Abstract
Severe acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are associated with significant poor outcomes including an increased risk of cardiovascular (CV) events and exercise intolerance. Endothelial dysfunction might contribute to an impaired vascular homeostasis and consequently to CV events and exercise capacity. This study aimed to evaluate the association between exercise capacity and endothelial function in patients with severe AECOPD. Forty-five COPD patients diagnosed with severe AECOPD and admitted to the University Hospital of São Carlos from 2017 to 2019 were enrolled in this observational clinical study. Endothelial Function was assessed by brachial artery ultrasonography (M-Turbo, Sonosite, Bottle, WA, USA) and Flow Mediated Dilatation (FMD) technique in absolute (mm) and percentage values (%). Walking distance (6MWD) obtained by six-minute walk test was considered to characterize the exercise capacity. Pearson’s correlation analysis and linear regression model were applied and a significance level of 5%. There was a significant positive correlation between exercise capacity and endothelial function. Pearson correlation coefficient were 0.36 (p = 0.02) and 0.40 (p = 0.01) between 6MWD and FMD in mm and %, respectively. Linear regression model revealed 6MWD (p = 0.007), accounting for 15% of FMD (%) variance (R2 adjusted). FMD (%) = 2.11 + (0.0081*6MWD). Exercise capacity is associated with endothelial function in patients with severe AECOPD. FMD was found to be increasing with increasing walked distance. Further research is needed to provide evidence of effectiveness of rehabilitation on exercise capacity and endothelial function in these patients and its prognostic value.
Subject terms: Cardiovascular diseases, Respiratory tract diseases, Diseases, Respiratory signs and symptoms
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death worldwide, affecting many people in low-income, middle-income, and wealthy countries1,2. On the course of the disease, many patients experience an acute exacerbation of COPD (AECOPD), characterized as an acute worsening of respiratory symptoms, which may require additional therapy3,4. Acute exacerbation also negatively affects patients’ health status5, exercise capacity6, lung function7 with a detrimental and prolonged impact in outcomes.
Severely distressing events requiring hospitalization impact greatly on physical activity capacity and functional state, which are markers of increased future risk8. Interestingly, a multicenter study involving more than 16,000 patients found that AECOPD also contributes to an increased risk of subsequent cardiovascular (CV) events (myocardial infarction, unstable angina and transient ischemic attack), with a tenfold increased hazard for CV events in those hospitalized with severe AECOPD9.
Some of the literature supports AECOPD as a trigger for CV events10–12. An more activated local and systemic inflammation, increased levels of fibrinogen and interleukin‐6, and hypoxemia resulting in a prothrombotic environment, higher arterial pressures, arterial stiffness and endothelial dysfunction may predispose to CV events during AECOPD11,12. In order to reduce the burden of COPD and given the acceptance of the prognostic significance of CV in AECOPD10, a heightened comprehensive investigation and an integrated approach for better management of this population at high risk is imperative.
As mentioned, dysfunctional endothelium is a well-established hallmark feature of CV disease13,14. Increased reactive oxygen species and inflammation, diminished nitric oxide (NO) production and bioavailability are the main mechanisms underlying the pathophysiology of endothelial dysfunction13. Unhealthy vascular endothelium is related to systemic inflammation and atherosclerosis resulting in higher cardiovascular risk in COPD15. Endothelial dysfunction has been widely recognized in stable and exacerbated COPD patients16–19.
Furthermore, endothelial dysfunction might contribute to dysregulation of muscle proteins balance and inadequate muscle perfusion, which may be related to a pathophysiological mechanism linking reduced exercise capacity, muscle weakness and CV disease in AECOPD20,21. Ideal blood flow to peripheral muscles is also endothelium-dependent and is crucial for normal perfusion of working muscle during activities22. Undeniably, exercise-induced increases in blood flow and shear stress are physiological stimuli for enhanced production and activity of NO, which has a key role in healthy functioning of the endothelium23.
In stable COPD patients, exercise capacity assessed by six-minute walking distance was independently related to endothelial dysfunction24. In this sense, the maintenance of functional capacity may play a role in preserving endothelial function in stable COPD patients24. Although severe acute exacerbation of COPD has a subsequent increase in CV risk and poor functional outcomes24, the relationship between endothelial function and exercise properties has not been investigated.
