Abstract
We investigated the potential of mid-regional pro-adrenomedullin (MR-proADM) for use as a novel biomarker for arterial stiffness as the criterion for vascular failure and cardiometabolic disease (obesity, hypertension, dyslipidemia, diabetes, and metabolic syndrome) compared with high-sensitivity C-reactive protein (hsCRP). Overall, 2169 individuals (702 men and 1467 women) were enrolled. Multiple regression analysis was performed to assess the association of MR-proADM and hsCRP with brachial-ankle pulse wave velocity (baPWV), adjusting for other variables. The diagnostic performance (accuracy) of MR-proADM with regard to the index of vascular failure was tested with the help of receiver operating characteristic curve analysis in the models. MR-proADM was significantly higher in participants with vascular failure, as defined by baPWV and/or its risk factors (obesity, hypertension, dyslipidemia, diabetes, and metabolic syndrome), than in control groups. Independent of cardiovascular risk factors (age, drinking, smoking, body mass index, systolic blood pressure, lipid and glycol metabolism), MR-proADM was significantly associated with baPWV, and MR-proADM showed higher areas under the curve of baPWV than hsCRP showed. MR-proADM is more suitable for the diagnosis of higher arterial stiffness as the criterion for vascular failure than hsCRP. Because vascular assessment is important to mitigate the most significant modifiable cardiovascular risk factors, MR-proADM may be useful as a novel biomarker on routine blood examination.
Subject terms: Cardiovascular biology, Cardiovascular diseases, Vascular diseases
Introduction
Vascular failure is defined as the integration of endothelial dysfunction, smooth muscle dysfunction, and metabolic abnormalities of the vessel wall1. In 2018, new physiological diagnostic criteria for vascular failure were proposed by the Physiological Diagnosis Criteria for Vascular Failure Committee of the Japan Society for Vascular Failure, according to the target vascular layers and areas, assessed by endothelial function and arterial stiffness (a marker integrating medial layer function) using universally available diagnostic tools2. Endothelial function can be measured by flow-mediated vasodilation (FMD) in the brachial artery and reactive hyperemia-peripheral arterial tonometry (RH-PAT) in the fingertip. Arterial stiffness, which is reflected by medial layer function, can be assessed by pulse wave velocity (PWV) and cardio-ankle vascular index (CAVI)2. However, no blood biomarker is known to be diagnostic of vascular failure.
Adrenomedullin (ADM) is a vasoactive peptide identified in human pheochromocytoma3. Although ADM is secreted from various organs and tissues, it is produced mainly by vascular endothelial cells and serves a number of physiological functions4,5. Of note, plasma ADM levels are elevated in patients with hypertension, congestive heart failure or myocardial infarction6,7, renal diseases8, diabetes mellitus9, the acute phase of stroke10, septic shock11, arterial stiffness assessed by PWV12, and the magnitude of the elevation is in proportion to the severity of the disease involving vascular damage.
However, reliable measurement of ADM levels is challenging due to its short half-life of 22 min13. The precursor molecule of ADM, mid-regional pro-ADM (MR-proADM), has higher stability, which allows it to be reliably measured as a surrogate biomarker for ADM14. In sum, the hypothesis holds that MR-proADM, like ADM, is associated with cardiometabolic diseases and arterial stiffness. Although the evidence for a classical cardiovascular biomarker, High-sensitivity C-reactive protein (hsCRP), in disease etiology has been initially promising, the evidence for a causal role in humans remains limited15. This study aimed the potential of MR-proADM for use as a novel biomarker for arterial stiffness assessed by PWV as the criterion for vascular failure and cardiometabolic disease (obesity, hypertension, dyslipidemia, diabetes, and metabolic syndrome) compared with hsCRP.
Results
Figure 1 shows the study participant flow chart. Table 1 showed the anthropometric measurements and participant characteristics in this study. The mean age was 59.8 ± 10.3 years in men and 57.3 ± 9.89 years in women. The MR-proADM level was significantly different between men and women, 0.468 ± 0.100 nmol/L in men versus 0.412 ± 0.082 nmol/L in women (P < 0.001).
Figure 1.
