Abstract
Recent results of high-altitude windborne mosquito migration raised questions about the viability of these mosquitoes despite ample evidence that many insect species, including other dipterans, have been known to migrate regularly over tens or hundreds of kilometers on high-altitude winds and retain their viability. To address these concerns, we subjected wild Anopheles gambiae s.l. Giles mosquitoes to a high-altitude survival assay, followed by oviposition (egg laying) and blood feeding assays. Despite carrying out the survival assay under exceptionally harsh conditions that probably provide the lowest survival potential following high altitude flight, a high proportion of the mosquitoes survived for 6- and even 11-h assay durations at 120- to 250-m altitudes. Minimal differences in egg laying success were noted between mosquitoes exposed to high altitude survival assay and those kept near the ground. Similarly, minimal differences were found in the female’s ability to take an additional bloodmeal after oviposition between these groups. We conclude that similar to other high-altitude migrating insects, mosquitoes are able to withstand extended high-altitude flight and subsequently reproduce and transmit pathogens by blood feeding on new hosts.
Keywords: altitude, disease-vector, egg-laying, long-range dispersal, wind
The recent report of windborne migrating mosquitoes at high-altitude (Huestis et al. 2019) marks a paradigm shift in our understanding of mosquito and pathogen dispersal. We follow the contemporary definition of insect migration as the persistent movement of individuals not driven by immediate cues for food, reproduction, or shelter, and which have a probability to land the migrator in a new environment suitable for survival and breeding (Gatehouse 1997, Dingle and Drake 2007, Chapman et al 2015). Many insect species, ranging in size from large locusts (Orthoptera; Acrididae), hoverflies (Diptera; Syrphidae), blackflies (Diptera; Simulidae), fruit flies (Diptera; Chloropidae), wheat midges (Diptera; Cecidomyiidae), and even minute Culicoides biting midges (Diptera; Ceratopogonidae) and aphids (Hemiptera; Aphididae) have been known to exploit high-altitude winds to migrate over tens or hundreds of kilometers (Johnson et al. 1962, Johnson 1969, Rainey 1973, Sellers 1980, Pedgley et al. 1995, Reynolds et al. 2006, Sanders et al. 2011, Miao et al. 2013, Wotton et al. 2019). However, despite anecdotal observations in support of similar migratory behavior (Glick 1939; Garrett-Jones 1950, 1962; Gillies and De Meillon 1968; Reynolds et al. 1996; Johansen et al. 2003), mosquitoes and especially malaria vectors were considered to migrate exclusively in the flight boundary layer, typically well below 10 m above ground level (agl) where the mosquito’s own flight is the key factor determining its speed and direction rather than the wind (Snow and Wilkes 1972, Gillies and Wilkes 1976, Snow 1982). Because high-altitude windborne migration in mosquitoes has long been considered accidental and thus of negligible significance (Service 1997), some vector biologists doubt the viability of the mosquitoes collected in altitude on grounds of desiccation pressure intensified by the wind speed.
In other high-altitude windborne migrant insects, questions about viability postmigration have been settled long ago by studies comparing survival and reproduction in a live collection of insects, including small Diptera (using nonsticky nets, at altitudes similar to our aerial sticky nets) with those captured on the ground or by simulated long flights (Taylor 1960, Cockbain 1961, Mcanelly and Rankin 1986). After finding similar survival and reproductive success, Taylor (1960) concluded that ‘This seems to establish the viability of high-level migrants beyond reasonable doubt’. The view that insect flight at high altitude is in itself harmful, and insects are subjected to physiological stresses not found in flight at low altitude, has become rare (Johnson 1969), at least among agricultural entomologists. This is partly due to small pest insects evidently migrating over very long distances and infesting crops on landing, such as the brown planthopper (Nilaparvata lugens [Hemiptera: Delphacidae]) which migrates about 700–1,000 km from eastern China to Japan every year (Rosenberg and Magor 1987). Evidence for the benefit of long-range windborne migration for the insect migrants has also recently come to light based on fourfold amplification of the spring migrants when compared with their returning offspring (Chapman et al. 2012).
Considering mosquitoes, specimens caught by aerial netting at altitude in China and India (Ming et al. 1993, Reynolds et al. 1996) were alive and active upon capture. Based on the distinct composition of the mosquito species, sexes, and female gonotrophic states at altitude compared with on the ground, Huestis et al. (2019) inferred that mosquitoes, like other insects (Drake and Reynolds 2012), deliberately ascend into the winds at altitude rather than being inadvertently ‘forced upwards’ by winds. For example, collections 100–290 m agl in Mali were dominated by secondary malaria vectors, e.g., Anopheles squamosus Theobald (Diptera: Culicidae) and Anopheles pharoensis Theobald (Diptera: Culicidae), whereas, on the ground using indoor collections, outdoor clay-pot traps, and larval collections in the vicinity of the same villages, >90% of Anopheles captured were Anopheles gambiae s.l. Giles. The difference between Anopheles coluzzii Coetzee & Wilkerson (Diptera: Culicidae) and Anopheles arabiensis (Patton) (Diptera: Culicidae) that share similar larval, biting, and resting sites (Toure et al. 1996, Lemasson et al. 1997, Lehmann and Diabate 2008, Dao et al. 2014) and are less affected by sampling bias, better demonstrates species-specific differences in high-altitude flight behavior because An. arabiensis has not been found at altitude. Additionally, aerial density of mosquitoes was higher when ground-level wind was slower (Huestis et al. 2019, Florio et al. 2020: Preprint).
