Summary
Anastomotic leakage is one of the most severe complications after esophagectomy and is associated with increased postoperative morbidity and mortality. Several projects ranging from small retrospective studies to large collaborations have aimed to identify potential pre- and perioperative risk factors and to improve the diagnostic processes and management. Despite the increase in available literature, many aspects of anastomotic leakage are still debated, without the existence of widely accepted guidelines. The purpose of this review is to provide a cutting edge overview of the recent literature regarding the definition and classification of anastomotic leakage, risk factors, novel diagnostic modalities, and emerging therapeutic options for treatment and prevention of anastomotic leakage following esophagectomy.
Keywords: anastomotic dehiscence, anastomotic leakage, esophageal cancer, esophageal resection, gastric tube reconstruction
INTRODUCTION
For patients with locally advanced esophageal cancer, a radical esophageal resection offers the best chance for cure. Anastomotic leakage (AL), one of the most severe complications, leads to significant morbidity, prolonged hospital stay, considerable use of healthcare resources, and increased risk of mortality.1 In the long term, AL has been associated with poorer quality of life, increased cancer recurrence rates, and subsequently worsened long-term survival. The incidence of AL ranges between 11.4 and 21.2%,2–5 with an associated mortality rate between 7.2 and 35%.1 In spite of the increasing research efforts, leakage pathophysiology and causal factors remain unclear. Even though AL has a multifactorial etiology, tissue perfusion seems to play a pivotal role in leakage development. Moreover, clinical symptoms for AL often only become manifest in a later stage or are nonspecific, while a large variability of diagnostic and treatment options are available, without a clear consensus on standardized procedures.
The aim of this review is to provide a cutting edge overview of the available literature for the definition and classifications of AL, main and emerging pre- and perioperative risk factors, available diagnostic modalities, and different therapeutic options.
SEARCH METHODOLOGY
The Scopus and PubMed electronic database were searched to identify original articles published from year 1995 to 2019 on AL after esophagectomy. The keywords used included the terms: ‘anastomotic leakage’, ‘esophageal carcinoma’, ‘cervical’, ‘intrathoracic’, ‘diagnosis’, ‘management’, ‘risk factors’, combined through the Boolean ‘OR’ function. References and citing articles of most relevant publications were searched for additional studies. English language restrictions were adopted.
DEFINITION OF AL
A clear definition of what constitutes an AL after esophagectomy has long been a matter of discussion. Several attempts have been made to establish a commonly accepted definition and classification of leakage and severity of the lesion in the perspective of an optimized transfer of information across centers. The absence of a standardized system for defining and recording complications and quality measures after an esophageal resection has determined certain variability in evaluating their impact and outcomes. The Clavien–Dindo classification, also integrated in the Comprehensive Complication Index,6 has been widely adopted, even though it is not specific for surgical complications. Despite the proposal of several classifications systems for AL, none has achieved wide acceptance. Based on a systematic review of 97 studies, a standard definition was proposed by Bruce et al., 20017 and later integrated by Lerut et al., 20027 and Price et al., 20137 (Table 1). In search for a clearer definition, a novel grading system has been recently proposed by Esophagectomy Complications Consensus Group (ECCG).8 The ECCG system defines ALs as a ‘full-thickness gastrointestinal defect involving esophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification’ and grades it into three severity types (Table 1). Consensus was also reached for three other post-esophagectomy complications: conduit necrosis, chyle leakage, and recurrent nerve palsy. The advantages of a standardized classification system have been clearly demonstrated in a large study reporting the outcome of 2704 esophagectomies,5 showing remarkably reduced variation in the incidence of complications across 24 contributing centers. Since this benchmarking paper, the ECCG complication system has been increasingly adopted for reporting on outcomes after esophagectomy.
Table 1.
Main classification systems of anastomotic leakage
| System | Leak classification | Grade | Signs and symptoms (or definition) | Management |
|---|---|---|---|---|
| Bruce et al., 2001* | Radiological | − | • Detected only on routine imaging; no clinical signs | • No change |
| Clinical minor | − | • Luminal contents through the drain or wound site (local inflammation) • Fever (>38°C) or leukocytosis (>10,000/L) • Leak may also be detected on imaging studies |
• Prolonged hospital stay and/or delay in resuming oral intake | |
| Clinical major | − | • As clinical minor with severe disruption to anastomosis • Leak may also be detected on imaging studies |
• Intervention required | |
| Lerut et al., 2002† | Radiological | − | • No clinical signs | • No change |
| Clinical minor | − | • Local inflammation cervical wound • X-ray contained leak (thoracic anastomosis) • Fever, > WBC, > CRP |
• Drain wound • Delay oral intake • Antibiotics |
|
| Clinical major | − | • Severe disruption on endoscopy • Sepsis |
• CT-guided drainage or reintervention | |
| Conduit necrosis | − | • Endoscopic confirmation | • Reintervention | |
| Price et al., 2013‡ | Radiological | I | • No clinical signs or symptoms • Purely radiological diagnosis |
• No change in management |
| Clinical minor | II | • Minor clinical signs (e.g. cervical wound inflammation or drainage) • Radiographically contained intrathoracic leak • Fever, leukocytosis |
• Delay oral intake • Antibiotics • Wound drainage • CT-guided drain placement |
|
| Clinical major | III | • Significant anastomotic disruption requiring surgical—revision • Minor anastomotic disruption with systematic sepsis |
• Esophageal stent placement • Surgical debridement • Anastomotic revision |
|
| Conduit necrosis | IV | • Conduit necrosis necessitating esophageal diversion | • Conduit resection with esophageal diversion | |
| Low et al., 2015§ | Anastomotic leakage | I | • Local defect | • No change in therapy or medical treatment or dietary modification |
| II | • Local defect | • Interventional radiology drain • Stenting or bedside opening • Packing of incision |
||
| III | • Local defect | • Surgical therapy | ||
| Conduit necrosis | I | • Focal (identified endoscopically) | • Additional monitoring or nonsurgical therapy | |
| II | • Focal (identified endoscopically, not associated with free anastomotic or conduit leakage) | • Surgical therapy without esophageal diversion | ||
| III | • Extensive | • Surgical therapy: conduit resection with diversion |
*Bruce, J., Krukowski, Z. H., Al-Khairy, G., Russell, E. M. & Park, K. G. M. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. British Journal of Surgery (2001) doi:10.1046/j.0007-1323.2001.01829.x.
†Lerut, T. et al. Anastomotic complications after esophagectomy. in Digestive Surgery (2002). doi:10.1159/000052018.
‡T.N., P. et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann. Thorac. Surg. (2013).
§Low, D. E. et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. (2015) doi:10.1097/SLA.0000000000001098.
RISK FACTORS
Identification of risk factors for AL is of critical importance for prevention and treatment, as well as for pre- and postoperative optimization. Several comorbidities, use of neoadjuvant treatment, anastomotic location, surgical technique, and perioperative monitoring techniques and therapies are among the most important and debated risk factors for AL development. Some of the risk factors are modifiable and, consequently, may guide a patient-tailored management of pre-, peri-, and postoperative strategies. Nevertheless, no overall agreement has been reached on which of them are the most decisive in AL development, leading to the lack of reliable predictive models and tools for a standardized preoperative risk assessment.
