Abstract
Beta-adrenergic blocking agents (abbreviated as beta-blockers) have been used for treating various cardiovascular diseases. However, the potential for asthma exacerbation is one of the major adverse effects of beta-blockers. This study aimed to compare the level of risk for an asthma attack in patients receiving various beta-blockers. We searched for randomized controlled trials (RCTs) of either placebo-controlled or active-controlled design. The current network meta-analysis (NMA) was conducted under a frequentist model. The primary outcome was the incidence of asthmatic attack. A total of 24 RCTs were included. Overall NMA revealed that only oral timolol [risk ratio (RR) = 3.35 (95% confidence interval (CI) 1.04–10.85)] and infusion of propranolol [RR = 10.19 (95% CI 1.29–80.41)] were associated with significantly higher incidences of asthma attack than the placebo, whereas oral celiprolol [RR = 0.39 (95% CI 0.04–4.11)], oral celiprolol and propranolol [RR = 0.46 (95% CI 0.02–11.65)], oral bisoprolol [RR = 0.46 (95% CI 0.02–11.65)], oral atenolol [RR = 0.51 (95% CI 0.20–1.28)], infusion of practolol [RR = 0.80 (95% CI 0.03–25.14)], and infusion of sotalol [RR = 0.91 (95% CI 0.08–10.65)] were associated with relatively lower incidences of asthma attack than the placebo. In participants with a baseline asthma history, in addition to oral timolol and infusion of propranolol, oral labetalol, oxprenolol, propranolol, and metoprolol exhibited significantly higher incidences of asthma attack than did the placebo. In conclusion, oral timolol and infusion of propranolol were associated with a significantly higher risk of developing an asthma attack in patients, especially in those with a baseline asthma history, and should be avoided in patients who present a risk of asthma.
Trial registration: PROSPERO CRD42020190540.
Subject terms: Respiratory tract diseases, Disease prevention
Introduction
Beta-adrenergic blocking agents (or beta-blockers) have been frequently used to treat various cardiovascular disorders such as hypertension, ischemic heart disease, cardiac arrhythmias, and congestive heart failure1–4. Clinicians often refrain from prescribing them for patients with an underlying disease of concern to adverse events, such as asthma, diabetes mellitus, and peripheral artery disease5. In fact, acute bronchoconstriction with leading asthma exacerbation is the most crucial side effect of beta-blockers, for which several review articles and practice guidelines have advised avoiding the use of beta-blockers in patients with asthma6–9. Furthermore, beta-blockers are one of the first-line treatment agents for thyrotoxicosis10 and essential tremor11, as well as for preventing variceal bleeding in patients with portal hypertension12 and aortic aneurysm in Marfan syndrome13. Although there is considerable evidence for the effectiveness and benefits of beta-blockers in treating these diseases, the associated adverse events such as an asthma attack create a dilemma for physicians considering treatment with beta-blockers for patients with asthma.
A large cohort study has demonstrated that the benefits outweigh the risks of cardioselective beta-blocker therapy in patients with asthma for long-term management of heart failure or decreased 1-year mortality rate after myocardial infarction14. Several randomized controlled trials (RCTs) or pairwise meta-analyses have reported the various influences of beta-blockers in pulmonary function, symptom changes, or asthma attack separately6,7,15,16. Salpeter et al. reported that patients with reactive airway disease who received a single dose of cardioselective beta-blockers presented a 7.46% decrease in forced expiratory volume in one second (FEV1)7. Another population-based nested case–control study demonstrated that nonselective beta-blockers were associated with a significantly increased risk of asthma exacerbation6. Moreover, the risk for asthma and adverse effects on pulmonary function with the use of non-cardioselective beta-blockers was found to be more prominent than that with the use of cardioselective beta-blockers17. Therefore, it is an important consideration in clinical practice to assess which beta-blockers have been shown to significantly increase the risk for serious asthma exacerbation and which have not. Nevertheless, the results could not be obtained by means of the traditional RCTs or meta-analysis studies in the past.
Network meta-analysis (NMA) of existing RCTs enables the estimation of the comparative efficacy or risk and the understanding of the relative merits of multiple interventions, which cannot be achieved in traditional pairwise meta-analysis18. Therefore, we conducted a comprehensive NMA to compare the risk of developing an adverse asthma attack in patients receiving treatment with various beta-blockers.
Methods
The detailed description of method is listed in eTable 1. In brief, it follows the preferred reporting items for systematic reviews and meta-analyses extension guideline (eTable 2)19 and follows the previous NMAs20–23. The current frequentist model-based NMA, which included only RCTs, was conducted to investigate the incidence of asthma attack after beta-blocking agent treatment in patients with and without a baseline asthma history. To examine the risk of asthma attack after treatment with various beta-blocking agents, we searched for RCTs specifically designed to assess the risk among asthmatics by using keywords of “asthma”, “dyspnea”, “bronchoconstriction, “bronchial constriction”, “bronchial hyperreactivity”, “respiratory sound”, “wheeze”, or “wheezing” (eTable 3). Because decreased pulmonary function associated with beta-blocking agents would not always result in clinical symptoms, we did not select for changes in pulmonary function as our primary outcome24. The primary outcome was the incidence of asthma attacks after treatment with beta-blocking agents compared with control conditions in patients with or without baseline asthma history. The definition of an asthma attack could be deterioration in symptoms, increased use of rescue bronchodilators, emergency room visits for asthma, and requiring systemic corticosteroids25. We estimated the summary risk ratios (RRs) with 95% confidence intervals (CIs) for categorical variables and further applied a 0.5 zero-cell correction during the procedure of the meta-analysis. To minimize the potential bias caused by imputing 0.5 to zero-cells in the data, we conducted a sensitivity test by removing trials with zero or 100% events in their treatment arms. Heterogeneity among the included studies was evaluated using the tau value, which is the estimated standard deviation of the effect across the included studies. To provide additional clinical application, we calculated the surface under the cumulative ranking curve (SUCRA) among the preventive effects of all treatments for the target outcomes to rank the potential superiority among the investigated treatments. We also performed a subgroup analysis focusing on patients with a definite baseline asthma history. Finally, we evaluated the potential inconsistency using the loop-specific approach, the node-splitting method, and the design-by-treatment model.
Results
After the initial screening procedure, a total of 183 articles were considered for full-text review (Fig. 1). However, 159 were excluded for various reasons (see Fig. 1 and eTable 4). Finally, 24 articles were included in the current study (eTable 5), among which 13 provided evidence related to patients with a definite baseline asthma history. Figure 2A depicts the entire geometric distribution of the treatment arms.
Figure 1.
Flowchart of the current network meta-analysis.
Figure 2.