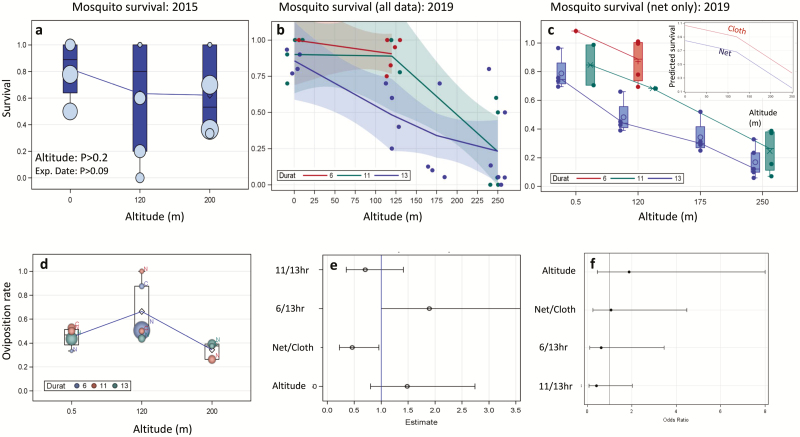
In addition to the exertion of sustained flight, presumably over several hours (Kaufmann and Briegel 2004, Huestis et al. 2019, Faiman et al. 2020: In Press), nightly high-altitude flight exposes mosquitoes to a combination of different temperatures, humidity (RH), and wind speeds than those conditions on the ground. Given the low sampling efficiency of mosquitoes at high altitude, evaluating the effects of these factors on their viability is not straightforward. In a preliminary analysis described in Huestis et al. (2019), survival of Anopheles gambiae s.l. collected indoors and placed individually, in modified 50-ml tubes (both ends covered with netting, Supp Fig. 1 [online only]) that were raised using the helium balloon to 120–190 m agl and subjected to wind passing through the tubes for 13 h was not statistically different from that of mosquitoes kept near the ground (altitude: 58%, n = 26 vs. ground: 71%, n = 17; P > 0.38, χ 21 = 0.75, Fig. 1a). Given that the mosquitoes at altitude were unable to ‘ride the wind’ but were tumbling against and abraded by the hard stretched net all night long, this assay provides the lowest survival limit of mosquitoes at altitude. Nonetheless, without a better alternative, here we utilized this conservative assay to measure the effect of altitude, duration of ‘flight’, and wind speed on the mosquito’s survival. Additionally, we evaluate her postflight capacity to lay eggs, and her ability to take another bloodmeal. Our new results, based on a larger sample size, demonstrate that mosquito migrants at high altitude can indeed survive, lay eggs, and thereafter take a new bloodmeal, thus enabling a new transmission encounter with the host after their migration.
Fig. 1.
The effect of altitude and assay duration on survival of An. gambiae s.l. mosquitoes. (a) The survival rates in July and October 2015 for 13-h assay duration described in Huestis et al. (2019); dot size signifies sample size per experimental date (lines connect the mean values) and statistical significance of altitude and Experimental date (Exp. Date) are shown (see text). (b) The survival rate in late October–November 2019 over different assay durations (pooling net and cloth covers of the tubes). (c) The survival rate (2019) over different assay times based on least square means (net only). Note: the least square mean survival of ground level for 6 h exceeds the maximum possible, reflecting the variance around the estimates of altitude and duration. Inset: The difference in survival between tubes covered with net (blue) or cloth (red) for the means of the 11- to 13-h exposure assays. (d) Oviposition rate (2019) among survivors from different altitudes, assay durations and net covers (N vs. C). (e) Odds ratios estimates (dot) and 95% CI of the probabilities to lay eggs between treatments denoted on the Y-axis based. Note: if the 95% CI intersects 1, the effect is not statistically significant. (f) Odds ratios estimates (dot) and 95% CI of the probability to blood feeding (after oviposition, see (e) above).
Materials and Methods
Study Location and Mosquitoes
This study was performed from October to November 2019 in Thierola, Mali (13°39′30.96″N, 7°12′52.92″W), a Sahelian village described previously (Lehmann et al. 2010, Dao et al. 2014). The area’s single wet season occurs between June and October (~550 mm). Rainfall is negligible (<50 mm) from November until May. By December, water is available only in deep wells.