The current study aimed to evaluate the association between exercise capacity and endothelial function in patients with severe AECOPD. We hypothesized that exercise capacity would be positively associated with endothelial function in AECOPD patients. This study may provide a valuable knowledge for future clinical studies focusing the effect of rehabilitative strategies to enhance exercise capacity on endothelial function, CV risk and outcomes during AECOPD.
Methods
Ethics aspects
All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). The study was approved by the Research Ethics Committee of the Federal University of São Carlos (Brazil, reference number 46431415.0.0000.5504) and University Hospital of São Carlos. All the subjects and/or responsible individual were informed about the study objectives, experimental procedures and insured the confidentiality of data collected. All subjects gave written informed consent before the study’s initiation.
Subjects
Forty-five COPD patients diagnosed with severe AECOPD and admitted to the University Hospital of São Carlos (HU-UFSCar) from 2017 to 2019 were enrolled in this observational clinical study. To confirm COPD diagnosis and stratification, all patients attended the laboratory pulmonary function testing (spirometry), thirty days after hospital discharge (stable condition). COPD diagnosis was confirmed with post-bronchodilator spirometry—FEV1/forced vital capacity ≤ 0.7 and post-bronchodilator FEV1, ≤ 80% predicted in stages I, II, III, or IV)25. AECOPD was clinically defined as an acute worsening of respiratory symptoms that results in additional therapy26 and classified as severe according GOLD as patients requiring hospitalization25.
Assessments during AECOPD occurred during hospitalization at least 24 h and within 48 h after starting standard therapy for AECOPD (beta-2 agonist, anticholinergics, oral corticosteroids, oxygen therapy and antibiotic treatment)25 at the University Hospital of São Carlos and then 30 days following AECOPD at Cardiopulmonary Laboratory of Federal University of São Carlos.
The exclusion criteria were: peripheral vascular disease, neurological conditions that would preclude participation in the required protocol, other concomitant respiratory diseases, mechanical ventilation, hemodynamic instability, unstable angina or myocardial infarction history in the last 6 months and patients over 80 years.
Protocol
During hospitalization: clinical characterization, endothelial function, 6 min Walking Distance (6MWD), modified Medical Research Council (mMRC) dyspnea scale and COPD Assessment Test (CAT) were assessed. Arterial blood gas and serum C-reactive protein (CRP) concentrations were measured at rest with ambient air or with a O2 catheter when the patient already used it.
After 30 days: lung function test (spirometry) was performed according to the American Thoracic Society/European Respiratory Society guidelines27.
Endothelial function
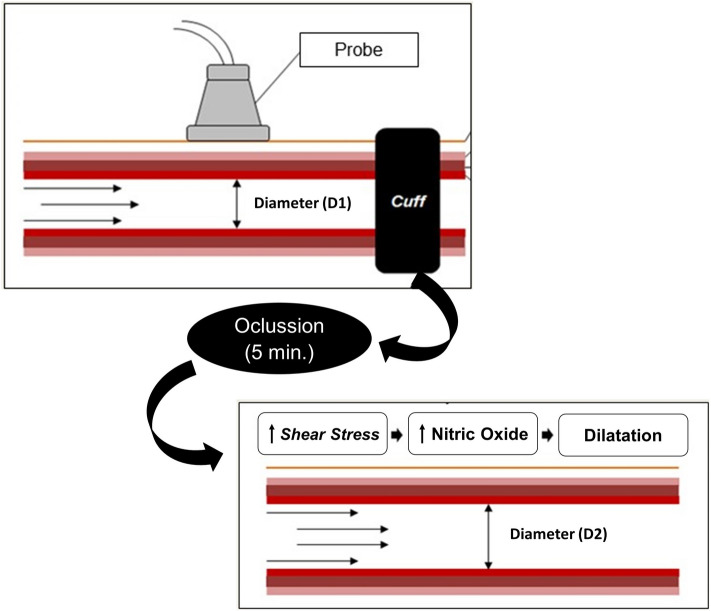
Endothelial function was assessed noninvasively by Flow Mediated Dilatation (FMD) of the brachial artery using ultrasonography (M-Turbo, Sonosite, Bottle, WA, EUA) based on endothelium-dependent vasomotor reactivity and according described previously28,29. Longitudinal images of the right brachial artery were obtained with a high-frequency probe (10 MHz) proximal to the antecubital fossa and the diameter and central flow velocity (pulsed Doppler) were measured. Reactive hyperemia (RH) was induced by the inflation of a cuff positioned around the forearm at 200 mmHg during 5 min. To assess FMD, peak and mean blood flow velocity they were measured within the first 10 s after cuff release, and the diameter was continuously recorded (at a rate of 7.5 images/sec) for 3 min (Fig. 1). Digitally recorded images were later analyzed using the software: Brachial Imager (Medical Imaging, Iowa City, IA, USA). FMD was expressed as the change in percentage: [(baseline diameter − diameter post RH)/ baseline diameter × 100] and absolute values (diameter post RH—baseline diameter)28,29. To perform an estimation of the shear stress (shear rate—SR) the following calculation was performed: SR = 8 × mean blood velocity (cm/s−1)/internal vessel diameter (mm)28. The normalized FMD by shear-rate was calculated using the following formula: FMD (%)/SR (s)30.