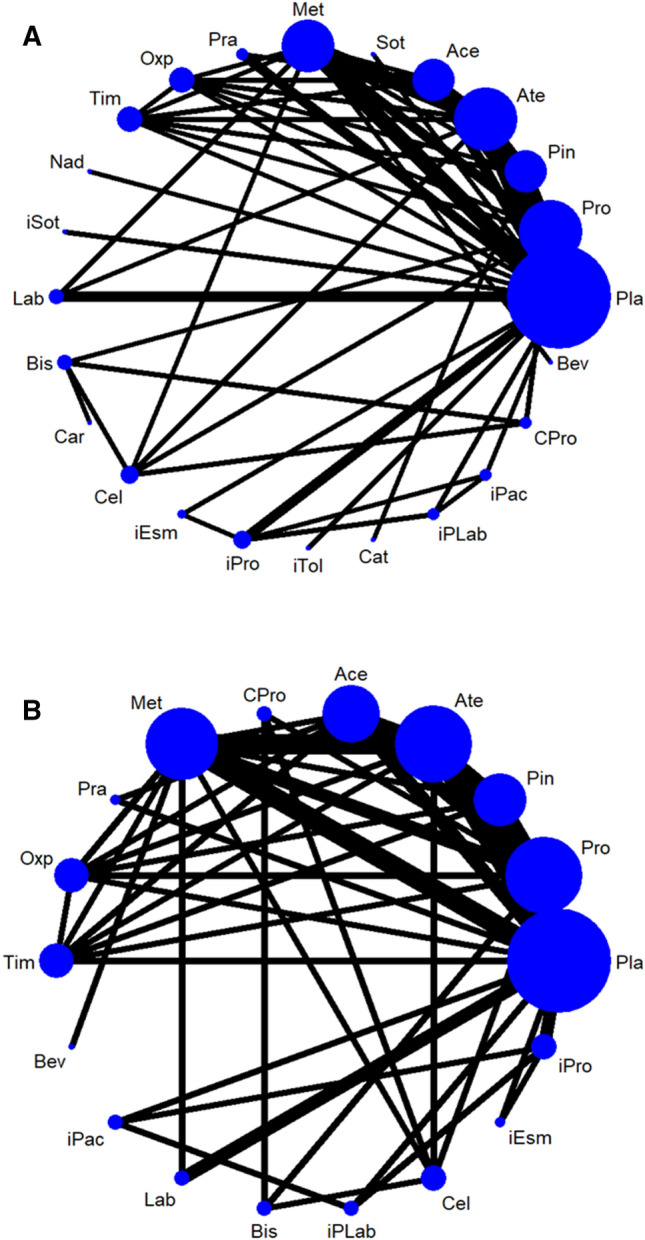
The network structure of (A) individual beta-blocking agents among the overall participants and (B) individual beta-blocking agents among participants with a baseline history of asthma. The lines between nodes represent direct comparisons in various trials, and the size of each circle is proportional to the size of the population involved in each specific treatment. The thickness of the lines is proportional to the number of trials connected to the network. Ace oral acebutolol, Ate oral atenolol, Bev oral bevantolol, Bis oral bisoprolol, Car oral carvedilol, Cat oral carteolol, Cel oral celiprolol, CI confidence interval, CPro oral celiprolol and propranolol, iEsm infusion of esmolol, iPac infusion of practolol, iPLab infusion of propranolol and labetalol, iPro infusion of propranolol, iSot infusion of sotalol, iTol infusion of tolamolol, Lab oral dilevalol or oral labetalol, Met oral metoprolol, Nad oral nadolol, Oxp oral oxprenolol, Pin oral pindolol, Pla Placebo/control, Pra oral practolol, Pro oral propranolol, Sot oral sotalol, Tim oral timolol.
Characteristics of the included studies
A total of 1301 participants were included. The mean age of the participants was 54.5 years (range 22.0–77.3 years, 25–75% interquartile = 39.6 and 61.0 years), and the mean female proportion was 22.6% (range 0.0–60.0%, 25–75% interquartile = 10.3% and 35.1%). The baseline characteristics of the included participants are listed in eTable 5.
The duration of beta-blocking agent prescription ranged from only once before evaluation through 14 weeks.
Overall incidence of asthma attack after receiving beta-blocking agents
A total of 24 articles with 24 treatment arms were investigated in the current NMA, including placebo or control, oral propranolol, oral pindolol, oral atenolol, oral acebutolol, oral sotalol, oral metoprolol, oral practolol, oral oxprenolol, oral timolol, oral nadolol, infusion of sotalol, oral labetalol, oral bisoprolol, oral carvedilol, oral celiprolol, infusion of esmolol, infusion of propranolol, infusion of tolamolol, oral carteolol, infusion of propranolol and labetalol, infusion of practolol, oral celiprolol and propranolol, and oral bevantolol.
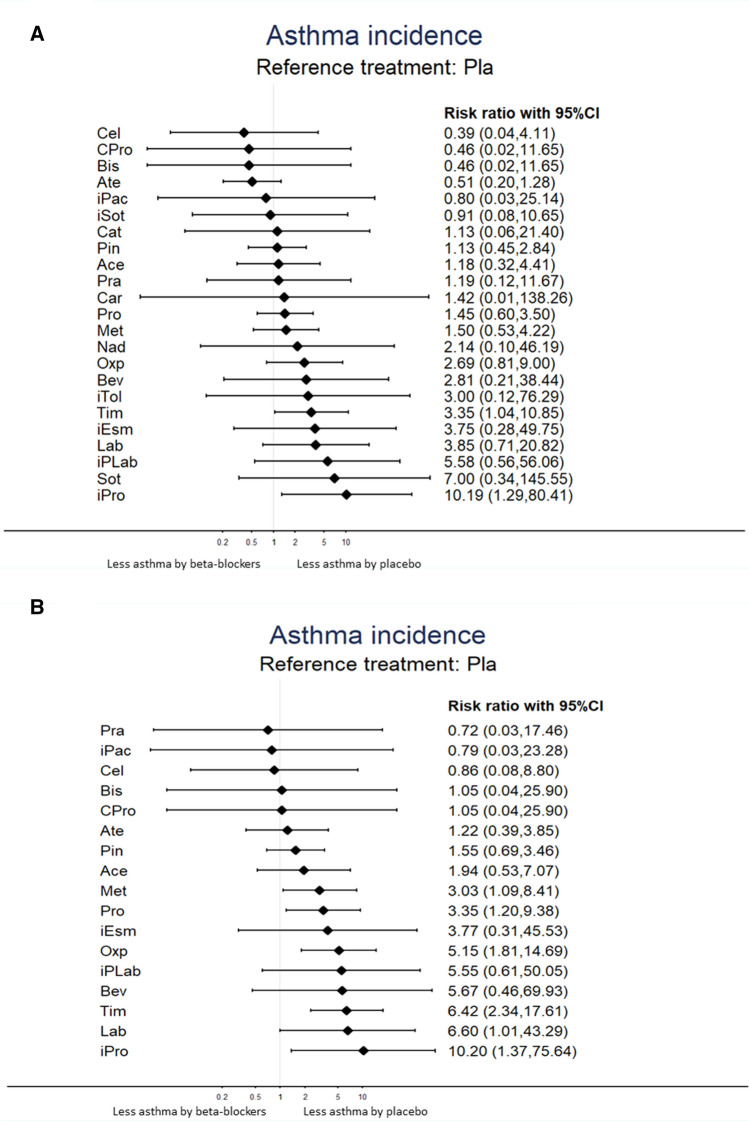
The NMA revealed that only oral timolol [RR = 3.35 (95% CI 1.04–10.85)] and infusion of propranolol [RR = 10.19 (95% CI 1.29–80.41)] were associated with a significantly higher incidence of asthma attack than the placebo or control groups. In contrast, oral celiprolol [RR = 0.39 (95% CI 0.04–4.11)], oral celiprolol and propranolol [RR = 0.46 (95% CI 0.02–11.65)], oral bisoprolol [RR = 0.46 (95% CI 0.02–11.65)], oral atenolol [RR = 0.51 (95% CI 0.20–1.28)], infusion of practolol [RR = 0.80 (95% CI 0.03–25.14)], and infusion of sotalol [RR = 0.91 (95% CI 0.08–10.65)] were associated with a relatively lower incidence of asthma attack than the placebo or control groups. However, the current evidence could not rule out the null hypothesis (Table 1 and Fig. 3A). The association of the beta-blocking agents and the incidence of asthma attack were ranked according to the SUCRA. In brief, oral atenolol was associated with the least risk of incidence of asthma attack after receiving beta-blocking agents, followed by oral celiprolol and oral bisoprolol (eTable 6A). In general, there was no detected significant heterogeneity (eTable 7). A meta-regression using restricted maximum likelihood estimators was performed to examine the potential effect of age and gender distribution on the incidence of asthma attack. The results of this meta-regression did not reveal a significant effect on the incidence of asthma attack when using a moderating variable, including age and gender distribution.
Table 1.