Wild An. gambiae s.l. females were collected in human dwellings between 08:00 and 10:00 in Thierola and the neighboring villages (<7 km away) using mouth aspirators. Female mosquitoes were provided with 10% sucrose solution in cages covered with wet towels, which were kept in a typical village house used as a field insectary (without climate control). Blood-fed and semigravid female mosquitoes were housed in the field insectary until they reached the gravid state (up to 2 d) and could be subjected to the high-altitude survival assay. Females had access to 10% sugar solution until 2 h before the survival assay. Because of limited availability of wild gravid females during the end of the wet season, when this experiment was done, we prioritized filling the most informative group-treatments; as a result, the survival experiment was not fully balanced.
The High-Altitude Survival Assay
Fully gravid females were randomly assigned to different altitude exposure treatments varying between a) 1 and 290 m agl, b) assay duration of 6, 11, or 13 h, and c) high versus low air flow. Each female was individually placed in 5-cm long and 3-cm diameter tubes made by cutting 50-ml Falcon tubes (Supp Fig. 1 [online only]). To control the air flow through the tubes, the openings were covered with net (hole diameter = 1.5mm) or cloth (hole diameter = 0.2 mm, Supp Fig. 1 [online only]). Groups of mosquitoes were launched after sunset and retrieved around sunrise, except the 6-h duration group, which were either launched and retrieved between 18:00 and midnight or between midnight and 06:00 as previously described (Huestis et al. 2019). Five to ten tubes containing mosquitoes were mounted on the rope using adhesive tape (Supp Fig. 1 [online only]) in set altitudes 1, 120, 180 (160 and 190 pooled), and 250 (220–280 pooled) m from the ground. Mosquitoes mounted 1 m from the ground and those kept in insectary were used as controls. Upon retrieval, typically around 07:00, mosquitoes were examined for mobility and recorded as live (mobile) or dead (immobile) within 1 h after retrieval. Live mosquitoes were further subjected to oviposition assay.
Oviposition Assay
Surviving mosquitoes were individually transferred into 50-ml tube with 5-ml water for oviposition on the afternoon of the same day they completed the survival assay. Every morning, during four consecutive days, each tube was inspected for eggs. The number of eggs laid was estimated and their hatching was noted in the following days. Females that died during the oviposition assay were scored to produce zero eggs. Females that did not lay eggs by the end of the oviposition assay were killed and immediately dissected and their spermatheca examined to determine their insemination status. Their ovaries were also examined to determine whether they were gravid and the number of developed eggs in their abdomen were counted. Due to logistical constraints, not all females that did not lay eggs were dissected.
Blood Feeding Assay
Females which laid eggs were subjected to a blood feeding assay the following night. They were provided with water only (no sugar solution) until 22:00, when they were placed in a pint size cage, against a chicken’s breast (under the wing) of an immobilized chicken for 20 min in accord with animal care guidelines (F20-00465 MRTC). Immediately afterward, females were scored as fully fed, partly fed, or unfed.
At the end of the blood feeding assay or after female mosquitoes died naturally (or accidentally), they were preserved in 80% ethanol. The sibling species of the An. gambiae complex were identified as previously described (Fanello et al. 2002).
Statistical Analysis
Mosquito survival, oviposition, and subsequent blood feeding are dichotomous variables. Their corresponding fractions in each category was computed and plotted. To increase group size and the power of the statistical analyses, adjacent altitudinal panels were pooled together, e.g., 160 and 190 m were pooled together in a class of 175 and 220–280 m were similarly pooled into 250-m class. Likewise, we pooled groups of mosquitoes that were exposed to altitude between 18:00 and midnight with those that were exposed from midnight to 06:00 in the 6-h duration group. Contingency tables and log-likelihood tests were used to examine the relationship of each treatment separately on the dependent variables (survival, oviposition, and blood feeding), including stratification across an additional variable using Cochran-Mantel–Haenszel test (SAS Inc. 2012). Multivariate analysis of the survival rate of mosquitoes was carried out using Proc Mixed (SAS Inc. 2012) on the fraction of surviving mosquitos per treatment (combination of altitude, duration, cover type, and date). Date was introduced in the model as a random variable because it captures variation in temperature, wind speed, and RH (below). To evaluate the variation among species, the analysis was repeated with and without the species effect. Finally, the nightly weather parameters were introduced into the model. Multivariate analyses of oviposition (egg laying) and blood feeding were carried out using logistic regression carried out by Proc Logistic (SAS Inc. 2012). Weather data including hourly temperature, RH, wind speed, and direction at 2 and 180 m agl were extracted from atmospheric reanalyzes of the global climate ERA5 (Copernicus Climate Change Service 2018) as previously described (Huestis et al. 2019). Nightly means of each parameter from 18:00 to 07:00 (Supp Fig. 2 [online only]) at corresponding experimental nights were used as predictors of mosquito survival (Table 1).
Table 1.