Comorbidities
Anatomical and physiological factors, including the intrinsic esophageal anatomy with the lack of an esophageal serosa and the negative pressure within the thoracic cavity, may contribute to the development of AL. A plethora of comorbidities, such as preoperative malnutrition (albumin <3.0 g/dL), obesity (body mass index [BMI] > 30 kg/m2) or underweight patients (BMI < 18.5 kg/m2), heart failure, hypertension, diabetes, renal insufficiency, steroids, and tobacco use, were significantly associated with an increased AL rate.1 Atherosclerotic calcification of the aorta and the arteries supplying the gastric tube has been identified as an emerging risk factor in both cervical and intrathoracic ALs. The calcification scoring system may aid in patient selection, leading to earlier diagnosis of this potentially fatal complication.9
Neoadjuvant treatment
Evidence about the effect of the extent and dosage of neoadjuvant chemoradiation on the occurrence of AL is conflicting, particularly regarding a ‘safe’ dose of radiation to the gastric fundus, the anastomotic region used in gastric tube reconstruction. No significant association was observed in cervical anastomosis for an average radiation dose of 24.2 Gy,10 while exceeding a dose of 31 Gy seems to increase AL incidence.11 In contrast, a large European multicenter study reported no impact of radiation up to 45 Gy on AL rate,12 as also confirmed by Nederlof,13 with an average of 41.1 Gy. Irrespective of AL incidence, irradiation of the upper mediastinum is associated with more severe complications.10
Anastomotic location
A cervical anastomosis has a five times greater risk of leakage when compared to intrathoracic location.14 The main causes include the need for a longer gastric conduit, more likely positioned in the fundus (where the vascularity is more compromised), and increased risk of tension and/or compression at the junction between thorax and neck. The higher AL risk in the neck may also be influenced by the indication for this procedure (more proximal tumors and/or lymph node metastases, a higher radiation field and a more extended resection).10 However, the mortality rate is unaffected by the site of the anastomosis, 2 although a cervical location may lead to increased recurrent laryngeal nerve paresis, wound infection, and longer hospital stay.15
Surgical technique
The minimally invasive esophagectomy (laparoscopy and thoracoscopy) or the hybrid approach (laparoscopy and thoracotomy) have been introduced to minimize surgical trauma and reduce perioperative complications (particularly pulmonary infections) as compared to open surgery.16 Most studies show no difference in the incidence of AL between open and either minimally invasive or hybrid techniques.16,17 If a higher AL rate is shown, this can most likely be explained by the proficiency gain curve-associated morbidity, since the minimally invasive approach is a technically challenging procedure that requires long and adequate training.18 Independently from the surgical approach, technical precautions are commonly considered important aspects to decrease AL risk, e.g. avoidance of excessive traction, compression or twist, and an incorrect number of sutures or incomplete donuts in a mechanical anastomosis. Omentoplasty seems an important surgical procedure to prevent leakage, both in cervical and intrathoracic anastomoses, as well as in manually and mechanically created anastomoses.19 The omentum will form adhesions with the underlying tissues, localizing potential inflammations and sealing microscopic leakage.
Anastomotic technique
Which of the most commonly performed esophageal anastomotic techniques has the lowest leakage rates remains controversial. In general, the cervical and intrathoracic anastomoses are more frequently performed hand-sewn and stapled, respectively.20 In the hand-sewn method, a single-layer continuous sutured anastomosis is the most commonly adopted technique, although some studies suggest a lower leakage rate following a two-layered anastomoses.21 Moreover, an end-to-end anastomosis seems to have a lower leakage incidence compared with an end-to-side technique, especially in cervical anastomosis.22 Although some studies support the superiority of the linear-stapled on hand-sewn technique, their comparison does not seem to unequivocally prove consistent differences in leakage rates and postoperative outcomes.14 Similar AL rates also emerge from the comparison of circular-stapled (both 25 and 29 mm), linear-stapled, and hand-sewn techniques.23–25 Therefore, irrespective of the quality of scientific evidence, the difference in anastomotic technique does not seem to influence AL rate.
Perioperative monitoring and therapy
Accurate monitoring of perioperative conditions has a significant impact on AL development. In particular, hemodynamic management is essential for maintaining an adequate perfusion of the anastomosis by preventing a reduction of the oxygen tension (pO2) in the gastric conduit, a major cause of leakage development. Intraoperative fluid administration and the use of vasopressors require particular caution. While restrictive fluid management is increasingly recommended to avoid postoperative pulmonary and anastomotic complications,26 an excessive fluid restriction can lead to hypotension and anastomotic dehiscence as well.27 To optimize intraoperative fluid administration, goal-directed therapy based on three hemodynamic parameters (stroke volume, mean arterial pressure, and cardiac output) has been proposed in noncardiac surgery.28 This algorithm has also been implemented in esophageal cancer surgery, particularly by focusing on the stroke volume parameter, observing a reduction of pneumonia, mediastinal abscesses, and gastric tube necrosis.29
Several studies have attested the beneficial effects of thoracic epidural analgesia (TEA) on intestinal perfusion during esophagectomy, due to improved microcirculation. TEA has positive effects on the reduction of AL incidence, albeit the activation of an extensive sympathetic block must be avoided due to the risk of reduced blood flow.30 Administration of ephedrine during the operation seems to increase tissue perfusion in the gastric tube and main arterial pressure (thereby potentially reducing ischemic conditions at the anastomotic site). This might be a potential coadjuvant in leakage prevention.31
INTRAOPERATIVE INVESTIGATIONS
Ischemia of the tip of the gastric conduit, defined as inadequate graft perfusion, is one of the major factors contributing to esophagogastric AL.32 Etiology of ischemia is complex but mostly arises from the inability of the isolated right gastroepiploic artery to ensure adequate blood supply to the whole conduit. In spite of the significant improvement of surgical techniques and operative procedures, perfusion-related complications (such as ischemia and necrosis) remain considerably high, ranging from 2.5% to 20%.33 Complete conduit necrosis is a rare but devastating complication occurring in less than 3% of esophagectomies.34 Intraoperative real-time monitoring of the status of conduit perfusion, eventually detecting early conduit ischemia (still reversible), is crucial for choosing the optimal site for the anastomosis based on vascular pattern. In case of gastric conduit reconstruction failure, alternative strategies for esophageal replacement (i.e. jejunal or colon transposition) should be adopted.35 A variety of analytical or biochemical methodologies have been proposed to evaluate tissue perfusion, mainly including conventional angiography, measurement of transmucosal oxygen saturation, and intraoperative esophagogastroduodenoscopy (as recently reviewed).36 None of them have achieved widespread acceptance.
Different optical techniques have also been developed to evaluate perfusion intraoperatively (real-time high-resolution imaging of blood flow), such as laser Doppler flowmetry, fluorescence imaging, near infra-red spectroscopy, laser speckle contrast imaging, optical coherence tomography (OCT), and sidestream darkfield microscopy (SDF), each with its own pros and cons as recently in depth reviewed by Jansen.37 None of them are able to combine all features of an ideal technique, such as ease of execution, contactless, wide field of view, depth-resolved imaging, and quantitative outcomes. Fluorescence imaging (also known as indocyanine green fluorescence angiography) is the most currently used, since this technique is easy to perform, is contactless, and has a wide field-of-view. Fluorescence imaging, based on intravenous injection of indocyanine green (ICG, a fluorescent molecule) and a near-infrared camera, has several advantages, including the assessment of the microvasculature network and a macroscopic view of regional organ perfusion; a short plasma half-life of ICG, allowing multiple readministrations during the same operation; and its easy elimination by bile excretion, permitting the use in patients with chronic renal disease. An increasing number of studies have demonstrated its safety, reliability, and suitability for intraoperative investigation (Fig. 1).38,39 The major drawback is the inability to directly measure perfusion (e.g. mL/min/gram) that can only be evaluated through technical-derived parameter(s). Indeed, the fluorescence intensity has been correlated with different qualitative outcomes, including microcirculation, blood flow, and routes. Furthermore, the distance of the demarcation point of the gastric tube toward the anastomosis was correlated with increased leakage risk (the longer this distance, the higher the risk).32 Also, the timing from initial ICG enhancement at the root of the right gastroepiploic artery until the gastric tube tip has been used as an estimator of perfusion, leading to the proposal of the 90-second rule.40 However, all derived parameters are subjective and cannot be translated into widely accepted and standardized protocols, while attempts to develop quantitative parameters have not still generated reliable results. Among other recently developed techniques, OCT and SDF are promising, but only preliminary evidence is available in literature. In particular, SDF evaluates not only the gastric tube perfusion microscopically but also the venous congestion in the fundus, which seems to play an important role in the development of ischemia (Jansen, submitted for publication).
Fig. 1.
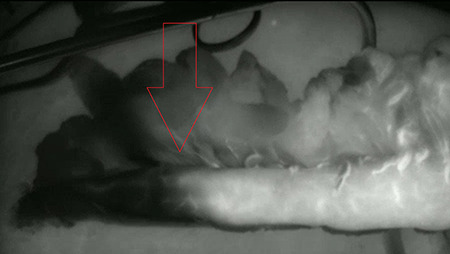
Gastric conduit before transfer to cervical region. This is a near infrared fluorescence view with the fluorescent signal displayed in white. A clear demarcation is noticed at the red arrow. The anastomosis was constructed within the fluorescent area.
