Abstract
Cyanase catalyzes the bicarbonate-dependent degradation of cyanate to produce ammonia and carbon dioxide, and ammonia is a considerable alternative nitrogen source. Strikingly, the cyanase from the thermophilic fungus Thermomyces lanuginosus (Tl-Cyn) has the highest catalytic efficiency reported among these enzymes. However, its molecular mechanism of action is not clearly understood, because currently there is no structural information available on fungal cyanases. Here we report the crystal structure of Tl-Cyn in complex with inhibitors malonate and formate at 2.2 Å resolution. The structure reveals extensive interactions at the subunit interfaces in a dimer, and a decamer is formed by a pentamer of these dimers. Our biochemical, kinetic and mutagenesis studies confirm the structural observations on the complex and provide further insights into its catalytic mechanism and inhibition. The structure has also aided the creation of a mutant enzyme with enhanced catalytic activity, and such enzymes may have the potential for biotechnological applications, including biotransformation and bioremediation. Moreover, other fungal cyanases with potentially high catalytic activity could also be predicted based on the Tl-Cyn structure, as the active site region among fungal cyanases are highly conserved.
Subject terms: Biocatalysis, Structural biology, Environmental sciences
Introduction
Cyanide is one of the most toxic chemicals widely used in mining industries for the extraction of metals1. Cyanide is also applied as an anticaking agent in road salt and fire retardants, where several hundred tonnes of cyanide is released into the environment annually2,3. Cyanate is an important cyanide derivative formed by the oxidation of cyanide1. Moreover, it is also generated spontaneously from urea and carbamoyl phosphate4,5. The toxicity of cyanate possibly arises from its reactivity with amino, sulfhydryl, carboxyl, phenolic hydroxyl, imidazole, and phosphate groups in proteins6. Additionally, cyanate is widely used to inhibit physiological reactions in plants as a herbicide7 and in mammals as an uremic toxin8. In contrast, cyanate has been assumed to serve as a nitrogen source for the growth of certain microorganisms (having a functional cyanase) under nitrogen limitation9,10, while microbes lacking a cyanase gene are not able to grow in the presence of cyanate11,12.
Cyanase (EC 4.2.1.104; also known as cyanate hydratase and cyanate lyase) catalyzes the decomposition of cyanate into ammonium and CO2. Cyanases are found in bacteria13–16, fungi17 and plants18, where they have important roles for nitrogen assimilation or cyanate detoxification. Despite these important functions, the production of this enzyme is low in most known organisms16–19. Although the cyanase from bacteria is characterized in detail and its structure is also known20,21, no attempt has been made to enhance its production.
Thermomyces lanuginosus, a thermophilic fungus, has been known to produce the highest amount of xylanase and it also produces several other hydrolytic enzymes22. In addition, it has a ubiquitin degradation pathway which plays an essential role in responses to various stress, such as nutrient limitation, heat shock, and heavy metal exposure23. Owing to these requisite properties, this fungus has been identified as one of the organisms of choice for industrial applications. Furthermore, whole genome sequencing and secretome analysis of T. lanuginosus23,24 unexpectedly revealed the presence of a cyanase (Tl-Cyn). We have successfully over-expressed Tl-Cyn and evaluated its potential in cyanate detoxification25–27. Notably, this cyanase (Tl-Cyn) showed ~ 250-fold higher catalytic activity compared to other cyanases25, suggesting that it could be used for large-scale applications.
To get a better understanding of Tl-Cyn function and a deeper insight into its molecular mechanism requires structural information on this enzyme. To date, biochemical and structural studies have been limited mainly to bacterial cyanases and no structural information is available on fungal cyanase. We report here the crystal structure of the cyanase from T. lanuginosus (Tl-Cyn) at 2.2 Å resolution. The structure reveals extensive interactions at the subunit interfaces in a dimer, and a decamer is formed by a pentamer of these dimers. The structure of the monomer and the overall architecture of the decamer show substantial differences to those of a bacterial cyanase20. We have also determined the binding modes of substrate-analog inhibitors in the active site of Tl-Cyn and characterized their inhibition of the enzyme by kinetic studies, which provide molecular insights into Tl-Cyn catalysis. In addition, we found that mutations in the active site region of Tl-Cyn can reduce enzyme activity. Furthermore, we found that the Y14A mutant has higher catalytic activity compared to the wild-type, due primarily to an increase in kcat.
Results
Overall structure of Tl-Cyn
To gain insight into the Tl-Cyn structure, activity and enable its rational design, we have determined the crystal structure of Tl-Cyn at 2.2 Å resolution. The full-length Tl-Cyn gene was expressed in E. coli BL21 Star (DE3) cells and purified (Supplementary Fig. S1a). The stability of the purified Tl-Cyn was evaluated using the thermal shift assay, and the Tm of the protein was ~ 65 °C (Supplementary Fig. S1b). The refined structure has excellent agreement with the crystallographic data and the expected bond lengths, bond angles, and other geometric parameters (Table 1). A total of 99.4% of the residues are in the favored region of the Ramachandran plot, and none are in the disallowed region.
Table 1.