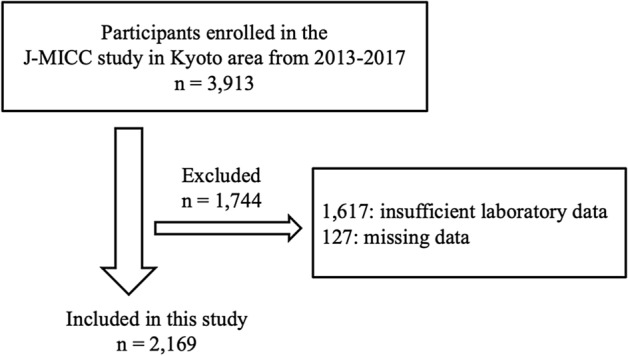
Flow chart of the study participants.
Table 1.
Participant characteristics at baseline according to sex.
| Men (n = 702) | Women (n = 1467) | All (n = 2169) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (years) | 59.8 ± 10.3 | 57.3 ± 9.89 | 58.1 ± 10.1 |
| Body mass index (kg/m2) | 23.5 ± 2.99 | 21.6 ± 3.15 | 22.2 ± 3.22 |
| Systolic blood pressure (mmHg) | 135 ± 17.3 | 125 ± 19.3 | 128 ± 19.1 |
| Diastolic blood pressure (mmHg) | 82.3 ± 10.5 | 76.4 ± 11.1 | 78.3 ± 11.2 |
| Triglycerides (mg/dL) | 124 ± 86.7 | 90.3 ± 63.9 | 101 ± 73.8 |
| Total cholesterol (mg/dL) | 204 ± 32.0 | 222 ± 37.4 | 216 ± 36.8 |
| Low-density lipoprotein cholesterol (mg/dL) | 121 ± 29.5 | 127 ± 31.7 | 125 ± 31.1 |
| High-density lipoprotein cholesterol (mg/dL) | 60.1 ± 14.4 | 73.9 ± 16.5 | 69.5 ± 17.1 |
| Blood sugar (mg/dL) | 94.9 ± 15.9 | 88.8 ± 12.8 | 90.8 ± 14.2 |
| A glycated hemoglobin (%) | 5.66 ± 0.55 | 5.59 ± 0.40 | 5.59 ± 0.46 |
| Waist circumstance (cm) | 85.1 ± 8.20 | 78.8 ± 8.91 | 80.8 ± 9.18 |
| Brachial-ankle pulse wave velocity (cm/s) | 1496 ± 334 | 1359 ± 316 | 1403 ± 329 |
| Mid-regional pro-adrenomedullin (nmol/L) | 0.468 ± 0.100 | 0.412 ± 0.082 | 0.430 ± 0.092 |
| High-sensitivity C-reactive protein | 0.055 ± 0.069 | 0.044 ± 0.064 | 0.047 ± 0.066 |
| n (%) | n (%) | n (%) | |
|---|---|---|---|
| Alcohol drinking | |||
| Current | 524 (74.6) | 745 (50.8) | 1269 (58.5) |
| Former | 11 (1.6) | 18 (1.2) | 29 (1.3) |
| Never | 167 (23.8) | 704 (48.0) | 871 (40.2) |
| Smoking | |||
| Current | 125 (17.8) | 68 (4.6) | 193 (8.9) |
| Former | 338 (48.1) | 184 (12.5) | 522 (24.1) |
| Never | 239 (34.0) | 1215 (82.8) | 1454 (67.0) |
| Drug treatment | |||
| Hypertension | 181 (25.8) | 174 (11.9) | 355 (16.4) |
| Dyslipidemia | 118 (16.8) | 227 (15.5) | 345 (15.9) |
| Diabetes | 51 (7.3) | 26 (1.8) | 77 (3.6) |
The means of MR-proADM, according to cardiometabolic disease and sex, are shown in Table 2. In both sexes, the mean MR-proADM levels were significantly higher in participants with vascular failure, as defined by baPWV and/or risk factors (obesity, hypertension, dyslipidemia, diabetes, and MS), than in control groups. Supplemental Table S1 shows the means of MR-proADM according to the sum of four cardiometabolic diseases (obesity, hypertension, dyslipidemia, and diabetes). In both sexes, the means of MR-proADM value tended to increase as the number of cardiometabolic diseases increased.
Table 2.