ISCHEMIC PRECONDITIONING
Ischemic preconditioning was previously proposed by Urschel41 with the aim to improve tissue perfusion of the gastric fundus to prevent occult ischemia.32 This approach consists of the occlusion (days to a week before the planned resection and anastomosis) of most or all of the gastric arteries, except for the right gastroepiploic artery, in order to provide time for the stomach to adapt to the reduced oxygenation occurring during its mobilization for the creation of the conduit.42 Devascularization is obtained through preoperative arterial embolization or laparoscopic ligation. In spite of some positive results reporting a reduction in AL, clinical evidence about the efficacy of ischemic preconditioning still remains largely contradictory.42,43 Gastric preconditioning does not seem to reduce the overall AL rate after an esophageal resection but seems to affect the severity of the leakage. This is probably due to an increased conduit resistance to ischemia rather than an improvement of perfusion due to neovascularization. Ischemic preconditioning is still an active research field, particularly regarding its exact role in the prevention of leakage.
DIAGNOSIS
An overall consensus on the AL diagnostic process is still lacking, particularly regarding the timing and the choice of the diagnostic strategy. The following paragraphs summarize the general procedures for AL diagnosis, which include the evaluation of clinical signs and biochemical analysis, such as blood and drain fluid tests, the support of diagnostic imaging tools, and the timing of the diagnostic process.
Clinical presentation
Clinical manifestations of a leakage range from no signs to fulminant sepsis. Many factors affect clinical presentation, such as the location of the anastomosis, the size of the defect, the ability to drain the fluid collection, and leakage containment. Common initial clinical AL signs include fever and wound abscess (in case of a cervical anastomosis); however, sometimes the first indicator of an anastomotic defect may only be a tachycardia, often in the form of atrial fibrillation.44 An erythema or induration along the neck incision, evident saliva-type fluid, pus, or air discharge from the wound is seen in cervical leakages. In certain cases, this infection can descend into the thorax and generate mediastinal abscesses, pleural emphysema, sepsis with an intrathoracic focus, and tracheaesophageal fistula.45 These manifestations are more common after a transthoracic esophagectomy than after a transhiatal approach, despite the fact that both anastomoses are located in the neck. This could be explained by differences in pleural dissection. During the transthoracic esophagectomy, the thoracic inlet is extensively dissected, while in a transhiatal esophagectomy, the parietal pleura remains intact in the superior mediastinum, where only the esophagus is stripped. This may effectively confine any infectious process and prevent extension into the pleural cavity. In addition, the negative thoracic pressure may facilitate leakage extension into the chest, particularly in the transthoracic approach.46 The clinical signs of an intrathoracic leakage may vary from chest pain and dyspnea to bronchopneumonia, mediastinitis, and respiratory failure. Sepsis can progress to multi-organ failure. Other signs include the presence of saliva or gastric contents in the drain (if present) and persistent cough, especially on swallowing.
Biochemical analysis
Apart from clinical signs, some tests can raise the suspicion of a leakage. A high level of blood inflammatory biomarkers (C-reactive protein (CRP), procalcitonin, and white blood cell counts) is one of the first indicators of AL. C-reactive protein content seems to be the most informative test, both in cervical and intrathoracic anastomoses.47 A CRP value around 17 mg/dL on postoperative day (POD) 3 has been identified as a significant diagnostic cutoff value for leakage development. Measurement of drain amylase levels is another useful tool for early diagnosis. A high amylase content in the external drain is a sign of salivary contamination and, therefore, indicative of leakage.48 A cutoff value for drain amylase level to indicate possible AL varies in literature from 125 to 250 UI/L on POD 4.49 Whether drain amylase values have a comparable accuracy for cervical and intrathoracic anastomoses has been not entirely clarified.
Diagnostic imaging
Several diagnostic modalities are available for AL detection, including contrast swallow examination (esophagography), computed tomography (CT) scan, and endoscopy.
Contrast swallow examination
Esophagography is a cost-effective and relatively safe approach to assess anastomotic integrity, providing information on the contour and emptying of the replacement conduit and the patency of the pylorus. It has a high specificity in AL detection, indicated by contrast material extravasating from the anastomosis or intrathoracic stomach into the mediastinum,50 but it is prone to false-negative test results. Sensitivity varies widely in literature and ranges between 33 and 52% with particularly poor results in cervical anastomoses, as reviewed by Jones et al.51 Other drawbacks include the requirement of an experienced radiologist for a reliable interpretation and the involvement of contrast agents (having well-known side effects and contraindications in patients with sepsis or altered swallowing function or altered consciousness and in sedated patients).52
CT scan
CT scanning is a noninvasive, safe-to-use, and widely available technique for investigating leakage.53 It has some intrinsic advantages, including simultaneous visualization of the neck, thorax, and abdomen during a single examination; applicability in postoperative patients with limited mobility or in critically ill patients; visualization of the extension and location of extraluminal fluid collections that might be accessible for percutaneous drainage; and the possibility to cover a broader range of diagnosis (e.g. pulmonary complications, abscess, pleural effusion, pneumothorax, or pulmonary abnormalities). The critical point of this diagnostic modality is the lack of consensus and objective criteria on radiographic findings associated with leakage, leading to low and variable accuracy 54. Leakage diagnosis by CT scanning is supported by visualization of free or contained extraluminal gas, fluid, and/or contrast material in the mediastinum or by visualization of a defect in the esophageal wall.50 However, the presence of mediastinal air near the anastomosis is not specific, since this can be often seen after esophagectomy without the presence of AL. The oral administration of a contrast agent improves CT sensitivity, although this is subjected to complications associated with aspiration as described before.48 The combination of different, well-recognizable CT findings (such as mediastinal fluid and air, anastomotic wall discontinuity, and fistula) in a CT-based risk score seems to improve diagnostic accuracy.54 Considering the well-known pitfalls of predictive models, the reliability of such approaches should be further validated, possibly across different centers. Recently, a new screening method based on the count of air bubble numbers (i.e. air density around anastomosis and mediastinal space larger than 2 mm) has been proposed to increase CT sensitivity.55 Further research is needed to validate whether this method may effectively improve diagnosis.
Endoscopy
Upper gastrointestinal endoscopy is a safe and useful approach to diagnose and treat the possible leakage in the same session. As demonstrated in several clinical model studies, the operating intraluminal pressure due to the endoscopy does not pose risks of anastomosis disruption. 52 Endoscopy is a reliable diagnostic modality, since specificity and sensitivity can reach almost 95%,56 although the diagnostic value seems to be lower in a cervical anastomosis (sensitivity 56%).57 It has many advantages: the ability to provide information about the vitality of the gastric conduit and to identify alterations of its integrity; the possibility to execute in an ICU setting in intubated patients; and the avoidance of oral administration of contrast agents. Apart from the requirement to have an experienced endoscopist, endoscopy has a crucial limitation: the inability to visualize the perianastomotic environment (e.g. pleural fluid/collections, relation of mediastinal collection to aorta and trachea, etc.). In addition, patients who are on the ward need to be sedated to undergo this investigation. Therefore, they generally need to be transferred to the ICU and temporarily intubated, with the associated risk of respiratory complications.
In summary, each diagnostic modality has its own pros and cons. A CT scan is often preferred as a first-level examination, while endoscopy is usually performed as a second-level investigation, to confirm uncertain CT diagnosis and possibly initiate treatment.58 Despite the lower sensitivity compared to the other diagnostic modalities, contrast swallow examination is still used in clinical practice but is progressively replaced by the other modalities. The combination of CT scan and endoscopy is emerging as the gold standard to diagnose AL, as both the mucosal integrity and the perianastomotic conditions can be examined.