Distribution of Anopheles gambiae s.l. across treatments and their survival rate
| Altitude | Duration (h) | Cover | N | Survival (%) |
|---|---|---|---|---|
| Ground | 6 | Net | 7 | 100 |
| Ground | 6 | Cloth | ND | ND |
| Ground | 11 | Net | 20 | 85 |
| Ground | 11 | Cloth | 20 | 95 |
| Ground | 13 | Net | 58 | 85 |
| Ground | 13 | Cloth | ND | ND |
| 120 m | 6 | Net | 127 | 91 |
| 120 m | 6 | Cloth | 10 | 100 |
| 120 m | 11 | Net | 9 | 78 |
| 120 m | 11 | Cloth | 10 | 100 |
| 120 m | 13 | Net | 33 | 49 |
| 120 m | 13 | Cloth | ND | ND |
| 175 m | 6 | Net | ND | ND |
| 175 m | 6 | Cloth | ND | ND |
| 175 m | 11 | Net | ND | ND |
| 175 m | 11 | Cloth | ND | ND |
| 175 m | 13 | Net | 65 | 19 |
| 175 m | 13 | cloth | ND | ND |
| 250 m | 6 | Net | ND | ND |
| 250 m | 6 | Cloth | ND | ND |
| 250 m | 11 | Net | 66 | 35 |
| 250 m | 11 | Cloth | 10 | 0 |
| 250 m | 13 | Net | 74 | 12 |
| 250 m | 13 | Cloth | 10 | 80 |
Due to insufficient numbers of mosquitoes not all combinations were carried out as indicated by ‘ND’ (see Materials and Methods).
Results
Survival After High-Altitude Exposure Assay
Over nine nights, a total of 519 wild An. gambiae s.l. females were subjected to the survival assay (Supp Fig. 1 [online only], see Materials and Methods) and maintained for 6 to 13 h in altitudes ranging from 1 to 280 m agl (Table 1). Because wind speed increases with altitude and the mosquitoes remain confined in their tubes against the wind, rather than fly almost stationary in relation to the parcel of air they would be carried by, we predicted that most stressful conditions occur in the longest assay at the highest altitude in tubes covered by net versus cloth.
Overall, the difference in survival due to altitude varied little between ground (91%, n = 105) and 120 m (84%, n = 188) but was large at higher altitudes (160–280 m: 25%, n = 225, Table 1). As expected, survival fell with exposure time: 92% (n = 144), 56% (n = 135), and 39% (n = 240) for 6, 11, and 13 h, respectively. Additionally, survival at altitude increased if the openings of the tubes were covered by a cloth of higher wind resistance (0.2-mm hole sizes: 78%, n = 60) compared with tubes covered by net of lower wind resistance (1.5-mm hole size: 56%, n = 411). However, because the experiments were not balanced, the similar survival rate between the ground and 120-m altitude compared with the lower survival at 160–280 m, probably was affected by the inclusion of short-duration exposure (6 h and to a lesser extent 11 h) at 120 m agl (Fig. 1b). To parse these effects, we analyzed them simultaneously using analysis of covariance with random variable (date) and fixed effects of duration, altitude, and net type. As expected, the results revealed that altitude, assay duration, and wind-resistance of the tube cover had significant effects on mosquito survival (P < 0.021, Table 2, Fig. 1c), whereas the variance among dates was nonsignificant (P < 0.067, Table 2). On average, 100-m increment of altitude was associated with 25% reduction in survival and an additional hour of the assay reduces survival by 6% (Table 1, Fig. 1c). Using higher wind resistant cover (cloth) over the tube’s opening instead of lower wind-resistant netting increased survival by 22% (Table 2, Fig. 1c, inset). The species composition at the time of the assay was dominated by An. coluzzii (76%), followed by An. arabiensis (14%) and An. gambiae s.s. (10%, n = 344 mosquitoes). The variation among the species in survival was not significant (P > 0.35, Table 2) and the other effects remained unchanged, indicating that the three Sahelian species responded similarly to the assay.
Table 2.