The means of mid-regional pro-adrenomedullin according to cardiometabolic disease and brachial-ankle pulse wave velocity.
| % | Men | p-value | % | Women | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||||
| Body Mass Index < 25 | n = 500 | 71.2 | 0.460 ± 0.096 | 0.001 | n = 1263 | 86.1 | 0.404 ± 0.077 | < 0.001 |
| Body Mass Index ≥ 25 | n = 202 | 28.8 | 0.487 ± 0.106 | n = 204 | 13.9 | 0.466 ± 0.093 | ||
| Hypertension (−) | n = 342 | 48.7 | 0.438 ± 0.077 | < 0.001 | n = 1038 | 70.8 | 0.396 ± 0.072 | < 0.001 |
| Hypertension (+) | n = 360 | 51.3 | 0.496 ± 0.110 | n = 429 | 29.2 | 0.451 ± 0.093 | ||
| Dyslipidemia (−) | n = 387 | 55.1 | 0.459 ± 0.099 | 0.007 | n = 803 | 32.2 | 0.400 ± 0.080 | 0.001 |
| Dyslipidemia (+) | n = 315 | 44.9 | 0.479 ± 0.099 | n = 664 | 67.8 | 0.428 ± 0.082 | ||
| Diabetes (−) | n = 619 | 88.2 | 0.463 ± 0.097 | 0.001 | n = 1413 | 96.3 | 0.411 ± 0.081 | < 0.001 |
| Diabetes (+) | n = 83 | 11.8 | 0.502 ± 0.110 | n = 54 | 3.7 | 0.464 ± 0.109 | ||
| Metabolic syndrome (−) | n = 485 | 69.1 | 0.456 ± 0.093 | < 0.001 | n = 1360 | 92.7 | 0.406 ± 0.078 | < 0.001 |
| Metabolic syndrome (+) | n = 217 | 30.9 | 0.497 ± 0.108 | n = 107 | 7.3 | 0.489 ± 0.098 | ||
| Brachial-ankle pulse wave velocity < 1400 | n = 331 | 47.2 | 0.431 ± 0.082 | < 0.001 | n = 908 | 61.9 | 0.394 ± 0.073 | < 0.001 |
| Brachial-ankle pulse wave velocity ≥ 1400 | n = 370 | 52.8 | 0.501 ± 0.103 | n = 559 | 38.1 | 0.443 ± 0.088 | ||
| Brachial-ankle pulse wave velocity < 1800 | n = 581 | 82.8 | 0.457 ± 0.096 | < 0.001 | n = 1338 | 91.2 | 0.407 ± 0.077 | < 0.001 |
| Brachial-ankle pulse wave velocity ≥ 1800 | n = 121 | 17.2 | 0.517 ± 0.104 | n = 129 | 8.8 | 0.470 ± 0.108 |
Category differences are analyzed by Welch’s t-tests.
Bold style represents p < 0.05.
Correlations between MR-proADM and the cardiometabolic parameters are shown in Table 3. Although most of the cardiometabolic parameters were significantly associated with MR-proADM, among them age and baPWV showed higher correlation coefficients for both sexes.
Table 3.
Correlations between mid-regional pro-adrenomedullin and cardiometabolic parameters.
| Men (n = 702) | Women (n = 1467) | |||
|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | |
| Age (years) | 0.510 | < 0.001 | 0.430 | < 0.001 |
| Body Mass Index | 0.123 | 0.001 | 0.287 | < 0.001 |
| Systolic blood pressure | 0.230 | < 0.001 | 0.324 | < 0.001 |
| Diastolic blood pressure | 0.058 | 0.123 | 0.220 | < 0.001 |
| Triglycerides | 0.233 | < 0.001 | 0.245 | < 0.001 |
| Total cholesterol | − 0.021 | 0.583 | 0.102 | < 0.001 |
| Low-density lipoprotein cholesterol | − 0.084 | 0.026 | 0.112 | < 0.001 |
| High-density lipoprotein cholesterol | − 0.025 | 0.505 | − 0.105 | < 0.001 |
| Blood sugar | 0.112 | 0.003 | 0.164 | < 0.001 |
| A glycated hemoglobin | 0.144 | < 0.001 | 0.166 | < 0.001 |
| Waist circumstance | 0.231 | < 0.001 | 0.343 | < 0.001 |
| Brachial-ankle pulse wave velocity | 0.426 | < 0.001 | 0.385 | < 0.001 |
| High-sensitivity C-reactive protein | 0.298 | < 0.001 | 0.274 | < 0.001 |
Correlations are analyzed by Spearman’s rank correlation analysis.
Bold style represents p < 0.05.