Diagnostic timing
Early and timely diagnosis of leakage is crucial to avoid potentially fatal complications, decrease hospital stay, and clinical burden. A delayed diagnosis negatively influences patient’s prognosis. The timing of imaging investigations to reach an early diagnosis is a topic of continuous debate, as the period of the manifestation of AL may vary considerably.59 The on-demand approach is prevalently based on the clinical observation of the patient during the postoperative course, assisted by the monitoring of the blood inflammatory index and, in some centers, amylase drain levels,48 while imaging investigations are reserved for patients displaying clinical signs of AL and/or biochemical tests above the normal range. This approach has been subjected to some criticism, since effectiveness depends on the many factors that may delay diagnosis, including the presence of clinical manifestations and the surgeon’s experience to recognize early clinical deterioration. The introduction of routine imaging post-esophagectomy to achieve an early diagnosis in asymptomatic patients has been questioned in several studies. Endoscopy has been the main proposed tool, as allowing both direct AL visualization and evaluation of mucosa degeneration (ischemia, necrosis) as an early predictor of leakage development.52,60,61 Only few studies evaluated the effectiveness of a CT scan or esophagography for this purpose. 55,58 The main issue of a routinely approach arises from the unpredictability of the onset of AL development, hence the difficulty of establishing a precise range for the diagnostic timing. Routine endoscopy seems to have a high predictive value if performed between POD 7 and 1460; however, some patients developed leakage before POD5.52 Fujiwara61 suggested very early and repeated endoscopy (POD1 and 3), based on the assessment of mucosal color change in the proximal gastric graft. In contrast, other studies reported no benefit from routinely performed imaging, since it led to the modification of management in only a few cases.62 On-demand assessment is also supported by the higher AL incidence found in symptomatic patients compared to asymptomatic patients (33 vs. 12%, respectively).63 Moreover, a routine check without clear pathological findings cannot exclude the development of a future leakage, thus may be ineffective for improving early diagnosis, as confirmed by the low sensitivity of routine imaging (endoscopy and esophagography) in asymptomatic patients.57
LEAKAGE MANAGEMENT
The broad and diversified clinical spectrum associated with AL manifestations has been the main cause for the lack of a standardized strategy for treatment. The basic principles of management strategies are the closure or coverage of the anastomotic defect, leakage containment, and drainage of fluid collections. However, the choice for a certain therapeutic strategy mainly depends on the localization and size of leakage, the severity of symptoms, the presence of conduit ischemia or necrosis, and the time after surgery when it becomes manifest. Prioritization of these factors is not well defined, and they may rank differently in the treatment algorithm depending on the study or center. Management strategies have evolved from early algorithms, prioritizing the severity of symptoms, anastomotic location or leakage size, and ischemic degree to more recent ones, primary considering ischemic degree and diagnostic timing.59 Even in the absence of a consensus guideline, strategies have progressively shifted from aggressive surgery toward more conservative approaches, along with an increasing adoption of endoscopic interventions. Agreement has generally been established to use conservative treatment for asymptomatic or minimally symptomatic leakage, surgery for early leakages and/or severe septic patients, and endoscopic techniques for all other cases. However, impressive differences exist between surgical practices, with treatment variations between aggressive surgical management for minimally symptomatic leakages and conservative management for gastric tube necrosis.64 In the following section, an overview of the different management options is provided, particularly focusing on common indications emerging in a growing number of studies from varying centers.
Conservative strategies
As a general principle, conservative strategies include a range of measures indicated for the treatment of late, asymptomatic, or minimally symptomatic cervical and intrathoracic AL. Nonsymptomatic or minimally symptomatic well-contained leakages (particularly cervical) are usually managed by a nil-by-mouth regimen combined with enteral (jejunostomy) or parenteral nutrition for a median period of 1–3 weeks; in nonspontaneously drained cervical AL, the neck wound should be opened and rinsed.65 In a minimally symptomatic intrathoracic AL, an accurate surveillance is suggested, with the possibility of interventional mediastinal drainage (in case of fluid collection). Systemic treatment usually consist of broad-spectrum antibiotic therapy (according to infectious parameters), by the use of anticholinergic medication (to reduce saliva), anti-acid drugs (PPI) and prokinetics (to decrease AL volume).62
Endoscopic treatment
The progressive development and improvement of endoscopic techniques has provided an alternative to surgery for those cases not manageable with a conservative approach, i.e. symptomatic leakage without sepsis and/or severe conduit ischemia. Endoscopic techniques include self-expandable metallic stents (SEMS), endoscopic vacuum therapy (EVAC), stent-over-sponge (SOS) therapy, clipping with the over-the-scope-clip (OTSC) system, suturing with overstitch, and the use of a sealant.
Stent
This technique consists of positioning a prosthesis in the esophageal lumen that covers the defect waiting for tissue healing. The choice for stenting strictly depends on the possibility to also apply drainage close to the leakage, in order to drain collected fluid.20 In relation to the two main complications (stent migration and tissue overgrowth), fully covered self-expanding metal stents (FSEMS) guarantee a better compromise, partially covered SEMS with uncoated terminal portions have a higher risk of tissue overgrowth, while self-expanding plastic stents have a higher risk of migration.66 The low anchoring capacity of FSEMS can be overcome by fixing the prosthesis through endoscopic suturing and clipping67 or using stents with larger diameter or colonic stents (up to 32 mm).68 The median stenting time to achieve healing is 4–8 weeks (or even shorter),69 as also demonstrated in animal model studies. Stent use is indicated for leakages extended less than 70% of the circumference20; although in recent studies, endoscopic treatment is not recommended for circumferences >30%.59 Stenting effectiveness improves in leakages with smaller luminal opening size and shorter time to diagnosis. 70 Failure risk is generally high in proximal cervical leakage, mainly due to the difficult fixation in residual esophagus and consequent tendency to stent migration. Also, the positioning close to the esophageal sphincter may cause airway compromise, globus sensation (that can be avoided by leaving at least 2 cm between the upper edge of the stent and the upper esophageal sphincter), pain, and aspiration pneumonia. Dedicated or custom-designed stents for the upper part of the esophagus are in development, characterized by smaller post-deployment diameters and shorter upper flared ends, but evidence of their effectiveness is still limited. 71 Apart from a cervical location, other risks of stent failure include esophageal injury longer than 6 cm and stent positioning more than 2 days after leakage development.72 The described complications from stenting are erosions or ulcers, bleeding, aspiration pneumonia, perforation, fistula, and reflux.
Endoscopic vacuum therapy
This technique requires a flexible endoscope to place an open-pored polyurethane sponge into the cavity (intracavitary) or within the esophageal lumen (intraluminal).73 By a nasogastric tube, this sponge is connected to a low vacuum drainage system. A negative pressure (about −100 to −125 mmHg)74 allows the suction of underlying tissue, providing a continuous wound drainage, removing secretions, and decreasing bacterial contamination and edema, as well as promoting granulation tissue proliferation and improving microcirculation. An intracavitary sponge is usually adopted for accessible paraesophageal extraluminal cavities; an intraluminal sponge is generally preferred for defects with diffuse local inflammation or shallow cavities, since these ensure a faster closure and a reduced risk of complications.75 The sponge is changed regularly every 3–4 days for intracavitary sponges (to prevent granulation tissue ingrowth that makes the removal of the sponge difficult) and up to 1-week interval for intraluminal sponges.76 EVAC terminates when stable granulation tissue covers a self-cleaning inner wound, without signs of necrosis or inflammation. Mean healing times range from 12 to 36 days. EVAC is indicated for large anastomotic breakdowns (limited only by the sponge size), local contamination, chronic fistula, or large abscess cavities. The therapy has been successfully used for a complete circumferential post-esophagectomy anastomotic breakdown, sparing the patient a cervical esophagostomy, as well as for acute lesions or leakage in advanced stages.77 The closure of an intrathoracic AL by EVAC has been described in many studies, with a success rate ranging from 86 to 100%,78 although most studies involve a limited number of patients. In contrast, EVAC treatment in cervical AL has rarely been described, and additionally, most of the evidence comes from single-center studies or small case reports, and studies are often heterogeneous.79 EVAC has some limitations, including the length of treatment and the number of interventions required for clinical success. The most common complication is stricture formation that requires dilation, due to vigorous formation of granulation tissue.80 Other complications are rare, approximately 10%, but may be severe and include bleeding from sponge erosion into a small or major cardiovascular structure, with even rupture of the descending aorta; bronchoesophageal fistula formation; mucosal tear caused by sponge removal managed endoscopically; and sponge dislocation and detachment.81,82A modified EVAC, in which the nasogastric tube is passed into the esophagus through an existing intrapleural drain tract using a rendezvous technique, has been recently described.78 The small residual fistula was amendable to fibrin glue embolization. This allows easier sponge placement and exchange compared to traditional EVAC and oral intake during treatment.