Summary of the results of the statistical models used to analyze survival, oviposition success, and blood feeding success
| Dep. Variable: Model (N) −2LL (Res)/AICa | Effectb | Fn:d/Z/Wc | P | Estimated |
|---|---|---|---|---|
| Survival: Mixed ANCOVA model (519), 8.2/12.2 | Altitude (m) | 67.21:26 | 0.0001 | −0.0025/m |
| Duration (h) | 16.01:26 | 0.0005 | −0.058/h | |
| Wind protection | 6.11:26 | 0.021 | −0.22 net vs. cloth | |
| Date [R] | 1.5 | 0.067 | 0.21 | |
| Residual [R] | 3.7 | 0.0001 | 0.28 | |
| Intercept | 1001:8 | 0.0001 | 1.77 | |
| Survival (with species): Mixed ANCOVA model (344), 53.9/57.9 | Altitude (m) | 47.01:68 | 0.0001 | −0.0026/m |
| Duration (h) | 1.41:68 | 0.0001 | −0.071/h | |
| Wind protection | 6.71:68 | 0.012 | −0.23 net vs. cloth | |
| Species | 1.12:68 | 0.35 | 0.065 S vs. M | |
| Date [R] | 1.6 | 0.057 | 0.38 | |
| Residual [R] | 5.8 | 0.0001 | 0.07 | |
| Intercept | 1001:8 | 0.0001 | 1.97 | |
| Survival (with weather): Mixed ANCOVA model (519), 15.6/17.6 | Altitude (m) | 66.41:32 | 0.0001 | −0.0025 |
| Duration (h) | 21.81:32 | 0.0001 | −0.049 | |
| Wind cover | 9.01:32 | 0.0053 | −0.278 | |
| RH | 6.71:32 | 0.0145 | 0.0037 | |
| Wind speed | 18.61:32 | 0.0001 | −00762 | |
| Residual [R] | 4 | 0.0001 | 0.029 | |
| Intercept | 1781:32 | 0.0001 | 1.91 | |
| Oviposition: Logistic regression (267) 361/368.5 Global Beta = 0: Wald = 9.13, P = 0.059 | Altitude (m) | 1.11 | 0.29 | −0.0022 (0.99) |
| Duration (h) | 4.71 | 0.029 | −0.10 (0.90) | |
| Wind protection | 2.51 | 0.11 | −0.27 (0.77) | |
| Intercept | 3.91 | 048 | 1.21 (3.34 | |
| Egg batch size: GLM ANCOVA (121), Global model: F3:117 = 1.8 P = 0.16, R2 = 0.043 | Altitude (m) | 0.31:116 | 0.58 | 0.05 |
| Duration (h) | 2.751:116 | 0.10 | 3.37 | |
| Wind protection | 2.651 | 0.11 | 21.4 | |
| Intercept | 3.761 | 0.055 | 54.4 | |
| Blood feeding: Logistic Regression (66) 88.4/97.3 Global Beta = 0: Wald = 2.03, P = 0.73 | Altitude (m) | 0.501 | 0.48 | −0.003 (0.99) |
| Duration (h) | 0.121 | 0.73 | 0.041 (1.04) | |
| Wind protection | 0.531 | 0.46 | 0.23 (1.26) | |
| Intercept | 0.0021 | 0.48 | 0.06 (1.06) |
aDependent variable and the statistical model used in the analysis; N denotes the number of mosquitoes used in the model. The residual −2 log likelihood value is followed by the Akaike information criterion (AIC). For logistic regression analyses, we provide the global Wald χ 2-test and P-value testing the null hypothesis that all effects are zero. For GLM, we list global model test and R2 values.
bIndependent variables, with random variable followed by [R]. ‘Wind protection’ refers to covering the tube with net versus cloth (see text).
c F-statistics with their corresponding numerator (n) and denominator (d) df for fixed effects and Z-statistics for random variables. The Wald χ 2-test for logistic regression is reported.
dEstimate for categorical variables compare the two categories, e.g., the estimated survival of An. gambiae s.s. (S) was higher by 6.5% than that of An. coluzzii (M), although the difference was not significant.
Oviposition of Mosquitoes That Survived High-Altitude Exposure Assay
To assess if gravid An. gambiae s.l. mosquitoes that withstood exposure to high altitude (above) were capable of laying eggs, they were kept with oviposition water for 4 d (see Materials and Methods). The water was examined daily for eggs during 4 d. Mosquitoes that did not lay eggs were dissected to determine insemination status. Overall, 46% of the 267 gravid females subjected to the assay laid eggs. However, 12% (n = 10) of 83 females that did not lay eggs and were dissected had no sperm and therefore could not lay eggs, thus indicating that overall oviposition rate among inseminated females was near 60% (the average egg batch size was 108 [n = 121, 95% CL = 97–119]). During October and November, oviposition rate and egg batch size of An. coluzzii are reduced (Yaro et al. 2012), and the observed values were similar to those observed previously during this time.
The effects of altitude, assay duration, and tube cover material on the likelihood of laying eggs were weak (Table 2, Fig. 1d and e). The effect of altitude was not significant (P > 0.2, Table 2, Fig. 1e). The effect of the assay duration was significant (P < 0.03, Table 2), but the 95% confidence limits of the highest odds ratio (6 versus 13 h) included 1 (Fig. 1e), questioning the significance of the effect. The effect of tube cover was statistically significant (P < 0.039, Table 2) and amounted for 32% higher egg laying probability compared with those housed in a net covered tube (Table 2). Considering egg batch size of females that laid eggs (excluding zeros, n = 121), the effects of altitude, assay duration, and tube cover material were all not significant (Table 2). For example, the mean egg batch size (and 95%CI) of females kept on the ground, at 120 and 200 m agl were 110.5 (92.9–128.0, n = 39), 108.9 (93.7–122.5, n = 66), and 101.7 (62.4–141.0, n = 16), respectively, and the largest egg batch size (340 eggs) was laid by a female kept at 200 m agl.