League table of association between asthma exacerbation and beta-blocking agent prescription: overall.
| Ate | 1.00 (0.02,47.85) | 0.77 (0.18,3.29) | 0.25 (0.03,2.23) | 0.25 (0.03,2.23) | 0.36 (0.05,2.71) | *0.38 (0.15,0.98) | *0.06 (0.00,0.97) | 0.33 (0.02,7.25) | *0.05 (0.00,0.77) | ||||||||||||||
| 1.29 (0.13,12.95) | Cel | 1.00 (0.02,46.05) | 1.00 (0.02,46.05) | 0.33 (0.02,7.32) | 0.20 (0.01,3.90) | ||||||||||||||||||
| 1.11 (0.04,28.19) | 0.86 (0.03,28.40) | Bis | 1.00 (0.02,46.05) | 0.32 (0.01,7.63) | 0.33 (0.02,7.32) | ||||||||||||||||||
| 1.11 (0.04,28.20) | 0.86 (0.03,28.41) | 1.00 (0.02,49.14) | CPro | 0.333 0.015 7.323 | |||||||||||||||||||
| 0.51 (0.20,1.28) | 0.39 (0.04,4.11) | 0.46 (0.02,11.65) | 0.46 (0.02,11.65) | Pla | 1.00 (0.02,47.18) | 0.91 (0.09,9.60) | 0.67 (0.27,1.61) | 0.24 (0.03,2.16) | 0.51 (0.05,5.81) | 0.56 (0.11,2.72) | *0.14 (0.02,0.82) | 0.47 (0.02,9.26) | 0.33 (0.01,7.81) | *0.06 (0.00,0.97) | 0.33 (0.02,7.30) | 0.18 (0.02,1.49) | *0.05 (0.00,0.77) | 0.14 (0.01,2.53) | 0.14 (0.01,2.73) | *0.10 (0.01,0.73) | |||
| 0.64 (0.02,22.67) | 0.49 (0.01,31.93) | 0.57 (0.01,64.96) | 0.57 (0.01,64.96) | 1.25 (0.04,39.52) | iPac | 0.14 (0.01,2.53) | 0.08 (0.01,1.25) | ||||||||||||||||
| 0.56 (0.04,7.81) | 0.43 (0.01,13.05) | 0.51 (0.01,29.52) | 0.51 (0.01,29.52) | 1.10 (0.09,12.97) | 0.88 (0.01,61.08) | iSot | |||||||||||||||||
| 0.45 (0.15,1.38) | 0.35 (0.03,3.80) | 0.41 (0.02,10.65) | 0.41 (0.02,10.65) | 0.88 (0.35,2.22) | 0.71 (0.02,25.03) | 0.80 (0.06,11.12) | Pin | 1.00 (0.24,4.24) | 1.00 (0.07,14.90) | 0.54 (0.17,1.74) | 0.56 (0.12,2.60) | 0.35 (0.08,1.47) | 0.28 (0.07,1.15) | ||||||||||
| 0.43 (0.11,1.62) | 0.33 (0.03,3.88) | 0.39 (0.01,10.72) | 0.39 (0.01,10.72) | 0.85 (0.23,3.16) | 0.67 (0.02,27.03) | 0.77 (0.05,12.53) | 0.96 (0.25,3.66) | Ace | 0.33 (0.02,7.58) | 0.54 (0.17,1.74) | 0.56 (0.12,2.60) | 0.35 (0.08,1.47) | 0.28 (0.07,1.15) | ||||||||||
| 0.45 (0.02,9.14) | 0.35 (0.01,13.74) | 0.41 (0.01,29.85) | 0.41 (0.01,29.85) | 0.88 (0.05,16.73) | 0.71 (0.01,65.56) | 0.80 (0.02,37.14) | 1.00 (0.06,16.33) | 1.04 (0.05,23.15) | Cat | ||||||||||||||
| 0.43 (0.04,4.69) | 0.33 (0.01,8.12) | 0.39 (0.01,19.03) | 0.39 (0.01,19.03) | 0.84 (0.09,8.24) | 0.67 (0.01,41.93) | 0.76 (0.03,21.91) | 0.95 (0.09,10.46) | 0.99 (0.09,10.58) | 0.95 (0.02,37.73) | Pra | |||||||||||||
| 0.36 (0.00,34.97) | 0.28 (0.00,32.72) | 0.32 (0.01,8.27) | 0.32 (0.00,51.31) | 0.70 (0.01,68.71) | 0.56 (0.00,173.48) | 0.64 (0.00,115.86) | 0.80 (0.01,79.57) | 0.83 (0.01,86.16) | 0.80 (0.00,173.76) | 0.84 (0.01,133.70) | Car | ||||||||||||
| *0.35 (0.14,0.88) | 0.27 (0.03,2.50) | 0.32 (0.01,7.19) | 0.32 (0.01,7.19) | 0.69 (0.29,1.68) | 0.55 (0.02,19.38) | 0.63 (0.05,8.60) | 0.78 (0.29,2.12) | 0.82 (0.26,2.58) | 0.78 (0.04,15.19) | 0.82 (0.08,8.64) | 0.98 (0.01,88.32) | Pro | 1.20 (0.55,2.61) | 0.69 (0.34,1.42) | 0.56 (0.29,1.07) | ||||||||
| *0.34 (0.14,0.82) | 0.26 (0.03,2.42) | 0.31 (0.01,7.45) | 0.31 (0.01,7.45) | 0.67 (0.24,1.87) | 0.53 (0.01,19.42) | 0.60 (0.04,8.74) | 0.75 (0.25,2.31) | 0.79 (0.23,2.74) | 0.75 (0.04,15.27) | 0.79 (0.07,8.79) | 0.95 (0.01,89.54) | 0.96 (0.44,2.10) | Met | 0.53 (0.05,5.29) | 0.62 (0.28,1.38) | 0.33 (0.01,7.63) | 0.50 (0.24,1.05) | ||||||
| 0.24 (0.01,5.87) | 0.18 (0.00,8.76) | 0.21 (0.00,18.51) | 0.21 (0.00,18.51) | 0.47 (0.02,10.06) | 0.37 (0.00,37.74) | 0.42 (0.01,21.68) | 0.53 (0.02,13.02) | 0.55 (0.02,15.57) | 0.53 (0.01,37.06) | 0.56 (0.01,25.49) | 0.66 (0.00,164.21) | 0.67 (0.03,16.47) | 0.70 (0.03,17.88) | Nad | |||||||||
| 0.18 (0.01,2.34) | 0.14 (0.01,3.68) | 0.16 (0.00,8.87) | 0.16 (0.00,8.87) | 0.36 (0.03,4.86) | 0.28 (0.00,21.45) | 0.32 (0.01,11.70) | 0.40 (0.03,5.68) | 0.42 (0.03,6.28) | 0.40 (0.01,18.88) | 0.42 (0.01,12.67) | 0.50 (0.00,86.57) | 0.51 (0.04,6.42) | 0.53 (0.05,5.89) | 0.76 (0.01,42.98) | Bev | ||||||||
| 0.17 (0.01,4.91) | 0.13 (0.00,7.14) | 0.15 (0.00,14.84) | 0.15 (0.00,14.84) | 0.33 (0.01,8.48) | 0.27 (0.00,30.13) | 0.30 (0.01,17.64) | 0.38 (0.01,10.90) | 0.39 (0.01,12.96) | 0.38 (0.00,29.87) | 0.40 (0.01,20.82) | 0.47 (0.00,128.82) | 0.48 (0.02,13.80) | 0.50 (0.02,14.94) | 0.71 (0.01,61.84) | 0.94 (0.01,60.10) | iTol | |||||||
| *0.19 (0.06,0.59) | 0.15 (0.01,1.51) | 0.17 (0.01,4.34) | 0.17 (0.01,4.34) | 0.37 (0.11,1.24) | 0.30 (0.01,11.42) | 0.34 (0.02,5.23) | 0.42 (0.12,1.41) | 0.44 (0.12,1.58) | 0.42 (0.02,8.83) | 0.44 (0.04,5.18) | 0.53 (0.01,51.47) | 0.54 (0.22,1.32) | 0.56 (0.22,1.44) | 0.80 (0.03,21.57) | 1.05 (0.08,13.82) | 1.11 (0.04,35.24) | Oxp | 0.80 (0.49,1.32) | |||||
| 0.14 (0.01,2.12) | 0.10 (0.00,3.45) | 0.12 (0.00,7.70) | 0.12 (0.00,7.70) | 0.27 (0.02,3.53) | 0.21 (0.01,6.57) | 0.24 (0.01,8.59) | 0.30 (0.02,4.69) | 0.31 (0.02,5.75) | 0.30 (0.01,15.14) | 0.32 (0.01,9.99) | 0.38 (0.00,72.84) | 0.39 (0.02,5.94) | 0.40 (0.02,6.50) | 0.57 (0.01,31.62) | 0.75 (0.02,29.73) | 0.80 (0.01,50.30) | 0.72 (0.04,12.50) | iEsm | 0.38 (0.06,2.28) | ||||