In multivariable logistic regression adjusting for known cardiovascular risk factors (age, drinking, smoking, BMI, SBP, lipid and glycol metabolism), MR-proADM was significantly associated with baPWV (men: beta = 0.111, p < 0.001, women: beta = 0.077, p < 0.001), and that most cardiometabolic parameters. However, hsCRP, the classical cardiovascular risk biomarker, was not significantly associated with baPWV (Table 4).
Table 4.
Multivariate analysis of association with brachial-ankle pulse wave velocity.
| Men (n = 702) | Women (n = 1467) | |||
|---|---|---|---|---|
| beta | p-value | beta | p-value | |
| Mid-regional pro-adrenomedullin | 0.111 | < 0.001 | 0.077 | < 0.001 |
| High-sensitivity C-reactive protein | 0.013 | 0.617 | 0.020 | 0.236 |
| Age | 0.335 | < 0.001 | 0.373 | < 0.001 |
| Body Mass Index | − 0.171 | < 0.001 | − 0.121 | < 0.001 |
| Systolic blood pressure | 0.477 | < 0.001 | 0.520 | < 0.001 |
| Triglycerides | 0.072 | 0.009 | 0.047 | 0.009 |
| High-density lipoprotein cholesterol | 0.024 | 0.417 | − 0.020 | 0.257 |
| Low-density lipoprotein cholesterol | − 0.035 | 0.174 | 0.007 | 0.660 |
| A glycated hemoglobin | 0.089 | 0.001 | 0.035 | 0.045 |
Adjusted for age, drinking and smoking status.
Analysis were performed by multiple regression analysis.
Bold style represents p < 0.05.
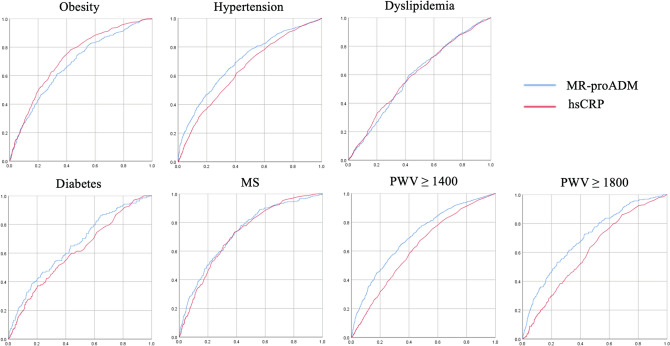
Since we demonstrated that MR-proADM might prove to be a significant biomarker for vascular failure, we calculated the receiver operating characteristic (ROC) curve (Fig. 2). ROC analysis showed that MR-proADM had both high sensitivity and specificity in predicting the baPWV, which is the cutoff value for vascular failure than hsCRP. The areas under the curve (AUC) of the model with MR-proADM and hsCRP were significantly associated with all variables (data not shown). Moreover, MR-proADM showed higher AUC of baPWV, which defined vascular failure, than did hsCRP.
Figure 2.
Receiver operating characteristic curve analysis classified cardiometabolic disease and arterial stiffness by MR-proADM and hsCRP.
Discussion
The main findings of the present study can be summarized as follows: first, MR-proADM was significantly higher in participants with vascular failure, as defined by baPWV and/or its risk factors (obesity, hypertension, dyslipidemia, diabetes, and MS), than in control groups. Second, independent of cardiovascular risk factors (age, drinking, smoking, BMI, SBP, lipid, and glycol metabolism), MR-proADM was significantly associated with baPWV, and MR-proADM showed higher AUC of baPWV than hsCRP showed. To our knowledge, this study is the first to report MR-proADM as a more suitable biomarker for vascular failure as defined by baPWV and its risk factors than hsCRP.
Our results showed that MR-proADM levels were significantly higher in participants with obesity, hypertension, dyslipidemia, diabetes, and MS than in control participants, and that most cardiometabolic parameters were significantly associated with MR-proADM. In agreement with many previous studies, our study confirmed that MR-proADM was associated with cardiovascular risk factors16–20.
ADM has also been shown also to have anti-inflammatory and anti-oxidative properties and to limit the arterial intimal hyperplasia, a response to protect organs from damage21–24. Indeed, the relationship between ADM signal and vascular integrity has been investigated in many experimental4,25–27 and some epidemiological studies28–30. As a consequence, the increase of ADM levels found in these pathological disorders affecting the vascular system may be seen as a counter-regulatory (compensatory) mechanism for vascular damage. In sum, MR-proADM is associated with cardiometabolic parameters and is an independent predictor for cardiovascular events in patients31–33.
