128/MCB.23.1.140-149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo C, et al. KMT2D maintains neoplastic cell proliferation and global histone H3 lysine 4 monomethylation. Oncotarget. 2013;4:2144–2153. doi: 10.18632/oncotarget.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herz HM, et al. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;26:2604–2620. doi: 10.1101/gad.201327.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu D, et al. The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol. Cell. Biol. 2013;33:4745–4754. doi: 10.1128/MCB.01181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang SY, et al. KMT2D regulates specific programs in heart development via histone H3 lysine 4 di-methylation. Development. 2016;143:810–821. doi: 10.1242/dev.132688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JE, et al. H3K4 mono- and di-methyltransferase MLL4 is required for enhancer activation during cell differentiation. Elife. 2013;2:e01503. doi: 10.7554/eLife.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creyghton MP, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tie F, et al. Trithorax monomethylates histone H3K4 and interacts directly with CBP to promote H3K27 acetylation and antagonize Polycomb silencing. Development. 2014;141:1129–1139. doi: 10.1242/dev.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SP, et al. A UTX-MLL4-p300 transcriptional regulatory network coordinately shapes active enhancer landscapes for eliciting transcription. Mol. Cell. 2017;67:308–321. doi: 10.1016/j.molcel.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, et al. Enhancer priming by H3K4 methyltransferase MLL4 controls cell fate transition. Proc. Natl. Acad. Sci. USA. 2016;113:11871–11876. doi: 10.1073/pnas.1606857113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai B, et al. MLL3/MLL4 are required for CBP/p300 binding on enhancers and super-enhancer formation in brown adipogenesis. Nucleic Acids Res. 2017;45:6388–6403. doi: 10.1093/nar/gkx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, et al. Crucial roles of mixed-lineage leukemia 3 and 4 as epigenetic switches of the hepatic circadian clock controlling bile acid homeostasis in mice. Hepatology. 2015;61:1012–1023. doi: 10.1002/hep.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps CJ, Romero MI, Hurley DL. Growth hormone-releasing hormone-producing and dopaminergic neurones in the mouse arcuate nucleus are independently regulated populations. J. Neuroendocrinol. 2003;15:280–288. doi: 10.1046/j.1365-2826.2003.01009.x. [DOI] [PubMed] [Google Scholar]
- 29.van den Pol AN, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J. Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 31.Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 32.Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor alpha in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145:360–366. doi: 10.1210/en.2003-0996. [DOI] [PubMed] [Google Scholar]
- 33.Dellovade TL, et al. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J. Comp. Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::AID-CNE4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Lee B, et al. Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat. Commun. 2018;9:2026. doi: 10.1038/s41467-018-04377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasif S, et al. Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc. Natl. Acad. Sci. USA. 2015;112:E1861–E1870. doi: 10.1073/pnas.1500672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee B, Lee S, Lee SK, Lee JW. The LIM-homeobox transcription factor Isl1 plays crucial roles in the development of multiple arcuate nucleus neurons. Development. 2016;143:3763–3773. doi: 10.1242/dev.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol. Endocrinol. 2006;20:1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- 38.Bailey TL, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS. Quantifying similarity between motifs. Genome Biol. 2007;8:R24. doi: 10.1186/gb-2007-8-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 42.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 43.Bjornsson HT, et al. Histone deacetylase inhibition rescues structural and functional brain deficits in a mouse model of Kabuki syndrome. Sci. Transl. Med. 2014;6:256ra135. doi: 10.1126/scitranslmed.3009278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee NO, et al. TTF-1 regulates growth hormone and prolactin transcription in the anterior pituitary gland. Biochem. Biophys. Res. Commun. 2007;362:193–199. doi: 10.1016/j.bbrc.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 46.Price M, et al. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992;8:241–255. doi: 10.1016/0896-6273(92)90291-K. [DOI] [PubMed] [Google Scholar]
- 47.Benjamin JS, et al. A ketogenic diet rescues hippocampal memory defects in a mouse model of Kabuki syndrome. Proc. Natl. Acad. Sci. USA. 2017;114:125–130. doi: 10.1073/pnas.1611431114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lasko LM, et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–132. doi: 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 51.Robertson G, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat. Methods. 2007;4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are provided within the paper and its supplementary information. A source data file is provided with this paper. The ChIP-seq dataset have been deposited in GEO (GSE149439). All additional information will be made available upon reasonable request to the authors. Source data are provided with this paper.