Abstract
Background
Dual antiplatelet therapy is the current standard of care after acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI). We intended to study the pattern of use of ticagrelor in patients with acute coronary syndrome undergoing PCI and the effect of switching over to other P2Y12 receptor inhibition on clinical outcomes.
Results
All patients aged > 18 years who had been admitted with acute coronary syndrome and had been provided ticagrelor as the second antiplatelet agent were included as study participants. The primary outcome of the study was the composite outcome of death, recurrent myocardial infarctions, re-intervention, and major bleeding.
We studied 321 patients (54 female patients, 16.82%). The mean age of the patients was 56.65 ± 11.01 years. Ticagrelor was stopped in 76.7% on follow-up. It was stopped in 6.3%, 13.5%, 13.1%, 21.9%, and 45.1% of patients during the first month but after discharge, between first and third months, between 3 and 6 months, between 6 and 12 months, and after 12 months, respectively. In the majority of patients, ticagrelor was replaced by clopidogrel (97.9%). It was stopped according to the physician’s discretion in 79.3% of patients, whereas it was the cost of the drug that made the patient to get swapped to another agent in 18.6%. No difference in the primary composite outcome was observed between the groups where ticagrelor was continued post 12 months and ticagrelor was continued and ticagrelor was switched-over to another agent. Similarly, no difference in death, recurrent myocardial infarctions, re-interventions, or major bleeding manifestations was observed between the two groups.
Conclusion
In patients with acute coronary syndrome who undergo PCI, we observed that early discontinuation of ticagrelor and switching over to other P2Y12 inhibitors after discharge did not affect clinical outcomes.
Keywords: Ticagrelor, Acute coronary syndrome, Bleeding, Clopidogrel, Switch over
Background
Coronary artery disease remains a major public health concern in India affecting people at their productive younger age. A recently published study from the state of Kerala estimated a prevalence of any CAD to be 12.5% (men 9.8%, women 14.3%) without any difference in urban and rural population [1]. Percutaneous coronary interventions (PCI) are increasingly used in our country. The role of PCI in patients with acute coronary syndrome especially ST-elevation MI is well established [2]. Anti-platelet drugs play a crucial role in the treatment of ACS. Dual antiplatelet therapy is the established mode of treatment in such scenarios. Until recently, it was clopidogrel that was available in treating such patients. At present, two more ADP receptor antagonists are available that include prasugrel and ticagrelor. It has been shown in TRITON TIMI-38, which was a randomized 13,608 patients with moderate-to-high-risk acute coronary syndromes with scheduled percutaneous coronary intervention to prasugrel or clopidogrel, that patients in the prasugrel arm had significantly reduced rates of ischemic events, including stent thrombosis. They also found that patients in the prasugrel arm had increased risk of major bleeding, including fatal bleeding [3]. Wallentin et al., in PLATO trial, studied 18,624 patients with acute coronary syndrome. They randomized the groups to clopidogrel or ticagrelor. They found that there was a significant reduction (absolute reduction of 1.9%) in the primary endpoint of the study that comprised of death from vascular causes, myocardial infarction, or stroke [4]. The rate of overall major bleeding was the same between the arms with an increase in the rate of non-procedure-related bleeding. Hence, it has been clearly shown in these two large randomized control trials that ticagrelor and prasugrel were superior to clopidogrel in patients with ACS [3, 4]. USFDA has approved both these agents in the treatment of patients with acute coronary syndrome who are getting intervened Though it is presumed that these drugs should act similarly in our Indian patients, there is a lack of indigenous evidence to prove the same. Similarly, the safety and efficacy of switching over from ticagrelor to clopidogrel is being addressed in many global studies. To our knowledge, no data is available from the sub-continent. Therefore, we intended to study the pattern of use of ticagrelor in patients with acute coronary syndrome undergoing PCI and the effect of switching over to other P2Y12 receptor inhibition on clinical outcomes in our study.