Endoscopic clips (OTSC)
Clips are an endoscopic treatment option for small ALs. This technique has faced a major improvement with the development of the novel OTSC (Ovesco Endoscopy AG, Tubingen, Germany) system and able to overcome the limitations of the previous through-the-scope clips (i.e. passage through the scope, therefore of small size and with low compression force). OTSC provide a full-thickness closure of the wall, because the avoidance of the working channel allows for a greater compressive force, a larger clip area and, therefore, closure of bigger lesions. Because of their wider mouth and ability to grasp larger amounts of tissue, they provide more durable closure.83 Adoption of OTSC is indicated in case of acute AL with minimal inflammation and for small perforations up to 15 mm, although increasing failure rates for lesions >13 mm have been reported.84 For optimal clip adherence and seal, the lesion should present reasonably healthy mucosal edges.84,85 The effectiveness and safety of this management approach have been confirmed in some studies, although involving a relative low number of patients.86 The success rate is higher in acute compared to chronic lesions (e.g. fistula).87 Furthermore, the long-term success was significantly improved when OTSCs were applied as the primary therapy (primary 69.1 vs. rescue 46.9%).85 Complications are rare and include detached clip necessitating a late surgical intervention; contralateral esophageal ulceration or esophageal perforation during system introduction; misplaced clip occluding the lumen, necessitating a surgical intervention; and tongue laceration.87
Sealant
Cyanoacrylates and fibrin glues are the two major groups of sealants. Studies about the use of sealants for treating postoperative AL and perforations are scarce. Lippert et al.88 report the efficacy of fibrin glue, while other studies underline the successful application of cyanoacrylate (even in the cervical area).89 Comparative studies assessing the efficacy of a particular sealant have not been performed yet. Moreover, a complete leakage closure requires multiple sealant applications. This approach appears more suitable for small leakages (<15 mm) or residual small fistulas after the use of EVAC. Some studies report the efficacy of combined treatments. For example, sealant with a vicryl plug seems to improve the effectiveness for leakages >15 mm,90 while Kotzampassi and Eleftheriadis91 successfully performed a combined treatment of sealants and clips.
Overstitch
The effectiveness of suturing techniques for closing leakages is unclear and poorly investigated. The recently introduced Overstitch system (Apollo Endosurgery, Inc, Austin TX) is based on a disposable, single-use device that uses a double channel therapeutic endoscope to apply continuous or intermittent stitches without the need to reload the needle and allow a full thickness suturing. Tissue approximation is facilitated by a tissue-retracting device or grasping forceps. The overstitch may represent an option for leakages occurring in the middle or distal third of the esophagus (in the cervical area technically difficult), although the results of its application in AL are still only anecdotal.92
Stent-over-sponge
Application of a SOS technique has been recently reported for complex leakages.93 SOS combines vacuum-assisted therapy with covered self-expanding stents. The endosponge-assisted device is covered by a SEMS that ensure sponge adherence to the underlying tissue, optimizing suction direction and efficacy. SOS is indicated for the treatment of uncontained leakages, after sponge failure. However, a recent study supports the use of this approach also as first-line treatment.94 Further studies should confirm its effectiveness for complex leakages, particularly regarding the limitations associated to the presence of an internal stent.
Overall considerations on endoscopic management
Endoscopic management of postoperative AL is undergoing a considerable evolution during the last decade, along with a growing body of evidence. Symptomatic and small (<15 mm) acute lesions, with healthy margins of the leakage site, can be sufficiently managed using clips, particularly OTSC. The use of sealants appears an alternative to the clips, although this technique should be further investigated. For acute lesions with nonviable margins or a size >15 mm, endoscopic stenting is still the most widespread endoscopic management, although EVAC is emerging as a powerful alternative. EVAC has a number of advantages compared to SEMS, such as visualization and access to the wound cavity on a regular basis, management of any variation and/or deterioration at an early stage, adequate drainage of the abscess cavity, and control of sepsis.74 The superiority of EVAC to SEMS (in terms of success rate in AL healing, incidence of major complication and in-hospital mortality rate) is supported by several comparative studies and confirmed by a recent meta-analysis.95 Furthermore, EVAC might be a superior tool for the management of cervical leakages, chronic lesions >15 mm, and in septic patients. The overstitch technique might be an alternative for the treatment of a viable acute lesion >15 mm in the middle or distal intrathoracic leakages. However, outcomes of this management strategy are currently poorly examined.
Surgery
Surgical treatment is indicated for early leakages (within 72 hours after resection), since these leakages are usually not contained and attributed to technical failure; for leakages that failed conservative and/or endoscopic treatment; and for severely septic patients. A surgical approach is generally required for both cervical and intrathoracic anastomoses that present with noncontained mediastinitis, empyema, a systemic sepsis, or necrosis of the gastric conduit. A preliminary evaluation of the patient’s symptomatology and the viability of the gastric tube mucosa are important to establish the type of intervention needed. Debridement and mediastinal drainage can be performed through a thoracoscopic approach in early leakage without thoracic empyema96; while in case of empyema or sepsis, open surgical exploration is mandatory: a rethoracotomy for decortication of the lung, drainage of the leakage, and effective assessment of gastric tube integrity.97
If disruption without conduit ischemia and necrosis is present, the gastric tube can usually be preserved and the defect can be sutured, particularly in an early leakage. In case of necrosis of the gastric fundus tip (local ischemia), resection of the necrotic tissue and immediate reanastomosis can be performed. Sometimes reconstruction of tissue defects may require employment of pedicle flaps to reinforce the anastomosis; they usually include a pedicled pleural, pericardial, or viable intercostal muscle flap for intrathoracic AL, and a sternocleidomastoid or pectoralis major muscle flap for cervical AL.98 Diffuse ischemia of the gastric conduit, or necrosis causing severe sepsis, requires a rethoracotomy or cervicotomy and the anastomosis takedown. In these rare cases, a gastric tube resection is performed, creating a temporary cervical esophagostomy. Only after full recovery, the gastrointestinal continuity can be restored with the interposition of colon or jejunum. Surgical treatment is usually patient-tailored, and randomized controlled trials on surgical treatment of AL are lacking. An overview of the postoperative management options for cervical and intrathoracic AL is summarized in Table 2.
Table 2.
Overview of the postoperative management strategies and options for cervical and intrathoracic anastomotic leakage, emerging from the literature review
| Site | Symptoms | Therapy | Management |
|---|---|---|---|
| Cervical | Asymptomatic or minimally symptomatic | Conservative | ✓ Nil-per-mouth
✓ Enteral nutrition through feeding tube ✓ Opening cervical wound and cleaning with isotonic fluid ± Nasogastric tube ± Antibiotic treatment ± Percutaneous drainage (pleura or mediastinum) |
| Symptomatic with local symptoms (neck inflammation) | Conservative | ✓ Nil-per-mouth
✓ Enteral nutrition through feeding tube ✓ Opening cervical wound and clearing with isotonic fluid ✓ Percutaneous drainage (pleura or mediastinum) ✓ Nasogastric tube ✓ Antibiotic treatment |
|
| Early leakage | Surgery | • Without ischemia
✓ Preserve gastric tube and suture defects ± Muscle flap repair • With local ischemia ✓ Resection of the ischemic area plus reanastomosis ± Muscle flap repair |
|
| Uncontrolled sepsis | Surgery | ✓ Resection of gastric tube plus creation of cervical esophagostomy
✓ Preserve gastric tube and suture defects ✓ Muscle flap repair |
|
| Necrosis | Surgery | ✓ Resection of gastric tube plus creation of cervical esophagostomy | |
| Intrathoracic symptoms | See below: intrathoracic anastomosis | ||
| Intrathoracic | Asymptomatic or minimally symptomatic | Conservative | ✓ Nil-per-mouth
✓ Enteral nutrition (see above) ✓ Nasogastric tube ✓ Antibiotic treatment ± Percutaneous drainage (pleura or mediastinum) |
| Symptomatic and/or with controlled sepsis | Drainage +/− Endoscopy | • Healthy AL margins and/or size <15 mm:
✓ Clip or sealant ± drainage ✓ or EVAC ✓ or STENT plus drainage • Inflamed/unhealthy AL margins and/or size >15 mm: ✓ EVAC ✓ or STENT plus drainage |
|
| Early leakage | Surgery | • Without or local ischemia
✓ Thoracotomy, washing plus drainage ✓ Resection of ischemic area plus reanastomosis ± Muscle flap repair |
|
| Uncontrolled sepsis or necrosis see above, split | Surgery | ✓ Resection of gastric tube plus creation of cervical esophagostomy | |
✓ Suggested treatment ± optional treatment.
COMMENTS
AL after esophagectomy has a multifactorial, complex etiology and can be a severe complication affecting postoperative outcome.
The clinical treatment is currently based on individualized approaches, while international, solid evidence-based guidelines would contribute to improve AL prevention and treatment and a better patient outcome. For such guidelines, more high-level evidence is urgently needed. The introduction of the ECCG system is configuring as a first milestone for providing contemporary international benchmarks to compare the outcomes of therapeutic strategies. This system could be further extended by including a standardized description of leakage characteristics (e.g. leakage length, circumference etc.) to improve comparison among studies.
Knowledge on several risk factors is increasing, although the prognostic values of each of them are still not clearly defined. The introduction of individual score-enabled risk stratification tools to predict perioperative outcomes may represent a first step toward a selective screening and follow-up. Remarkable progresses have been achieved in the real-time intraoperative monitoring of conduit perfusion, a major factors contributing to AL development. Indocyanine green fluorescence angiography is the most promising technique, although further improvements are needed to derive standardized quantitative parameters guiding operative protocols.