Blood Feeding of Mosquitoes That Survived High-Altitude Exposure Assay
To assess if gravid An. gambiae s.l. mosquitoes that withstood exposure to high altitude were capable of taking a bloodmeal after laying eggs, females were subjected to a blood feeding assay (see Materials and Methods). Overall, 56% of the 66 females subjected to the assay took a bloodmeal. Differences between treatments were minimal and statistically not significant (Table 2. Fig. 1f). The rates of blood feeding on the ground versus at altitude were 65% and 50%, respectively; at assay times of 6, 11, and 13 h were 52%, 50%, and 73%, respectively; and under net versus cloth were 58% and 50%, respectively.
Discussion
These experiments extend previous results obtained in 2015 (Huestis et al. 2019). In addition to larger sample size for the survival analysis, surviving mosquitoes were subjected to an oviposition assay followed by a blood feeding assay to evaluate the capacity of anopheline mosquitoes to survive the exposure to high altitude, and subsequently to lay eggs and take an additional bloodmeal. Despite carrying out these experiments during the transition from the wet to the dry season (October–November), after peak migration (Huestis et al. 2019), when RH decreases and wind speed increases – conditions that reduce mosquito survival (Clements 1992, Huestis and Lehmann 2014, Arcaz et al. 2016) and despite using an exceptionally harsh survival assay that arguably provides the lowest limit of survival following high altitude flight, a high proportion of the mosquitoes survived for 11-h assay duration. Furthermore, minimal differences in egg laying and in their ability to take another bloodmeal were found between mosquitoes exposed to high altitudes overnight and those near the ground. We conclude that similar to all other insect species that have been evaluated (Taylor 1960, Cockbain 1961, Mcanelly and Rankin 1986), mosquitoes are able to withstand high-altitude flight and subsequently reproduce and transmit pathogens by blood feeding on new hosts.
A close examination of the results reveals that under this conservative survival assay, females of An. coluzzii, An. Gambiae, and An. arabiensis can survive >13 h at high altitude. Highest survival (>90%) was shown when exposure was 6 h or up to 11 h at 120 m when the wind force was attenuated by a cloth (pores of 0.2-mm diameter) instead of a net (pores of 1.5-mm diameter). Mean nightly windspeed at 150 m agl (5–7 m/s) was more than fivefold greater than at 2 m agl (Supp Fig. 2 [online only]), and more than sevenfold at 250 m agl (7–9 m/s, not shown), explaining the harsh conditions mosquitoes experience in tubes covered by nets. The small pore size of the cloth allows rapid equilibration of temperature and RH with the surroundings, so the protective effect of the cloth operates solely by wind attenuation. This is also corroborated by the negative effect of altitude on survival because wind speed increases with altitude. The survival assay is extremely harsh because the mosquito is pummeled by strong wind against the rough surface of the stretched net, probably resulting in desiccation and physical damage that increase mortality. This effect does not occur in natural high-altitude flight when the mosquito is more or less stationary with respect to the air parcel it is carried in. Additionally, the 2019 experiments were performed in the transition between the wet (October) and the dry season (November) when nightly relative humidity drops from 80 to 30% (Supp Fig. 2 [online only]) and mean nightly windspeed at altitude increased during November to 9–10 m/s compared with 5–6 m/s in August–September (Supp Fig. 2 [online only]), during peak migration (Huestis et al. 2019). Importantly, even under these exaggerated, taxing conditions, 70% and 30% of the mosquitoes survived for 11 h at 120 or 250 m agl, respectively, compared with 90% at ground level.
The typical duration of mosquito windborne flight and whether it continues over multiple nights are presently unknown (Huestis et al. 2019). Tethered flight studies (Kaufman and Briegel 2004, Faiman et al. 2020: In Press) recorded total flight over a single night that exceeded 4 and even 10 h in members of the An. gambiae complex (and in An. atroparvus). Because these studies evaluated capacity of nightly flight without providing external cues such as headwind and visual movement of the background, Huestis et al. (2019) have conservatively estimated nightly flight to vary between 2 and 9 h and assumed that mosquitoes engage in windborne migration over a single night per lifetime. Future experiments using tethered flight may provide more direct answers on these questions.
Supplementary Material
Acknowledgments
We are grateful to the residents of Thierola for their permission to work near their homes and for their wonderful assistance and hospitality. We thank Drs. Sekou F Traore and Thomas Wellems, and Margie Sullivan, and Samuel Moretz (National Institutes of Health, USA) for logistical support. Dr. Malla Rao (NIH) has highlighted the importance of the question addressed by this study to the vector biology community in a discussion with Dr. Yan Guiyun (University of California, Irvine, CA). We thank Drs. Don Reynolds (University of Greenwich, United Kingdom) and Jason Chapman (University of Exeter, United Kingdom and Nanjing Agricultural University, China) for reading earlier versions of this manuscript and providing us with helpful suggestions. This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Grant: ZIA AI001196-06).