Figure 1.
Measurement of FMD. The baseline diameter (D1) between the proximal and distal intima was recorded before RH. The Cuff was inflated at 200 mmHg during 5 min. Immediately after brachial artery ischemia, the diameter post RH (D2) was recorded. The release of cuff should cause increased flow to the region, promoting increased shear stress, greater release of nitric oxide and, consequently, vasodilation. FMD was expressed as the change in percentage: [(baseline diameter − diameter post RH)/baseline diameter × 100] and absolute (diameter post RH − baseline diameter).
Six-minute walk test (6MWT)
The 6MWT was performed following American Thoracic Society/European Respiratory Society guidelines to evaluate exercise capacity31. Subjects were asked to walk as far as possible along a 30-m corridor in 6 min. The following measurements were taken at the beginning, during (second and fourth minute) and at the end of the test: blood peripheral oxygen saturation (SpO2) using an oximeter, heart rate (HR) using a heart rate monitor (Polar, Oulu, Finland), and dyspnea sensation. Blood pressure (BP) was measured at the beginning and at the end of testing with a sphygmomanometer (Welch Allyn1, Skaneateles Falls, New York, USA) and stethoscope (Littmann, Saint Paul, Minnesota, USA). Patients with hypoxemia or who presented with SpO2 < 85% during the test were supplemented with oxygen. Physical therapist walked beside the patient pulling the portable cylinder trolley. The walking distance in meters was used for the analyses. Predicted values were calculated according to reference for Brazilian population: 6MWDpred = 890.46 − (6.11 × age) + (0.0345 × age2) + (48.87 × gender) − (4.87 × BMI)32.
Statistical analysis
The Shapiro–Wilk test was used to verify the normality of data. Clinical and endothelial function data, continuous variables, were expressed as mean (± standard deviation); categorical variables were quantified as numbers and percentages compared to the entire population. Pearson’s correlations coefficients were used to test the association between 6MWD and FMD. The magnitude of correlations was determined considering the following classification scheme for r-values: low (0–0.25), moderate (> 0.25–0.50), strong (> 0.50–0.75), and very strong (> 0.75). Linear regression model was generated to determine the association of the exercise capacity (6MWD), as an independent variable on dependent variable of vascular function (FMD%). All the assumptions (independence of values, linearity of the means, data normality and homoscedasticity in variance values) of the linear regression model were verified. Statistical analysis were performed using SigmaPlot Version 11.0 (SyStat Software, Inc., San Jose, CA) and the accepted level of significance was p ≤ 0.05. Sample size was previously calculated using G*Power 3.1, in order to predict exercise capacity using FMD (%), considering a statistical power of 80%, assuming a medium effect size of 0.2, with an alpha value at 0.05.
Results
One hundred and twenty-six were initially recruited for this study, forty-five (68.2 ± 8.0 years) patients completed the study and were included for the analysis (Fig. 2). The clinical data and physiological parameters, lung function, main comorbidities and medications of the patients are shown in Table 1. A gender balance, GOLD classification of airway limitation severity staged II-IV and reduced exercise capacity (6MWD, predicted %) were observed.
Figure 2.