Early diagnosis is a crucial and challenging clinical target to decrease leakage-associated complications and mortality. However, the shift from on-demand assessment toward the adoption of a routinely imaging approach appears not justified, particularly considering its limited clinical value in asymptomatic patients and the required resource allocation. Diagnosis timing still remains a bottleneck for an efficient management.
The diversity of presentation and severity of leakage, in combination with a number of available diagnostic and therapeutic techniques, make optimal management challenging. Recent trends in AL treatment notice a shift toward a more conservative management compared to the past, along with an increasing adoption of endoscopic intervention (mainly EVAC technique), with surgery reserved for the most severe cases.
ABBREVIATIONS
AL, anastomotic leakage; BMI, body mass index; CT, computed tomography; ECCG, Esophagectomy Complications Consensus Group; EVAC, endoscopic vacuum therapy; FSEMS, fully covered self-expanding metal stents; ICG, indocyanine green; OCT, optical coherence tomography; OTSC, over-the-scope-clip; POD, postoperative day; SDF, sidestream darkfield microscopy; SEMS, self-expandable metallic stents; SOS, stent-over-sponge; TEA, thoracic epidural analgesia
Competing interests
The authors declare no competing interests.
ACKNOWLEDGMENTS
No acknowledgements.
References
- 1. Kassis E S, Kosinski A S, Ross P et al. Predictors of anastomotic leak after Esophagectomy: an analysis of the Society of Thoracic Surgeons general thoracic database. Ann Thorac Surg. 2013; 96: 1919–26. [DOI] [PubMed] [Google Scholar]
- 2. van Workum F, van der Maas J, van den Wildenberg F J H et al. Improved functional results after minimally invasive Esophagectomy: Intrathoracic versus cervical anastomosis. Ann Thorac Surg. 2017; 103(1): 267–73. [DOI] [PubMed] [Google Scholar]
- 3. Seesing M F J, Gisbertz S S, Goense L et al. A propensity score matched analysis of open versus minimally invasive transthoracic Esophagectomy in the Netherlands. Ann Surg. 2017; 266: 839–46. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt H M, Gisbertz S S, Moons J et al. Defining benchmarks for transthoracic Esophagectomy: a Multicenter analysis of Total minimally invasive Esophagectomy in Low risk patients. Ann Surg. 2017 Nov; 266(5): 814–21. [DOI] [PubMed] [Google Scholar]
- 5. Low D E, Kuppusamy M K, Alderson D et al. Benchmarking complications associated with Esophagectomy. Ann Surg. 2019; 269: 291–8. [DOI] [PubMed] [Google Scholar]
- 6. Slankamenac K, Graf R, Barkun J, Puhan M A, Clavien P A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013 Jul; 258(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 7. Bruce J, Krukowski Z H, Al-Khairy G et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. British Journal of Surgery. 2001 Sep; 88(9): 1157–68. [DOI] [PubMed] [Google Scholar]
- 8. Low D E, Alderson D, Cecconello I et al. International consensus on standardization of data collection for complications associated with Esophagectomy: Esophagectomy complications consensus group (ECCG). Ann Surg. 2015; 262: 286–94. [DOI] [PubMed] [Google Scholar]
- 9. Goense L, Van Rossum P S N, Weijs T J et al. Aortic calcification increases the risk of anastomotic leakage after Ivor-Lewis Esophagectomy. Ann Thorac Surg. 2016 Jul; 102(1): 247–52. [DOI] [PubMed] [Google Scholar]
- 10. Koeter M, Kathiravetpillai N, Gooszen J A et al. Influence of the extent and dose of radiation on complications after Neoadjuvant Chemoradiation and subsequent Esophagectomy with gastric tube reconstruction with a cervical anastomosis. Int J Radiat Oncol Biol Phys. 2017; 97: 813–21. [DOI] [PubMed] [Google Scholar]
- 11. Goense L, van Rossum P S N, Ruurda J P et al. Radiation to the gastric fundus increases the risk of anastomotic leakage after Esophagectomy. Ann Thorac Surg. 2016 Dec; 102(6): 1798–804. [DOI] [PubMed] [Google Scholar]
- 12. Gronnier C, Tréchot B, Duhamel A et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection results of a european multicenter study. Ann Surg. 2014; 260: 764–71. [DOI] [PubMed] [Google Scholar]
- 13. Nederlof N, Slaman A E, van Hagen P et al. Using the comprehensive complication index to assess the impact of Neoadjuvant Chemoradiotherapy on complication severity after Esophagectomy for cancer. Ann Surg Oncol. 2016 Nov; 23(12): 3964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markar S R, Arya S, Karthikesalingam A et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Annals of Surgical Oncology. 2013 Dec; 20(13): 4274–81. [DOI] [PubMed] [Google Scholar]
- 15. Gooszen J A H, Goense L, Gisbertz S S et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg. 018 Apr; 105(5): 552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biere S S, van Berge Henegouwen M I, Maas K W et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012; 379: 1887–92. [DOI] [PubMed] [Google Scholar]
- 17. Straatman J, Wielen N, Cuesta M A et al. Minimally invasive versus open Esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017 Aug; 266(2): 232–6. [DOI] [PubMed] [Google Scholar]
- 18. van Workum F, Berkelmans G H, Klarenbeek B R et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis. 2017 Jul; 9(Suppl 8): S826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou D, Liu Q X, Deng X F et al. Anastomotic reinforcement with omentoplasty reduces anastomotic leakage for minimally invasive esophagectomy with cervical anastomosis. Cancer Manag Res. 2018 Feb 7; 10: 257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turkyilmaz A, Eroglu A, Aydin Y et al. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus. 2009; 22: 119–26. [DOI] [PubMed] [Google Scholar]
- 21. Zhu Z J, Zhao Y F, Chen L Q et al. Clinical application of layered anastomosis during esophagogastrostomy. World J Surg. 2008 Apr; 32(4): 583–8. [DOI] [PubMed] [Google Scholar]
- 22. Nederlof N, Tilanus H W, Tran T C K et al. End-to-end versus end-to-side esophagogastrostomy after esophageal cancer resection: a prospective randomized study. Ann Surg. 2014 Jan; 259(1): e6. [DOI] [PubMed] [Google Scholar]
- 23. Rostas J W, Graffree B D, Scoggins C R et al. Long-term outcomes after hand-sewn versus circular-stapled (25 and 29 mm) anastomotic technique after esophagogastrectomy for esophageal cancer. J Surg Oncol. 2018 Mar; 117(3): 469–72. [DOI] [PubMed] [Google Scholar]
- 24. Price T N1, Nichols F C, Harmsen W S et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg. 2013 Apr; 95(4): 1154–60. [DOI] [PubMed] [Google Scholar]
- 25. Honda M, Kuriyama A, Noma H et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Annals of Surgery. 2013 Feb; 257(2): 238–48. [DOI] [PubMed] [Google Scholar]
- 26. Klevebro F, Boshier P R, Low D E. Application of standardized hemodynamic protocols within enhanced recovery after surgery programs to improve outcomes associated with anastomotic leak and conduit necrosis in patients undergoing esophagectomy. Journal of Thoracic Disease. 2019 Apr; 11(Suppl 5): S692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fumagalli U, Melis A, Balazova J et al. Intra-operative hypotensive episodes may be associated with post-operative esophageal anastomotic leak. Updates Surg. 2016 Jun; 68(2): 185–90. [DOI] [PubMed] [Google Scholar]
- 28. Feldheiser A, Conroy P, Bonomo T et al. Development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgery. J Int Med Res. 2012; 40(4): 1227–41. [DOI] [PubMed] [Google Scholar]
- 29. Veelo D P, van Berge Henegouwen M I, Ouwehand K S et al. Effect of goal-directed therapy on outcome after esophageal surgery: a quality improvement study. PLoS One. 2017; 12: e0172806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feltracco P, Bortolato A, Barbieri S et al. Perioperative benefit and outcome of thoracic epidural in esophageal surgery: a clinical review. Diseases of the esophagus? official journal of the International Society for Diseases of the Esophagus. 2018 May 1; 31(5). [DOI] [PubMed] [Google Scholar]