Methods
Our study was a non-randomized, retrospective, single-center, observational study. It was an investigator-initiated, non-funded study. All patients aged > 18 years who had been admitted with acute coronary syndrome and had been provided ticagrelor as the second antiplatelet agent in the Department of Cardiology were included as study participants. The study was approved by Institutes Ethics committee, and patients provided their informed consent for the participation in the study. Study participants were identified from medical records through copyrighted software. Those patients who fulfilled the requirements were called individually by a research coordinator for detailed clinical assessment by their respective physicians. In case they were not able to make it in person, the necessary information was obtained from them over the phone. Baseline characteristics of the included but deidentified patients like age, sex, and presence of traditional risk factors for CAD including diabetes, hypertension, and dyslipidemia were studied. Patients were categorized into those with unstable angina, non-ST elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI). All available laboratory parameters were noted including electrocardiogram, transthoracic echocardiogram, angiographic findings, and interventional procedural along with clinically significant bleeding that required transfusions, reintervention, and recurrent myocardial infarction. Patients were specifically asked about their symptoms and adherence to antiplatelet agents. Details about the continuation of ticagrelor after procedure were also noted. Based on the same, they were divided into two groups. Group 1 where ticagrelor was discontinued and group 2 where it was continued. If it was stopped or swapped to a different p2Y12 inhibitor before one year, an attempt was made to analyze the reason for the same. The primary outcome of the study was the composite outcome of death, recurrent myocardial infarctions, re-intervention, and major bleeding requiring transfusions. We also intended to study the reason for the switch-over and its relation with clinical outcomes.
Statistical analysis
Descriptive data were expressed in terms of ratio, proportion, or percentage; mean and median (interquartile range) were used for discrete quantitative data. Continuous variables were analyzed by t test. Categorical variables were analyzed by chi-squared test. A p value < 0.05 was considered significant. SPSS v20 (IBM) was used for statistical analysis.
Results
We identified 336 patients. Out of this, we studied 321 patients (54 female patients, 16.8%) after the exclusion of 15 patients who could not be reached. The mean age of the patients was 56.65 ± 11.01 years (Table 1). The median duration of follow-up was 22 months (interquartile range 18). History of diabetes and hypertension were present in 56.7% and 52.3%, respectively. Prior history of CAD was present in 43.9%. Most of the patients had STEMI (47.4%) while 39.9% had unstable angina, and 12.8% had NSTEMI. The majority of the patients had good LV systolic function with an ejection fraction of > 55% in 57.9%. Mild LV dysfunction (LVEF45–55%), moderate LV dysfunction (30–45%), and severe LV dysfunction (< 30%) were present in 19.9%, 18.7%, and 3.4%, respectively. Nearly one third of the patients (35.2%) were taking aspirin before the index procedure. Some of the patients were receiving other antiplatelet agents that included 26.8% of patients with clopidogrel, 2.2% of patients with prasugrel, and 9.3% of patients with ticagrelor. More than 40% of individuals were using a statin. The history of prior bleeding was noted in 0.9%. The majority of the patients had left anterior descending artery (LAD) territory involvement. Most patients (98.8%) received stents, while 1.2% received plain old balloon angioplasty. Only two patients had received bare-metal stents. The mean stent used per patient was 1.3. Most of the patients had received Gp2b/3a inhibitor (82.9%). Non-culprit vessel intervention was done in 25.5% of patients during the index procedure or the same admission.
Table 1.
Baseline characteristic of patient populations.