In this study, we used only baPWV as a diagnostic of vascular failure. However, physiological diagnostic criteria of vascular failure were based on vascular functional parameters: endothelial function and arterial stiffness. Endothelial function was measured by FMD and/or RH-PAT2. One study, the Gutenberg Health Study, showed that MR-proADM was significantly associated with RH-PAT after adjusting for cardiovascular risk; however, results have been inconsistent for FMD. In brief, MR-proADM was not significantly associated with FMD after adjusting for cardiovascular risk factors: age, sex, BMI, diabetes, current smoking, logarithmically transformed pulse pressure, dyslipidemia, and a positive family history of myocardial infarction34. The relationship between ADM and endothelial function from laboratory data is well known4 and it was required further study of endothelial function and ADM using a large number of human samples.
ADM has potential for therapeutic applications, but its use is limited by its short half-life in the bloodstream. On the other hand, MR-proADM has higher stability14. In this study, MR-proADM plasma concentrations were measured by an automated KRYPTOR analyzer, which is used commercially in some countries14. Using UPLC-MS/MS, we recently established a sensitive method for determining plasma MR-proADM concentration35. Our novel UPLC-MS/MS assay for determining MR-proADM concentration can be used in the clinical setting and may have better selectivity than the immunoassay method. This means that MR-proADM can be measured on routine examination.
The limitations of our study include its cross-sectional design and the inclusion of only Japanese participants. Recall bias is unavoidable because we use the self-administered questionnaires study. Therefore, the value of the self-reported lifestyle factor may not have been accurate enough. If present, however, such information is non-specific and can be prevented by the large sample size. The strength of this study is the inclusion of a large number of Japanese participants, which prevented sample bias. Another limitation is that the mean cut-off value for MR-proADM is yet to be established, and we were not able to categorize the MR-proADM of the participants. On the other hand, MR-pro ADM measurement in many Japanese is novel and study strength.
Our results show that MR-proADM is more suitable for the diagnosis of higher arterial stiffness as the criterion for vascular failure than hsCRP. Because vascular assessment is important to mitigate the most significant modifiable cardiovascular risk factors, MR-proADM may be useful as a novel biomarker on routine blood examination.
Methods
Participants
The Japan Multi-Institutional Collaborative Cohort Study, was launched in 2005 to investigate gene–environmental interactions in lifestyle-related diseases36,37. This study included individuals who were enrolled in the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study second survey in the Kyoto area from 2013 to 2017. A total of 3913 participants were eligible for analyses. All subjects underwent a routine health checkup. Among these 3913 participants, 1744 were excluded, 1617 due to insufficient laboratory data and 127 due to the absence of other data. After these exclusions, 2169 individuals (702 men and 1467 women) were eligible for analyses. Inclusion criteria was all participants with no missing variables.
The study was approved by the Institutional Ethics Committee of Kyoto Prefectural University of Medicine (approval number: RBMR-E-36-8 at 2013) and was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent before participation.
Clinical and biochemical analysis
The following lifestyle and medical information obtained through self-administered questionnaires which used in J-MICC Study were evaluated: alcohol consumption and smoking status (current, former, never) and current medications. In addition, anthropometric data obtained from the health check-ups were collected38. Waist circumstance (WC) was measured at the umbilical level in minimal respiration in a standing position. Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). The cut-off point for obesity was set at a BMI of 25 kg/m2 according to the definition of obesity in Japan39.
Anamnesis and medication history were assessed using self-administered questionnaires38. Hypertension was defined as a systolic/diastolic blood pressure (SBP/DBP) ≥ 140/90 mmHg and/or current use of medication for hypertension. Dyslipidemia was defined as low-density lipoprotein cholesterol (LDL-C) ≥ 140 mg/dL and/or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL and/or current use of medication for dyslipidemia. Diabetes was defined as a glycated hemoglobin (HbA1c) level ≥ 6.5% and/or blood sugar (BS) ≥ 126 and/or current use of medication for diabetes. The Japanese criteria of metabolic syndrome (MS) was defined as follows: if a man has a WC ≥ 85 cm (in the case of a woman, ≥ 90 cm) in addition to two or more of the following: lipid abnormality: high triglyceride level (≥ 150 mg/dL), and/or HDL-cholesterol level (≤ 40 mg/dL), or the use of lipid-modifying drugs; elevated blood pressure: SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg, or the use of antihypertensive drugs; and elevated blood glucose: HbA1c 5.6% and/or BS ≥ 100, or the use of drugs for diabetes40.