| *0.13 (0.02,0.75) | 0.10 (0.01,1.65) | 0.12 (0.00,4.27) | 0.12 (0.00,4.28) | 0.26 (0.05,1.41) | 0.21 (0.00,9.65) | 0.24 (0.01,4.67) | 0.29 (0.05,1.88) | 0.31 (0.04,2.33) | 0.29 (0.01,8.41) | 0.31 (0.02,5.12) | 0.37 (0.00,46.14) | 0.38 (0.06,2.23) | 0.39 (0.07,2.32) | 0.56 (0.02,18.52) | 0.73 (0.04,14.55) | 0.78 (0.02,30.00) | 0.70 (0.10,4.79) | 0.98 (0.04,21.41) | Lab | ||||
| *0.15 (0.05,0.46) | 0.12 (0.01,1.19) | 0.14 (0.01,3.44) | 0.14 (0.01,3.44) | *0.30 (0.09,0.96) | 0.24 (0.01,9.06) | 0.27 (0.02,4.14) | 0.34 (0.10,1.10) | 0.35 (0.10,1.23) | 0.34 (0.02,6.99) | 0.35 (0.03,4.09) | 0.42 (0.00,40.95) | 0.43 (0.18,1.01) | 0.45 (0.18,1.11) | 0.64 (0.02,17.10) | 0.84 (0.06,10.93) | 0.89 (0.03,27.96) | 0.80 (0.34,1.91) | 1.12 (0.07,19.21) | 1.15 (0.17,7.70) | Tim | |||
| 0.09 (0.01,1.09) | 0.07 (0.00,1.89) | 0.08 (0.00,4.35) | 0.08 (0.00,4.35) | 0.18 (0.02,1.80) | 0.14 (0.01,2.76) | 0.16 (0.01,4.75) | 0.20 (0.02,2.42) | 0.21 (0.01,3.00) | 0.20 (0.00,8.49) | 0.21 (0.01,5.47) | 0.25 (0.00,42.75) | 0.26 (0.02,3.05) | 0.27 (0.02,3.35) | 0.38 (0.01,17.87) | 0.50 (0.02,16.41) | 0.54 (0.01,28.59) | 0.48 (0.04,6.48) | 0.67 (0.07,6.55) | 0.69 (0.04,12.00) | 0.60 (0.05,7.95) | iPLab | 0.54 (0.18,1.59) | |
| 0.07 (0.00,1.74) | 0.06 (0.00,2.61) | 0.07 (0.00,5.53) | 0.07 (0.00,5.53) | 0.14 (0.01,2.97) | 0.11 (0.00,11.28) | 0.13 (0.00,6.45) | 0.16 (0.01,3.85) | 0.17 (0.01,4.61) | 0.16 (0.00,11.05) | 0.17 (0.00,7.58) | 0.20 (0.00,49.27) | 0.21 (0.01,4.87) | 0.21 (0.01,5.29) | 0.31 (0.00,22.95) | 0.40 (0.01,22.07) | 0.43 (0.01,36.20) | 0.38 (0.01,10.07) | 0.54 (0.01,28.86) | 0.55 (0.02,17.71) | 0.48 (0.02,12.40) | 0.80 (0.02,36.07) | Sot | |
| *0.05 (0.01,0.48) | *0.04 (0.00,0.88) | 0.04 (0.00,2.09) | 0.04 (0.00,2.09) | *0.10 (0.01,0.77) | 0.08 (0.00,1.38) | 0.09 (0.00,2.22) | 0.11 (0.01,1.07) | 0.12 (0.01,1.34) | 0.11 (0.00,4.04) | 0.12 (0.01,2.54) | 0.14 (0.00,21.16) | 0.14 (0.01,1.34) | 0.15 (0.01,1.48) | 0.21 (0.01,8.51) | 0.28 (0.01,7.73) | 0.29 (0.01,13.69) | 0.26 (0.02,2.89) | 0.37 (0.06,2.46) | 0.38 (0.03,5.44) | 0.33 (0.03,3.54) | 0.55 (0.15,1.99) | 0.69 (0.02,26.99) | iPro |
Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of asthma exacerbation incidence rate. Interventions are reported in order of mean ranking of incidence of asthma exacerbation, and outcomes are expressed as odds ratio (OR) (95% confidence intervals). For the pairwise meta-analyses, OR of less than 1 indicate that the treatment specified in the row got less incidence of asthma exacerbation than that specified in the column. For the network meta-analysis (NMA), OR of less than 1 indicate that the treatment specified in the column got less incidence of asthma exacerbation than that specified in the row. Bold results marked with * indicate statistical significance.
Ace oral acebutolol, Ate oral atenolol, Bev oral bevantolol, Bis oral bisoprolol, Car oral carvedilol, Cat oral carteolol, Cel oral celiprolol, CI confidence interval, CPro oral celiprolol and propranolol, ES effect size, iEsm infusion of esmolol, iPac infusion of practolol, iPLab infusion of propranolol and labetalol, iPro infusion of propranolol, iSot infusion of sotalol, iTol infusion of tolamolol, Lab oral dilevalol or oral labetalol, Met oral metoprolol, Nad oral nadolol, NMA network meta-analysis, OR odds ratio, Oxp oral oxprenolol, Pin oral pindolol, Pla Placebo/control, Pra oral practolol, Pro oral propranolol, Sot oral sotalol, SUCRA surface under the cumulative ranking curve, Tim oral timolol.
Figure 3.
Forest plot of the incidence of asthma attack by (A) individual beta-blocking agents among the overall participants and (B) individual beta-blocking agents among participants with a baseline history of asthma. An effect size (presented as risk ratio) of < 1 corresponds to a lower incidence of asthma attack by specified beta-blocking agent compared with that by the placebo or control group. Ace oral acebutolol, Ate oral atenolol, Bev oral bevantolol, Bis oral bisoprolol, Car oral carvedilol, Cat oral carteolol, Cel oral celiprolol, CI confidence interval, CPro oral celiprolol and propranolol, iEsm infusion of esmolol, iPac infusion of practolol, iPLab infusion of propranolol and labetalol, iPro infusion of propranolol, iSot infusion of sotalol, iTol infusion of tolamolol, Lab oral dilevalol or oral labetalol, Met oral metoprolol, Nad oral nadolol, Oxp oral oxprenolol, Pin oral pindolol, Pla Placebo/control, Pra oral practolol, Pro oral propranolol, Sot oral sotalol, Tim oral timolol.
Sensitivity test
After the removal of trials with zero event in their treatment arms, there were 11 remaining studies for the NMA (eFigure 1), which compared 10 treatments, including placebo, oral propranolol, oral pindolol, oral atenolol, oral acebutolol, oral bevantolol, oral metoprolol, oral carteolol, oral oxprenolol, and infusion of sotalol. The primary results of the NMA remained largely unchanged, except that oral atenolol was associated with a significantly lower incidence of asthma attack [RR = 0.33 (95% CI 0.14–0.74)] than the placebo or control groups (Table 2, eFigure 2 and eTable 6C).
Table 2.