| Characteristics | Number-321 | N % |
|---|---|---|
| Age | 56.65 ± 11.01 years | |
| Female | 54 | 16.80 |
| Diabetes mellitus | 182 | 56.70 |
| Insulin-dependent diabetes mellitus | 27 | 8.40 |
| Hypertension | 168 | 52.30 |
| Prior CAD | 141 | 43.90 |
| Unstable angina | 128 | 39.90 |
| NSTEMI | 41 | 12.80 |
| STEMI | 152 | 47.40 |
| EF ≤ 30% | 11 | 3.40 |
| EF ≥ 55% | 186 | 57.90 |
| EF-30–45% | 60 | 18.70 |
| EF-45–55% | 64 | 19.90 |
| Clopidogrel | 86 | 26.80 |
| Prasugrel | 7 | 2.20 |
| Aspirin | 113 | 35.20 |
| Ticagrelor | 30 | 9.30 |
| Statin | 131 | 40.80 |
| Prior bleeding | 3 | 0.90 |
| LAD | 177 | 55.10 |
| LCx-OM | 51 | 15.90 |
| RCA | 88 | 27.40 |
| Left main | 5 | 1.60 |
| Plain old balloon angioplasty (POBA) | 4 | 1.20 |
| DES | 315 | 98.2 |
| BMS | 2 | 0.60 |
| YES | 266 | 82.90 |
| Non-culprit vessel intervention | 81 | 25.50 |
| Recurrent MI | 4 | 1.20 |
| Re-intervention | 3 | 0.90 |
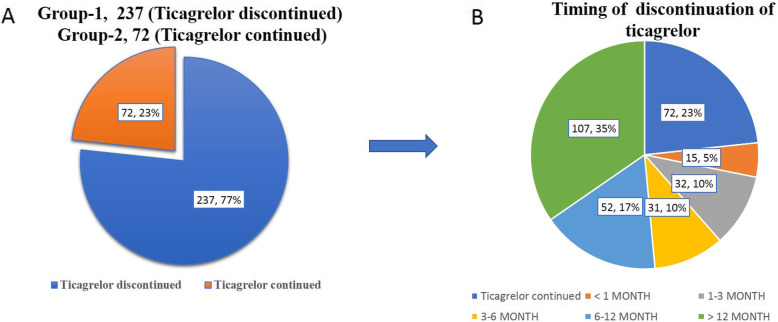
We excluded 12 patients who died before discharge for further analysis, as all patients were on ticagrelor during that period, i.e., before discharge from index hospital admission. This resulted in 309 patient populations for further analysis. No patient died during the follow-up study period. They were classified into group 1 (ticagrelor discontinued) and group 2 (ticagrelor continued) (Fig. 1 and Table 2). Ticagrelor was stopped and switched-over to other P2Y12 inhibitor in 76.7% of patients. Insulin-dependent diabetes status and hypertensive status were significantly more common in the group where ticagrelor was continued (Table 2). The primary composite event happened in 4.6% in the group where ticagrelor was discontinued and 8.3% where ticagrelor was continued (p-0.23, Table 3). Three out of 237 patients in whom ticagrelor was stopped early had recurrent MI while 1 out of 72 patients in the other arm had recurrent MI (P-non-significant). Similarly, no difference was seen in re-interventions and major bleeding between the studied groups (Table 3). The primary composite event happened in 5.5% in the group where ticagrelor was discontinued and 8.3% where ticagrelor was continued (p-0.229, Table 3). Three out of 237 patients in whom ticagrelor was stopped early had recurrent MI, while 1 out of 72 patients in the other arm had recurrent MI (P-non-significant). Similarly, no difference was seen in re-interventions and major bleeding between the studied groups (Table 3). There was no difference in the treated culprit vessel between the groups.
Fig. 1.
a Percentage of patients who discontinued ticagrelor. b Timing of discontinuation of Ticagrelor.
Table 2.
Clinical outcomes in ticagrelor discontinued group and ticagrelor continued group.
| Group 1, n-237 | Group 2, n-72 | p value | |||
|---|---|---|---|---|---|
| n | n% | n | n% | ||
| Female | 44 | 18.60 | 7 | 9.70 | 0.077 |
| Insulin-dependent diabetes mellitus | 13 | 5.50 | 10 | 13.90 | 0.017 |
| Prior bleeding | 7 | 3.00 | 5 | 6.90 | 0.125 |
| Hypertension | 113 | 47.70 | 46 | 63.90 | 0.016 |
| Prior CAD | 101 | 42.60 | 31 | 43.10 | 0.947 |
| Unstable angina | 101 | 42.60 | 24 | 33.30 | 0.16 |
| NSTEMI | 25 | 10.50 | 12 | 16.70 | |
| STEMI | 111 | 46.80 | 36 | 50.00 | |
| EF ≤ 30% | 7 | 3.00 | 1 | 1.40 | 0.234 |
| EF ≥ 55% | 133 | 56.10 | 48 | 66.70 | |
| EF-30–45% | 44 | 18.60 | 14 | 19.40 | |
| EF-45–55% | 53 | 22.40 | 9 | 12.50 | |
| Left anterior descending artery (LAD) | 126 | 53.20 | 41 | 56.90 | 0.709 |
| LCX-OM | 41 | 17.30 | 10 | 13.90 | |
| RCA | 67 | 28.30 | 19 | 26.40 | |
| Left main | 3 | 1.30 | 2 | 2.80 | |
| Recurrent MI | 3 | 1.30 | 1 | 1.40 | 0.936 |
| Re-intervention | 3 | 1.30 | 0 | 0.00 | |
Table 3.