Crystallographic data collection and refinement statistics.
| Structure | Tl-Cyn |
|---|---|
| Data collection | |
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (Å) | 75.37, 157.64, 163.87 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution (Å) | 50.00–2.20 (2.28–2.20)* |
| Rmerge | 9.6 (38.1) |
| Completeness (%) | 99.90 (99.50) |
| Redundancy | 4.4 (4.3) |
| Refinement | |
| Resolution (Å) | 50.00—2.20 (2.28 – 2.20) |
| Rwork/Rfree | 15.44 / 20.18 |
| R.m.s deviations | |
| Bond lengths (Å) | 0.018 |
| Bond angles (°) | 1.9 |
| Ramachandran plot statistics | |
| Most favoured regions (%) | 99 |
| Additionally allowed regions (%) | 1 |
| Outliers (%) | 0.0 |
| PDB entry code | 6XGT |
*Highest resolution shell is shown in parenthesis.
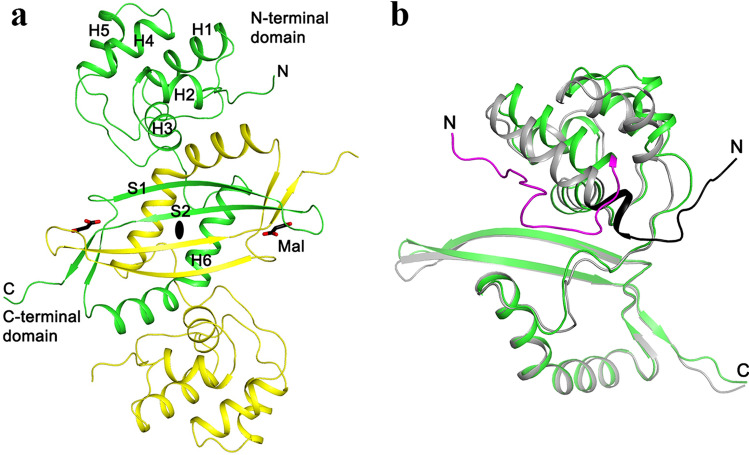
The structure of cyanase contains an N-terminal α-helical domain (residues 1–91; helices H1–H5) and a C-terminal α + β domain (residues 92–161; two antiparallel β-strands S1–S2 and a helix H6) (Fig. 1a). There are extensive interactions between the C-terminal domains of two subunits of a dimer, which together form a four-stranded anti-parallel β-sheet that is flanked on one face with the two H6 helices (Fig. 1a).
Figure 1.
Structure of fungal cyanase dimer. (a) An intertwined C-terminal domain (residues 92–160) in the dimer structure of T. lanuginosus cyanase (Tl-Cyn). The two monomers are colored in yellow and green, and the two-fold axis of the dimer is indicated by the black oval. The two malonate molecules bound to the active sites of the dimer are shown as sticks, colored according to atom types (carbon black, and oxygen red) and labeled Mal. Each monomer contains six α-helices and two anti-parallel β-strands, labeled as H1–H6 and S1–S2, respectively. (b) Overlay of the structure of Tl-Cyn monomer (green) with that of E. coli cyanase (gray). Large structural differences are seen for the N-terminal domain. Especially, the N-terminal segments run in opposite directions in the two structures and are highlighted in magenta and black. The structure figures were produced using PyMOL (http://www.pymol.org).
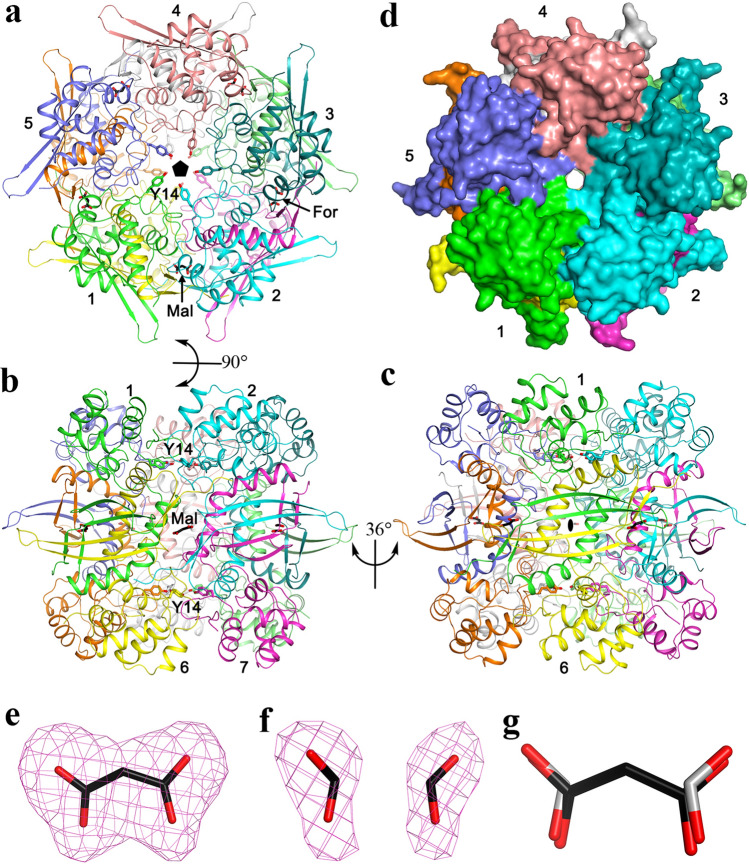
A decamer of Tl-Cyn is formed by a pentamer of these dimers, with intimate contacts among them (Fig. 2a–d). The interfaces in this decamer primarily involve the N-terminal domain and the H6 helix, while the four-stranded β-sheet in the center of the dimer interface is located near the ‘equator’ of the decamer. There is a small channel through the entire decamer, along its five-fold symmetry axis (Fig. 2d).