References Cited
- Arcaz A. C., Huestis D. L., Dao A., Yaro A. S., Diallo M., Andersen J., Blomquist G. J., and Lehmann T.. . 2016. Desiccation tolerance in Anopheles coluzzii: the effects of spiracle size and cuticular hydrocarbons. J. Exp. Biol. 219: 1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. W., Bell J. R., Burgin L. E., Reynolds D. R., Pettersson L. B., Hill J. K., Bonsall M. B., and Thomas J. A.. . 2012. Seasonal migration to high latitudes results in major reproductive benefits in an insect. Proc. Natl. Acad. Sci. U. S. A. 109: 14924–14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. W., Reynolds D. R., and Wilson K.. . 2015. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18: 287–302. [DOI] [PubMed] [Google Scholar]
- Clements A. N. 1992. The biology of mosquitoes. Chapman & Halll, London, United Kingdom. [Google Scholar]
- Cockbain A. J. 1961. Viability and fecundity of alate alienicolae of aphis fabae scop. after flights to exhaustion. J. Exp. Biol. 38: 181–187. [Google Scholar]
- Copernicus Climate Change Service. 2018. ERA5. (C3S) (2017): ERA5: Fifth generation of ECMWF atmospheric reanalyses of the global climate. Copernicus Climate Change Service Climate Data Store (CDS). (https://cds.climate.copernicus.eu/cdsapp#!/home) (accessed 6 March 2020). [Google Scholar]
- Dao A., Yaro A. S., Diallo M., Timbiné S., Huestis D. L., Kassogué Y., Traoré A. I., Sanogo Z. L., Samaké D., and Lehmann T.. . 2014. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature. 516: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle H., and Drake A.. . 2007. What is migration? Bioscience. 57: 113+. [Google Scholar]
- Drake V. A., and Reynolds D. R.. . 2012. Radar entomology : observing insect flight and migration. CAB International., Wallingford, United Kingdom. [Google Scholar]
- Faiman R., Yaro A. S., Diallo M., Dao A., Djibril S., Sanogo Z. L., Sullivan M., Krishna A., Krajacich B. J., and Lehmann T.. . 2020. Quantifying flight aptitude variation in wild A. gambiae s.l. in order to identify long-distance migrants. bioRxiv. 2020.03.03.975243. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanello C., Santolamazza F., and della Torre A.. . 2002. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 16: 461–464. [DOI] [PubMed] [Google Scholar]
- Florio J., Verú L., Dao A., Yaro A., Diallo M., Sanogo Z., Samaké D., Huestis D., Yossi O., Talamas E., . et al. 2020. Massive windborne migration of Sahelian insects: Diversity, seasonality, altitude, and direction. bioRxiv. 2020.02.28.960195. [Google Scholar]
- Garrett-Jones C. 1950. A dispersion of mosquitoes by wind. Nature. 165: 285. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. 1962. The possibility of active long-distance migrations by Anopheles pharoensis Theobald. Bull. World Health Organ. 27: 299–302. [PMC free article] [PubMed] [Google Scholar]
- Gatehouse A. G. 1997. Behavior and ecological genetics of wind-borne migration by insects. Annu. Rev. Entomol. 42: 475–502. [DOI] [PubMed] [Google Scholar]
- Gillies M. T., and De Meillon B.. . 1968. The Anophelinae of Africa south of the Sahara, 2nd edn.South African Institute for Medical Research, Johannesburg, South Africa. [Google Scholar]
- Gillies M. T., and Wilkes T. J.. . 1976. The vertical distribution of some West African mosquitoes (Diptera, Culicidae) over open farmland in a freshwater area of the Gambia. Bull. Entomol. Res. 66: 5. [Google Scholar]
- Glick P. A. 1939. The distribution of insects, spiders, and mites in the air, United States Dep. Agric. Tech. Bull., Washington, DC. [Google Scholar]
- Huestis D. L., and Lehmann T.. . 2014. Ecophysiology of Anopheles gambiae s.l.: persistence in the Sahel. Infect. Genet. Evol. 28: 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis D. L., Dao A., Diallo M., Sanogo Z. L., Samake D., Yaro A. S., Ousman Y., Linton Y. M., Krishna A., Veru L., . et al. 2019. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature. 574: 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. G. 1969. Migration and dispersal of insects by flight. Methuen, London, United Kingdom. [Google Scholar]
- Johnson C. G., Taylor L. R., and Southwood T. R. E.. . 1962. High altitude migration of Oscinella frit L. (Diptera: Chloropidae). J. Anim. Ecol. 31: 373. [Google Scholar]
- Johansen C. A., Farrow R. A., Morrisen A., Foley P., Bellis G., Van Den Hurk A. F., Montgomery B., Mackenzie J. S., and Ritchie S. A.. . 2003. Collection of wind-borne haematophagous insects in the Torres Strait, Australia. Med. Vet. Entomol. 17: 102–109. [DOI] [PubMed] [Google Scholar]