Comparison of baseline characteristics between ticagrelor discontinued and ticagrelor continued arms.
| S. no. | Outcomes | Ticagrelor discontinued (237) | Ticagrelor continued (72) | p value |
|---|---|---|---|---|
| 1 | Composite outcome(n,%) | 11 (4.6%) | 6 (8.3) | 0.23 |
| 2 | Death | 0 | 0 | NA |
| 3 | Recurrent MI | 3 (1.3%) | 1 (1.4%) | 0.94 |
| 4 | Re-intervention | 3 (1.2%) | 0 | 0.34 |
| 5 | Major bleeding | 7 (3%) | 5 (6.9) | 0.12 |
In the majority of those patients, it was stopped before 6 months. Period of stopping was classified empirically into five periods (Table 4 and Fig. 1). They were before the first month but after discharge from index hospitalization, between the first and third months, between 3 and 6 months, between 6 and 12 months, and after 12 months. It was stopped in 6.3%, 13.5%, 13.1%, 21.9%, and 45.1% of patients during the first month, between first and third months, between 3 and 6 months, between 6 and 12 months, and after 12 months, respectively (Table 4). We classified the basis for early dis-continuation into four possible causes (Table 3 and Fig. 2). They were (1) stopped due to the high cost of the drug, (2) stopped due to physician-based discretion, (3) stopped due to non-availability of drugs, and (4) stopped due to side effects. They were stopped according to physician’s discretion in 79.3% of patients, whereas it was the cost of the drug that made the patient to get swapped to another agent in 18.6% (Table 4). Only in a very small number of patients, it was stopped due to non-availability or side effect. Dyspnea was the reason to stop the drug in one patient during follow-up. In the majority of patients, ticagrelor was replaced by clopidogrel (97.9%), while in the remaining prasugrel was used. Most of the patients were loaded with a 300 mg loading dose of clopidogrel followed by 75 mg of maintenance dose while switching-over from ticagrelor. We assessed the effect various factors like diabetes, ACS, LV ejection fraction, reason for stopping ticagrelor, time of stopping ticagrelor, and nature of new drug added instead of ticagrelor on our primary outcome and found no significant association between any of the factors and the primary outcome (Table 5).
Table 4.
Reason for discontinuation of ticagrelor and its timing
| Characteristics | Group-1, n-237 | % | |
|---|---|---|---|
| Reason for stopping ticagrelor | Reason not known | 1 | 0.40 |
| Cost of the drug | 44 | 18.60 | |
| Physician’s discretion | 188 | 79.30 | |
| Non-availability of the drug | 3 | 1.30 | |
| Side effect | 1 | 0.40 | |
| Timing of discontinuation | Group 1, n-237 | ||
| Timing of discontinuation of ticagrelor | < 1 month | 15 | 6.30 |
| 1–3 months | 32 | 13.50 | |
| 3–6 months | 31 | 13.10 | |
| 6–12 months | 52 | 21.90 | |
| > 12 months | 107 | 45.10 |
Fig. 2.
Reason for stopping ticagrelor and switching-over to other P2Y12 agents.
Table 5.