Figure 2.
Crystal structure of the fungal cyanase decamer. (a) Overall structure of the Tl-Cyn decamer (top view). Each monomer is depicted in a different color. The side chains of Y14 are shown as sticks, pointing towards the center of the structure. The malonate (labeled Mal) and formate (labeled For) molecules bound to the active sites of the decamer are shown as sticks. The five-fold symmetry axis of the decamer is indicated with the black pentagon. (b) Overall structure of the Tl-Cyn decamer viewed after a 90° rotation around the horizontal axis. (c) Overall structure of the Tl-Cyn viewed after a 36° rotation around the vertical axis from panel (b). A two-fold axis of the decamer is indicated with the black oval. (d) Molecular surface of the Tl-Cyn decamer, viewed in the same orientation as in (a). (e) Omit Fo − Fc electron density at 2.2 Å resolution for malonate, contoured at 3 s. (f) Omit Fo − Fc electron density at 2.2 Å resolution for two formate molecules, contoured at 3σ. g, Overlay of the binding modes of malonate (black carbon atoms) and two formate molecules (gray carbon atoms).
The structure of Tl-Cyn shows substantial differences to that of E. coli cyanase20 (Fig. 1b), even though the two proteins share 40% sequence identity. With the C-terminal domains of the two structures in overlay, large differences in the positions of the helices in the N-terminal domain are observed. In particular, the N-terminal segment of the two enzymes run in nearly opposite directions. On the other hand, the overall appearance of the two decamers is similar (Supplementary Fig. S2a,b).
Active site of Tl-Cyn
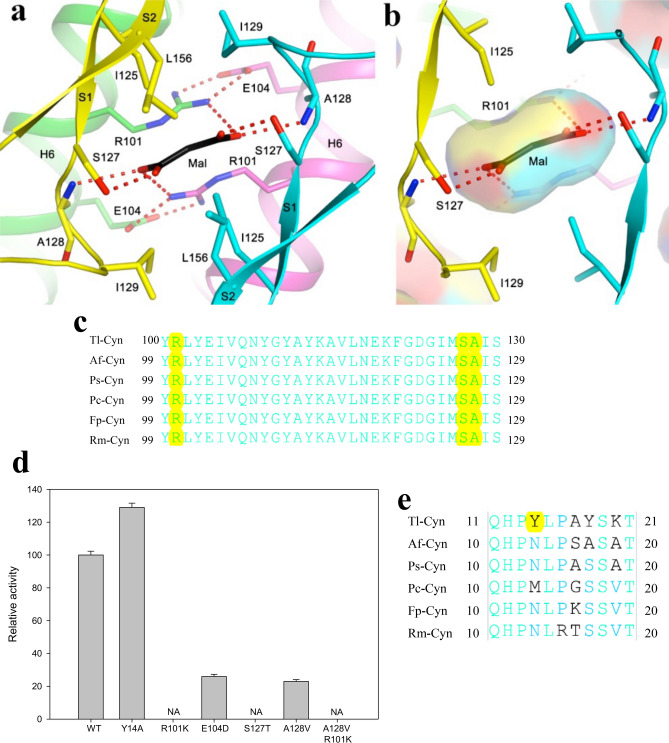
The active site of Tl-Cyn is located in the C-terminal domain (Fig. 1a), at the interface between neighboring dimers of the decamer (Fig. 2a). This suggests that Tl-Cyn needs to be a decamer to be active. Malonate (Mal) is situated on a two-fold symmetry axis in some of the dimers of the decamer, while two formates (For) are observed in the other dimers (Fig. 2a). Both Mal and For are present in the Tacsimate solution used to crystallize Tl-Cyn and have good electron density (Fig. 2e,f). The carboxylate groups of Mal are recognized by six hydrogen bonds from residues of four monomers (Fig. 3a,b), and formate has the same interactions as it has essentially the same binding mode as the carboxylates of malate (Fig. 2g). Specifically, the carboxylate is hydrogen-bonded to the side chain of Ser127 and the main-chain amide of Ala128 from one monomer (Fig. 3a). One of the carboxylate oxygen atoms is also hydrogen-bonded to the side chain of Arg101 from another monomer, which is also involved in a bidentate ion-pair with Glu104 from a third monomer. The carboxylate group of For is recognized in a similar fashion.
Figure 3.