- Kaufmann C., and Briegel H.. . 2004. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. J. Vector Ecol. 29: 140–153. [PubMed] [Google Scholar]
- Lehmann T., and Diabate A.. . 2008. The molecular forms of Anopheles gambiae: a phenotypic perspective. Infect. Genet. Evol. 8: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T., Dao A., Yaro A. S., Adamou A., Kassogue Y., Diallo M., Sékou T., and Coscaron-Arias C.. . 2010. Aestivation of the African malaria mosquito, Anopheles gambiae in the Sahel. Am. J. Trop. Med. Hyg. 83: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson J. J., Fontenille D., Lochouarn L., Dia I., Simard F., Ba K., Diop A., Diatta M., and Molez J. F.. . 1997. Comparison of behavior and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera:Culicidae) in Barkedji, a Sahelian area of Senegal. J. Med. Entomol. 34: 396–403. [DOI] [PubMed] [Google Scholar]
- Mcanelly M. L., and Rankin M. A.. . 1986. Migration in the grasshopper Melanoplus sanguinipes (Fab.). II. Interactions between flight and reproduction. Biol. Bull. 170: 378–392. [Google Scholar]
- Miao J., Wu Y.-Q., Gong Z.-J., He Y.-Z., Duan Y., and Jiang Y.-L.. . 2013. Long-distance wind-borne dispersal of Sitodiplosis mosellana Géhin (Diptera:Cecidomyiidae) in Northern China. J. Insect Behav. 26: 120–129. [Google Scholar]
- Ming J. G., Jin H., Riley J. R., Reynolds D. R., Smith A. D., Wang R. L., Cheng J. Y., and Cheng X. N.. . 1993. Autumn southward ‘return’ migration of the mosquito Culex tritaeniorhynchus in China. Med. Vet. Entomol. 7: 323–327. [DOI] [PubMed] [Google Scholar]
- Pedgley D. E., Reynolds D. R., and Tatchell G. M.. . 1995. Long-range insect migration in relation to climate and weather: Africa and Europe, pp. 3– 30. InDrake V. A., Gatehouse A. G. (eds) Insect migration: tracking resources through space and time. Cambridge University Press, New York. [Google Scholar]
- Rainey R. C. 1973. Airborne pests and the atmospheric environment. Weather. 28: 224–239. [Google Scholar]
- Reynolds D. R., Smith A. D., Mukhopadhyay S., Chowdhury A. K., De B. K., Nath P. S., Mondal S. K., Das B. K., and Mukhopadhyay S.. . 1996. Atmospheric transport of mosquitoes in northeast India. Med. Vet. Entomol. 10: 185–186. [DOI] [PubMed] [Google Scholar]
- Reynolds D. R., Chapman J. W., and Harrington R.. . 2006. The migration of insect vectors of plant and animal viruses. Adv. Virus Res. 67: 453–517. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. J., and Magor J. I.. . 1987. Predicting windborne displacements of the brown planthopper, nilaparvata lugens from synoptic weather data. 1. Long-distance displacements in the North-East Monsoon. J. Anim. Ecol. 56: 39. [Google Scholar]
- Sanders C. J., Selby R., Carpenter S., and Reynolds D. R.. . 2011. High-altitude flight of Culicoides biting midges. Vet. Rec. 169: 208. [DOI] [PubMed] [Google Scholar]
- SAS Inc., I. 2012. SAS for Windows Version 9.4. SAS Inc., Cary, NC. [Google Scholar]
- Sellers R. F. 1980. Weather, host and vector–their interplay in the spread of insect-borne animal virus diseases. J. Hyg. (Lond). 85: 65–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service M. W. 1997. Mosquito (Diptera: Culicidae) dispersal–the long and short of it. J. Med. Entomol. 34: 579–588. [DOI] [PubMed] [Google Scholar]
- Snow W. F. 1982. Further observations on the vertical distribution of flying mosquitoes (Diptera: Culicidae) in West African savanna. Bull. Entomol. 72: 695–708. [Google Scholar]
- Snow W. E., and Wilkes T. J.. . 1972. The vertical distribution, and age, of mosquito populations in West African savanna. Trans. R. Soc. Trop. Med. Hyg. 66: 536–537. [PubMed] [Google Scholar]
- Taylor L. R. 1960. Mortality and viability of insect migrants high in the air. Nature. 186: 410.14412572 [Google Scholar]
- Toure Y. T., Traore S. F., Sankare O., Sow M. Y., Coulibaly A., Esposito F., and Petrarca V.. . 1996. Perennial transmission of malaria by the Anopheles gambiae complex in a north Sudan Savanna area of Mali. Med. Vet. Entomol. 10: 197–199. [DOI] [PubMed] [Google Scholar]
- Wotton K. R., Gao B., Menz M. H. M., Morris R. K. A., Ball S. G., Lim K. S., Reynolds D. R., Hu G., and Chapman J. W.. . 2019. Mass seasonal migrations of hoverflies provide extensive pollination and crop protection services. Curr. Biol. 29: 2167–2173.e5. [DOI] [PubMed] [Google Scholar]
- Yaro A. S., Traoré A. I., Huestis D. L., Adamou A., Timbiné S., Kassogué Y., Diallo M., Dao A., Traoré S. F., and Lehmann T.. . 2012. Dry season reproductive depression of Anopheles gambiae in the Sahel. J. Insect Physiol. 58: 1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.