Effect of various factors like diabetes, ACS, EF, reason for stopping ticagrelor, time of stopping ticagrelor, and nature of new drug added instead of ticagrelor on our primary outcome
| Primary outcome happened, N-17 | Primary outcome did not happen, N-292 | p value | |||
|---|---|---|---|---|---|
| n | n% | n | n% | ||
| Diabetes | 7 | 41.2 | 130 | 44.5 | 0.79 |
| Acute coronary syndrome group | |||||
| USA | 7 | 41.2 | 118 | 40.4 | 0.99 |
| NSTEMI | 2 | 11.8 | 35 | 12.0 | |
| STEMI | 8 | 47.1 | 139 | 47.6 | |
| Ejection fraction | |||||
| EF ≤ 30% | 0 | 0.0 | 8 | 2.7 | 0.8 |
| EF ≥ 55% | 11 | 64.75 | 170 | 58.2 | |
| EF-30–45% | 3 | 17.6 | 55 | 18.8 | |
| EF-45–55% | 3 | 17.6 | 59 | 20.2 | |
| Culprit vessel | |||||
| Left anterior descending artery (LAD) | 7 | 41.2 | 160 | 54.8 | 0.32 |
| Circumflex (LCX) | 2 | 11.85 | 49 | 16.8 | |
| Right coronary artery (RCA) | 8 | 47.1 | 78 | 26.7 | |
| Left main or triple vessel disease | 0 | 0.0 | 5 | 1.7 | |
| Reason for stopping | 0.70 | ||||
| Ticagrelor - not stooped | 6 | 35.6 | 67 | 22.9 | |
| Ticagrelor stopped - physician-based decision | 1 | 5.9 | 1 | 14.7 | |
| Ticagrelor stopped - cost | 10 | 58.8 | 178 | 61.0 | |
| Ticagrelor stopped - non-availability of drug | 0 | 0.0 | 3 | 1.0 | |
| Ticagrelor stopped - side effects | 0 | 0.0 | 1 | 0.3 | |
| Nature of drug that was used during switch-over from ticagrelor | |||||
| Ticagrelor continued | 6 | 35.3 | 67 | 22.9 | 0.45 |
| Clopidogrel | 11 | 64.7 | 219 | 75.0 | |
| Prasugrel | 0 | 0.0 | 6 | 2.1 | |
| Timing of ticagrelor stopped and event | |||||
| Not stopped | 6 | 35.3 | 66 | 22.6 | |
| Stopped < 1 month | 2 | 11.8 | 13 | 4.5 | 0.44 |
| Stopped 1–3 months | 1 | 5.9 | 31 | 10.6 | |
| Stopped 3–6 months | 2 | 11.8 | 29 | 9.9 | |
| Stopped 6–12 months | 3 | 17.6 | 49 | 16.8 | |
| Stopped > 12 months | 3 | 17.6 | 104 | 35.6 | |
Discussion
In this real-world single-center experience study, we observed early discontinuation of ticagrelor after discharge, and switching-over to other P2Y12 agents in patients with acute coronary syndrome did not affect clinical outcomes. It was found that ticagrelor was stopped early, i.e., before the end of the first year in the majority of patients. It happened more frequently after 6 months post PCI. Though the cost of ticagrelor remained an important factor in the discontinuation of the drug, it was stopped at the discretion of the physician in the majority of patients. Newer oral P2Y12 receptor blockers like ticagrelor and prasugrel have been shown to have increased bleeding risk as compared to clopidogrel [2, 3]. Similar to the PLATO trial, a large prospective registry from Sweden has shown better outcomes with ticagrelor as compared to clopidogrel [4]. Though few case reports from India attributed increased risk of bleeding to newer antiplatelet agents like ticagrelor [5], large observational studies have documented the safety of ticagrelor and prasugrel in the Indian subset of patients [6–8]. Similar to the other two studies from India, we observed ticagrelor to be safe in Indian patients. Major societal guidelines recommend continuing ticagrelor at least 12 months post-acute coronary syndrome interventions [9–11] based on the PLATO trial. In contrary to the above findings, the CHANGE-DAPT study has shown that ticagrelor was associated with increased events as compared to clopidogrel [12]. They categorized the period into the clopidogrel period (2012–2014) and ticagrelor period (2014–2015). They studied more than 2000 patients with ACS. The primary outcome of their study was net adverse cardiac and cerebral events (NACCE) that included all-cause death, any myocardial infarction, stroke, or major bleeding. They found that the 1-year NACCE rate was significantly higher during the ticagrelor period (5.1% vs. 7.8%; HR 1.53 [95% CI 1.08–2.17]; p = 0.02) that was attributed to more bleeding in these patients without any benefit in ischemic benefits. Cuisset et al. described the benefit of de-escalation of p2y12 inhibitors [13]. They studied 646 patients and found that switching DAPT strategy after a month of PCI in patients with acute coronary syndrome was superior to an unchanged DAPT strategy without any raise in ischemic events following ACS. In their subgroup analysis related to platelet reactivity study [14], they observed that benefit was seen in all groups irrespective of their platelet reactivity as assessed by vasodilator-stimulated phosphoprotein (VASP) assay. It was also found that greater benefits were seen in patients with lower platelet reactivity. In our study, we found no increased clinical events in patients who were continued on ticagrelor as compared to those who had been switched over to other P2Y12 agents. During swapping to clopidogrel, most of our patients were loaded with 300 mg of clopidogrel. In a recently presented study, it was found that loading with 600 mg of clopidogrel appeared a better strategy than 300 mg loading dosage [15].