League table of association between asthma exacerbation and beta-blocking agent prescription: sensitivity test of removal of zero event.
| Ate | 0.46 (0.15,1.48) | *0.31 (0.12,0.82) | |||||||
| 0.74 (0.15,3.71) | Ace | 0.45 (0.07,3.00) | 0.44 (0.10,1.85) | 1.00 (0.16,6.14) | 0.27 (0.04,1.61) | ||||
| 0.43 (0.17,1.04) | 0.58 (0.13,2.57) | Met | 0.82 (0.37,1.83) | 0.45 (0.07,3.00) | 0.53 (0.05,5.29) | 0.59 (0.26,1.36) | |||
| *0.37 (0.15,0.90) | 0.50 (0.12,2.03) | 0.86 (0.42,1.76) | Pro | 0.44 (0.10,1.85) | 0.77 (0.41,1.45) | 0.67 (0.32,1.39) | |||
| 0.36 (0.02,6.60) | 0.48 (0.02,10.96) | 0.84 (0.05,15.14) | 0.97 (0.06,16.68) | Cat | 1.00 (0.07,14.90) | ||||
| 0.36 (0.03,4.39) | 0.49 (0.03,7.91) | 0.85 (0.07,10.28) | 0.98 (0.09,11.14) | 1.01 (0.03,39.75) | iSot | 0.91 (0.09,9.60) | |||
| 0.36 (0.12,1.07) | 0.48 (0.10,2.31) | 0.84 (0.30,2.37) | 0.97 (0.41,2.34) | 1.00 (0.07,14.90) | 0.99 (0.08,11.94) | Pin | 1.33 (0.53,3.36) | 0.27 (0.04,1.61) | |
| *0.33 (0.14,0.74) | 0.44 (0.10,1.94) | 0.77 (0.34,1.74) | 0.89 (0.51,1.57) | 0.91 (0.05,15.27) | 0.91 (0.09,9.60) | 0.91 (0.41,2.02) | Pla | ||
| 0.23 (0.02,2.66) | 0.31 (0.02,4.75) | 0.53 (0.05,5.29) | 0.62 (0.06,6.87) | 0.64 (0.02,25.59) | 0.63 (0.02,18.74) | 0.64 (0.05,7.90) | 0.70 (0.06,7.95) | Bev | |
| *0.23 (0.08,0.63) | 0.31 (0.07,1.31) | 0.54 (0.26,1.14) | 0.63 (0.32,1.22) | 0.64 (0.04,11.52) | 0.64 (0.05,7.75) | 0.64 (0.23,1.77) | 0.70 (0.31,1.59) | 1.01 (0.09,11.28) | Oxp |
Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of asthma exacerbation incidence rate. Interventions are reported in order of mean ranking of incidence of asthma exacerbation, and outcomes are expressed as odds ratio (OR) (95% confidence intervals). For the pairwise meta-analyses, OR of less than 1 indicate that the treatment specified in the row got less incidence of asthma exacerbation than that specified in the column. For the network meta-analysis (NMA), OR of less than 1 indicate that the treatment specified in the column got less incidence of asthma exacerbation than that specified in the row. Bold results marked with * indicate statistical significance.
Ace oral acebutolol, Ate oral atenolol, Bev oral bevantolol, Bis oral bisoprolol, Car oral carvedilol, Cat oral carteolol, Cel oral celiprolol, CI confidence interval, CPro oral celiprolol and propranolol, ES effect size, iEsm infusion of esmolol, iPac infusion of practolol, iPLab infusion of propranolol and labetalol, iPro infusion of propranolol, iSot infusion of sotalol, iTol infusion of tolamolol, Lab oral dilevalol or oral labetalol, Met oral metoprolol, Nad oral nadolol, NMA network meta-analysis, OR odds ratio, Oxp oral oxprenolol, Pin oral pindolol, Pla Placebo/control, Pra oral practolol, Pro oral propranolol, Sot oral sotalol, SUCRA surface under the cumulative ranking curve, Tim oral timolol.
Subgroup analysis of participants with a definite baseline history of asthma
A total of 13 RCTs provided evidence related to patients with a baseline asthma history and 18 treatment arms, including placebo or control, oral propranolol, oral pindolol, oral atenolol, oral acebutolol, oral metoprolol, oral practolol, oral oxprenolol, oral timolol, oral labetalol, oral bisoprolol, oral celiprolol, infusion of esmolol, infusion of propranolol, infusion of propranolol and labetalol, infusion of practolol, oral celiprolol and propranolol, and oral bevantolol (Fig. 2B and Table 3).
Table 3.
League table of association between asthma exacerbation and beta-blocking agent prescription: patients with baseline asthma.
| Pla | 1.00 (0.02,47.38) | 1.00 (0.02,47.18) | 0.57 (0.12,2.68) | 0.67 (0.27,1.61) | 0.24 (0.03,2.16) | *0.14 (0.02,0.82) | 0.49 (0.02,14.93) | 0.33 (0.02,7.30) | 0.14 (0.01,2.53) | *0.06 (0.00,0.97) | 0.18 (0.02,1.49) | *0.05 (0.00,0.77) | *0.10 (0.01,0.73) | ||||
| 1.17 (0.11,12.02) | Cel | 1.00 (0.02,47.85) | 1.00 (0.02,46.05) | 1.00 (0.02,46.05) | 0.20 (0.01,3.90) | 0.33 (0.02,7.32) | |||||||||||
| 1.39 (0.06,33.84) | 1.19 (0.03,53.95) | Pra | 0.33 (0.02,7.58) | ||||||||||||||
| 1.26 (0.04,37.06) | 1.08 (0.02,65.52) | 0.91 (0.01,94.63) | iPac | 0.14 (0.01,2.53) | 0.08 (0.00,1.25) | ||||||||||||
| 0.82 (0.26,2.58) | 0.70 (0.07,6.78) | 0.59 (0.02,15.24) | 0.65 (0.02,23.05) | Ate | 0.25 (0.03,2.23) | 0.25 (0.03,2.23) | *0.38 (0.15,0.98) | 0.36 (0.05,2.71) | *0.06 (0.00,0.97) | *0.05 (0.00,0.77) | |||||||
| 0.95 (0.04,23.51) | 0.82 (0.03,25.23) | 0.68 (0.01,55.57) | 0.75 (0.01,79.63) | 1.16 (0.05,28.16) | Bis | 1.00 (0.02,46.05) | 0.33 (0.02,7.32) | ||||||||||
| 0.95 (0.04,23.51) | 0.82 (0.03,25.23) | 0.68 (0.01,55.57) | 0.75 (0.01,79.63) | 1.16 (0.05,28.16) | 1.00 (0.02,46.05) | CPro | 0.33 (0.02,7.32) | ||||||||||
| 0.65 (0.29,1.45) | 0.55 (0.05,5.63) | 0.46 (0.02,11.46) | 0.51 (0.02,16.54) | 0.79 (0.24,2.55) | 0.68 (0.03,16.52) | 0.68 (0.03,16.52) | Pin | 1.00 (0.24,4.24) | 0.56 (0.12,2.60) | 0.54 (0.17,1.74) | 0.35 (0.08,1.47) | 0.28 (0.07,1.15) | |||||
| 0.51 (0.14,1.87) | 0.44 (0.04,4.78) | 0.37 (0.02,7.81) | 0.41 (0.01,15.20) | 0.63 (0.16,2.39) | 0.54 (0.02,13.73) | 0.54 (0.02,13.73) | 0.80 (0.23,2.81) | Ace | 0.56 (0.12,2.60) | 0.54 (0.17,1.74) | 0.35 (0.08,1.47) | 0.28 (0.07,1.15) | |||||
| *0.33 (0.12,0.92) | 0.28 (0.03,2.45) | 0.24 (0.01,5.79) | 0.26 (0.01,8.95) | *0.40 (0.17,0.95) | 0.35 (0.02,7.77) | 0.35 (0.02,7.77) | 0.51 (0.19,1.41) | 0.64 (0.20,2.07) | Met | 0.83 (0.38,1.80) | 0.53 (0.05,5.29) | 0.62 (0.28,1.38) | 0.33 (0.01,7.63) | 0.50 (0.24,1.05) | |||
| *0.30 (0.11,0.84) | 0.26 (0.03,2.21) | 0.21 (0.01,5.15) | 0.24 (0.01,8.10) | *0.36 (0.13,0.98) | 0.31 (0.01,6.60) | 0.31 (0.01,6.60) | 0.46 (0.17,1.23) | 0.58 (0.19,1.76) | 0.90 (0.45,1.83) | Pro | 0.69 (0.34,1.42) | 0.56 (0.29,1.07) | |||||
| 0.26 (0.02,3.20) | 0.23 (0.01,6.87) | 0.19 (0.00,10.90) | 0.21 (0.01,5.66) | 0.32 (0.02,5.02) | 0.28 (0.00,16.11) | 0.28 (0.00,16.11) | 0.41 (0.03,5.62) | 0.52 (0.03,8.51) | 0.80 (0.05,11.83) | 0.89 (0.06,13.14) | iEsm | 0.38 (0.06,2.28) | |||||
| 0.18 (0.02,1.63) | 0.15 (0.01,3.80) | 0.13 (0.00,6.24) | 0.14 (0.01,2.53) | 0.22 (0.02,2.63) | 0.19 (0.00,9.24) | 0.19 (0.00,9.24) | 0.28 (0.03,2.90) | 0.35 (0.03,4.49) | 0.55 (0.05,6.17) | 0.60 (0.05,6.85) | 0.68 (0.09,5.37) | iPLab | 0.54 (0.18,1.59) | ||||
| 0.18 (0.01,2.17) | 0.15 (0.01,3.52) | 0.13 (0.00,6.46) | 0.14 (0.00,9.42) | 0.22 (0.02,2.49) | 0.19 (0.00,8.82) | 0.19 (0.00,8.82) | 0.27 (0.02,3.35) | 0.34 (0.03,4.50) | 0.53 (0.05,5.29) | 0.59 (0.05,6.51) | 0.67 (0.02,22.87) | 0.98 (0.03,27.58) | Bev | ||||