Structural insights into the catalytic mechanism of Tl-Cyn. (a) Schematic drawing of the detailed interactions between Tl-Cyn and the malonate molecule bound in the active site (sticks model and labeled Mal). Hydrogen-bonding interactions with the malonate are indicated with dashed lines (red). (b) Close-up view to that in (a), and the internal cavity for malonate is shown with semi-transparent surface. (c) Conserved sequence near the active site region of the C-terminal domain in fungal cyanases are shown in cyan. Residues that interact with Mal are shown with yellow background. Tl: Thermomyces lanuginosus, Af: Aspergillus flavus, Ps: Penicillium subrubescens, Pc: Phaeomoniella chlamydospora, Fp: Fonsecaea pedrosoi, Rm: Rhinocladiella mackenziei. (d) Catalytic activities of wild-type (WT) and mutant Tl-Cyn. The cyanate concentration is at 2 mM. The error bars represent the standard deviation from three independent measurements. NA, no activity observed under the condition tested. (e) Sequence alignment for the N-terminal domain of the fungal cyanases. Conserved residues are in cyan and a unique Y14 residue in Tl-Cyn is shown with yellow background.
Residues in the active site region of Tl-Cyn are highly conserved among fungal cyanases, sharing 100% amino-acid sequence identity (Fig. 3c). Thus, elucidation of the Tl-Cyn structure could be beneficial to improve the catalytic properties of fungal cyanases and also allow for the creation of novel enzymes for biotechnological applications.
Mutagenesis studies to provide insights into the catalytic mechanism
We designed a series of point mutations that are expected to perturb the hydrogen-bonding interactions with Mal based on the structural observations and evaluated their effects on catalysis (Fig. 3d). When the arginine (R101) and serine (S127) residues (in Tl-Cyn) were mutated to lysine and threonine, respectively, the catalytic activity was completely inhibited (Fig. 3d). We also found that the A128V and E104D mutations greatly reduced the catalytic activity by 77 and 74%, respectively, compared to the wild-type (Fig. 3d). The Glu104 residue is not directly involved in the hydrogen-bonding interactions with Mal; however, it stabilizes the catalytic residue Arg101, which resulted in the loss of catalytic activity. We also observed that, the A128V/R101K double mutation completely eliminated the catalytic activity of the enzyme. These data strongly support the structural observation on the active site of Tl-Cyn in which Arg101, Ser127, and Ala128 residues are involved in the interaction with inhibitor (Mal) residue by hydrogen bonds (Fig. 3a).
In addition, a unique feature of Tl-Cyn is the Tyr14 residue (Fig. 3e), which is located in the center of the structure and helps to reduce the size of the central channel (Fig. 2a,c). Surprisingly, the Y14A mutant displayed ~ 1.3-fold higher catalytic activity as compared to the wild-type Tl-Cyn (Fig. 3d). This could be due to the bulky side chain of Tyr14 residue sterically hindering substrate access to and/or product release from the active site of the Tl-Cyn, while mutating Tyr14 to a smaller amino acid (Ala) might have facilitated more substrate binding to the active site of the mutant. Because of the structural differences in the N-terminal segment (Fig. 1b), the bacterial enzyme does not have an equivalent to Tyr14, and the residue closest to Tyr14 in the structure is Asn9.
We next used kinetic experiments to further characterize the effects of mutations on the catalytic efficiency (kcat/Km) of Tl-Cyn. We observed that the catalytic efficiency of the E104D and A128V mutants was 2.79 ± 0.025 × 107 s−1 M−1 and 2.93 ± 0.12 × 107 s−1 M−1, which is ~ 3.5 and ~ 3.3-fold lower than that for the wild-type enzyme, respectively (Table 2). In contrast, the catalytic efficiency of the Y14A mutant was 12.04 ± 0.69 × 107 s−1 M−1, which is ~ 1.2-fold higher compared to the wild-type enzyme (9.8 ± 0.4 × 107 s−1 M−1), due mostly to a higher kcat.
Table 2.
Summary of kinetic data for wild-type and mutant cyanases.
| Protein | kcat (s−1) | Km (mM) (for cyanate) | kcat/Km (s−1 M−1) |
|---|---|---|---|
| Wild-type | 3.56 ± 0.19 × 104 | 0.36 ± 0.04 | 9.8 ± 0.4 × 107 |
| Y14A | 4.81 ± 0.11 × 104 | 0.40 ± 0.01 | 12.04 ± 0.69 × 107 |
| R101K | NAα | NAα | NAα |
| E104D | 5.84 ± 0.026 × 103 | 0.21 ± 0.003 | 2.79 ± 0.025 × 107 |
| S127T | NAα | NAα | NAα |
| A128V | 5.72 ± 0.029 × 103 | 0.20 ± 0.007 | 2.93 ± 0.12 × 107 |
| A128V/R101K | NAα | NAα | NAα |
αNo activity was observed under the same conditions.