- 31. Jansen S M, De Bruin D M, Van Berge Henegouwen M I et al. Effect of ephedrine on gastric conduit perfusion measured by laser speckle contrast imaging after esophagectomy: a prospective in vivo cohort study. Dis Esophagus. 2018 Oct; 1: 31(10). [DOI] [PubMed] [Google Scholar]
- 32. Zehetner J, DeMeester S R, Alicuben E T et al. Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Annals of Surgery. 2015 Jul; 262(1): 74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jansen S M, de Bruin D M, van Berge Henegouwen M I et al. Can we predict necrosis intra-operatively? Real-time optical quantitative perfusion imaging in surgery: study protocol for a prospective, observational, in vivo pilot study. Pilot Feasibility Stud. 2017 Nov 25; 3: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferri L E, Law S, Wong K H et al. The influence of technical complications on postoperative outcome and survival after esophagectomy. Ann Surg Oncol. 2006 Apr; 13(4): 557–64. [DOI] [PubMed] [Google Scholar]
- 35. Bakshi A, Sugarbaker D J, Burt B M. Alternative conduits for esophageal replacement. Ann Cardiothorac Surg. 2017 Mar; 6(2): 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grimminger P P, Goense L, Gockel I et al. Diagnosis, assessment, and management of surgical complications following esophagectomy. Ann N Y Acad Sci. 2018 Dec; 1434(1): 254–73. [DOI] [PubMed] [Google Scholar]
- 37. Jansen S M, De Bruin D M, Van Berge Henegouwen M I et al. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: a review of technologies and thresholds. Diseases of the Esophagus. 2018 Jun; 1: 31(6). [DOI] [PubMed] [Google Scholar]
- 38. Hodari A, Park K U, Lace B et al. Robot-assisted minimally invasive Ivor Lewis Esophagectomy with real-time perfusion assessment. Ann Thorac Surg. 2015; 100: 947–52. [DOI] [PubMed] [Google Scholar]
- 39. Ladak F, Dang J T, Switzer N et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surgical Endoscopy. 2019 Feb; 33(2): 384–94. [DOI] [PubMed] [Google Scholar]
- 40. Kumagai Y, Hatano S, Sobajima J et al. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus. 2018 Dec; 1: 31(12). [DOI] [PubMed] [Google Scholar]
- 41. Urschel J D. Ischemic conditioning of the stomach may reduce the incidence of esophagogastric anastomotic leaks complicating esophagectomy: a hypothesis. Dis Esophagus. 1997 Jul; 10(3): 217–9. [DOI] [PubMed] [Google Scholar]
- 42. Kechagias A, Van Rossum P S N, Ruurda J P et al. Ischemic conditioning of the stomach in the prevention of Esophagogastric anastomotic leakage after Esophagectomy. Annals of Thoracic Surgery. 2016 Apr; 101(4): 1614–23. [DOI] [PubMed] [Google Scholar]
- 43. Heger P, Blank S, Diener M K et al. Gastric preconditioning in advance of Esophageal resection-systematic review and meta-analysis. J Gastrointest Surg. 2017 Sep; 21(9): 1523–32. [DOI] [PubMed] [Google Scholar]
- 44. Stippel D L1, Taylan C, Schröder W, Beckurts K T, Hölscher A H. Supraventricular tachyarrhythmia as early indicator of a complicated course after esophagectomy. Diseases of the Esophagus. 2005; 18(4): 267–73. [DOI] [PubMed] [Google Scholar]
- 45. Van Heijl M, Van Wijngaarden A K S, Lagarde S M et al. Intrathoracic manifestations of cervical anastomotic leaks after transhiatal and transthoracic oesophagectomy. Br J Surg. 2010 May; 97(5): 726–31. [DOI] [PubMed] [Google Scholar]
- Skorst R J, Port J L, Lee P C et al. Intrathoracic manifestations of cervical anastomotic leaks after transthoracic esophagectomy for carcinoma. Ann Thorac Surg. 2010 May; 97(5): 726–31. [DOI] [PubMed] [Google Scholar]
- 47. Park J K, Kim J J, Moon S W. C-reactive protein for the early prediction of anastomotic leak after esophagectomy in both neoadjuvant and non-neoadjuvant therapy case: a propensity score matching analysis. J Thorac Dis. 2017 Oct; 9(10): 3693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller D L, Helms G A, Mayfield W R. Evaluation of Esophageal anastomotic integrity with serial pleural amylase levels. Ann Thorac Surg. 2018 Jan; 105(1): 200–6. [DOI] [PubMed] [Google Scholar]
- 49. Jiang B, Ho V P, Ginsberg J et al. Decision analysis supports the use of drain amylase-based enhanced recovery method after esophagectomy. Dis Esophagus. 2018 Oct; 1: 31(10). [DOI] [PubMed] [Google Scholar]
- 50. Lantos J E, Levine M S, Rubesin S E et al. Comparison between esophagography and chest computed tomography for evaluation of leaks after esophagectomy and gastric pull-through. J Thorac Imaging. 2013; 28: 121–8. [DOI] [PubMed] [Google Scholar]
- 51. Jones C M, Clarke B, Heah R et al. Should routine assessment of anastomotic integrity be undertaken using radiological contrast swallow after oesophagectomy with intra-thoracic anastomosis? Best evidence topic (BET). Int J Surg. 2015 Aug; 20: 158–62. [DOI] [PubMed] [Google Scholar]
- 52. Page R D, Asmat A, McShane J et al. Routine endoscopy to detect anastomotic leakage after esophagectomy. Ann Thorac Surg. 2013 Jan; 95(1): 292–8. [DOI] [PubMed] [Google Scholar]
- 53. Kim TH, Kim JH, Shin C Il, et al. CT findings suggesting anastomotic leak and predicting the recovery period following gastric surgery. Eur Radiol. 2015 Jul;25(7):1958–66. [DOI] [PubMed] [Google Scholar]
- 54. Goense L, Stassen P M C, Wessels F J et al. Diagnostic performance of a CT-based scoring system for diagnosis of anastomotic leakage after esophagectomy: comparison with subjective CT assessment. Eur Radiol. 2017 Oct; 27(10): 4426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shoji Y, Takeuchi H, Fukuda K et al. Air bubble sign: a new screening method for anastomotic leakage after Esophagectomy for Esophageal cancer. Ann Surg Oncol. 2018 Apr; 25(4): 1061–8. [DOI] [PubMed] [Google Scholar]
- 56. Hogan B A, Winter D C, Winter D et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc. 2008; 22: 767–71. [DOI] [PubMed] [Google Scholar]
- 57. Nederlof N, de Jonge J, de Vringer T et al. Does routine endoscopy or contrast swallow study after Esophagectomy and gastric tube reconstruction change patient management? J Gastrointest Surg. 2017 Feb; 21(2): 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strauss C, Mal F, Perniceni T et al. Computed tomography versus water-soluble contrast swallow in the detection of intrathoracic anastomotic leak complicating esophagogastrectomy (Ivor Lewis): a prospective study in 97 patients. Ann Surg. 2010; 251: 647–51. [DOI] [PubMed] [Google Scholar]
- 59. Messager M, Warlaumont M, Renaud F et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol. 2016; 43: 258–69. [DOI] [PubMed] [Google Scholar]
- 60. Nishikawa K, Fujita T, Yuda M et al. Early postoperative endoscopy for targeted management of patients at risks of anastomotic complications after esophagectomy. Surgery. 2016; 160: 1294–301. [DOI] [PubMed] [Google Scholar]
- 61. Fujiwara H, Nakajima Y, Kawada K et al. Endoscopic assessment 1 day after esophagectomy for predicting cervical esophagogastric anastomosis-relating complications. Surg Endosc. 2016 Apr; 30(4): 1564–71. [DOI] [PubMed] [Google Scholar]
- 62. Struecker B, Andreou A, Chopra S et al. Evaluation of anastomotic leak after Esophagectomy for Esophageal cancer: typical time point of occurrence, mode of diagnosis, value of routine Radiocontrast agent studies and therapeutic options. Dig Surg. 2018; 35(5): 419–26. [DOI] [PubMed] [Google Scholar]
- 63. Schaible A, Ulrich A, Hinz U et al. Role of endoscopy to predict a leak after esophagectomy. Langenbeck’s Arch Surg. 2016 Sep; 401(6): 805–12. [DOI] [PubMed] [Google Scholar]
- 64. Hagens E R C, Anderegg M C J, van Berge Henegouwen M I et al. International survey on the Management of Anastomotic Leakage after Esophageal resection. Ann Thorac Surg. 2018 Dec; 106(6): 1702–8. [DOI] [PubMed] [Google Scholar]