The brachial-ankle pulse wave velocity (baPWV) used to evaluate arterial stiffness was measured with a volume-plethysmographic apparatus (BP-203RPE II form PWV/ABI, Omron Healthcare Co. Ltd., Kyoto, Japan). We simultaneously measured baPWV on both the right and left sides, and the average values in each individual were subjected to statistical analysis. The cut-off point of baPWV was using 1400 cm/s and 1800 cm/s, its definition of vascular failure2.
MR-proADM plasma concentrations were measured by an automated KRYPTOR analyzer, using a time-resolved amplified cryptate emission (TRACE) technology assay (Thermo Fisher Diagnostics K.K., Japan), according to the manufacturer’s instruction manuals14. Briefly, TRACE is measured by a sandwich immunoassay, wherein the Europium Cryptate, and Cyanine 5 are bound to specific antibodies. This homogeneous assay uses time-resolved measurements and spectral selection to eliminate the background signal of the media and select the specific signal. The plasma sampling was from an antecubital vein.
Statistical analysis
Continuous variables are expressed as means ± standard deviation (SD) and categorical data are expressed as sums and percentages. Since MR-proADM shows gender differences, we analyzed by gender. Inter-group comparisons were performed using Welch’s t-tests for continuous variables. Tests for linear trends (e.g., P trend tests) were conducted by the sum of four cardiometabolic diseases (obesity, hypertension, dyslipidemia, and diabetes). Spearman’s rank correlation analysis was performed to assess the relationship between the baPWV and other variables, including anthropometric and blood chemistry data, because MR-proADM does not show a normal distribution. Multiple regression analysis was performed to assess the association of MR-proADM and hsCRP, a classical cardiovascular biomarker, with baPWV, adjusting for other variables. The diagnostic performance (accuracy) of MR-proADM with regard to the index of vascular failure was tested with the help of ROC curve analysis in the models. For comparative purposes, the corresponding AUC were calculated and reported. Using G*Power version 3.1.9.6 (http://www.gpower.hhu.de), we verified that the sample size was sufficient. A sample size was calculated with a 0.80 power at the 0.05 alpha level. All statistical analyses were performed using JMP version 13 software (SAS Institute Inc., Cary, NC, USA), and p < 0.05 was considered statistically significant.
Supplementary Information
Acknowledgements
This study was supported by Takeda Science Foundation, Kondou Kinen Medical Foundation, Grant-in-Aid for Scientific Research on Priority Areas of Cancer (No. 17015018) and Innovative Areas (No. 221S0001), Japan Society for the Promotion of Science Fellows (19J00106), AMED under Grant Numbers 17ek0210098h0001 and 19lk0201096h0001, and by JSPS KAKENHI Grant Numbers 15K19185, 16H06277 and 18K17387 from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We thank Drs Etsuko Ozaki, Daisuke Matsui and Isao Watanabe for their work for acquisition of data, and Yoshiyuki Watanabe for initiating and organizing the J-MICC study in the Kyoto area as former principal investigator.
Author contributions
T.K. analysed the data and wrote the main manuscript text. N.K., Y.S., S.S., R.T., M.I., T.M., H.I., M.I. and R.U. designed the study. M.T. and T.S. provided critical comments on the manuscript. All authors contributed to and have approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in Tables 3 and 4. “Low-density lipoprotein cholesterol” was incorrectly given as “Low-sensitivity C-reactive protein”. “High-density lipoprotein cholesterol” was incorrectly given as “High-sensitivity C-reactive protein”.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/30/2021
A Correction to this paper has been published: 10.1038/s41598-021-96984-3
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79525-2.