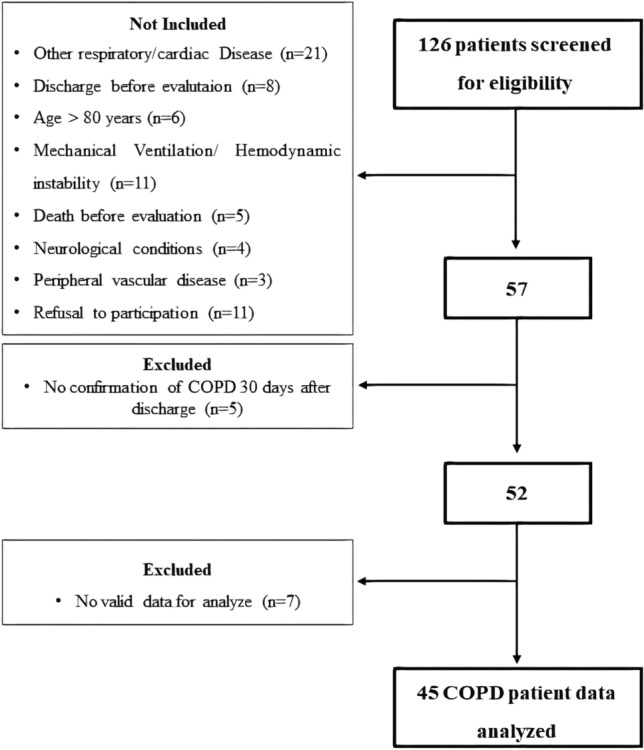
Study flow. COPD chronic obstructive pulmonary disease;
Table 1.
Baseline patients’ features.
| Clinical data | n = 45 |
|---|---|
| Age, years | 68.2 ± 8.0 |
| Male, n (%) | 23 (51) |
| BMI, kg/m2 | 24.7 ± 5.5 |
| Exacerbation, n/ per year | 1.9 ± 1.3 |
| Smoking, never/ex/current | 1(3%)/29(64%)/15(33%) |
| Pack-years | 60.0 ± 51.5 |
| HR, bpm | 85.8 ± 18.9 |
| RR, rpm | 21.7 ± 3.4 |
| SBP, mmHg | 125.6 ± 19.6 |
| DBP, mmHg | 76.2 ± 10.6 |
| SpO2, % | 92.4 ± 3.3 |
| PaO2, mmHg | 70.5 ± 24.7 |
| PaCO2, mmHg | 44.7 ± 11.6 |
| CRP, mg/dl | 6.1 ± 6.0 |
| Supplemental O2, n (%) | 35 (78) |
| mMrC | 3 (2–3) |
| CAT | 23.7 ± 8.7 |
| 6MWD, meters | 230.5 ± 107.5 |
| 6MWD, predicted (%) | 38.7 ± 18.2 |
| Main comorbidities, n (%) | |
| Systemic arterial hypertension | 24 (53) |
| Type 2 diabetes | 8 (18) |
| Chronic stroke | 5 (11) |
| Previous myocardium infarction | 6 (13) |
| Lung function | |
| Forced vital capacity, % predicted | 71.9 ± 18.8 |
| FEV1/forced vital capacity | 0.56 ± 0.19 |
| FEV1, % predicted | 48.5 ± 16.2 |
| COPD Gold Stage II, n (%) | 13 (29) |
| COPD Gold Stage III, n (%) | 25 (55) |
| COPD Gold Stage IV, n (%) | 7 (16) |
| Medications, n (%) | |
| Antibiotic therapy | 41 (91) |
| Short-acting beta agonist (saBa) | 28 (62) |
| Long-acting beta agonist (laBa) | 4 (9) |
| Anticholinergic (saMa and laMa) | 37 (82) |
| Systemic corticosteroids (SCs) | 38 (84) |
| Systemic arterial hypertension | 26 (58) |
| Others | 37 (82) |
Data are presented as mean ± SD or median (interquartile).
BMI body mass index; HR heart rate; RR respiratory rate; SBP systolic brachial pressure; DBP Diastolic Brachial Pressure; sPO2 oxygen saturation; PaO2 partial pressure of oxygen; PaCO2 partial pressure of carbon dioxide; CRP C-reactive protein; mMrC modified Medical Research Council; CAT COPD Assessment Test; 6MWD six minute walking distance; FEV1 forced expiratory volume in first second; laBa long-acting Beta2-Agonist; SCs systemic corticosteroids; laMa long-acting anticholinergics; saBa short-acting Beta2-agonist; saMa Short-acting anticholinergics.