| *0.19 (0.07,0.55) | 0.17 (0.02,1.51) | 0.14 (0.01,3.37) | 0.15 (0.00,5.30) | *0.24 (0.09,0.65) | 0.20 (0.01,4.57) | 0.20 (0.01,4.57) | *0.30 (0.11,0.82) | 0.38 (0.12,1.16) | 0.59 (0.29,1.19) | 0.65 (0.34,1.26) | 0.73 (0.05,10.92) | 1.08 (0.09,12.31) | 1.10 (0.10,12.16) | Oxp | 0.80 (0.49,1.32) | ||
| *0.15 (0.02,0.99) | 0.13 (0.01,2.37) | 0.11 (0.00,4.25) | 0.12 (0.00,5.75) | 0.19 (0.02,1.50) | 0.16 (0.00,6.14) | 0.16 (0.00,6.14) | 0.23 (0.03,1.73) | 0.29 (0.03,2.65) | 0.46 (0.06,3.38) | 0.51 (0.07,3.88) | 0.57 (0.03,12.97) | 0.84 (0.05,15.20) | 0.86 (0.04,18.01) | 0.78 (0.10,6.02) | Lab | ||
| *0.16 (0.06,0.43) | 0.13 (0.01,1.19) | 0.11 (0.00,2.67) | 0.12 (0.00,4.20) | *0.19 (0.07,0.50) | 0.16 (0.01,3.62) | 0.16 (0.01,3.62) | *0.24 (0.09,0.63) | *0.30 (0.10,0.90) | *0.47 (0.25,0.90) | *0.52 (0.29,0.95) | 0.59 (0.04,8.64) | 0.86 (0.08,9.72) | 0.88 (0.08,9.60) | 0.80 (0.49,1.32) | 1.03 (0.14,7.77) | Tim | |
| *0.10 (0.01,0.73) | 0.08 (0.00,1.81) | 0.07 (0.00,3.05) | 0.08 (0.00,1.26) | 0.12 (0.01,1.21) | 0.10 (0.00,4.51) | 0.10 (0.00,4.51) | 0.15 (0.02,1.32) | 0.19 (0.02,2.07) | 0.30 (0.03,2.81) | 0.33 (0.03,3.13) | 0.37 (0.06,2.18) | 0.54 (0.18,1.60) | 0.56 (0.02,13.83) | 0.51 (0.05,4.85) | 0.65 (0.04,10.11) | 0.63 (0.07,5.94) | iPro |
Pairwise (upper-right portion) and network (lower-left portion) meta-analysis results are presented as estimate effect sizes for the outcome of asthma exacerbation incidence rate. Interventions are reported in order of mean ranking of incidence of asthma exacerbation, and outcomes are expressed as odds ratio (OR) (95% confidence intervals). For the pairwise meta-analyses, OR of less than 1 indicate that the treatment specified in the row got less incidence of asthma exacerbation than that specified in the column. For the network meta-analysis (NMA), OR of less than 1 indicate that the treatment specified in the column got less incidence of asthma exacerbation than that specified in the row. Bold results marked with * indicate statistical significance.
Ace oral acebutolol, Ate oral atenolol, Bev oral bevantolol, Bis oral bisoprolol, Car oral carvedilol, Cat oral carteolol, Cel oral celiprolol, CI confidence interval, CPro oral celiprolol and propranolol, ES effect size, iEsm infusion of esmolol, iPac infusion of practolol, iPLab infusion of propranolol and labetalol, iPro infusion of propranolol, iSot infusion of sotalol, iTol infusion of tolamolol, Lab oral dilevalol or oral labetalol, Met oral metoprolol, Nad oral nadolol, NMA network meta-analysis, OR odds ratio, Oxp oral oxprenolol, Pin oral pindolol, Pla Placebo/control, Pra oral practolol, Pro oral propranolol, Sot oral sotalol, SUCRA surface under the cumulative ranking curve, Tim oral timolol.
In the subgroup NMA of patients with a baseline asthma history, besides oral timolol [RR = 6.42 (95% CI 2.34–17.61)] and infusion of propranolol [RR = 10.20 (95% CI 1.37–75.64)], there were additional beta-blockers that were associated with a significantly higher incidence of asthma attack than the placebo or control groups, including oral labetalol [RR = 6.60 (95% CI 1.01–43.29)], oral oxprenolol [RR = 5.15 (95% CI 1.81–14.69)], oral propranolol [RR = 3.35 (95% CI 1.20–9.38)], and oral metoprolol [RR = 3.03 (95% CI 1.09–8.41)]. In contrast, only oral practolol, infusion of practolol, and oral celiprolol retained their association with a relatively lower incidence of asthma attack than did the placebo or control groups, although not reaching statistical significance. The relative safety of oral bisoprolol, oral atenolol, oral celiprolol and propranolol, and infusion of sotalol did not persist in patients with a definite baseline asthma history. However, the current evidence could not rule out the null hypothesis (Table 3 and Fig. 3B). The association with the beta-blocking agents and the incidence of asthma attack in patients with a definite baseline history of asthma were ranked according to the SUCRA. In brief, the placebo or control group was associated with the least risk of incidence of asthma attack, followed by oral celiprolol and oral practolol (eTable 6B). A meta-regression was performed using restricted maximum likelihood estimators to analyze the potential effect of age and gender distribution on the incidence of asthma attack. The results of this meta-regression did not demonstrate a significant effect on the incidence of asthma attack when using a moderating variable, including age and gender distribution.
Risk of bias and publication bias
We found that 43.4% (76/175 items), 49.7% (87/175 items), and 6.9% (12/175 items) of the included studies had an overall low, unclear, and high risk of bias, respectively. The ambigious results of randomization procedures or blindness of the studies further contributed to the potential bias (eFigures 3A,B).
Funnel plots of publication bias across the included studies (eFigures 4A–F) revealed a general symmetry, and the results of Egger’s test indicated no significant publication bias among the articles included in the NMA. In general, NMAs did not demonstrate inconsistency, in terms of either local inconsistency, as assessed using the loop-specific approach and the node-splitting method, or global inconsistency, as determined using the design-by-treatment method (eTables 8–9). In brief, the overall quality of evidence of the NMA, direct evidence, and indirect evidence were low to medium according to GRADE ratings (eTable 10).
Discussion
To the best of our knowledge, this is the first NMA addressing the risk of asthma attack in conjunction with different beta-blocker treatments in the general and asthma population. Our findings suggest that across the entire sample, only oral timolol and infusion of propranolol were associated with a significantly higher risk of asthma attack than placebo, whereas the other beta-blockers such as oral celiprolol, oral celiprolol and propranolol, oral bisoprolol, oral atenolol, infusion of practolol, and infusion of sotalol exhibited a relative lower risk of asthma exacerbation than did placebo with no statistically significant differences. When focusing on participants with a baseline diagnosis of asthma, in addition to oral timolol and infusion of propranolol, oral labetalol, oxprenolol, propranolol, and metoprolol were also associated with a significantly higher incidence of asthma attack than placebo.