References
- 1.Inoue T, Node K. Vascular failure: A new clinical entity for vascular disease. J. Hypertens. 2006;24:2121–2130. doi: 10.1097/01.hjh.0000249684.76296.4f. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka A, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72:1060–1071. doi: 10.1161/HYPERTENSIONAHA.118.11554. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura K, et al. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 4.Koyama T, et al. Adrenomedullin-RAMP2 system in vascular endothelial cells. J. Atheroscler. Thromb. 2015;22:647–653. doi: 10.5551/jat.29967. [DOI] [PubMed] [Google Scholar]
- 5.Cheung BM, Tang F. Adrenomedullin: Exciting new horizons. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012;6:4–17. doi: 10.2174/187221412799015263. [DOI] [PubMed] [Google Scholar]
- 6.Nagaya N, et al. Cardiac adrenomedullin gene expression and peptide accumulation after acute myocardial infarction in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R1019–1026. doi: 10.1152/ajpregu.2000.278.4.R1019. [DOI] [PubMed] [Google Scholar]
- 7.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 8.Ishimitsu T, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J. Clin. Investig. 1994;94:2158–2161. doi: 10.1172/JCI117573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi M, et al. Plasma adrenomedullin in diabetes. Lancet. 1997;350:1449–1450. doi: 10.1016/S0140-6736(05)64211-0. [DOI] [PubMed] [Google Scholar]
- 10.Kikumoto K, et al. Increased plasma concentration of adrenomedullin in patients with subarachnoid hemorrhage. Anesth. Analg. 1998;87:859–863. doi: 10.1097/00000539-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Hirata Y, et al. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. J. Clin. Endocrinol. Metab. 1996;81:1449–1453. doi: 10.1210/jcem.81.4.8636349. [DOI] [PubMed] [Google Scholar]
- 12.Levy D, et al. Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: The Framingham heart study. Circulation. 2007;115:3079–3085. doi: 10.1161/CIRCULATIONAHA.106.652842. [DOI] [PubMed] [Google Scholar]
- 13.Meeran K, et al. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: A pharmacokinetic study. J. Clin. Endocrinol. Metab. 1997;82:95–100. doi: 10.1210/jcem.82.1.3656. [DOI] [PubMed] [Google Scholar]
- 14.Caruhel P, Mazier C, Kunde J, Morgenthaler NG, Darbouret B. Homogeneous time-resolved fluoroimmunoassay for the measurement of midregional proadrenomedullin in plasma on the fully automated system B.R.A.H.M.S KRYPTOR. Clin. Biochem. 2009;42:725–728. doi: 10.1016/j.clinbiochem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Aleksandrova K, Mozaffarian D, Pischon T. Addressing the perfect storm: Biomarkers in obesity and pathophysiology of cardiometabolic risk. Clin. Chem. 2018;64:142–153. doi: 10.1373/clinchem.2017.275172. [DOI] [PubMed] [Google Scholar]
- 16.Lim SC, et al. The relationship between adrenomedullin, metabolic factors, and vascular function in individuals with type 2 diabetes. Diabetes Care. 2007;30:1513–1519. doi: 10.2337/dc06-1899. [DOI] [PubMed] [Google Scholar]
- 17.Neumann JT, et al. Association of MR-proadrenomedullin with cardiovascular risk factors and subclinical cardiovascular disease. Atherosclerosis. 2013;228:451–459. doi: 10.1016/j.atherosclerosis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Ohlsson T, Nilsson PM, Persson M, Melander O. Midregional proadrenomedullin predicts reduced blood pressure and glucose elevation over time despite enhanced progression of obesity markers. J. Hypertens. 2019;37:590–595. doi: 10.1097/HJH.0000000000001893. [DOI] [PubMed] [Google Scholar]
- 19.Krintus M, et al. Plasma midregional proadrenomedullin (MR-proADM) concentrations and their biological determinants in a reference population. Clin. Chem. Lab. Med. 2018;56:1161–1168. doi: 10.1515/cclm-2017-1044. [DOI] [PubMed] [Google Scholar]
- 20.Seissler J, et al. Vasoregulatory peptides pro-endothelin-1 and pro-adrenomedullin are associated with metabolic syndrome in the population-based KORA F4 study. Eur. J. Endocrinol. 2012;167:847–853. doi: 10.1530/EJE-12-0472. [DOI] [PubMed] [Google Scholar]