Endothelial function values at AECOPD are demonstrated in Table 2.
Table 2.
Endothelial function parameters by brachial artery measurement.
| AECOPD patients, n = 45 | |
|---|---|
| Baseline diameter, mm | 4.51 ± 0.63 |
| FMD, mm | 0.19 ± 0.10 |
| FMD, % | 4.20 ± 2.12 |
| Baseline velocity, cm/sec | 19.64 ± 9.49 |
| RH flow velocity, cm/sec | 37.56 ± 16.55 |
| RH Shear-rate, s | 66.76 ± 35.06 |
Data are presented as mean ± SD.
AECOPD acute exacerbation of chronic obstructive pulmonary disease; mm millimeter; FMD flow-mediated dilatation; RH reactive hyperemia; % relative change.
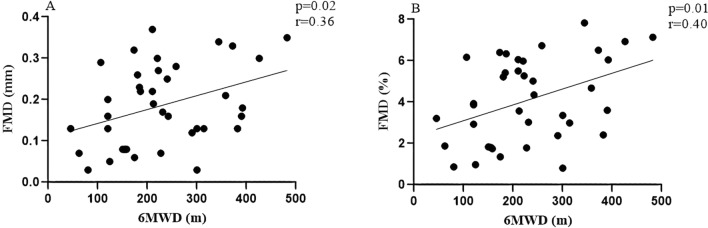
Statistically significant associations were found between exercise capacity and endothelial function in severe acute exacerbation of COPD (Fig. 3). Pearson correlation test showed coefficients of 0.36 (p = 0.02) and 0.40 (p = 0.01) between 6MWD and FMD in mm and %, respectively. Linear regression analysis resulted in a significant correlation between 6MWD and endothelial function (%), p = 0.007, ß = 0.0081, t = 2.83, accounting for 15% of the variance (adjusted R2). The simple linear regression model was FMD (%) = 2.11 + (0.0081*6MWD) (Table 3). The regression coefficient associated with 6MWD demonstrated that each one-unit increase in walking distance is associated with a 0.0081 unit increase in FMD.
Figure 3.
Correlations between exercise capacity and endothelial function. Data are presented as the correlation coefficient (r) and p < 0.05. Relationship between exercise capacity and endothelial function: (A) The FMD (mm) was positively associated with the 6MWD; (B) The FMD (%) was positively associated with the 6MWD. FMD (mm): absolute values; 6MWD was expressed in meters. The figure was create using SigmaPlot version 11.0, from Systat Software, Inc., San Jose California USA, www.systatsoftware.com.
Table 3.
Linear regression model between exercise capacity and FMD (%).
| Variables | β coefficient | Std. Error | p-value |
|---|---|---|---|
| Constant | 2.11 | 0.747 | 0.007 |
| 6MWD (m) | 0.0081 | 0.0028 | 0.007 |
Adjusted R2 = 0.15; F = 8.04 (p = 0.007).
Discussion
This study aimed to evaluate the association between exercise capacity and endothelial function in patients with severe AECOPD. The present study findings revealed that FMD was found to be increasing with increasing walked distance. Exercise capacity was able to explain 15% of the FMD variance, which is a recognized causative factor of cardiovascular disease during exacerbation period.
Exercise capacity and endothelial function in AECOPD
In COPD patients, 6WMD has been considered a potentially useful biomarker of disease severity, evaluating pulmonary, cardiovascular and muscular systems integrated responses33,34. The 6MWD below 350 m has been used to identify subsets of the COPD population at higher risk of exacerbation-related admission or death35. As previously reported, AECOPD is marked by important functional injuries. Bed rest during hospitalization and reduced physical activity, poses a potent threat to muscles and consequently to functional capacity. In agreement with the literature, we found a reduced distance covered of 38% of the predicted value (230 m) and 43% (268 m) was found in the study of Pitta et al.8. Another previous study, aimed to evaluate effects of aerobic exercise also find a distance of 224 m 48 h after exacerbation in patients during hospitalization for exacerbation of COPD36. Therefore, exercise capacity deserves attention at this moment independently, however with promising association with vascular health.