Malonate and formate are cyanase inhibitors
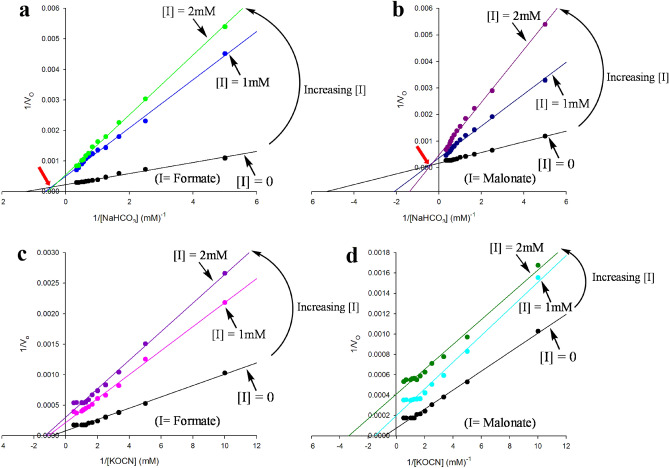
In order to confirm the structural observations that malonate and formate are present in the active site of Tl-Cyn (Fig. 2a), kinetic experiments were performed to determine the inhibition mechanisms. The data are plotted in the form of 1/v0 against 1/[S], where v0 is the initial velocity of the enzyme-catalyzed reaction (µmoles mg−1 min−1) and [S] is the substrate concentration (mM) (Fig. 4). Based on the double-reciprocal plots, formate and malonate have direct inhibitory effect on the catalysis of Tl-Cyn28. Furthermore, the point of intersections observed in the double-reciprocal plots showed that it is a mixed-type of inhibition with respect to bicarbonate HCO3− (Fig. 4a,b). In contrast, no point of intersections were observed with respect to cyanate OCN−, and the presence of parallel lines in the double-reciprocal plots are indicative of the uncompetitive-type of inhibitions (Fig. 4c,d). The inhibitory constants (Ki) for malonate and formate are in the low mM range (Table 3). Our kinetic results confirmed that formate and malonate could inhibit the catalytic activity of Tl-Cyn, which further support the structural observations. Overall, mutagenesis and kinetic studies confirmed the presence of malonate and formate inhibitors at the active site of the Tl-Cyn. We propose a possible mechanism for the decomposition of cyanate and kinetic inhibition of the Tl-Cyn (Supplementary Fig. S3a,b). A similar mechanism for the decomposition of cyanate has been proposed the kinetic studies of another cyanase14.
Figure 4.
Kinetic studies on the inhibition of Tl-Cyn by malonate and formate. (a,b) A set of double-reciprocal plots, one obtained in the absence of inhibitor and two at different concentrations of inhibitor, formate and malonate, respectively in the presence of varying concentrations of NaHCO3. The location of the intersection point of the lines is indicated with the red arrow. (c,d) A set of double-reciprocal plots, one obtained in the absence of inhibitor and two at different concentrations of inhibitor, formate and malonate, respectively in the presence of varying concentrations of KOCN. Data points are means taken from three representative set of experiments.
Table 3.
Summary of inhibition kinetics of Tl-Cyn.
| Substrate | Inhibitor | Type of inhibition | Inhibitory constant (Ki) (mM) | |
|---|---|---|---|---|
| Kiaβ | Kibγ | |||
| KOCN | Formate | Uncompetitive | – | 0.70 ± 0.06 |
| KOCN | Malonate | Uncompetitive | – | 0.54 ± 0.12 |
| NaHCO3 | Formate | Mixed | 0.41 ± 0.11 | 1.19 ± 0.39 |
| NaHCO3 | Malonate | Mixed | 0.23 ± 0.01 | 1.08 ± 0.20 |
βThe Ki for binding to the free enzyme.
γThe Ki for binding to the enzyme–substrate complex.
Discussion
Rapid industrialization and proliferative development of mining industries have resulted in increased global pollution and environmental deterioration, due to the release of toxic chemicals such as cyanide or cyanate29,30. Although the role of Tl-Cyn in the bioremediation of cyanurated-waste is known25–27, the molecular mechanism by which Tl-Cyn carries out decomposition of cyanate is unclear. The structure of the inhibitor-bound Tl-Cyn has elucidated the molecular mechanism of enzyme catalysis, in which the inhibitor interacts with the catalytic residues of the Tl-Cyn and explains the binding affinity between them (Fig. 3 a,b). In particular, it also provides a better understanding for the interpretation of the kinetic data.
Based on results from our mutagenesis studies, it is clear that Arg101 and Ser127 are crucial for the catalytic activity of Tl-Cyn, as highlighted by our structural observations (Fig. 3a,b). This is supported by a previous report wherein it has been shown that Ser122 (equivalent to Ser127) is responsible for the binding of substrates (OCN- and HCO3−) and guanidinium group of the Arg96 (equivalent to Arg101) stabilizes the negative charge of the substrates20. In addition, we performed structure-based protein engineering by replacing Tyr14 (a residue located in the center of structure) to Ala. The Y14A mutant showed higher catalytic activity and the structure also indicates a possible mechanism for the enhanced catalytic activity. This is a key finding as the structure-based engineering of a Tyr14 residue (N-terminal), which is located away from the catalytic site (C-terminal), gives rise to increased cyanase activity. Further mutagenesis studies are needed to enhance the substrate specificity of fungal cyanases, and our structure would provide insights on the effect of mutations with respect to substrate binding.