The major finding of the current NMA was that only oral timolol and infusion of propranolol were associated with a significantly higher risk of asthma attack than placebo, especially in participants with a baseline asthma diagnosis. The result that oral timolol [RR = 3.35 (95% CI 1.04–10.85)] had a substantially increased risk of developing an asthma attack was primarily reported in only one single-blind, randomized, crossover study that was included in our NMA26. The subjects showed a reduction in FEV1 of 53.3% from baseline after 2 h of 10 mg oral timolol. The serious adverse reaction of topical administration, ophthalmic timolol in glaucoma, has been discussed previously27,28. Oral propranolol is one nonselective beta-blocker, extensively used in the treatment of hypertension and ischemic heart disease due to its negative inotropic and chronotropic effects. Although chronic oral propranolol use in patients with asthma had no significant effect on airway hyper-responsiveness or no change in asthma control questionnaire (ACQ)29, intravenous infusion of propranolol resulted in marked symptomatic bronchoconstriction even at the lowest dose (1 mg)30. Therefore, oral timolol and infusion of propranolol definitively increase the risk of developing an asthma attack and are contraindicated for use in patients with asthma.
Although management of comorbidity in the primary care setting is the norm in modern medicine, clinical uncertainty still exists around whether to prescribe beta-blockers to people with asthma and cardiovascular disease. Timothy et al.5 reviewed seven studies and advised against the routine use of beta-blockers in patients with asthma and hypertension because of the increased adverse events of decline in FEV1 or asthma exacerbation. A retrospective cohort study using Veterans Administration databases in Iowa and Nebraska demonstrated that the hazard ratio of hospital admission for asthma was comparable for patients taking or not taking beta-blockers and that there was no difference between selective and nonselective beta-blockers31. Despite these observations, one population-based nested case–control study conducted in the UK demonstrated that nonselective beta-blockers were associated with a significantly increased risk of asthma exacerbation and that, in contrast, cardioselective beta-blocker exposure was not15. In our study, oral timolol and infusion of propranolol, both of which are nonselective beta-blockers, demonstrated a statistically significant risk for asthma exacerbation. Furthermore, oral labetalol, oxprenolol and propranolol, all nonselective, demonstrated a significantly higher incidence of asthma attack in patients with underlying asthma history. Therefore, these findings reveal that additional respiratory adverse events may be observed more consistently with nonselective beta-blocker use.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses6,7. Patients with reactive airway disease who received one single dose of cardioselective beta-blockers had a 7.46% decrease in FEV1, an effect of 4.63% that was reversed by treatment with a beta-agonist inhaler. Patients who received continuous cardioselective beta-blockers experienced no significant reduction in FEV1, and no new symptoms developed. In addition, there were differences in the adverse effect on FEV1 with acute exposure of each of the other beta-blockers. Compared with placebo, celiprolol did not cause a change in FEV1, whereas metoprolol and atenolol did. In our study results, oral celiprolol, oral bisoprolol, oral atenolol and infusion of practolol showed a relatively lower risk of asthma exacerbation than did placebo without statistically significant differences. Moreover, the incidence of asthma attack was found to differ with cardioselective or nonselective beta-blockers according to the SUCRA in the current study. It is possible that the selectivity of beta1-adrenoceptor (calculated by beta 1-/beta 2-affinity ratios) varies, for example, from 13.5 for bisoprolol, 4.7 for atenolol, to 2.3 for metoprolol32,33. Celiprolol is a beta-blocker with a partial agonist activity and a greater selectivity than atenolol and bisoprolol. In our NMA, the sensitivity test revealed that oral atenolol demonstrated a significantly lower risk of asthma attack than did placebo, as studies on bisoprolol and celiprolol were removed due to no event being observed in their trials.
Although the Global Initiative for Asthma (GINA)34 guideline does not mention beta-blocker use in patients with asthma, other clinical guidelines for the treatment of asthma around the world provide various recommendations. The British Thoracic Society’s guideline recommends that all beta-blockers, including eye drops, be contraindicated35. However, the guideline of the National Heart, Lung, and Blood Institute in the USA recommends avoiding nonselective beta-blocker use in patients with asthma36. Correspondingly, guidelines from Australia37 and Japan38 suggest choosing cardioselective beta-blockers when possible. Our data support the additional recommendation that the use of the nonselective beta-blockers oral timolol and infusion of propranolol should be avoided. Furthermore, the cardioselective beta-blockers atenolol, bisoprolol, and celiprolol could be considered for use in patients with asthma and cardiovascular diseases.
There are several limitations that must be considered while interpreting our results. First, some of the analyses in this study were limited by underpowered statistics and small sample sizes, including heterogeneity in the characteristics of the participants (i.e., age, underlying diseases, initial severity of asthma, and trial duration) and the small numbers for some treatment arms. Also, the most included RCTs were published between the 1980s and 2000s. Second, differences in the dosing schemes and the route of administration (i.e., oral versus intravenous) of medications across the included studies may limit the comparability of outcomes in the present NMA. Third, variability in the definition of acute asthma attack of the included studies, including symptoms of wheezing, dyspnea, and symptomatic bronchospasm, may also limit the comparability of outcomes in our study. Fourth, the wide range of treatment durations among the investigated medications may limit the interpretation of the current study results. Fifth, the connection of the overall network structure was weak, so some of the interventions did not have additional direct evidence to support the primary result of the current NMA (i.e., infusion of sotalol, infusion of tolamolol, oral bevantolol, oral carteolol, oral carvedilol, and oral nadolol). In addition, although the superiority of oral celiprolol and oral bisoprolol in terms of preventing asthma exacerbation was ranked as 2nd and 3rd, respectively, according to SUCRA, the primary evidence was based only on a limited number of RCTs (i.e., two RCTs for oral celiprolol39,40 and another two RCTs for oral bisoprolol)39,41. Therefore, clinicians should be careful when applying the results of the current NMA in their clinical practice. Finally, although we tried to include as many RCTs as possible by including early RCTs in 1976, it is possible that some RCTs were missed because of the use of the keyword “asthma” in our search strategy.
Conclusion
This study showed that oral timolol and infusion of propranolol were associated with a significant risk of developing asthma attacks in patients with or without asthma history. Alternatively, oral celiprolol, oral celiprolol and propranolol, oral bisoprolol, oral atenolol, infusion of practolol, and infusion of sotalol might be associated with a lower incidence of asthma exacerbation. However, as some of the intervention comparisons were based only on a limited number of RCTs, clinicians should select specific treatments with caution and avoid the “one-size-fits-all” treatment for all clinical conditions.
Supplementary Information
Acknowledgements
Brendon Stubbs is supported by a Clinical Lectureship (ICA-CL-2017-03-001) jointly funded by Health Education England (HEE) and the National Institute for Health Research (NIHR). Brendon Stubbs is part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Brendon Stubbs is also supported by the Maudsley Charity, King’s College London and the NIHR South London Collaboration for Leadership in Applied Health Research and Care (CLAHRC) funding. This paper presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the acknowledged institutions. The work of Kuan-Pin Su is supported by the following Grants: MOST 106-2314-B-039-027-MY3; 106-2314-B-038-049; 106-2314-B-039-031; 106-2314-B-039-035; and 105-2918-I-039-001 from the Ministry of Science and Technology, Taiwan; and CMU106-S-33, CRS-106-063, DMR-107-202, DMR-107-204, DMR-107-091, DRM-107-097, DRM-108-091, CRS-108-048 and the Chinese Medicine Research Center from the China Medical University, Taiwan. The work by Pao-Yen Lin is supported by the following Grants: MOST 106-2314-B-182A-085 -MY2 and 105-2314-B-182A-057 from the Ministry of Science and Technology, Taiwan; and CMRPG8F1371, CMRPG8E1061 from Kaohsiung Chang Gung Memorial Hospital, Taiwan. The work of Yu-Kang Tu was supported by a grant from the Ministry of Science and Technology, Taiwan (Grant no: 106‐2314‐B‐002‐098‐MY3). These research sponsors have no role in study design, in the collection, analysis, and interpretation of data, in the writing of this report, and in the decision to submit the paper for publication.