A properly functioning endothelium normally plays a crucial role in providing appropriate hemostatic balance. Nonetheless, a dysfunctional endothelium is seen during exacerbation related mainly to high inflammation and oxidative stress imbalance37. This abnormal condition is characterized by reduced vasodilation ability, and both pro-inflammatory and pro-thrombic states, creating favorable conditions for platelet, leukocyte and cytokines activation and adhesion towards damage of the arterial wall38,39 which can favor CV events. In this study, we used the FMD method, which is a widely accepted evaluation method of the vasodilator function of the endothelium by stimulating reactive hyperemia.
To our knowledge, no previous study investigated the association between endothelial function and exercise capacity status during severe AECOPD. Minet et al.24 found that the 6MWD was independently associated with reactive hyperemia by peripheral arterial tonometry (RH-PAT) ( 0.00768; SE: 0.00249; p = 0.0040) suggesting that an impaired functional capacity may be a main predictor of endothelial dysfunction in COPD patients24. Clarenbach et al. showed that patients with severe airflow obstruction who were physically active were less prone to more severe impairments of FMD, compared to inactive COPD patients with severe airflow obstruction, suggesting that physical activity may attenuate the progression of vascular dysfunction in COPD40. Vaes et al.41 also demonstrated a significant association between reduced maximal aerobic capacity (VO2 peak) and impaired peripheral endothelial function in patients with stable COPD.
In this way, our findings revealed significant associations between exercise capacity and endothelial function in severe acute exacerbation of COPD. FMD was found to be increasing with increasing walked distance. The coefficient of determination observed was of 0.15, which means that 15% of the variance in FMD is due to exercise capacity in patients with COPD and AECOPD. The remaining 85% could be due to individual variation and might be explained by factors that were not taken into account in the analysis, such speculative factors as previous comorbities and endothelial function healthy, previous exercise and rehabilitation effects, inflammation profile and medications.
Regarding shear stress, it is well accepted as an important stimulus for the vascular dilation reactivity response42. Shear stress is able to stimulate a quiescent endothelial cell phenotype that is anti-inflammatory, vasodilatory, antithrombotic and pro-atherogenic42. In this context, exercise-induced increases in blood flow and shear stress have been observed to enhance vascular function. Increased blood flow enhances endothelium-dependent vasodilatation by increased expression of endothelial NO synthase and the release of NO and prostacyclin43. NO and prostacyclin inhibit multiple processes involved in atherogenesis. In addition, increase flow modulates the expression of a panoply of paracrine substances, including endothelial growth factors43. All of these processes may contribute to the beneficial effects of exercise-induced vascular remodeling and reactivity43.
Thus, it may be expected that patients who have a better capacity for exercise during hospitalization, may present with some benefits already accumulated in vascular health, causing this endothelium to be less affected in exacerbation of the disease, while patients who walk a minor distance may have a worse vascular prognosis. In general, physical exercise has been shown to improve exercise capacity and arterial function in particular endothelium-dependent vasodilation44, which seems to be an important key to upgrade vascular health and CV events prevention at AECOPD.
The increased inflammatory state associated with AECOPD may even further accelerate cardiovascular diseases and place patients at an increased risk of death due to cardiac events at this moment45. Our findings on endothelial function values are close to those from Marchetti et al.17, that demonstrated FMD values of 2.8%, while in present study the values were 4.0%. In the study of Ozben et al.46, patients presented about 6% of FMD, though the exacerbation was not severe. The exercise is also responsible for decreasing levels of inflammatory markers such as cytokines and C-reactive protein44. A study described for the first time the association between airway inflammation and endothelial dysfunction related to NO activity in patients with COPD47. They demonstrated that endothelial NO function was compromised by suppressing airway inflammation. We emphasize that this study may further reinforce our findings that a lower exercise capacity in hospitalization would be related to a worse vascular outcome of patients with acute exacerbation of COPD.
A recent review showed that worsening of muscle strength, physical activity, and exercise performance during hospitalization due to exacerbation may be a marker for further worsening in the future. Given the evidence, the AECOPD period represents a crucial time to explore interventions that can reverse these processes and reduce the morbidity associated with hospitalization for AECOPD48.