Our kinetic studies also corroborate the presence of inhibitor molecules viz., malonate and formate at the active site of Tl-Cyn, which is in agreement with the structural observations. Although there is no direct inhibition (uncompetitive-type) observed with respect to cyanate by these inhibitors, it however showed competitive- as well as uncompetitive-type inhibition with respect to bicarbonate. Further, it has been shown that bicarbonate is necessary for the binding of cyanate to the active site of cyanase for its degradation12, hence, inhibition of bicarbonate leads to the inhibition of cyanate binding at the active site of Tl-Cyn. It has also been observed that bicarbonate and cyanate have a competitive relationship for a catalytic binding site12. In addition, inhibition of cyanase activity by malonate in E. coli was also previously reported31, which supports our findings. Our structural analysis would therefore be useful for protein engineering to design biocatalysts with enhanced catalytic properties, which would be valuable for industries focused on sustainable bio-remediation.
Methods
Protein expression and purification
The Tl-Cyn gene was sub-cloned from the previous clone25, into the pET28a vector (Novagen), using NdeI and XhoI restriction sites, which introduced an N-terminal hexa-histidine tag. The recombinant protein was over-expressed in E. coli BL21 Star (DE3) cells (Novagen). The cell culture was grown in LB medium at 37 °C with shaking until the OD600 reached ~ 0.6, which was induced with 0.8 mM IPTG and allowed to grow at 20 °C for 16 h. The culture was then harvested by centrifugation and cells were resuspended in lysis buffer, consisting of 20 mM Tris–HCl (pH 8.0), 250 mM NaCl and lysed by sonication. The soluble protein was bound to nickel-charged immobilized-metal affinity resin (Qiagen) and eluted using lysis buffer supplemented with 250 mM imidazole. The protein was further purified by gel filtration chromatography (Sephacryl S-300, GE Healthcare)32 using buffer consisting of 20 mM Tris–HCl (pH 8.0) and 150 mM NaCl. The purified protein was concentrated using10 kDa ultrafiltration membrane cartridge (Millipore) to 13 mg/mL in a buffer containing 20 mM Tris–HCl (pH 8.0) and 150 mM NaCl, flash-frozen in liquid nitrogen and stored at − 80 °C. The N-terminal hexa-histidine tag was not removed for crystallization.
Thermal shift assay
Thermal stability of the Tl-Cyn at 10 µM concentration was analyzed at various temperatures using the Mx3005P Real-Time PCR system (Stratagene). Tl-Cyn was mixed with the fluorescence dye (SYPRO orange; Invitrogen) for monitoring protein unfolding. All assays were performed in duplicate and contained final concentrations of 20 mM Tris–HCl (pH 8.0) and 150 mM NaCl. The temperature was increased from 25 to 99 °C in 1 °C intervals over a 75 min period. Fluorescence values for the curve were normalized to the maximum and the minimum of the curve33.
Protein crystallization
Crystals were grown by the sitting-drop vapour diffusion method at 20 °C from a protein concentration of 13 mg/mL34. The reservoir solution contained 2% (w/v) Tacsimate (pH 8.0), 0.1 M Tris (pH 8.5), and 16% (w/v) polyethylene glycol 3,350. Fully-grown crystals were obtained after 24 h of set-up. The crystals were cryo-protected in the reservoir solution supplemented with 20% (v/v) glycerol and flash-frozen in liquid nitrogen for data collection at 100 K.
Data collection and processing
X-ray diffraction data were collected at the Advanced Photon Source beamline NE-CAT 24-ID-E using an ADSC Q315r detector and at the X25 beamline at the National Synchrotron Light Source at Brookhaven National Laboratory using a Pilatus 6 M detector. The diffraction images were processed and scaled with the HKL2000 package35.
Structure determination and refinement
The structure of Tl-Cyn was solved by the molecular replacement method with the programme Phaser36, using the structure of E. coli cyanase (PDB code 1dw9) as the search model20. Manual model rebuilding was carried out with Coot37 and refinement with the programme Refmac38.
Site directed mutagenesis
Site directed mutagenesis was carried out according to PCR-based methods. A set of overlapping oligonucleotide primer pairs with the desired mutations were designed to generate the mutant constructs and were synthesized from Integrated DNA Technologies (IDT), the sequences of which are shown in Supplementary Table S1. The recombinant plasmid (pET28a-Tl-Cyn) was used as a template for PCR amplification and the entire plasmid was amplified, using specific primers with the desired mutations (Supplementary Table S1). The PCR products were treated with DpnI (methylation-dependent endonuclease) to remove the parent template and then transformed in E. coli DH5α. Further, plasmids were isolated from the transformed clones and verified by sequencing for successful mutagenesis. The mutants were expressed and purified following the same protocol as that for the wild-type protein.
Cyanase assay
The cyanase assay was performed as previously described25. One unit (U) of cyanase is defined as the amount of enzyme that liberates 1 µmol of ammonium per minute under the defined assay conditions.