Author contributions
K.Y.H., P.T.T., P.Y.L., and C.H.L. designed the study. K.Y.H., P.T.T., C.W.H., and Y.W.C. collected and organized the data. P.T.T., Y.C.W., Y.K.T., and C.W.H. performed statistical analysis. K.Y.H. and P.T.T. wrote the manuscript. P.T.T., K.P.S., B.S., C.H.L., Y.J.M., and P.Y.L. interpreted the data and critically revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kuo-Yang Huang and Ping-Tao Tseng.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79837-3.
References
- 1.James PA, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Kukin A, Noel ZR, Watson K. Through the decades: Beta-blocker use and outcomes in acute coronary syndromes. Cardiol. Rev. 2018;26:157–166. doi: 10.1097/CRD.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 3.Prystowsky EN, Padanilam BJ, Fogel RI. Treatment of atrial fibrillation. JAMA. 2015;314:278–288. doi: 10.1001/jama.2015.7505. [DOI] [PubMed] [Google Scholar]
- 4.Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–1995. doi: 10.1016/S0140-6736(17)31071-1. [DOI] [PubMed] [Google Scholar]
- 5.Self TH, Wallace JL, Soberman JE. Cardioselective beta-blocker treatment of hypertension in patients with asthma: When do benefits outweigh risks? J. Asthma. 2012;49:947–951. doi: 10.3109/02770903.2012.719252. [DOI] [PubMed] [Google Scholar]
- 6.Morales DR, Jackson C, Lipworth BJ, Donnan PT, Guthrie B. Adverse respiratory effect of acute beta-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145:779–786. doi: 10.1378/chest.13-1235. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: A meta-analysis. Ann. Intern. Med. 2002;137:715–725. doi: 10.7326/0003-4819-137-9-200211050-00035. [DOI] [PubMed] [Google Scholar]
- 8.British Thoracic Society & Scottish Intercollegiate Guidelines Network British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192. [PubMed] [Google Scholar]
- 9.Christiansen SC, Zuraw BL. Treatment of hypertension in patients with asthma. N. Engl. J. Med. 2019;381:1046–1057. doi: 10.1056/NEJMra1800345. [DOI] [PubMed] [Google Scholar]
- 10.Ross DS, et al. 2016 American thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 11.Shanker V. Essential tremor: Diagnosis and management. BMJ. 2019;366:l4485. doi: 10.1136/bmj.l4485. [DOI] [PubMed] [Google Scholar]
- 12.Triantos C, Kalafateli M. Primary prevention of bleeding from esophageal varices in patients with liver cirrhosis. World J. Hepatol. 2014;6:363–369. doi: 10.4254/wjh.v6.i6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiratzka LF, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J. Am. Coll. Cardiol. 2010;55:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J. Am. Coll. Cardiol. 2001;37:1950–1956. doi: 10.1016/S0735-1097(01)01225-6. [DOI] [PubMed] [Google Scholar]
- 15.Morales DR, Lipworth BJ, Donnan PT, Jackson C, Guthrie B. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: Population-based nested case control study. BMC Med. 2017;15:18. doi: 10.1186/s12916-017-0781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst. Rev. 2002;2:2992. doi: 10.1002/14651858.CD002992. [DOI] [PubMed] [Google Scholar]
- 17.Loth DW, Brusselle GG, Lahousse L, Hofman A, Leufkens HG, Stricker BH. beta-Adrenoceptor blockers and pulmonary function in the general population: The Rotterdam study. Br. J. Clin. Pharmacol. 2014;77:190–200. doi: 10.1111/bcp.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Welton NJ. Network meta-analysis: A norm for comparative effectiveness? Lancet. 2015;386:628–630. doi: 10.1016/S0140-6736(15)61478-7. [DOI] [PubMed] [Google Scholar]
- 19.Hutton B, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 20.Wu YC, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: A network meta-analysis. JAMA Psychiatry. 2019;76:526–535. doi: 10.1001/jamapsychiatry.2018.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng BS, et al. Prevention of postdental procedure bacteremia: A network meta-analysis. J. Dent. Res. 2019;98:1204–1210. doi: 10.1177/0022034519870466. [DOI] [PubMed] [Google Scholar]
- 22.Yang CP, et al. Melatonergic agents in the prevention of delirium: A network meta-analysis of randomized controlled trials. Sleep Med. Rev. 2020;50:101235. doi: 10.1016/j.smrv.2019.101235. [DOI] [PubMed] [Google Scholar]
- 23.Tseng PT, et al. The association between melatonin and episodic migraine: A pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J. Pineal Res. 2020;69:e12663. doi: 10.1111/jpi.12663. [DOI] [PubMed] [Google Scholar]
- 24.Osborne ML, Vollmer WM, Pedula KL, Wilkins J, Buist AS, O'Hollaren M. Lack of correlation of symptoms with specialist-assessed long-term asthma severity. Chest. 1999;115:85–91. doi: 10.1378/chest.115.1.85. [DOI] [PubMed] [Google Scholar]
- 25.Reddel HK, et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 26.Decalmer PB, Chatterjee SS, Cruickshank JM, Benson MK, Sterling GM. Beta-blockers and asthma. Br. Heart J. 1978;40:184–189. doi: 10.1136/hrt.40.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoene RB, Martin TR, Charan NB, French CL. Timolol-induced bronchospasm in asthmatic bronchitis. JAMA. 1981;245:1460–1461. doi: 10.1001/jama.1981.03310390060024. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman TJ. Topical ophthalmic beta blockers: A comparative review. J. Ocul. Pharmacol. 1993;9:373–384. doi: 10.1089/jop.1993.9.373. [DOI] [PubMed] [Google Scholar]
- 29.Short PM, Williamson PA, Anderson WJ, Lipworth BJ. Randomized placebo-controlled trial to evaluate chronic dosing effects of propranolol in asthma. Am. J. Respir. Crit. Care Med. 2013;187:1308–1314. doi: 10.1164/rccm.201212-2206OC. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard D, et al. Effects of esmolol on airway function in patients with asthma. J. Clin. Pharmacol. 1986;26:169–174. doi: 10.1002/j.1552-4604.1986.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 31.Barnett MJ, Milavetz G, Kaboli PJ. beta-Blocker therapy in veterans with asthma or chronic obstructive pulmonary disease. Pharmacotherapy. 2005;25:1550–1559. doi: 10.1592/phco.2005.25.11.1550. [DOI] [PubMed] [Google Scholar]
- 32.Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology. 2010;113:585–592. doi: 10.1097/ALN.0b013e3181e73eea. [DOI] [PubMed] [Google Scholar]
- 33.Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br. J. Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.[Internet] go: The Global Initiative for Asthma. In. California; 2019.
- 35.[Internet] SIGNS: British Guideline on the Management of Asthma. SIGN 158. In. Edinburgh; 2019.
- 36.National Asthma Education and Prevention Program Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J. Allergy Clin. Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 37.[Internet] NACAN: Australian Asthma Handbook. In. Melbourne; 2019.
- 38.Ichinose M, et al. Japanese guidelines for adult asthma 2017. Allergol. Int. 2017;66:163–189. doi: 10.1016/j.alit.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Pujet JC, Dubreuil C, Fleury B, Provendier O, Abella ML. Effects of celiprolol, a cardioselective beta-blocker, on respiratory function in asthmatic patients. Eur. Respir. J. 1992;5:196–200. [PubMed] [Google Scholar]
- 40.Schindl R, Wurtz J, Hoffmann H. The effect of the cardioselective beta blocker celiprolol on pulmonary function in asthmatic patients. J. Cardiovasc. Pharmacol. 1986;8(Suppl 4):S99–101. doi: 10.1097/00005344-198608004-00021. [DOI] [PubMed] [Google Scholar]
- 41.Lainscak M, Podbregar M, Kovacic D, Rozman J, von Haehling S. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir. Med. 2011;105(Suppl 1):S44–49. doi: 10.1016/S0954-6111(11)70010-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.