Although our results have demonstrated a positive association between exercise capacity and endothelial function, in which 6MWD accounting for 15% of the FMD variance in severe AECOPD patients, a causal relationship cannot be established. However, our findings should encourage researchers to elucidate the effect of rehabilitative strategies to enhance exercise capacity have on endothelial function, CV risk and clinically relevant outcomes to optimally manage this high-risk population. In addition, the prognostic value of impaired exercise capacity regarding endothelial function, cardiovascular health and functional status in hospitalized patients with AECOPD also deserve to be explored.
Limitation of the study
The present study has some limitations. For instance, its cross-sectional design, which does not allow the establishment of a causal relationship. Furthermore, only one method to assess endothelial function was used and endothelial-independent dilation was not measured.
Conclusion
The findings of this study confirmed our hypothesis that exercise capacity is associated with endothelial function in patients with chronic obstructive pulmonary disease and severe AECOPD. Endothelial function was found to be increasing with increasing walked distance. These results highlight a possible and valuable potential of rehabilitation strategies focusing on improving exercise capacity translate into vascular and CV risk benefits. However, further research is required to provide evidence of effectiveness of this rehabilitation programs.
Author contributions
E.Z.K., A.B.S. and R.G.M. contributed to the conceptualization, design and methodology of the study. E.Z.K. and A.D.H. contributed to the investigation and data acquisition. E.Z.K., C.L.G. and R.G.M. contributed to the formal data analysis and resources and interpretation. E.Z.K., C.L.G. and R.G.M. contributed to the Writing—Original draft preparation. E.Z.K., R.G.M., A.D.H., C.L.G., V.A.P.L. and S.A.P. contributed to the Writing—Review & Editing. All authors provided critical revision for intellectual content and final approval of the manuscript.
Funding
This work was supported by FAPESP—process nº 2015/12763–4; FAPESP—process nº 2015/26501–1 and Coordination for the Improvement of Higher Education Personnel (CAPES – Brazil/Finance code 001).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth GA, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir. Care. 2003;48:1204–1213. [PubMed] [Google Scholar]
- 4.Soler-Cataluña JJ, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58:589–593. doi: 10.1136/thorax.58.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbation on patient-centered outcomes. Chest. 2007;131:696–704. doi: 10.1378/chest.06-1610. [DOI] [PubMed] [Google Scholar]
- 7.Celli BR, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: Results from the TORCH study. Am. J. Respir. Crit. Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 8.Pitta F, et al. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 9.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: A meta-analysis and statistical modelling approach. Eur. Respir. J. 2011;37:508–515. doi: 10.1183/09031936.00043710. [DOI] [PubMed] [Google Scholar]
- 10.Morgan A, Zakeri R, Quint J. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018;12:175346581775052. doi: 10.1177/1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto T, Shimada Y, Faridi M, Camargo C, Hasegawa K. Incidence of acute cardiovascular event after acute exacerbation of COPD. J. Gen. Int. Med. 2018;33:1461–1468. doi: 10.1007/s11606-018-4518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laratta C, van Eeden S. Acute exacerbation of chronic obstructive pulmonary disease: Cardiovascular links. BioMed. Res. Intern. 2014;2014:528789. doi: 10.1155/2014/528789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanhoutte PM, Shimokawa H, Tang EHC, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunisaki KM, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events a post hoc cohort analysis from the SUMMIT randomized clinical trial. Am. J. Respir. Crit. Care Med. 2018;198:51–57. doi: 10.1164/rccm.201711-2239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebron Lipovec N, et al. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: A systematic review. COPD J. Chronic Obstr. Pulm. Dis. 2016;13:399–406. doi: 10.3109/15412555.2016.1140732. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, et al. Increased circulating endothelial microparticles in COPD patients: A potential biomarker for COPD exacerbation susceptibility. Thorax. 2012;67:1067–1074. doi: 10.1136/thoraxjnl-2011-201395. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti N, et al. Hospitalized acute exacerbation of COPD impairs flow and nitroglycerin-mediated peripheral vascular dilation. COPD J. Chronic Obstr. Pulm. Dis. 2011;8:60–65. doi: 10.3109/15412555.2011.558541. [DOI] [PubMed] [Google Scholar]
- 18.Stone IS, Barnes NC, Petersen SE. Chronic obstructive pulmonary disease: A modifiable risk factor for cardiovascular disease? Heart. 2012;98:1055–1062. doi: 10.1136/heartjnl-2012-301759. [DOI] [PubMed] [Google Scholar]