Kinetic studies
The enzyme kinetics studies were performed by determining the velocities of the enzyme reactions at different concentrations of potassium cyanate (0.05 to 3.0 mM—Sigma). The apparent Michaelis constant (Km) and the maximal velocity (Vmax) of the enzyme activities were calculated by fitting the initial velocities and substrate concentrations into the Lineweaver–Burk plots. The catalytic efficiency of the enzyme was estimated as kcat/Km ratio. Essentially the same values for Km and kcat were obtained from curve-fitting to the Michaelis–Menten equation (Supplementary Figs. S4, S5 and Supplementary Table S2).
Inhibition kinetics by Formate and Malonate
To characterize the nature of the inhibition by formate and malonate, kinetic assays were carried out with varying concentrations of cyanate and bicarbonate along with different concentrations of inhibitors (one at a time). The inhibitory constants for uncompetitive and mixed inhibitions were analyzed by the Eqs. (1), (2) and (3), (4), respectively.
| 1 |
| 2 |
| 3 |
| 4 |
where Kmapp is the apparent Michaelis constant (measured values of Km in the presence of the inhibitor), [I] the inhibitor concentration, Kia and Kib are the inhibitory constants for binding to the free enzyme and enzyme–substrate complex, respectively, and Vmaxapp is the apparent maximal velocity (measured values of Vmax in the presence of inhibitor). SigmaPlot 10.0 was used to generate the plots.
Sequence alignment
The alignment of selected sequences of cyanase was produced with Vector NTI Advance 11.5 and modified manually to include additional information.
Supplementary Information
Acknowledgments
This research is supported by grants from the National Research Foundation (NRF–UID 103232), Technology Innovation Agency and the Durban University of Technology, Durban, South Africa (to SS). We thank K. Perry and R. Rajashankar for access to the NE-CAT beamline at the Advanced Photon Source. This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, funded by the NIH (P41 GM103403). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author contributions
S.S., L.T., B.R. conceived the project and designed the experiments; B.R. performed experiments; B.R., P.H.C. and L.T. carried out data collection and analysis, performed model building and structure refinement. S.S., L.T., K.P. and S.P. supervised the research and analyzed the data. B.R. drafted the paper L.T., S.S. and S.P. revised and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liang Tong, Email: ltong@columbia.edu.
Suren Singh, Email: singhs@dut.ac.za.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79489-3.
References
- 1.Luque-Almagro VM, Moreno-Vivian C, Roldan MD. Biodegradation of cyanide wastes from mining and jewellery industries. Curr. Opin. Biotechnol. 2016;38:9–13. doi: 10.1016/j.copbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Baxter J, Cummings SP. The current and future applications of microorganism in the bioremediation of cyanide contamination. Antonie Van Leeuwenhoek. 2006;90:1–17. doi: 10.1007/s10482-006-9057-y. [DOI] [PubMed] [Google Scholar]
- 3.Mudder TI, Botz MM. Cyanide and society: A critical review. Eur. J. Miner. Process. Environ. Prot. 2004;4:62–74. [Google Scholar]
- 4.Qian M, Eaton JW, Wolff SP. Cyanate-mediated inhibition of neutrophil myeloperoxidase activity. Biochem. J. 1997;326:159–166. doi: 10.1042/bj3260159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purcarea C, et al. Aquifex aeolicus aspartate transcarbamoylase, an enzyme specialized for the efficient utilization of unstable carbamoyl phosphate at elevated temperature. J. Biol. Chem. 2003;278:52924–52934. doi: 10.1074/jbc.M309383200. [DOI] [PubMed] [Google Scholar]
- 6.Stark GR. Modification of proteins with cyanate. Methods Enzymol. 1967;11:590–594. doi: 10.1016/S0076-6879(67)11074-4. [DOI] [PubMed] [Google Scholar]
- 7.Koshiishi I, Mamura Y, Imanari T. Cyanate causes depletion of ascorbate in organisms. Biochim. Biophys. Acta. 1997;1336:566–574. doi: 10.1016/S0304-4165(97)00073-1. [DOI] [PubMed] [Google Scholar]
- 8.Kraus LM, Kraus AP., Jr The search for the uremic toxin: The case for carbamoylation of amino acids and proteins. Wien Klin Wochenschr. 1998;110:521–530. [PubMed] [Google Scholar]
- 9.Palatinszky M, et al. Cyanate as an energy source for nitrifiers. Nature. 2015;524:105–108. doi: 10.1038/nature14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocap G, et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature. 2003;424:1042–1047. doi: 10.1038/nature01947. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PM, Sung Y-C, Fuchs JA. The cyanase operon and cyanate metabolism. FEMS Microbiol. Lett. 1990;87:247–252. doi: 10.1111/j.1574-6968.1990.tb04920.x. [DOI] [PubMed] [Google Scholar]