In addition to the effect of early discontinuation of ticagrelor, we intended to study the reason behind the same. In the Paris registry, patients who had PCI were studied about the effect of cessation of DAPT, the reason behind the same, and its effect on clinical events [16]. We observed that ticagrelor was stopped in 237 patients (76.7%) of patients. They were stopped according to the physician’s discretion in 79.3% of patients, whereas it was the cost of the drug that made the patient to get swapped to other agents in 18.6%. Non-availability of the drug and the side effects were the reason for discontinuation in very few patients only. Clopidogrel was used as the replacement antiplatelet agent in the majority of patients. Ease of availability, long-term safety data, lesser bleeding complications, and cost of clopidogrel might have been the reason for swapping to clopidogrel instead of prasugrel. In one fifth of the patients, the cost of the drug was the reason behind the discontinuation. The availability of generic versions of ticagrelor may change this pattern of practice. We also observed no difference in outcomes of the patients according to the reason for stopping the drug.
Conclusion
In patients with acute coronary syndrome who undergo PCI, we observed that early discontinuation of ticagrelor and switching over to other P2Y12 inhibitors after discharge did not affect the composite outcome of death, recurrent myocardial infarctions, re-intervention, and major bleeding requiring transfusions. More than 75%, ticagrelor was switched-over to another P2Y12 agent. Switching-over to other drug was performed frequently after 6 months post PCI. Around 80% of patients, the change was made by their physician. Clopidogrel was the primary replacement agent.
Limitations
It was a retrospective study. Hence, all possible limitations due to retrospective study hold for this study. Though we arbitrarily categorized the reason for stopping the drug into different categories including the physician’s discretion, this might not be perfectly correct due to the retrospective nature of the study. Period of overlap between the groups cannot be excluded absolutely considering retrospective nature of the study. Events that happened in our study were few. That might be the reason for not having any significant difference between the groups. We could not contact 15 patients. It was a single-center experience. Whether it could be generalized to other centers remains a question.
Acknowledgements
All patients and patients’ advisors who have agreed to participate in this trial. We also thank the Apollo Hospitals, Vanagram, Chennai.
Declarations
For the purposes of submission, please see below:
1) None of the paper’s contents have been previously published;
2) All authors have read and approved the manuscript;
3) There is an agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved;
4) There is full disclosure of any relationship with industry or no such relationship exists.
Abbreviations
- TRITON TIMI-38
Prasugrel versus clopidogrel in patients with acute coronary syndromes
- PLATO
Ticagrelor versus clopidogrel in patients with acute coronary syndromes
- CHANGE DAPT
Clopidogrel or ticagrelor in acute coronary syndrome patients treated with newer-generation drug-eluting stents
Authors’ contributions
NBS: Conceptualization, designing, interpretation supervision, writing the original draft, review, and editing. RS-Interpretation, review, and editing. YR-Acquisition, analysis, review, and editing. DT-Acquisition, analysis, review, and editing. AM−Acquisition, analysis, review, and editing. DM−Acquisition, analysis, review, and editing. DK−Acquisition, analysis, Review & Editing. MJK-Formal analysis and software. SKS-Acquisition, analysis, review, and editing. AS-Acquisition, analysis, review, and editing. PKJ-Acquisition, analysis, review, and editing. HP-Acquisition, analysis, review, and editing. AL-Acquisition, analysis, review, and editing. AG-Acquisition, analysis, review, and editing. KNR-Interpretation, review, and editing. KAA-Interpretation, guarantor, review, and editing. In addition, all authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
None.
Availability of data and materials
If required, we can submit the data sheet.