- 21.Abe M, et al. Adrenomedullin augments collateral development in response to acute ischemia. Biochem. Biophys. Res. Commun. 2003;306:10–15. doi: 10.1016/S0006-291X(03)00903-3. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, et al. Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1506–H1514. doi: 10.1152/ajpheart.00270.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kawai J, et al. Endogenous adrenomedullin protects against vascular response to injury in mice. Circulation. 2004;109:1147–1153. doi: 10.1161/01.CIR.0000117231.40057.6D. [DOI] [PubMed] [Google Scholar]
- 24.Isumi Y, Kubo A, Katafuchi T, Kangawa K, Minamino N. Adrenomedullin suppresses interleukin-1beta-induced tumor necrosis factor-alpha production in Swiss 3T3 cells. FEBS Lett. 1999;463:110–114. doi: 10.1016/S0014-5793(99)01615-4. [DOI] [PubMed] [Google Scholar]
- 25.Koyama T, et al. Vascular endothelial adrenomedullin-RAMP2 system is essential for vascular integrity and organ homeostasis. Circulation. 2013;127:842–853. doi: 10.1161/CIRCULATIONAHA.112.000756. [DOI] [PubMed] [Google Scholar]
- 26.Xian X, et al. Vasoprotective activities of the adrenomedullin-RAMP2 system in endothelial cells. Endocrinology. 2017;158:1359. doi: 10.1210/en.2016-1531. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa-Shindo Y, et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J. Clin. Investig. 2008;118:29–39. doi: 10.1172/JCI33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottsater M, et al. Adrenomedullin is a marker of carotid plaques and intima-media thickness as well as brachial pulse pressure. J. Hypertens. 2013;31:1959–1965. doi: 10.1097/HJH.0b013e328362fe99. [DOI] [PubMed] [Google Scholar]
- 29.Kita T, Kitamura K, Hashida S, Morishita K, Eto T. Plasma adrenomedullin is closely correlated with pulse wave velocity in middle-aged and elderly patients. Hypertens. Res. 2003;26:887–893. doi: 10.1291/hypres.26.887. [DOI] [PubMed] [Google Scholar]
- 30.Koyama T, et al. Genetic variants of RAMP2 and CLR are associated with stroke. J. Atheroscler. Thromb. 2017;24:1267–1281. doi: 10.5551/jat.41517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wild PS, et al. Midregional proadrenomedullin for prediction of cardiovascular events in coronary artery disease: Results from the AtheroGene study. Clin. Chem. 2012;58:226–236. doi: 10.1373/clinchem.2010.157842. [DOI] [PubMed] [Google Scholar]
- 32.Peacock WF. Novel biomarkers in acute heart failure: MR-pro-adrenomedullin. Clin. Chem. Lab. Med. 2014;52:1433–1435. doi: 10.1515/cclm-2014-0222. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Navero JL, et al. Evaluation of the vasoactive-inotropic score, mid-regional pro-adrenomedullin and cardiac troponin I as predictors of low cardiac output syndrome in children after congenital heart disease surgery. Med. Intens. 2019;43:329–336. doi: 10.1016/j.medin.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Schnabel RB, et al. Multiple endothelial biomarkers and noninvasive vascular function in the general population: The Gutenberg health study. Hypertension. 2012;60:288–295. doi: 10.1161/HYPERTENSIONAHA.112.191874. [DOI] [PubMed] [Google Scholar]
- 35.Iwao M, et al. Sensitive and selective quantification of mid-regional proadrenomedullin in human plasma using ultra-performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020;183:113168. doi: 10.1016/j.jpba.2020.113168. [DOI] [PubMed] [Google Scholar]
- 36.Wakai K, et al. Profile of participants and genotype distributions of 108 polymorphisms in a cross-sectional study of associations of genotypes with lifestyle and clinical factors: A project in the Japan multi-institutional collaborative cohort (J-MICC) study. J. Epidemiol. 2011;21:223–235. doi: 10.2188/jea.JE20100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi K, et al. Study profile of the Japan multi-institutional collaborative cohort (J-MICC) study. J. Epidemiol. 2020 doi: 10.2188/jea.JE20200147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyama T, Kuriyama N, Uehara R. Midregional proadrenomedullin can reflect the accumulation of visceral adipose tissue—A key to explaining the obesity paradox. Int. J. Environ. Res. Public Health. 2020;17:3968. doi: 10.3390/ijerph17113968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Examination Committee of Criteria for 'Obesity Disease' in Japan. Japan Society for the Study of Obesity New criteria for 'obesity disease' in Japan. Circ. J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 40.Yamagishi K, Iso H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health. 2017;39:e2017003. doi: 10.4178/epih.e2017003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.