- 12.Kozliak EI, Fuchs JA, Guilloton MB. Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J. Bacteriol. 1995;177:3213–3219. doi: 10.1128/JB.177.11.3213-3219.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung YC, Parsell D, Anderson PM, Fuchs JA. Identification, mapping, and cloning of the gene encoding cyanase in Escherichia coli K-12. J. Bacteriol. 1987;169:2639–2642. doi: 10.1128/JB.169.6.2639-2642.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson WV, Anderson PM. Bicarbonate is a recycling substrate for cyanase. J. Biol. Chem. 1987;262:9021–9025. doi: 10.1016/S0021-9258(18)48040-4. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PM, Little RM. Kinetic properties of cyanase. Biochemistry. 1986;25:1621–1626. doi: 10.1021/bi00355a026. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PM. Purification and properties of the inducible enzyme cyanase. Biochemistry. 1980;19:2882–2888. doi: 10.1021/bi00554a010. [DOI] [PubMed] [Google Scholar]
- 17.Elleuche S, Pöggeler S. A cyanase is transcriptionally regulated by arginine and involved in cyanate decomposition in Sordaria macrospora. Fungal Genet. Biol. 2008;45:1458–1469. doi: 10.1016/j.fgb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Qian D, Jiang L, Lu L, Wei C, Li Y. Biochemical and structural properties of cyanases from Arabidopsis thaliana and Oryza sativa. PLoS ONE. 2011;6:1–10. doi: 10.1371/journal.pone.0018300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harano Y, et al. Identification and nitrogen regulation of the cyanase gene from the cyanobacteria Synechocystis sp. Strain PCC 6803 and Synechococcus sp. Strain PCC 7942. J. Bacteriol. 1997;179:5744–5750. doi: 10.1128/JB.179.18.5744-5750.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh MA, Otwinowski Z, Perrakis A, Anderson PM, Joachimiak A. Structure of cyanase reveals that a novel dimeric and decameric arrangement of subunits is required for formation of the enzyme active site. Structure. 2000;8:505–514. doi: 10.1016/S0969-2126(00)00134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butryn A, Stoehr G, Linke-Winnebeck C, Hopfner KP. Serendipitous crystallization and structure determination of cyanase (CynS) from Serratia proteamaculans. Acta Crystallogr. Sect. F. 2015;71:471–476. doi: 10.1107/S2053230X15004902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Madlala AM, Prior BA. Thermomyces lanuginosus: Properties of strains and their hemicellulases. FEMS Microbiol. Rev. 2003;27:3–16. doi: 10.1016/S0168-6445(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 23.Mchunu NP, et al. Xylanase Superproducer: Genome sequence of a compost-loving thermophilic fungus, Thermomyces lanuginosus Strain SSBP. Genome Announc. 2013;1:4–5. doi: 10.1128/genomeA.00388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winger AM, et al. Secretome analysis of the thermophilic xylanase hyper-producer Thermomyces lanuginosus SSBP cultivated on corn cobs. J. Ind. Microbiol. Biotechnol. 2014;41:1687–1696. doi: 10.1007/s10295-014-1509-1. [DOI] [PubMed] [Google Scholar]
- 25.Ranjan B, Pillai S, Permaul K, Singh S. Expression of a novel recombinant cyanate hydratase ( rTl-Cyn ) in Pichia pastoris, characteristics and applicability in the detoxification of cyanate. Bioresour. Technol. 2017;238:582–588. doi: 10.1016/j.biortech.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 26.Ranjan B, Pillai S, Permaul K, Singh S. A novel strategy for the efficient removal of toxic cyanate by the combinatorial use of recombinant enzymes immobilized on aminosilane modified magnetic nanoparticles. Bioresour. Technol. 2018;253:105–111. doi: 10.1016/j.biortech.2017.12.087. [DOI] [PubMed] [Google Scholar]
- 27.Ranjan B, Pillai S, Permaul K, Singh S. Simultaneous removal of heavy metals and cyanate in a wastewater sample using immobilized cyanate hydratase on magnetic-multiwall carbon nanotubes. J. Hazard. Mater. 2019;363:73–80. doi: 10.1016/j.jhazmat.2018.07.116. [DOI] [PubMed] [Google Scholar]
- 28.Lehninger A, Nelson D, Cox M. Principles of Biochemistry. New York: W.H. Freeman; 2005. [Google Scholar]
- 29.Dash RR, Gaur A, Balomajumder C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009;163:1–11. doi: 10.1016/j.jhazmat.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 30.Luque-Almagro VM, et al. Characterization of the Pseudomonas pseudoalcaligenes CECT5344 cyanase, an enzyme that is not essential for cyanide assimilation. Appl. Environ. Microbiol. 2008;74:6280–6288. doi: 10.1128/AEM.00916-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson PM, Johnson WV, Endrizzi JA, Little RM, Korte JJ. Interaction of mono- and dianions with cyanase: Evidence for apparent half-site binding. Biochemistry. 1987;26:3938–3943. doi: 10.1021/bi00387a029. [DOI] [PubMed] [Google Scholar]
- 32.Choi PH, et al. Structural and functional studies of pyruvate carboxylase regulation by cyclic di-AMP in lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 2017 doi: 10.1073/pnas.1704756114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Molecular basis for the role of oncogenic histone mutations in modulating H3K36 methylation. Sci. Rep. 2017;7:43906. doi: 10.1038/srep43906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi PH, et al. A distinct holoenzyme organization for two-subunit pyruvate carboxylase. Nat. Commun. 2016;7:12713. doi: 10.1038/ncomms12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otwinowski Z, Minor W, Mode O. Processing of X-Ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 36.McCoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 38.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.