- 19.Simsolo C, Blum A. Vascular responsiveness in patients with chronic obstructive pulmonary disease (COPD) Chest. 2014;145:398A. doi: 10.1378/chest.1783150. [DOI] [PubMed] [Google Scholar]
- 20.Chin SO, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE. 2013;8:1–6. doi: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Matthaeis A, et al. Effects of hypercapnia on peripherain elderly patients with acute exacerbation of chronic obstructive pulmonary disease. Clin. Interv. Aging. 2014;9:871–878. doi: 10.2147/CIA.S57548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo J, et al. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J. Cachexia. Sarcopenia Muscle. 2018;9:1034–1041. doi: 10.1002/jcsm.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francescomarino SD, Sciartilli A, Valerio VD, Baldassarre AD, Gallina S. The effect of physical exercise on endothelial function. Sport. Med. 2009;39:797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Minet C, et al. Reduced six-minute walking distance, high fat-free-mass index and hypercapnia are associated with endothelial dysfunction in COPD. Respir. Physiol. Neurobiol. 2012;183:128–134. doi: 10.1016/j.resp.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Singh D, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 26.Vestbo J. Clinical assessment, staging, and epidemiology of chronic obstructive pulmonary disease exacerbations. Proc. Am. Thorac. Soc. 2006;3:252–256. doi: 10.1513/pats.200510-107SF. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, et al. ATS/ERS task force: Standardisation of lung function testing. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Thijssen DHJ, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019;40:2534–2547. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 29.Anderson TJ, Phillips SA. Assessment and prognosis of peripheral artery measures of vascular function. Prog. Cardiovasc. Dis. 2015;57:497–509. doi: 10.1016/j.pcad.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J. Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland AE, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur. Respir. J. 2014 doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 32.Britto RR, et al. Reference equations for the six-minute walk distance based on a Brazilian multicenter study. Braz. J. Phys. Ther. 2013;17:556–563. doi: 10.1590/S1413-35552012005000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issues S, Test MW, Equipment R, Preparation P. American Thoracic Society ATS statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 34.Polkey MI, et al. Six-minute-walk test in chronic obstructive pulmonary disease: Minimal clinically important difference for death or hospitalization. Am. J. Respir. Crit. Care Med. 2013;187:382–386. doi: 10.1164/rccm.201209-1596OC. [DOI] [PubMed] [Google Scholar]
- 35.Spruit MA, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J. Am. Med. Direc. Assoc. 2012;13:291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Knaut C, et al. Assessment of aerobic exercise adverse effects during COPD exacerbation hospitalization. Can. Respir. J. 2017;2017:5937908. doi: 10.1155/2017/5937908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 38.Pinto-Plata VM, et al. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest. 2007;131:37–43. doi: 10.1378/chest.06-0668. [DOI] [PubMed] [Google Scholar]
- 39.Hurst JR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;174:867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 40.Clarenbach CF, et al. Determinants of endothelial function in patients with COPD. Eur. Respir. J. 2013;42:1194–1204. doi: 10.1183/09031936.00144612. [DOI] [PubMed] [Google Scholar]
- 41.Vaes AW, Spruit MA, Theunis J, Wouters EFM, De Boever P. Peripheral endothelial function is positively associated with maximal aerobic capacity in patients with chronic obstructive pulmonary disease. Respir. Med. 2018;142:41–47. doi: 10.1016/j.rmed.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Chiu JJ, Chiu CC. NIH Public Access. Physiol Rev. 2011;91(1):2011. doi: 10.1152/physrev.00047.2009.Effects.Physiolrev91. [DOI] [Google Scholar]
- 43.Niebauer J, Cooke JP. Cardiovascular effects of exercise: Role of endothelial shear stress. J. Am. Coll. Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 44.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc. Sport. Sci. Ver. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 45.Shimbo D, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192:197–203. doi: 10.1016/j.atherosclerosis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Özben B, et al. Acute exacerbation impairs endothelial function in patients with chronic obstructive pulmonary disease. Turk Kardiyol. Dern. Ars. 2010;38:1–7. [PubMed] [Google Scholar]
- 47.Csoma B, et al. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir. Res. 2019;20:1–10. doi: 10.1186/s12931-019-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdulai RM, et al. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018;197:433–449. doi: 10.1164/rccm.201703-0615CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.