Ethics approval and consent to participate
Institute’s Ethics committee clearance was obtained; IEC-Clincal studies; Apollo hospitals, Chennai; IEC-AVH-002/10–16. Written informed consent to participate was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nagendra Boopathy Senguttuvan, Email: nagendraboopathy@sriramachandra.edu.in, Email: drsnboopathy@gmail.com.
Ramesh Sankaran, Email: sankaran_ramesh2000@yahoo.co.in.
Yashasvi Rajeev, Email: Rajeev_jrsh@yahoo.co.in.
Devi Thaiyal, Email: devithaiyal@yahoo.com.
Angel Mathew, Email: mathew.angel7@gmail.com.
K. Dharsini, Email: Dharslovely94@gmail.com
Divya Marcelene, Email: Pulse2802@gmail.com.
Maria Jusler Kalsingh, Email: kalsingh1989@gmail.com.
Sujit Kumar Sahu, Email: drsujitsahu@gmail.com.
Aravind Sampath, Email: draravindsampath@yahoo.co.in.
K. J. Prem Kumar, Email: drkjprem@gmail.com
Harikrishnan Parthasarathy, Email: krishnankala@hotmail.com.
Amal Louis, Email: dramallouis@gmail.com.
Anand Gnanaraj, Email: gnanaraj.anand@gmail.com.
K. N. Reddy, Email: drknr2003@yahoo.co.in
K. A. Abraham, Email: drabraham_ka@apollohospitals.com
References
- 1.Krishnan MN, Zachariah G, Venugopal K, Mohanan PP, Harikrishnan S, Sanjay G, et al. Prevalence of coronary artery disease and its risk factors in Kerala, South India: a community-based cross-sectional study. BMC Cardiovasc Disord. 2016;16:12. doi: 10.1186/s12872-016-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, CH MC, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 4.Sahlén A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37(44):3335–3342. doi: 10.1093/eurheartj/ehw284. [DOI] [PubMed] [Google Scholar]
- 5.Suryanarayana Sharma PM, Tekkatte Jagannatha A, Javali M, Hegde AV, Mahale R, Null M, et al. Spontaneous subdural hematoma and antiplatelet therapy: does the efficacy of Ticagrelor come with added risk? Indian Heart J. 2015;67(Suppl 3):S30–S35. doi: 10.1016/j.ihj.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaul U, Sethi A, Arambam P, Omar AK, Keshava R, Roy S, et al. Safety of Prasugrel in Indian patients - multicentric registry of 1000 cases. Indian Heart J. 2014;66(6):598–601. doi: 10.1016/j.ihj.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalal JJ, Digrajkar A, Gandhi A. Oral antiplatelet therapy and platelet inhibition: an experience from a tertiary care center. Indian Heart J. 2016;68(5):624–631. doi: 10.1016/j.ihj.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Kumari K, Talwar KK, Prasad D, Agarwal S, et al. Clinical safety profile of ticagrelor compared to clopidogrel in 1208 patients: real-world evidence. Egypt Heart J EHJ Off Bull Egypt Soc Cardiol. 2018;70(4):375–378. doi: 10.1016/j.ehj.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–e155. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 10.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 11.Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 12.Zocca P, van der Heijden LC, Kok MM, Löwik MM, Hartmann M, Stoel MG, et al. Clopidogrel or ticagrelor in acute coronary syndrome patients treated with newer-generation drug-eluting stents: CHANGE DAPT. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2017;13(10):1168–1176. doi: 10.4244/EIJ-D-17-00634. [DOI] [PubMed] [Google Scholar]
- 13.Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070–3078. doi: 10.1093/eurheartj/ehx175. [DOI] [PubMed] [Google Scholar]
- 14.Deharo P, Quilici J, Camoin-Jau L, Johnson TW, Bassez C, Bonnet G, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome according to on-treatment platelet reactivity: the TOPIC-VASP pre-specified analysis of the TOPIC randomized study. JACC Cardiovasc Interv. 2017;10(24):2560–2570. doi: 10.1016/j.jcin.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 15.Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382(9906):1714–1722. 10.1016/S0140-6736(13)61720-1. Epub 2013 Sep 1. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If required, we can submit the data sheet.