- 65. Verstegen M H P, Bouwense S A W, Van Workum F et al. Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World Journal of Emergency Surgery. 2019 Apr 4; 14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dasari B V M, Neely D, Kennedy A et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Annals of Surgery. 2014 May; 259(5): 852–60. [DOI] [PubMed] [Google Scholar]
- 67. Law R, Prabhu A, Fujii-Lau L et al. Stent migration following endoscopic suture fixation of esophageal self-expandable metal stents: a systematic review and meta-analysis. Surg Endosc. 2018 Feb; 32(2): 675–81. [DOI] [PubMed] [Google Scholar]
- 68. Sousa P, Castanheira A, Martins D et al. Treatment of postoperative leaks of the upper gastrointestinal tract with colonic self-expandable metal stents. GE Port J Gastroenterol. 2017 Jul; 24(4): 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Freeman R K, Ascioti A J, Dake M et al. An assessment of the optimal time for removal of Esophageal stents used in the treatment of an Esophageal anastomotic leak or perforation. Ann Thorac Surg. 2015 Aug; 100(2): 422–8. [DOI] [PubMed] [Google Scholar]
- 70. Van Halsema E E, WFW K, BLAM W et al. Stent placement for benign esophageal leaks, perforations, and fistulae: a clinical prediction rule for successful leakage control. Endoscopy 2018. doi: 10.1055/s-0043-118591. [DOI] [PubMed] [Google Scholar]
- 71. Wu G, Yin M, Zhao Y S et al. Novel esophageal stent for treatment of cervical anastomotic leakage after esophagectomy. Surg. Endosc 2017. doi: 10.1007/s00464-017-5545-6. [DOI] [PubMed] [Google Scholar]
- 72. Persson S, Rouvelas I, Irino T, Lundell L. Outcomes following the main treatment options in patients with a leaking esophagus: a systematic literature review. Diseases of the Esophagus 2017. doi: 10.1093/dote/dox108. [DOI] [PubMed] [Google Scholar]
- 73. Newton N J, Sharrock A, Rickard R, Mughal M. Systematic review of the use of endo-luminal topical negative pressure in oesophageal leaks and perforations. Dis. Esophagus 2017. doi: 10.1111/dote.12531. [DOI] [PubMed] [Google Scholar]
- 74. Mennigen R, Senninger N, Laukoetter M G. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J. Gastroenterol. 2014. doi: 10.3748/wjg.v20.i24.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gubler C, Vetter D, Schmidt H M et al. Preemptive endoluminal vacuum therapy to reduce anastomotic leakage after esophagectomy: a game-changing approach? Dis. Esophagus 2019. doi: 10.1093/dote/doy126. [DOI] [PubMed] [Google Scholar]
- 76. Schniewind B, Schafmayer C, Both M, Arlt A, Fritscher-Ravens A, Hampe J. Ingrowth and device disintegration in an intralobar abscess cavity during endosponge therapy for esophageal anastomotic leakage. Endoscopy 2011. doi: 10.1055/s-0030-1255799. [DOI] [PubMed] [Google Scholar]
- 77. Schorsch T, Müller C, Loske G. Endoscopic vacuum therapy of anastomotic leakage and iatrogenic perforation in the esophagus. Surg. Endosc. 2013. doi: 10.1007/s00464-012-2707-4. [DOI] [PubMed] [Google Scholar]
- 78. Pines G, Bar I, Elami A et al. Modified endoscopic vacuum therapy for nonhealing esophageal anastomotic leak: technique description and review of literature. J. Laparoendosc. Adv. Surg. Tech 2018. doi: 10.1089/lap.2017.0318. [DOI] [PubMed] [Google Scholar]
- 79. Schniewind B, Schafmayer C, Voehrs G et al. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg. Endosc 2013. doi: 10.1007/s00464-013-2998-0 Available form URL LK- http://rug.on.worldcat.org/atoztitles/link/?sid=EMBASE&issn=14322218&id=doi:10.1007%2Fs00464-013-2998-0&atitle=Endoscopic+endoluminal+vacuum+therapy+is+superior+to+other+regimens+in+managing+anastomotic+leakage+after+esophagectomy%3A+A+comparative+retrospective+study&stitle=Surg.+Endosc.+Interv.+Tech.&title=Surgical+Endoscopy&volume=27&issue=10&spage=3883&epage=3890&aulast=Schniewind&aufirst=Bodo&auinit=B.&aufull=Schniewind+B.&coden=&isbn=&pages=3883-3890&date=201. [DOI] [PubMed] [Google Scholar]
- 80. Watkins J R, Farivar A S. Endoluminal therapies for esophageal perforations and leaks. Thoracic Surgery Clinics 2018. doi: 10.1016/j.thorsurg.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 81. Laukoetter M G, Mennigen R, Neumann P A et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg. Endosc 2017. doi: 10.1007/s00464-016-5265-3. [DOI] [PubMed] [Google Scholar]
- 82. Pournaras D J, Hardwick R H, Safranek P M et al. Endoluminal vacuum therapy (E-vac): a treatment option in Oesophagogastric surgery. World J. Surg 2018. doi: 10.1007/s00268-018-4463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Von Renteln D, Vassiliou M C, Rothstein R I. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy 2009. doi: 10.1055/s-0029-1215241. [DOI] [PubMed] [Google Scholar]
- 84. Hagel A F, Naegel A, Lindner A S et al. Over-the-scope clip application yields a high rate of closure in gastrointestinal perforations and may reduce emergency surgery. J. Gastrointest. Surg 2012. doi: 10.1007/s11605-012-1983-6. [DOI] [PubMed] [Google Scholar]
- 85. Haito-Chavez Y, Law J K, Kratt T et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest. Endosc. 2014. doi: 10.1016/j.gie.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 86. Voermans R P, Le Moine O, Renteln D et al. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin. Gastroenterol. Hepatol 2012. doi: 10.1016/j.cgh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 87. Donatelli G, Cereatti F, Dhumane P et al. Closure of gastrointestinal defects with Ovesco clip: long-term results and clinical implications. Therap. Adv. Gastroenterol 2016. doi: 10.1177/1756283X16652325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lippert E, Klebl F H, Schweller F et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int. J. Colorectal Dis. 2011. doi: 10.1007/s00384-010-1104-5. [DOI] [PubMed] [Google Scholar]
- 89. Ojima T, Nakamura M, Nakamori M et al. Successful treatment of esophageal fistulas with endoscopic injection of alpha-cyanoacrylate monomer. Endoscopy 2014. doi: 10.1055/s-0033-1359159. [DOI] [PubMed] [Google Scholar]
- 90. Böhm G, Mossdorf A, Klink C et al. Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Endoscopy 2010. doi: 10.1055/s-0029-1244165. [DOI] [PubMed] [Google Scholar]
- 91. Kotzampassi K, Eleftheriadis E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surg. (United States) 2015. doi: 10.1016/j.surg.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 92. Gaur P, Lyons C, Malik T M, Kim M P, Blackmon S H. Endoluminal suturing of an anastomotic leak. Ann. Thorac. Surg. 2015. doi: 10.1016/j.athoracsur.2014.01.089. [DOI] [PubMed] [Google Scholar]
- 93. Gubler C, Schneider P M, Bauerfeind P. Complex anastomotic leaks following esophageal resections: the new stent over sponge (SOS) approach. Dis. Esophagus 2013. doi: 10.1111/dote.12005. [DOI] [PubMed] [Google Scholar]
- 94. Valli P V, Mertens J C, Kröger A et al. Stent-over-sponge (SOS): a novel technique complementing endosponge therapy for foregut leaks and perforations. Endoscopy 2018. doi: 10.1055/s-0043-120442. [DOI] [PubMed] [Google Scholar]
- 95. Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis. Esophagus 2018. doi: 10.1093/dote/doy060. [DOI] [PubMed] [Google Scholar]
- 96. Straatman J, Wielen N, Cuesta M A et al. Minimally invasive versus open Esophageal resection. Ann. Surg 2017. doi: 10.1097/SLA.0000000000002171. [DOI] [PubMed] [Google Scholar]
- 97. Schaheen L, Blackmon S H, Nason K S. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am. J. Surg. 2014. doi: 10.1016/j.amjsurg.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kotzampassakis N, Christodoulou M, Krueger T et al. Esophageal leaks repaired by a muscle Onlay approach in the presence of Mediastinal sepsis. Ann. Thorac. Surg. 2009. doi: 10.1016/j.athoracsur.2009.05.011. [DOI] [PubMed] [Google Scholar]


